Abstract
A three-step sequential extraction procedure, which was based on the BCR method, was used to analyse Guanabara Bay sediment samples to evaluate the potential mobility of some metals and metalloids. Zn and Cd were found to be the most labile metals because these were recovered in the first extraction stage and were associated with the non-residual fraction of the sediment. In contrast, Cr, Cu, Ni, and Hg were the least labile metals, and 62 to 84 % of the concentrations of these metals were found in the organic residual fraction of the sediment samples. The spatial distribution of the metal concentrations in Guanabara Bay led to the identification of various sources of contamination. The continental sources, which were related to Fe and Al, were distributed in the northern and central sectors, although lower concentrations were found in the mouths of the rivers that flow into the estuarine system of Guanabara Bay. The urban emission sources are related to the discharges of the São João de Merití River, which exhibit significant levels of Ba, Cd, Ni, Zn, and Hg. The São João de Merití River and harbour activities also show a predominance of Pb and Cu. The system formed by the Iguaçu River and the Sarapuí River is mainly characterised by V and Cr. The metals associated with biogeochemical processes, such as As and Mn, were found in the central sector of Guanabara Bay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal sediments are the main accumulation compartment of materials transported from continent to the marine environment, as demonstrated by trace metals of anthropogenic origin (Lacerda et al. 1992; Salomons and Förstner 1984). Although the role of estuarine systems as biogeochemical barriers in metal transport is evident, natural processes and human activities cause variations in the efficiency of the retention of these elements in coastal sediments (Machado et al. 2002a, b). Thus, understanding the behaviour of trace metals in sedimentary environments is necessary for the establishment of preventive measures against the deleterious effects of contamination by these potentially toxic elements, in order to best manage and even restore contaminated areas, particularly when seeking to prevent the remobilisation of already withheld metals (Perin et al. 1997).
Despite the development of pollution control plans, the conditions of the water quality and sediments in the Bay have worsened (JICA 1994). Large amounts of suspended solids, organic matter from sewage, organotin compounds and hydrocarbon (Kjerfve et al. 1997; Fernandez et al. 2005; Wagener et al. 2012), as well as metals (Pfeiffer et al. 1982; Rebello et al. 1986; de Souza et al. 1986; Rego et al. 1993; Perin et al. 1997; Wasserman et al. 2000; Machado et al. 2004; Kehrig et al. 2003; Kfouri et al. 2005; Vilela et al. 2004; Monteiro et al. 2011) are released into the bay and accumulate in the sediments (Table 1). Guanabara Bay has undergone an occupational process characterised by low investments in sewage treatment and industrial impact control.
In recent decades, a great variety of extraction schemes has been developed, and although some have been widely used, none has been unreservedly accepted by the scientific community (Fuentes et al. 2008). Consequently, the Community Bureau of Reference (BCR) began a programme in 1987 to harmonise the methodology utilised in the sequential extraction schemes used for the determination of metals in soils and sediments (Ure et al. 1993). Sahuquillo et al. (1999) recommend a sequential extraction protocol by using three fractions. The first fraction (F1) is the acid-soluble fraction. This fraction exhibits the most labile link to the sediment (Usero et al. 1998) and thus the most dangerous from the ecotoxicological point of view. The second fraction (F2) is the reducible fraction, which theoretically represents the contents of each metal bound to iron and manganese oxides that would be released if the sediment were subjected to more reductive conditions (Panda et al. 1995). The third fraction (F3), which is the oxidable fraction, shows the amount of metal bound to organic matter and sulphur that would be released into the environment if conditions became oxidative.
In order to evaluate the retention processes for metals Al, Ba, Cd, Cr, Cu, Mn, Ni, Pb, V, Zn, Hg, and arsenic from estuarine sediments collected from Guanabara Bay, the present study applies the BCR sequential extraction procedure. The multivariate statistical approach (PCA factor and cluster analyses) was adopted to assist the interpretation of the geochemical and anthropogenic sources of the studied elements. Combining geographical information system using the distribution of the principal components of the geochemical data permitted to integrate and identify pollution sources and to distinguish natural vs. anthropic contributions.
Environmental setting
The Guanabara Bay drainage system is inserted into the Guanabara rift which is part of a tertiary series of depressions in the south-eastern Brazilian coast, and its current configuration is linked to the Holocene sea-level variations (Suguio et al. 1985), which are conditioned by flooding of an ancient Pleistocene river valley (Amador 1997). Guanabara Bay is an eutrophic coastal bay with an area of approximately 384 km2, with a narrow entrance that measures 1.6 km, widening to 28 km in the inner bay (de Melo et al. 2014) and a drainage basin of approximately 4,080 km2 (Kjerfve et al. 1997). The cities around the Bay have approximately 11.7 million inhabitants and contain approximately 6,000 industries. There are a total of 35 rivers and streams of which six accounts for 85 % of the discharge into the bay. The net annual average discharge into the Bay is approximately 100 ± 59 m3 s−1, which values oscillating around 40 m3 s−1 in winter and 190 m3 s−1 in summer (Kjerfve et al. 1997). The sedimentation rates in Guanabara Bay increased during the last 500 year (Figueiredo et al. 2014), but particularly during the last 40 years, and enhanced by anthropogenic influence (Godoy et al. 1998; Figueiredo et al. 2014).
Methods
Sampling
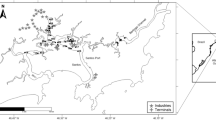
Considering the environmental features of Guanabara Bay ecosystem and taking into account its different areas, twenty-eight sampling stations were chosen (Fig. 1). 2-cm surface sediments were sampled with the help of scuba divers (Wagener et al. 2012) using a 7.5 cm diameter plexiglas core or using a specially designed van Veen sampler to avoid losses during sample retrieval (Wagener et al. 2012). Samples were stored in plastic bags, transported to the laboratory under refrigeration (ice bath), and maintained at 4 °C until analysis. The sediment collection was performed in two sampling campaigns (February 2006 and December 2006). The data sets from each sampling campaigns were statistically compared, and the average values for each station were used to obtain a more representative and concise environmental characterisation.
Sequential extraction procedure
Measurements of the elements were made in dry weight basis. In order to avoid volatilisation of susceptible elements, samples used for the extractions were dried at 40° C until constant weight is obtained. A sequential extraction procedure according to Sahuquillo et al. (1999) was used. Ultra-pure water obtained by a Elix and Synergy system (Millipore, Bedford, USA) was used throughout. All reagents used were of analytical grade and were employed without further purification. An aliquot of each sample was weighted (0.1 mg) directly in the extraction flasks. The extractions were performed in pre-cleaned low-density 100-mL polypropylene centrifuge tubes using an end-over-end mechanical shaker at room temperature (20–28 °C). Twenty millilitres of a 0.11 mol L−1 acetic acid solution was added to 1 g of the sediment sample in a 100-mL centrifuge tube. The mixture was shaken for 16 h at room temperature. The supernatant was separated from the solid residue by centrifugation (2,500 rpm, 10 min). This supernatant was stored in a polyethylene container at 4 °C until analysis. The residue was washed by adding 20 mL of water, shaken for 15 min, and centrifuged. The supernatant was discharged carefully to ensure that no solid residue was lost. The solid residue was submitted to a second extraction step, in which 40 mL of hydroxylamine hydrochloride (0.1 mol L−1) solution was added, and the extraction was performed as described above. The supernatant (containing the elements extracted in the second step) was separated and stored, and the residue was washed with water and further treated with 10 mL of an 8.8 mol L−1 hydrogen peroxide solution, which was carefully added in small aliquots to avoid losses due to a possibly violent reaction. The tube was covered with a small watch glass, and its contents were digested at room temperature for 1 h with occasional manual shaking. The digestion was continued by heating the covered tube for 1 h at 85 °C in a water bath; the cover was then removed, and the volume reduced to a few mL. An additional volume (5 mL) of the hydrogen peroxide solution (8.8 mol L−1) was then added. The tube was heated again (85 °C for 1 h) until the volume was reduced to 1 mL. Then, 50 mL of an ammonium acetate solution (1 mol L−1, pH 2) was added to the cool residue, which was extracted as described above. After that, the supernatant was separated and used by the determination of elements concentration extracted in the third step. Blank extractions using the same reagents were conducted throughout the complete procedure for each set of analyses.
Determination of major and trace element concentration in the extracts were performed by using an Ultima 2 sequential and radial view inductively coupled plasma optical emission spectrometer (ICP OES, Horiba Jobin–Yvon, Longjumeau, France), equipped with a cyclonic spray chamber and a MiraMist parallel path nebuliser (Mira Mist CE, Burgener Research Inc., Ontario, Canada). To arsenic and mercury determinations, the cyclonic spray chamber was replaced by Concomitant Metals Analyser (CMA, Horiba Jobin–Yvon, Longjumeau, France) for the generation of arsenic volatile hydride and to reduce the ionic mercury to Hg0, also volatile. For the generation of these volatile species, solutions of NaBH4 1 % w/v in NaOH 0.5 mol L−1 and HCl 6 mol L−1 were used. The certified reference material BCR-701® (Freshwater Lake Sediment) was used to validate the methodology and for quality control (Table 2).
Statistical analyses procedures
The software Statistica for Windows, version 8.0 (StatSoft, Inc., 2007) was utilised for the multivariate statistical analysis, and for descriptive and correlation analyses. The comparison of results between the two campaigns as inter-annual comparison was performed using the t test between groups. The probability used in the tests was p < 0.05. The correlation index data were calculated from the Pearson, considering the normality of the data employed.
Multivariate analysis was performed using the values of the sum of the concentrations of the elements in the three analysed fractions. As the metal concentrations exhibit different magnitudes, was performed standardization of the data [Standard value = (measured value − average value)/standard deviation values)]. This is important when using statistical analysis based on criteria of distance in order to minimise the influence values of different orders of magnitude. To the cluster analyses, grouping method employed was the method of Ward using the Euclidean distances. To make the results more easily interpretable, the PCA with Varimax normalised rotation was applied, maximising the variances of the factor loadings across variables for each factor. The maps of the four-principal factors were made based on the loading of principal components factorial analyses related to the (Varimax normalised).
Results
The statistics of the concentration results obtained for each extraction step and \(\sum\) F1 − F3 are shown in Table 3. The analysis of thèe average distributions of the metals in the different extraction fractions showed that the elements exhibit different behaviours.
Temporal changes in the distribution of the elements
The distribution of each element between the two sampling campaigns exhibited the same tendencies, with a high and statistically significant correlation coefficient (Table 4). No significant differences are found when comparing the average concentration of each element in the two different sampling campaigns. Low correlation values found in cadmium concentrations can be attributed to the differential distribution of this metal and can be explained by changes in the retention capacity and release of metals that may occur due to the sensitivity of cadmium compounds to redox conditions, seasonal changes, physical disturbances and the resuspension of sediments (Cooper and Morse 1998). Wagener et al. (2012) interpreted polycyclic aromatic hydrocarbon (PAH) concentrations in Guanabara Bay sediments as a variable in space and time. This random temporal variability was hypothesised to derive from the combined effect of constant inputs of different magnitudes, the weathering of light PAH, and the remobilisation by tidal currents, bioturbation and other mechanisms that facilitate sediment drift. Because cadmium is mainly linked to the more labile fraction, the mechanisms described by Wagener et al. (2012) likely promoted the high cadmium concentrations variability between the two sampling campaigns. Fonseca et al. (2013) observed that heavy metal concentration and fractionation in rainy and dry seasons present high seasonal influence on the heavy metal fractionation within the sediments of Guanabara Bay.
Behaviour and spatial distribution of elements
The results shown in Fig. 2 are obtained from the evaluation of the similarities in the distributions of the different elements in the different extraction fractions. These results show the various trends in the distributions of the elements according to different sources and processes that affect sediment scattering in the environment.
Al, Fe, V: metals linked to lithogenic source
The concentrations of aluminium, iron and vanadium are highly correlated, and their distribution patterns are related to their deposition in low-energy environments far from both the mouths of rivers and marine influences. The geochemical fractionation of aluminium showed a low contribution to the F1-exchangeable fraction with an average of approximately 4.85 ± 6.57 % and a maximum of 28.4 % in São João do Meriti river mouth (BG-36 station) (see Table 3; Fig. 3). The average contribution of Al in F2 fraction was approximately 31.8 %, and F3 fraction exhibited an average contribution of 63.4 %. The average sum of the three analysed fractions in the 28 sampling stations resulted in an average value of 2,581 ± 1,468 µg/g. The highest concentrations (5,200 and 4,685 µg g−1) were found in northwest sector, BG-31 and the BG-32 sampling stations, respectively, located in a low hydrodynamic area with low water circulation and high input of untreated sewage.
The prevalence of iron was also found to exhibit a high variability between the F2 and F3 fractions (oxide and organic fractions). The contribution of F1 was always low, with a relative average of 3.28 %. This relative value is significantly smaller than the average contributions obtained from F2 and F3, which were 50.8 and 45.9 %, respectively (Fig. 3). The sum of the three analysed fractions in the 28 sampling stations studied gave an average value of 12,660 ± 6,940 µg g−1. As observed with aluminium, the highest values (26,600 and 22,800 µg g−1) were found in the BG-31 and the BG-32 sampling stations, respectively (Fig. 3).
The geochemical fractionation of V has predominance observed in F2 fraction, which showed an average relative contribution of 78.8 %. The average contribution of F1 fraction was also low (5.78 %). The average V contribution of F3 fraction was 15.4 %. The sum of the three analysed fractions in the 28 sampling stations gave an average concentration of 14.8 ± 8.3 µg.g−1. As observed with Al, the highest concentrations (29.0 and 29.3 µg g−1) were obtained in the BG-37 sampling station in the mouth of the São João de Meriti River and the BG-19 sampling station in the central sector of Guanabara Bay, respectively (Fig. 3). De Melo et al. (2015) also observed high correlation between V and Fe in suspended sediments observed in central sector of Guanabara Bay.
Cr and Ba: metals linked to multiple sources
The distribution of Cr in Guanabara Bay appears to show multiple sources in different sectors of the bay mainly at the mouth of the Iguaçu river, with the exception of the north-eastern sector, which receives inputs from rivers with low anthropogenic impact that drain the Guapimirim conservation area and the area that communicates with the external bay sector. The fractionation of Cr was also different in different sedimentary environments, and the greater contribution, which exhibited less variability, was obtained from the F3 fraction. In contrast to the fractionation of Cd, the contribution of the F1 fraction to the Cr concentration showed a low average of 2.05 %. The F2 fraction exhibited a relatively high contribution of 31.8 %, and the F3 fraction presented the highest average value of 69.3 %. The largest sum of all of the fractions was observed at the following stations in the northwest sector of Guanabara Bay (BG-27, BG-28, BG-30, BG-31 and BG-32). The average Cr concentration distribution in the bottom sediment was 44.6 ± 46.1 µg g−1, and the maximum sum of the fractions (156 µg g−1) was observed at the BG-32 sampling station (Fig. 4).
Fractionation of Ba exhibited an average participation of the F1 fraction of 18.2 %. Contributions higher than 50 % were observed at the Guanabara Bay entrance sector (BG-02 and BG-03 sampling stations). The highest relative contribution (76.5 %) was observed in station in the mouth of the São João de Merití River (BG-37). This element was predominantly found in the F2 fraction, with an average contribution of 53.5 % and a maximum contribution of 86.2 % in the BG-08 sampling station. The F3 fraction showed a mean relative contribution of 28.2 %. The maximum relative value of 72.5 % was observed in the BG-14 station. The station with the highest sum of the three fractions (116 µg g−1) was the BG-06 at the Niterói Harbour sampling station, and the average values of barium considering the sum of the fractions were 24.8 ± 25.9 µg g−1 (Fig. 4).
As and Mn: elements linked to oxyhydroxides precipitation
The sum of the concentrations of As in the three analysed fractions from all of the sampling stations revealed an average value of 2.43 ± 1.59 µg g−1, and the highest value of 5.65 µg g−1 was found in the BG-16 station (Fig. 5). In addition, the As distribution exhibited an elevated and significant correlation coefficient with the Mn distribution (R = 0.76, n = 28, p < 0.05). The association is higher (R = 0.86, n = 26, p < 0.05) when the As values obtained in harbour areas that are high in As and low in Mn were subtracted. These observations show that there is a secondary source of As in harbour areas. In general, the geochemical fractionation of As was dominated by the F2 fraction, as was the case in the fractionations of V and Pb, with a relative average of 57.2 %. The relative contribution of the F1 fraction was 14.4 %, whereas the average relative contribution of F3 to the As concentration was 26.7 % (Fig. 5).
Mn exhibited a geochemical fractionation that was characterised by its predominance in the F1 fraction with an average relative contribution of 31.5 %. The highest relative contribution of this fraction was observed in the BG-27 station, which exhibited a maximum value of 58.3 %. The average relative contributions of the F2 and F3 fractions were 29.9 and 38.7 %, respectively. The highest concentration (1,309 µg g−1) of Mn was observed in the central sector (BG-27), which is most likely a sector with a high primary productivity that favours a high oxidative environment with an intense Mn precipitation. As observed in the Mn distribution, As was found in high concentrations in the central-north sector of Guanabara Bay.
These results demonstrate an association between As and Mn in the oxyhydroxide phase (F2 fraction), which supports the hypothesis that the distribution of As is correlated with the distribution of Mn in the central sector of Guanabara Bay. Moore et al. (1988) showed that the arsenic solid phase was predominantly controlled by manganese oxyhydroxide in oxidised zones. In Lake Biwa sediments (Japan), As is present at high concentrations in the upper layer and exhibits a depth profile similar to that of Mn. Adsorption experiments of As onto synthetic hydrous Mn oxide (HMO) in the presence of Mn2+ and the speciation of Mn in the sediment cores suggest that one of the most important factors that determine the accumulation of As at the sediment surface is its adsorption onto Mn2+-rich HMO (Takamatsu et al. 1985). This finding demonstrates the highly dependent biogeochemical processes that occur in the central sector of Guanabara Bay.
Cd, Zn, Ni and Hg: metals associated with the São João de Meriti River mouth
The fractionation of Cd exhibited a large relative contribution to the F1 fraction with an average value of 54.6 % and a mean concentration of 0.234 µg g−1 (Fig. 6). This result implies that this metal exhibits a general tendency for higher bioavailability compared with other metals. The concentration of Cd in the F1 fraction generally exceeded that in the F3 fraction. In addition, the highest relative value of 80.3 % (which corresponded to the highest absolute value of 0.375 µg g−1) in the F1 fraction was observed in the BG-10 sampling station in Governador Island. The BG-37 station at the mouth of the São João de Meriti River presented the highest concentration value of 0.869 µg g−1. The F2 fraction showed a mean of Cd concentration of 0.131 µg g−1 with a mean relative value of 31.8 % and was thus the second most important fraction for cadmium. The F3 fraction exhibited an average concentration of 13.7 % with an average absolute value of 0.054 µg g−1. The average value of the sum of the three fractions analysed was 0.419 ± 0.362 µg g−1, and the highest value (1.82 µg g−1) was found in the BG-37 sampling station located in the mouth of the São João de Meriti River (Fig. 6).
As previously shown for Cd, the Zn fractionation also exhibited a high concentration in the F1 fraction with an average relative contribution of 40.6 %, which indicates the high potential bioavailability of this metal. The oxide fraction (F2) exhibited an average relative contribution of 41.7 %. The average relative contribution of F3 (the organic fraction) was 17.7 %. The average sum of the three fractions was 145 ± 136 µg g−1, and the highest value of 691 µg g−1 was observed in the BG-37 sampling station (Fig. 6).
The analysis of the fractionation of Ni revealed that this metal was predominantly found in F3 with an average of 62.6 %. The contribution from the oxide fraction (F2) was low with a relative average of 21.2 %. It is noteworthy that the contribution of the F1 fraction, which corresponds to the phase that exhibits the greatest potential bioavailability, was more significant with a mean value of 18.4 % at the BG-37 site in the mouth of the São João de Meriti River. The sum of these fractions gave a value of 38.9 µg g−1. The average value of the sum of the three fractions in the 28 sampling stations was 8.61 ± 7.23 µg g−1 (Fig. 6).
The distribution of Hg is linked principally to the large contaminant entrance from northwest sector. The fractionation of Hg showed a dominance of the F3 fraction, which exhibited an average contribution of 83.7 % in all of the sampling stations. The contribution of the exchangeable weak-acid-soluble fraction (F1) was generally negligible with a mean value of 5.42 %. The contribution of the F2 fraction reached a relative mean value of 10.9 %. As observed by Baptista Neto et al. (2000) and Wasserman et al. (2000), the distribution of Hg in Guanabara Bay is mainly linked to the mouth of the João de Meriti River, which exhibits the highest value of 7.79 µg g−1 for the sum of the fractions (Fig. 7). The spatial distribution trends showed an increasing concentration at the mouth of the São João de Meriti River and high values in the Rio de Janeiro harbour area (BG-05 site) as observed by Baptista Neto et al. (2005, 2006). The sum of these three fractions (approximately 1.68 µg g−1) extends to the BG-08 station, which exhibited a high value of 1.33 µg g−1. The Niterói harbour presented high Hg concentrations with a maximum of 1.02 µg g−1. The distribution of Hg obtained in the present study shows concentrations that are similar to those obtained by Covelli et al. (2012) in the surface sediments of Guanabara Bay, which ranged from 0.10 to 3.22 µg g−1 (average of 0.917 ± 0.79 µg g−1, n = 47). However, the configuration obtained by Covelli et al. (2012) differs from that obtained in the present study, which used sampling stations closer to the mouth of the São João de Meriti River in Guanabara Bay (Fig. 7).
Cu and Pb: metals linked to the São João de Meriti River mouth and harbour areas
The geochemical fractionation of copper is highly variable with a predominance of the F3 fraction in most of the sampling sites. The mean contribution of F3 fraction to the Cu concentration was 62.5 %. An important exception was found in the BG-22 sampling station in Guapimirim protection area. In this station, a large contribution from the F1 fraction was observed (approximately 69.5 %). The sum of the three fractions reached the maximum values of 62.7 and 90.2 µg/g at the BG-32 and the BG-37 sampling stations, respectively, at the mouth of the São João de Meriti River. In the harbour area, sampling stations BG-05 and BG-06 exhibited maximum values of 77.4 and 72.1 µg g−1, respectively (Fig. 7).
Fractionation of Pb was usually dominated by the F2 fraction with mean values of approximately 81.2 %. The averaged relative contribution of the F1 fraction was very low (approximately 3.2 %), and the F3 fraction presented an average contribution of 15.6 %. Analysis of the sum of the concentrations of Pb in the three fractions shows that higher concentrations of this metal are found in the harbour sectors: 107 µg g−1 in the harbour of Rio de Janeiro (BG-05), 92.6 µg/g in the harbour of Niterói (BG-06) and 76.0 µg g−1 in the mouth of the São João de Meriti River (Fig. 7).
Discussion
The trace element contamination of Guanabara Bay has been widely recognised. It is therefore necessary to consider the mechanisms that are involved in the retention of these elements by the sediments to predict their behaviour and ultimately prevent their remobilisation. The sediments of this bay, such as those found in the mouth of the Iguaçu River (De Souza et al. 1986) and the São João de Meriti River (Rego et al. 1993), originate from a eutrophic water column that is influenced by the inflow of sewage effluents that favour the predominance of anaerobic conditions (Kjerfve et al. 1997; Carreira et al. 2002) and are associated with the massive inflow of materials from sources located at the urban and industrial watersheds of these areas. This process, which predominantly occurs in the northwest sector of the bay, represents the first factor obtained through the principal components analysis (PCA; Table 5; Figs. 8, 9). The first factor map represents the association of elements primarily at the mouth of the rivers of the northwest sector (São João de Meriti and Iguaçu/Sarapuí River system). The association between Cd, Zn, Ni was linked principally to the São João do Meriti River (Figs. 2, 6, 9); Hg and principally Cu, Pb also present a strong source in the Harbour areas (Figs. 2, 7, 9); Cr linked also to the mouth of Iguaçu River; V present a good correlation with a lithogenic elements, however, with high concentration in São João do Meriti mouth. The sources associated with these elements are the following: the mouth of the São João de Meriti River, harbours of Rio de Janeiro and Niterói, and the mouth of the Iguaçu/Sarapuí River system. These areas can be considered the most polluted with Cd, Zn, Ni and Hg (Fig. 6, 7) and thus became a hot spot of metal contamination due to the low amount of government investments for sewage treatment in the area. The São João de Meriti River and the Iguaçu River drain an area that is densely populated and exhibits reduced domestic sewage treatment, and the samplings station at the mouth of these rivers exhibited increased relative values for these metals, particularly in the most labile fraction. In contrast, mainly Cu, and Pb exhibited two main sources: the São João de Meriti River and the harbour areas of Rio de Janeiro and Niterói (Fig. 7). The very high concentrations of these elements in sewage sludge show that the lack of basic sanitation, in addition to the consequent discharge of “in natura” domestic sewage, may be an important source of Zn, Cu, and Hg in this environment (Pereira and Kuch 2005).
The Fe and Al concentrations measured at the sampling stations near the mouths of rivers presented low values, likely due to the dilution of quartz-rich sedimentary material from rivers that drain into Guanabara Bay because the large output regime of these rivers can transport quartz and coarse material. High factor loadings were observed for Al, Fe, Cr, and V and thus reveal an important association between these metals. This process is represented by the second factor obtained from the principal components analysis (Fig. 8; Table 5). The highest factor loadings obtained from the PCA can be attributed to the Fe input from the mouth of the Iguaçu River and Guapimirim River (Table 5). The third factor demonstrate an association between As and the oxides and hydroxides of manganese (F2), which reinforces the hypothesis that the As distribution is correlated with the manganese distribution in the middle sector of Baia de Guanabara Bay. In areas far from the mouths of the rivers with low hydrodynamics, the biological oxygen demand (generated by the decomposition of the large biomass in domestic sewage) probably maintains the manganese in solution, which limits its precipitation to the sediment. However, in the central sector of Guanabara Bay, the high water circulation and high productivity increase the amount of dissolved oxygen, which promotes the precipitation of manganese principally in the second sediment fraction. In a transect through a gradient between river stations and estuarine area, Fonseca et al. (2014) observed the highest concentrations of As in the estuarine area, while more elevated concentrations of the metals Cu, Pb, Cd, Zn, and Ni were found in the river stations (continental source). Many studies show association between As and Mn. In the lakes of Saskatchewan, which is a prairie province in Canada, Oscarson et al. (1981) found evidence that indicates that Mn is the primary sediment component responsible for the oxidation of different arsenic forms. In major French rivers/estuaries (the Rhône, Gironde, and Loire), Seyler and Martin (1990) interpreted that reduction and solubilisation of hydrous iron and manganese oxides that occur in the sediment/water interface and vice versa process can mobilise As and transport between these compartments through repeated cycles of deposition and resuspension. In a complex system as Guanabara Bay, biogeochemical process can be a major factor in the distribution of As.
The fourth factor, which is related to Ba, shows an increase in two distinct areas: in the mouth of the Guapimirim River and in the sector that is influenced by the harbour and shipyard region of Niterói. A decreasing gradient from these regions to the central sector, which corresponds to sampling stations BG-06, BG-35, and BG-08 was observed.
The identification of various pollution sources is in agreement with previous published study where different Pb isotope compositions were observed in two important tributaries, Iriri and Surui rivers, resulting from two active pollutants which are transported to the Guanabara Bay (Geraldes et al. 2006).
Conclusions
The results obtained in the present study verify that the distribution of the sum of the three extraction phases generally indicated a lack of enrichment of elements of environmental concern in the central sector of the Guanabara Bay. This result is in agreement with the literature and indicates that the trace element contamination is determined by river inflows. In addition to the contributions of the main contaminated rivers, the obtained results confirmed the trend that was previously observed and that the harbour areas of Guanabara Bay exhibit an increased level of various metals, such as Pb and Cu.
Various sources of contamination were identified from the spatial distribution of the metal concentrations in Guanabara Bay. The continental sources, which are related to Fe and Al, are distributed in the mouth of Macacu and mainly Iguaçu River in northern sector, although lower concentrations were found in the mouths of the rivers that flow into the estuarine system of Guanabara Bay. The sources from urban emissions are related to the river discharges mainly the São João de Meriti River, which exhibits significant levels of Ba, Cd, Ni, Zn, and Hg. The Pb and Cu levels in Guanabara Bay are attributable to urban emissions, which are associated with discharges from the São João de Merití River and harbour activities. The V and Cr sources were related to the Iguaçu/Sarapuí River system. The elements associated with biogeochemical processes in the central sector of Guanabara Bay, such as arsenic and manganese, appear to be conditioned by physicochemical processes. The precipitation of Mn may be associated with a high primary productivity, and this metal is likely co-precipitated with As.
Cd and Zn were predominantly found in the most labile fraction of the samples obtained from the mouth of the São João de Merití River and, consequently, the northwest sector of Guanabara Bay. This finding shows the high toxic potential of these metals. The amounts of Ba, Pb, V, and As in the F2 fraction, which is bound to oxides and hydroxides of iron and manganese, denote higher potential for toxicity when the sediment becomes reduced. In addition, Al, Cr, Cu, Ni, and Hg are linked to the more refractory fraction.
References
Amador ES (1997) Baía da Guanabara e Ecossistemas Periféricos: Homem e Natureza. Reproarte Gráfica e Editora, Rio de Janeiro
Baptista Neto JA, Smith BJ, Mcallister JJ (2000) Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba Sound, Southeast Brazil. Environ Poll 109:1–9
Baptista Neto JA, Crapez M, Mcalister JJ, Vilela CG (2005) Concentration and bioavailability of heavy metals in sediments from Niterói harbour (Guanabara Bay/S.E. Brazil). J Coastal Res 21:811–817
Baptista Neto JA, Gingele FX, Leipe T, Brehme I (2006) Spatial distribution of heavy metals in surficial sediments from Guanabara Bay: Rio de Janeiro, Brazil. Environ Geol 49:1051–1063
Barbosa MC, de Almeida MSS, Mariz DG, de Almeida JLDSS (2004) Studies of channel sediments contaminated with organics and heavy metals. J Hazar Mat 110:29–38
Borges AC, Dias JC, Machado W, Patchineelam SR (2007) Distribuição espacial de ferro, cobre e chumbo em sedimentos de manguezal em um gradiente de degradação na Baía de Guanabara (Estado do Rio de Janeiro). Quím Nova 30:66–69
Carreira RS, Wagener ALR, Readman JW, Fileman TW, Macko SA, Veiga A (2002) Changes in the sedimentary organic carbon pool of a fertilized tropical estuary, Guanabara Bay, Brazil: an elemental, isotopic and molecular marker approach. Mar Chem 79:207–227
Cooper DC, Morse JW (1998) Biogeochemical controls on trace metal cycling in anoxic marine sediments. Environ Sci Technol 32:327–330
Covelli S, Protopsalti I, Acquavita A, Sperle CM, Bonardi M, Emili A (2012) Spatial variation, speciation and sedimentary records of mercury in the Guanabara Bay (Rio de Janeiro, Brazil). Cont Shelf Res 35:29–42
De Melo GV, Baptista Neto JA, Malm O, Fernandez MAS, Patchineelam SM (2015) Composition and behaviour of heavy metals in suspended sediments in a tropical estuarine system. Environ Earth Sci 73:1331–1344
De Souza CMM, Pestana MHD, Lacerda LD (1986) Geochemical partitioning of heavy metals in sediments of three estuaries along the coast of Rio de Janeiro (Brazil). Sci Total Environ 58:63–72
Faria MAM, Sanchez BA (2001) Geochemistry and mineralogy of recent sediments of Guanabara Bay (NE sector) and its major rivers—Rio de Janeiro State—Brazil. An Acad Bras Ciênc 73:121–133
Fernandez MA, Wagener ALR, Limaverde AM, Scofield AL, Pinheiro FM, Rodrigues E (2005) Imposex and surface sediment speciation: a combined approach to evaluate organotin contamination in Guanabara Bay, Rio de Janeiro, Brazil. Mar Environ Res 59:435–452
Figueiredo AG Jr, de Toledo MB, Cordeiro RC, Godoy JMO, da Silva FT, Vasconcelos SC, dos Santos RA (2014) Linked variations in sediment accumulation rates and sea-level in Guanabara Bay, Brazil, over the last 6000 years. Palaeogeogr Palaeoclimatol Palaeoecol 415(2014):83–90
Fonseca EM, Baptista Neto JA, Silva CG, McAlister JJ, Smith BJ, Fernandez MA (2013) Stormwater impact in Guanabara Bay (Rio de Janeiro): evidences of seasonal variability in the dynamic of the sediment heavy metals. Estuar Coast Shelf Sci 130:161–168
Fonseca EM, Baptista Neto JA, Pereira MPS, Silva CG Arantes, Junior JD (2014) Study of pollutant distribution in the Guaxindiba Estuarine System—SE, Brazil. Mar Pollut Bull 82:45–54
Fuentes A, Lloréns M, Sáez J, Aguilar MI, Ortunõ JF, Meseguer VF (2008) Comparative study of six different sludges by sequential speciation of heavy metals. Bioresour Technol 99:517–525
Geraldes MC, Paula AH, Godoy JM, Valeriano CM (2006) Pb isotope signatures of sediments from Guanabara Bay, SE Brazil: evidence for multiple anthropogenic sources. J Geochem Explor 88:384–388
Godoy JM, Moreira I, Bragança MJ, Wanderley C, Mendes LB (1998) A study of Guanabara Bay sedimentation rates. J Radioanal Nucl Chem 227:157–160
JICA (1994) The study on recuperation of the Guanabara Bay ecosystem. Japan International Cooperation Agency, Tokio
Kehrig HA, Pinto FN, Moreira I, Malm O (2003) Heavy metals and methylmercury in a tropical coastal estuary and a mangrove in Brazil. Org Geochem 34:661–669
Kfouri PBP, Figueira RCL, Figueiredo AMG, Souza SHM, Eichler BB (2005) Metal levels and foraminifera occurrence in sediment cores from Guanabara Bay, Rio de Janeiro, Brazil. J Radioanal Nucl Chem 265:459–466
Kjerfve B, Ribeiro CHA, Dias GTM, Filippo AM, Quaresma VS (1997) Oceanographic characteristics of an impacted coastal bay: Baía de Guanabara, Rio de Janeiro, Brazil. Cont Shelf Res 17:1609–1643
Lacerda LD, Fernandez MA, Calazans CF, Tanizaki KF (1992) Bioavailability of heavy metals in sediments of two coastal lagoons in Rio de Janeiro, Brazil. Hydrobiologia 228:65–70
Machado W, Moscatelli M, Rezende LG, Lacerda LD (2002a) Mercury, zinc, and copper accumulation in mangrove sediments surrounding a large landfill in southeast Brazil. Environ Pollut 120:455–461
Machado W, Silva-Filho EV, Oliveira RR, Lacerda LD (2002b) Trace metal retention in mangrove ecosystems in Guanabara Bay, SE Brazil. Mar Pollut Bull 44:1277–1280
Machado W, Carvalho MF, Santelli RE, Maddock JEL (2004) Reactive sulfides relationship with metals in sediments from an eutrophicated estuary in southeast Brazil. Mar Pollut Bull 49:89–92
Monteiro FF, Cordeiro RC, Santelli RE, Machado W, Evangelista H, Villar LS, Viana LCA, Bidone ED (2011) Sedimentary geochemical record of historical anthropogenic activities affecting Guanabara Bay (Brazil) environmental quality. Environ Earth Sci 65:1661–1669
Moore JN, Flcklln WH, Johns C (1988) Partitioning of Arsenic and Metals in Reducing Sulfidic Sediments. Environ Sci Technol 22:432–437
Moraes RBC, Pfeiffer WC, Guimaraes JRD, Borges ALN, Campos AN (2000) Development of sediment toxicity test with tropical peneid shrimps. Environ Toxicol Chem 19:1881–1884
Oscarson DW, Huang PM, Liaw WK (1981) Role of manganese in the oxidation of arsenite by freshwater lake sediments. Clays Clay Miner 29:219–225
Panda D, Subramanian V, Panigrahy RC (1995) Geochemical fractionation of heavy metals in Chilka Lake (east coast of India)—a tropical coastal lagoon. Environ Geol 26:199–210
Pereira MS, Kuch B (2005) Heavy metals, PCDD/F and PCB in sewage sludge samples from two wastewater treatment facilities in Rio de Janeiro State, Brazil. Chemosphere 60:844–853
Perin G, Fabris R, Manente S, Rebello Wagener A, Hamacher C, Scotto S (1997) A five-year study on the heavy metal pollution of Guanabara Bay sediments (Rio de Janeiro, Brazil) and evaluation of the metal bioavailability by means of geochemical speciation. Water Res 12:3017–3028
Pfeiffer WC, Lacerda LD, Van Weerelt M, Carbonell N (1982) Chromium in water, suspended particles, sediments and biota in the Irajá River estuary. Environ Pollut 4:193–205
Rebello AL, Haekel W, Moreira I, Santelli R, Schroeder F (1986) The fate of heavy metals in an estuarine tropical system. Mar Chem 18:215–225
Rego VS, Pfeiffer WC, Barcellos CC, Rezende CE, Malm O, Souza CMM (1993) Heavy metal transport in the Acarí-São João de Merití river system, Brazil. Environ Technol 14:167–174
Sahuquillo A, López-Sánchez JF, Rubio R, Rauret G, Thomas RP, Davidson CM, Ure AM (1999) Use of a certified reference material for extractable trace metals to assess sources of uncertainty in the BCR three-stage sequential extraction procedure. Anal Chim Acta 382:317–327
Salomons W, Förstner U (1984) Metals in the hydrocycle. Springer, Berlin
Seyler P, Martin JM (1990) Distribution of arsenite and total dissolved arsenic in major French estuaries: Dependence on biogeochemical processes and anthropogenic inputs. Mar Chem 29:277–294
Suguio K, Martin L, Bittencourt ACSP, Dominguez JML, Flexor JM, Azevedo AEG (1985) Flutuações do nível relativo do mar durante o Quaternário superior ao longo do litoral brasileiro e suas implicações na sedimentação costeira. Rev Bras Geosci 15:273–286
Takamatsu T, Kawashima M, Koyama M (1985) The role of Mn2+-rich hydrous manganese oxide in the accumulation of arsenic in lake sediments. Water Res 19:1029–1032
Ure AM, Quevauviller PH, Muntau H, Griepink B (1993) Speciation heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of European Communities. Int J Environ Anal Chem 51:135–151
Usero J, Gamero M, Morillo J, Gracia I (1998) Comparative study of three sequential extraction procedure. Environ Int 24:487–496
Vilela CG, Batista DS, Baptista-Neto JA, Crapez M, Mcallister JJ (2004) Benthic foraminifera distribution in high polluted sediments from Niterói Harbor (Guanabara Bay), Rio de Janeiro, Brazil. An Acad Bras Ciênc 76:161–171
Wagener ALR, Meniconi MFG, Hamacher C, Farias CO, Silva GC, Gabardo IT, Scofield AL (2012) Hydrocarbons in sediments of a chronically contaminated bay: the challenge of source assignment. Mar Pollut Bull 64:284–294
Wasserman JC, Freitas-Pinto AAP, Amouroux D (2000) Mercury concentrations in sediment profiles of a degraded tropical coastal environment. Environ Technol 21:297–305
Acknowledgments
The authors are grateful to PETROBRAS for funding the Environment Assessment of Guanabara Bay Project, which was coordinated by CENPES. They also thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ Edição: Apoio a Grupos Emergentes de Pesquisa do Rio de Janeiro 2010 (Process number E-26/111.625/2011)) for financial support and fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cordeiro, R.C., Machado, W., Santelli, R.E. et al. Geochemical fractionation of metals and semimetals in surface sediments from tropical impacted estuary (Guanabara Bay, Brazil). Environ Earth Sci 74, 1363–1378 (2015). https://doi.org/10.1007/s12665-015-4127-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4127-y