Abstract
In this investigation the heavy metals (Cu, Zn, Mn, Cr and Ni) remediation potential of Eisenia fetida was studied in the crude oil polluted soil. The potential of E. fetida was evaluated based on the decrease in concentrations of Cu, Zn, Mn, Cr and Ni, and improvement in the soil enzyme activities at the end of 90 days of experimental trials. Moreover, soil health quality, inter-relationship between the enzyme activities and the growth parameters of E. fetida and synergistic relation among the enzyme activities were also evaluated through G-Mean and T-QSI indices, chord plot analysis and principal component analysis (PCA) to confirm the performance of E. fetida during vermiremediation. The results revealed that the soil treated with E. fetida showed a reduction in the concentration of Cu, Zn, Mn, Cr and Ni by 17.4% 19.45%, 9.44%, 23.8% and 9.6% respectively by end of the experimental trials. The cellulase, amylase, polyphenol oxidase, peroxidase, urease, dehydrogenase and catalase activities in the E. fetida-treated soil were enhanced by 89.83%, 99.17%, 142%, 109.9%, 92.9%, 694.3% and 274.5% respectively. The results of SEM-EDS revealed enhancement in the O, K, Na, Mg and P content by 62.36%, 96.2%, 97.9%, 93.7% and 98.2% respectively by the end of the experimental trial. The G-Mean and T-QSI indices also confirmed the improvement in soil enzyme activities thereby indicating the positive influence of E. fetida on soil decontamination process. The chord plot indicated the interrelationship between the earthworm’s growth parameters and enzyme activities of the soil as indicated by the high linkage between the nodes. Finally, the PCA confirmed the negative effect of the heavy metals on the soil enzyme activities and synergistic interrelationship between the enzyme activities during the vermiremediation process. Thus, this study demonstrated the changes in the soil enzyme activities and their interconnected influences during vermiremediation of crude oil sourced heavy metals from polluted soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crude oil sourced heavy metals (HMs) contamination is still a persistent problem that has received more attention globally. The HMs are the highly hazardous pollutants in crude oil-contaminated sites (Borah and Deka 2023) as it is associated with toxic organic and inorganic pollutants. The HMs are known to have a negative effect on the soil ecosystem and act as a major sink for other organisms. The HMs contamination already caused a devastating effect in the crude oil polluted agricultural lands, grazing fields, tea gardens and residual areas (Hussain Qaiser et al. 2019). Moreover, the HMs loaded crude oil can penetrate through the soil and deteriorates the underground water reservoirs and ultimately affects the food chain (Rashmi et al. 2020). In addition, the enhanced level of HMs such as Zn, Ni, Cr, Cu, Mn, Fe, Ag and others can inhibit activities of soil urease, phosphatase, dehydrogenase, cellulase and others by binding to its active sites and thus alter soil properties (Tang et al. 2020). Moreover, it has been reported that HMs interfere with the physiological and metabolic functions of soil microbes, which play a vital role in supporting the biogeochemical equilibrium in soil (Ameen and Al-Homaidan 2022; Zeng et al. 2020). Henceforth, looking at this disturbing scenario caused by crude oil associated HMs remediation strategies for HMs-contaminated soil have become an urgent necessity.
Several techniques including physical, chemical and biological are already in use for decontamination of crude oil and/or HMs from contaminated soil. However, these approaches have numerous limitations. For example, the physical and chemical methods are costly and generate hazardous waste byproduct and secondary pollution that can bring potential damage to the soil structure and ecosystem (Patel et al. 2020). Even, biological techniques such as phytoremediation although found to be promising are less effective when HMs concentration in soil exceeded the plant’s tolerance limits (Cleophas et al. 2022). In addition, slow growth and low biomass of the plant are also considered to be the major limiting factors for removal of toxic HMs during the phytoremediation process (Yan et al. 2020). When considering rhizoremediation, the effectiveness of HMs removal is inhibited by the extent and depth of the plant root system. In case of bacterial remediation generation of toxic intermediate forms limits the success of HMs decontamination in soil (Ghazal et al. 2022). Hence, effective and environmentally sound approaches are still required to tackle the HMs contamination problems in land.
At present, the vermiremediation that relies on the potential of earthworms to counter the HMs problem has received more attention globally as it is cost-effective, sustainable and possesses the ability to remove HMs from soil even at very high concentrations (Singh et al. 2022). Several studies have confirmed earthworm’s neutralization and accumulation abilities for HMs (Cheng et al. 2021) and thus help in mitigation of HMs issue in the contaminated lands. The earthworms make the HMs available for the metal remover microbes in the soil by their unique crawling and borrowing movement. Besides, the earthworm secret mucus and other enzymes which stimulate the microbes of the contaminated soil by promoting the mineralization of organic matters (Medina-Sauza et al. 2019). Moreover, the earthworms themselves have the ability to change the biological and physicochemical properties of the soil by improving the aeration of the soil (Xiao et al. 2022). The most commonly used earthworm in the vermiremediation of heavy metals is the Eisenia fetida. For example, Paul et al. (2018) employed E. fetida in HMs-contaminated soil and established the HMs removal efficacy of the earthworm in terms of detoxification and physico-chemical improvement in soil condition. Similarly, Ukalska-Jaruga et al. (2022) had also reported the HMs reduction by means of E. fetida. Therefore, E. fetida is an efficient earthworm to be used in the vermiremediation trials. Furthermore, the assessment of alterations in soil biological properties, specifically the enzyme activities, holds significance as they serve as the vital indicators of soil health (Adetunji et al. 2017). The connection between the soil enzyme activities and metal concentrations in soil is a crucial factor for evaluation of the effectiveness of vermiremediation techniques. This aspect still remains unexplored in the context of crude oil sourced HMs contamination in soil thereby emphasizing the need for further investigation.
Therefore, in this investigation it is aimed to bridge the gap in research leading to a scientific breakthrough and significant advancements in the field of HMs remediation in soil by employing earthworm. Here, the potential of E. fetida has been assessed to reduce the HMs content and enhancement in the biological properties, particularly enzyme activities, in crude oil-contaminated soil. The effectiveness of E. fetida in improvement of soil quality was further confirmed by employing indices like G-Mean and T-QSI, which provides a quantitative understanding of impact of the earthworm on soil enzyme profiles. Besides, the correlation matrix PCA was used to examine the synergistic interactions among soil enzyme activities, while a chord plot was used to illustrate the interrelationship between enzyme activities and E. fetida’s growth parameters during the remediation process.
Materials and methods
Collection of soil, cowdung and earthworm (Eisenia fetida)
The crude oil-contaminated soil samples were collected from the nearby agricultural land of Lakwa Oil fields in Sivasagar, Assam, India. The soil samples were neatly labeled and packaged in sterilized polythene bags for further processing. The soil samples were then carefully crushed and homogenized, air dried in the shade for use in the experimental trials. A 100g of processed soil was kept at 4 °C for analysis of initial physico-chemical parameters. The urine free fresh cowdung (7 days old) was collected locally from nearby farm of Gauhati University, Assam India. The cowdung was added to the experimental chambers as a bulking agent to the soil (Rich et al. 2018). The initial physicochemical properties of the soil and cowdung samples were also analyzed. The earthworm species E. fetida was collected from institutional vermicomposting station maintained by Assam Agricultural University, Kahikuchi, Guwahati, Assam, India. Stock cultures of the collected earthworms were maintained in laboratory to generate sufficient numbers of uniform size individuals for use in the experiment.
Experimental setup
The laboratory experiment was carried out in plastic chambers (18.5L×12W×4H). For drainage and aeration, perforations were built into the chamber’s bottom and lid. The experiment was carried out in crude oil-contaminated soil that contains Cu, Zn, Mn, Cr and Ni beyond permissible limits. The experimental plastic chambers were filled up with 100g of such crude oil-contaminated soil samples. The crude oil-contaminated soil with the application of E. fetida was denoted as T1, while the treatment without the implement of earthworm was denoted as T2. For effective monitoring of earthworm during vermiremediation process, ten individuals of Eisenia fetida were put in the experimental chambers (Rajadurai et al. 2022), with average lengths of 3 cm and biomass of 0.75g. Prior to the experimental trial, the experimental earthworms were starved for a day to allow the cleansing of their gut contents. Subsequently, the earthworms were placed within petri plates that were lined with moist filter paper, facilitating the removal of their gut contents. The filter paper underwent replacement after a 24-hour interval to prevent the earthworms from coprophagy. After that the earthworms were then allowed to acclimatize in the soil system to adapt the new habitat. The duration of the experiment was fixed for 90 days. The moisture levels in the experimental chamber were maintained at 40–60% by sprinkling sterilized distilled water. The experimental chambers were kept in the dark at ambient condition (temperature—22–25 °C and humidity—70%). As cowdung supports the initial microbial population for survival of the earthworms in adverse condition, therefore cowdung was blended with the contaminated soil at a ratio of 1:1 (Sohal et al. 2021). Three replicas were maintained, and average results were compared.
Heavy metals (Cu, Zn, Mn, Cr and Ni) assessments in soil
For the analysis of HMs (Cu, Zn, Mn, Cr and Ni) content in soil samples, the method outlined by Kotoky et al. (2003) and Borah and Deka (2023) was used with slight modifications. For the soil sample, in brief, 10 ml of HNO3, 6 ml of perchloric acid and 6 ml of hydrofluoric acid were added to 1 g of soil samples to digest them. The digested soil is then heated for around 4 hours in platinum crucibles in a fume hood at a temperature of 95–100 °C. Following the digestion, the acquired residues were filtered, and the total volume was then brought to 50ml in a volumetric flask using deionized water. The HMs content were then estimated using Atomic Absorption Spectrometer (AAS) (PerkinElmer, Pinaacle 900 series).
Assessment of soil enzyme activities
The activities of cellulase, phosphatase, catalase, urease, dehydrogenase, amylase, peroxidase and polyphenol oxidase were investigated in the soil samples. The soil cellulase activities were determined by employing the Pancholy et al. (1975) method. To measure the phosphatase activity, Tabatabai and Bremner’s (1969) method was applied. Additionally, soil catalase activity was measured using the Johnson and Temple (1964). Furthermore, the urease activities of the soil were determined by the Roberge (1978) approach. The dehydrogenase activities of the soil were examined based on decrease in the triphenyl formazan (TPF) from 2, 3, 5-triphenyltetrazolium chloride (TTC), and soil amylase activity was determined by using the starch hydrolysis procedure following the methods prescribed in Borah and Deka (2023). The method of Cao et al. (2018) was used to measure the soil’s peroxidase and polyphenol oxidase activities.
Geometric mean (G-Mean) and treated soil quality index (T-QSI)
The geometric mean (G-Mean) was applied to estimate the total enzymatic activity of the soil (Zhou et al. 2022). The analysis was carried out among all the studied soil enzymes that include cellulase, phosphatase, catalase, urease, dehydrogenase, amylase, peroxidase and polyphenol oxidase. The geometric mean (G-Mean) was computed as follows:
where, CELL, PHOS, CAT, URE, DEH, AMY, PERO and POLY.OXI are cellulase, phosphatase, catalase, urease, dehydrogenase, amylase, peroxidase and polyphenol oxidase respectively. This logarithm has been performed to understand the soil quality in different treatments.
The treated soil quality index (T-QSI) was performed to know the quality of the soil after spiking the soil with earthworms. The T-QSI was calculated using the following equation:
where, m denotes the reference soil, i.e. the mean value of enzymatic activity, which was set to 100%; n denotes the mean value for each enzyme activity in the earthworm-treated soil as percentages of the reference soil.
The T-QSI is basically used to measure the direction and magnitude (increase or inhibition) of changes caused by the environmental stresses (environmental contaminants) on soil enzyme activities compared those from a reference soil (Sanchez-Hernandez et al. 2018).
Growth parameters of Eisenia fetida
The growth parameters of Eisenia fetida were evaluated during the experimental trial. The parameters include survival rate (SR), growth rate (GR), mortality rate (MR), cocoon production rate (CPR), juvenile production rate (JPR). The evaluated parameters help in the selection of effective earthworm species in the removal of HMs from the soil (Koolivand et al. 2020).
Toxicity assay through seed germination
The HMs toxicity in the soil was evaluated with two metal sensitive seeds Brassica juncea (brown mustard) and Solanum lycopersicum (tomato). For the estimation of HMs toxicity, the soil extract was prepared with 0.5g of soil mixed in 5ml of deionized water. The extract was filtered through Whatman no. 1 filter paper. The filtrate thus obtained was then applied to the petri plates, where filter papers were already fixed. Deionized water was used as a control for comparison. Ten seeds in total were used in the experiment. The plates were incubated at a temperature of 25 °C (Baruah et al. 2019; Warman 1999). The moisture content of the papers was kept constant throughout the experiment by lightly sprinkling the plates with distilled water. After seven days of incubation, the relative seed germination (RSG), relative root growth (RRG) and germination index (GI) were computed by using the equations listed below
Scanning electron microscopy and energy dispersive spectroscopy (SEM-EDS) analysis
For the SEM-EDS analysis, the soil samples were dried at 80±2°C constantly so that all the moisture content was removed completely. The dried soil samples were then processed by initially fixing the samples on a metallic sample holder with the help of double-sided adhesive carbon tape. Then the fixation was followed by sample coating, which was done with gold through sputter coater for clear visibility of picture (Boruah et al. 2019). The micrographs of surface morphology of the samples were recorded at different magnification of scanning electron microscopy (Gemini, Sigma-300 series).
Principal component analysis (PCA) and chord plot analysis
To investigate the changing trends in enzyme activities and their interrelationships a principal component analysis was performed in OriginLab Software (Version: 2022), with the variables categorized according to the studied treatments. For PCA, the method as outlined by Ordoñez-Arévalo et al. (2018) was followed.
The chord plot was used to analyze the correlation of changing trend in the soil enzyme activities and Eisenia fetida’s growth parameters. The analysis was carried out by following the method of Zhou et al. (2022). The chord plot analysis was performed in OriginPro Software (Version: 2021), with the variables categorized according to the studied treatments.
Statistical analysis
The software used for the statistical comparison was SPSS (2018 version) and Origin Pro (2019). The values showed a significant difference in all the soil samples which was performed by one-way analysis of variance (ANOVA), paired t test and LSD test. The association between the soil biological activities and heavy metal concentrations was determined through bivariate correlations analysis. In each case the significance was accepted at p< 0.05.
Results and discussions
Heavy metals (Cu, Zn, Mn, Cr and Ni) dynamics in soil
The values of Cu, Zn, Mn, Cr and Ni concentrations in different treatments are presented in Table 1. The results revealed that there was a significant reduction (ANOVA, LSD, p ≤ 0.05) in the concentration of all the studied heavy metals in T1 when compared to the initial levels. Besides, there was a metal-wise variation in the concentrations in T1 at the end of the experimental period. In T1, the concentrations of Cu, Zn, Mn, Cr and Ni are found to be 152.7±1.0 mg/kg, 164.0±1.5 mg/kg, 365.1±1.3 mg/kg, 164.2±1.2 mg/kg and 132.4±1.5 mg/kg respectively at the beginning of the experiment, whereas, the values were recorded as 126.1±0.5 mg/kg, 132.1±1.5 mg/kg, 330.6±0.5 mg/kg, 125.1±0.5 mg/kg and 119.6±2.5 mg/kg, after treatment by E. fetida for Cu, Zn, Mn, Cr and Ni respectively. In case of T2, there was a marginal reduction in the HMs content of the soil, and the values were not statistically different (Table 1). The decrease in the Cu, Zn, Mn, Cr and Ni content in T1 could have been associated with several factors such as (i) ability of the earthworm’s gut microbes to tolerate and remove the HMs from the soil (Zhang et al. 2020); (ii) the ability of earthworms to alter the physical and chemical properties of the soil affecting the fractionation of metals (Cheng et al. 2021); (iii) bioaccumulation of Cu, Zn, Mn, Cr and Ni in their tissue (Parelho et al. 2021) and (iv) presence of metal binding proteins like metallothionein which helps in detoxification and biotransformation of the metals (Yuvaraj et al. 2021; Ekperusi et al. 2016). Moreover, the above results could be corroborated with previous studies of Shameema and Chinnamma (2018), where decrease in As, Cd, Cr, Pb, Hg, Ni, Zn and Cu contents in the range of 54.46–95.5% was found to be feasible by employing earthworms in the contaminated soil. Likewise, Ekperusi et al. (2016), reported about 60% decrease in the concentration of Zn, Mn, Cu, Ni, Cd, Cr, Pb, As, Hg from soil in 90 days of experimental trial. Further, the unique feeding nature and activities of E. fetida also in turn reduce the concentration of the HMs in soil (Cui et al. 2023; Xiao et al. 2022). Moreover, it has also been suggested that E. fetida has some cellular adaptations such as formation of inclusion bodies when metals bind to the nuclear proteins in their body and thus decreases the HMs concentration in the soil (Ekperusi et al. 2016). Further, the HMs content is directly proportional to the pH level of the soil (Borah and Deka 2023). Therefore, pH could be the other factor behind the higher reduction of Cu, Zn, Mn, Cr and Ni by E. fetida. Since, Eisenia fetida is known for its ability to improve soil conditions, it raises the soil’s pH level, which in turn could have reduced the concentrations of Cu, Zn, Mn, Cr and Ni in the soil. This happens because at higher pH level HMs become less soluble and highly immobile, helping to mitigate HM contamination in the soil (Xu et al. 2020). Finally, the marginal changes in Cu, Zn, Mn, Cr and Ni content in T2as revealed in this study could be attributed to the activities of the native microbial population (Feria-Cáceres et al. 2022) which could have decreased the HMs concentration in the soil during the experimental trial.
Changes in the soil enzymatic activities
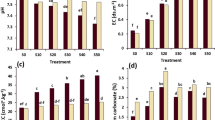
The results of the soil enzymes activities of treatments T1 and T2 have been presented graphically in Figs. 1, 2 and 3. The soil enzymes including cellulase, amylase, polyphenol oxidase, peroxidase, urease, dehydrogenase and catalase showed significant enhancement except phosphatase after the 90 days of experimental trial (ANOVA, LSD, p ≤ 0.05). In T1, the cellulase, amylase, polyphenol oxidase, peroxidase, urease, dehydrogenase and catalase activities of E. fetida-treated soil was enhanced by 63.6%, 65.25%, 86.23%, 65.49%,58.87%, 633.3% and 227.0% when compared with the initial levels. However, there was a contrasting result observed in case of phosphatase activities in the treatments, where the activities declined at the end of the experimental trial. However, no such significant changes were observed in T2.
The findings demonstrated that, E. fetida exhibited a remarkable ability to enhance the soil cellulase activities. This advantage could be attributed to its robust physiological capabilities that enable to endure oxidative stress which in turn amplifies the cellulase activities in the contaminated soil (Xiao et al. 2022) after treatment. Furthermore, several factors are linked with amelioration in amylase and catalase activities in the E. fetida applied contaminated soil. For example, the E. fetida possesses a distinctive capacity to absorb toxic heavy metals, as highlighted by Ukalska-Jaruga et al. (2022) which in turn increases the microbial population responsible for enhancement of associated enzyme activities. Additionally, E. fetida improves the aeration and porosity in soil (Xiao et al. 2022) which provides a conducive habitat for beneficial microorganisms to thrive that could also accelerate enzyme activities after the treatments (Cao et al. 2016). Moreover, the fragmentation in earthworm’s biomass and mineralization process could have contributed to the soil moisture and promoted microbial growth by breaking down organic matter into nutrient-rich components. This mineralization process could have also enriched the soil quality and triggers an enhancement in the activities of enzymes such as polyphenol oxidase and peroxidase. In addition, earthworm-associated mechanisms in the gut can enhance bacterial diversity and population, which raises the soil’s urease activity as seen in the cast that formed during the study period (Cao et al. 2017). Moreover, this increase in bacterial diversity and population may have stimulated catalase activity to keep the amount of H2O2 at its ideal level in heavy metal-contaminated soil (Xu et al. 2021). Dehydrogenase activity may rise as the earthworm and its active micro biomes speed up the metabolic process that produces ATP, whereas phosphatase activity may decrease as the earthworm increases the mineralization of organic phosphorous (Wang et al. 2021). As a whole, the application of E. fetida considerably enhanced the enzymatic activities in the heavy metal-contaminated soil thereby confirming the improvement in soil condition by end of the experimental trials.
G-Mean index and treated soil quality index (T-QSI)
The results of G-Mean index and treated soil quality index are presented in Fig. 4. The results of G-Mean index offer a comprehensive single numerical value for quantifying all the enzymatic activities. It was observed that the G-Mean values of soil enzyme activities prior to the addition of earthworms were considerably lower, with a mean value of 0.47±0.001. However, following the introduction of Eisenia fetida, the overall enzyme activities improved significantly, with values reaching up to 4.86±0.03. However, the G-Mean values of T2 suggested a marginal change at the end of the experiment (Fig. 4a). The augmentation in enzyme activities could be attributed to the presence of earthworm casts, which are rich in gut enzymes. The earthworm’s casts essentially served as a nutrient-rich substrate that could have promoted the proliferation of specific groups of microbes in the soil and enhance the overall enzymatic processes (Sanchez-Hernandez et al. 2018). The G-Mean index was performed in this research because it has reduced variability as compared to the individual enzyme activities, and hence it is considered to be more acceptable index for analyzing the soil quality than individual enzyme activities (Paz-Ferreiro and Fu 2016).
a G-Mean index of soil enzymes activities treated with earthworms in treatments T1 and T2. b T-SQI index of soil treated with E. fetida in the soil treatments T1, where T1= crude oil-contaminated soil with earthworm and T2= crude oil-contaminated soil without earthworm. Values are mean, n=3, error bars indicate SD. Different letters above error bars stand for significant differences in the values (ANOVA, LSD test, p≤0.05)
The treated soil quality index (T-QSI) was performed to understand the magnitude and direction of the changes caused by the earthworms’ behavior on the soil enzyme activities compared with those from a reference soil (control soil). The results revealed that there was a significant increase in the enzyme activities of the soil at the end of the experimental trial (ANOVA, LSD, p ≤ 0.05). The T-QSI values of soil in T1 employed with E. fetida were found to be enhanced by 4.6-fold (Fig. 4b). In accordance with the insights from Xu et al. (2021), earthworms cause alterations to soil enzyme activities via a dual-path approach. First, as earthworms burrow through the soil, they secreted substances that come into direct contact with the enzyme proteins. This interaction leads to either the stimulation or inhibition of these enzymes. This sequence of events creates a pathway denoted as “earthworm-secretions-enzyme,” where the earthworm’s secretions act as a direct mediator of enzyme behavior in soil. Secondly, the earthworm’s influence on soil dynamics involved the transformation of soil bacterial populations, culminating in shifts within secretion and metabolism processes. This phenomenon introduces an additional layer of complexity to the relationship between earthworms and enzyme activities, elucidating a pathway termed “earthworm-secretions-bacteria-enzyme” (Van Elsas et al. 2019).
Changes in the growth parameters of earthworms
The changes in the growth parameters such as growth rate (GR), survivability rate (SR), mortality rate (MR), cocoon production rate (CPR), juvenile production rate (JPR) of E. fetida were evaluated during the experimental trial. The results revealed that E. fetida showed a significant enhancement in the growth in all the studied parameters (ANOVA, LSD, p ≤ 0.05). The GR, SR, MR, CPR and JPR of E. fetida were found to be 189.1±0.2%, 80±1.5%, 20±1.2%, 12.40±1.5% and 16.5±2.0% respectively. The results shared a similarity with the previous findings of Lemtiri et al. (2016), where higher GR and SR were reported in case of E. fetida. These higher GR and SR of E. fetida could be due to their ability to respond to the stress condition and thereby increase the production of their offspring to offset the mortality. Furthermore, the reduced mortality rate observed in E. fetida could be linked to the earthworm’s physiological adaptation to the stress conditions exerted by crude oil sourced heavy metals contamination (Cheng et al. 2021). Moreover, previous workers such as Chachina et al. (2015) had also confirmed that E. fetida seems to be very resistant to oil contamination. The increased cocoon production rate and juvenile production rate in E. fetida could be linked to certain specific metals, such as zinc, which has been reported to stimulate cocoon production in the soil (Lemtiri et al. 2016).
Seed germination assay
The results of seed germination assay of Brassica juncea and Solanum lycopersicum represented as seed germination index (GI) for the treatments T1 and T2. The results revealed that the seeds of both the species (B. juncea and S. lycopersicum) germinated effectively in the soil treated with E. fetida. The initial GI for B. juncea and S. lycopersicum were recorded as 11.1±0.2% and 10.2±0.1% respectively. The GI value in T1 after the experimental trial was found to be 178.5±0.3 and 250.1±0.03 for B. juncea and S. lycopersicum respectively. The values for T2 were finally recorded as 150±0.01 for B. juncea, while it was found to be 161±0.15 in S. lycopersicum. Hence in T1 the GI values were enhanced by 13.5 and 16-folds in E. fetida-treated soil for B. juncea and S. lycopersicum respectively. Thus, the results showed an enhancement in the seed germination index in the contaminated soil after treatments by E. fetida. This improvement in the seed germination index could be attributed to various activities carried out by E. fetida, which ultimately improved the quality of the soil. These activities include enhancement in the soil structure and promotion of beneficial microorganisms and also foster a favorable environment for seed germination and plant growth (Gusain and Suthar 2020). Additionally, E. fetida, as demonstrated by Boruah et al. (2019), enhances soil nutrient levels, particularly nitrogen, phosphate and potassium which are the crucial factors for the seed germination. On the other hand, Al-Moaikal et al. (2012) suggested that inorganic contaminants like crude oil found in the soil can delay the seed emergence and hinder the seed aeration abilities, which could have been the reason for the reduced germination in T2.
Scanning electron microscopy and energy dispersive spectroscopy (SEM-EDS)
The results of SEM-EDS provide significant information about the surface profile of the soil samples before and after the implement of the earthworms. The surface morphology of the initial soil samples showed a compact mass and a robust structure without any pores and fragmentation in the soil. On the other hand, the soil samples after treating with E. fetida were converted to porous, fragmented and disintegrated structure as revealed in the SEM micrographs. The EDS profiling of the soil samples showed a significant variation in the composition of the soil during the experimental trial. The EDS of initial soil sample in T1 showed the weight percentage of C, O, K, Na, Mg and P as 57.65%, 20.38%, 0.38%, 0.27%, 0.41% and 0.19% while it was 4.78%, 54.15%, 10.17%, 13.37%, 6.59% and 13.65% at the end of the experiment respectively. Hence a significant variation was observed in the EDS profile of the soil samples in the treatment during the experimental trial.
These superficial changes in the soil structure could be attributed due to the crawling and grinding action by E. fetida which destructed the compact soil surface and produced a looser one. The granulated soil structures thus formed lead to an improved soil texture and better aeration of the soil. The SEM showed an increased in the surface area of the soil particle, which could decline the mobility of the heavy metals as suggested by Mallampati et al. (2013). Besides, the heavy metal’s fractions in the soil surface also got disturbed due to the broken soil particles; even the minerals hosting the metals also got distressed due to the fragmentation (Wang et al. 2020). Moreover, the porousness and coarseness soil help the native heavy metal-tolerant bacteria and other beneficial microbes to flourish freely which ultimately helps in the decontamination of the soil. The fragmented soil structures favor a strong binding force between the heavy metals and the HMs-tolerant bacteria (Sun et al. 2021). In addition, the microbial activities in the aerated soil thus enhance the nutrient profile of the soil such as Na, Mg, P and K as confirmed from the EDS analysis (Lai et al. 2022). The decrease in the carbon percentage at the end of the experiment advocates the reduction of hydrocarbon from the soil. The rise in the oxygen level in the soil leads to the formation of metal oxides like Mn oxides, thus converting the toxic metals to a non-toxic, non-bioavailable form. Again, the high oxygen level in the soil also proposes the formation oxygen functional groups such as carboxyl and hydroxyl groups resulting in more negative charges, and ultimately this may aid to the immobilization of heavy metals (Wang et al. 2019a, 2019b). The formation of phosphorus in the EDS suggests the metal phosphate formulation, which could lead to immobilization of the metal (Cao et al. 2020).
Synergistic changes of enzyme activities through PCA
The principal component analysis (Fig. 5) was carried out to determine the overall synergistic relationship between the enzymatic activities and the HMs content of the soil samples. Four distinct directions were used to conduct the analysis. The interactions between the enzymes and HMs content in T1 containing E. fetida are shown in Fig. 5. The variables were represented by the vectors pointing outwards from the origin. The length and proximity of the lines to the circle indicate the magnitude of variable representation in principal component analysis (Yano et al. 2019). The angle between the two variables can be used to estimate the correlation between them. A very small angle indicates a positive correlation between the vectors, whereas an angle of 90° indicates no correlation; additionally, an angle closer to 180° among the variables indicates a negative correlation (Jahirul et al. 2021).
Principal component analysis of T1 containing E. fetida. The enzymes and HMs content were considered variables of PCA; the size and angle of the vectors were used for the interpretation of synergistic interaction. Alphabets A=Cellulase, B=Amylase, C=Polyphenol oxidase, D=Peroxidase, E=Urease, F=Dehydrogenase, G=Catalase and H=Phosphatase respectively, while “I” denotes the average HMs content
The PCA studies revealed that, in T1 employed with E. fetida, the enzymes such as cellulase, amylase, polyphenol oxidase, peroxidase, urease, dehydrogenase and catalase are positively correlated as the lines lie in the same plot and are pointing toward the same direction with minimal angles, while the phosphatase enzyme along with the HMs content showed a negative correlation with the other studied enzymes and lies in the different plot. The positive correlation among various enzyme activities could be due to a collaborative action among related microbes. This shows that certain groups of enzymes tend to move in the same direction synergistically during an efficient remediation process. Moreover, this synergistic relation among the enzymes also leads to a speedy remediation of the heavy metals content by altering the oxidation state of the HMs in the soil (Munir et al. 2021). Besides, HMs show a strong negative effect on most of the enzymes except phosphatase. The negative effect of HMs on enzyme activities could be due to various factors such as (1) inhibitory effect of the HMs on enzyme activities which compete with its active site to form metal-organo-mineral complexes; (2) increasing HMs contents inside microbial cells cause protein denaturation of endoenzymes like dehydrogenase, urease and others; (3) the enzymes activities decrease with HMs content because of the changes of chemical conformation mainly due to coordination reaction. These factors lead to enzyme deactivation and thus show an antagonist effect toward the heavy metals content (Aponte et al. 2020).
Interrelation of enzyme activities and earthworm’s growth parameters through chord plot
To gain a deeper insight into the correlation between soil enzyme activities and earthworm growth parameters, chord plot analysis was performed, as depicted in Fig. 6. The circular arrangement of nodes on the chord plot represents the links between points connected through arcs, with the size of the arcs reflecting the assigned values of each connection (Zhou et al. 2022). The chord plot analysis for soil treated with E. fetida showed a significant link between the soil enzyme activities and earthworm’s growth parameters, which includes survivability rate, mortality rate, growth rate, cocoon production rate and juvenile production rate. This relationship highlighted how enzyme activities in the soil impact the health and dynamics of earthworm populations, influencing their survival, reproduction and overall well-being as evidenced by the high degree of interconnectedness between nodes across different parameters. The higher node data value of the chord plot among each parameter represents higher connectedness in case of E. fetida. The use of chord plot in this study facilitated the interpretation of data trends and patterns.
Practical implications of the study
The vermiremediation stands out as a promising choice for the decontamination of crude oil sourced HMs pollution in soil. Earthworms including E. fetida have high tolerance toward elevated heavy metals concentration and shown the ability to break apart the compacted soil aggregates formed by the waxy nature of crude oil contamination. Moreover, E. fetida, with its unique crawling movement, significantly improved soil aeration and fostered soil porosity, creating a favorable environment for the soil microbes. These soil microbes, in turn, played a crucial role in enhancing the soil quality by effectively mitigating the toxicity of heavy metals and hence promoted the enzymatic activities of the soil. All of which contributed to sustainable and overall well-being of the land. Thus, field application of E. fetida in the crude oil-contaminated land is a feasible approach to mitigate crude oil associated HMs burdens. Nevertheless, several factors such as massive field trials, analysis of ecosystem effects and services are crucial steps to be addressed prior to the practical implementation of this strategy in field environment.
Conclusion
The E. fetida was found to be effective for reduction of Mn, Zn, Ni, Cu and Cr concentrations in the crude oil-contaminated soil. Besides, E. fetida has also shown improvement in soil structure and enzyme activities in the oil-contaminated land and thus ameliorates the soil quality indices. The PCA established significant synergistic interactions among soil enzyme activities and heavy metals, while chord plot showed a complex inter-relationship between the enzyme’s activities and the earthworms’ growth parameters. Finally, the toxicity assay has confirmed a higher germination index in the E. fetida-treated soil, indicating the positive impact of the earthworm in decreasing heavy metals contents in soil after treatments (Fig. 7).
Data availability
The data reported in the current study have been obtained in original upon experimentation. The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adetunji AT, Lewu FB, Mulidzi R, Ncube B (2017) The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: a review. J Soil Sci Plant Nutr 17(3):794–807. https://doi.org/10.4067/S0718-95162017000300018
Al-Moaikal RMS, Shukry WM, Azzoz MM, Al-Hawas GHS (2012) Effect of crude oil on germination, growth and seed protein profile of Jojoba (Simmodsiachinensis). Plant Sci J 1:20–35. https://doi.org/10.13140/2.1.3537.4087
Ameen F, Al-Homaidan AA (2022) Treatment of heavy metal–polluted sewage sludge using biochar amendments and vermistabilization. Environ Monit Assess 194(12):861. https://doi.org/10.1007/s10661-022-10559-x
Aponte H, Meli P, Butler B, Paolini J, Matus F, Merino C et al (2020) Meta-analysis of heavy metal effects on soil enzyme activities. Sci Total Environ 737:139744. https://doi.org/10.1016/j.scitotenv.2020.139744
Baruah N, Mondal SC, Farooq M, Gogoi N (2019) Influence of heavy metals on seed germination and seedling growth of wheat, pea, and tomato. Water Air Soil Pollut 230(12):1–15. https://doi.org/10.1007/s11270-019-4329-0
Borah G, Deka H (2023) Crude oil associated heavy metals (HMs) contamination in agricultural land: understanding risk factors and changes in soil biological properties. Chemosphere 310:136890. https://doi.org/10.1016/j.chemosphere.2022.136890
Boruah T, Barman A, Kalita P, Lahkar J, Deka H (2019) Vermicomposting of citronella bagasse and paper mill sludge mixture employing Eisenia fetida. Bioresour Technol 294:122147. https://doi.org/10.1016/j.biortech.2019.122147
Cao J, Wang C, Ji D (2016) Improvement of the soil nitrogen content and maize growth by earthworms and arbuscular mycorrhizal fungi in soils polluted by oxytetracycline. Sci Total Environ 571:926–934. https://doi.org/10.1016/j.scitotenv.2016.07.077
Cao X, Bi R, Song Y (2017) Toxic responses of cytochrome P450 sub-enzyme activities to heavy metals exposure in soil and correlation with their bioaccumulation in Eisenia fetida. Ecotoxicol Environ Saf 144:158–165. https://doi.org/10.1016/j.ecoenv.2017.06.023
Cao X, Cai C, Wang Y, Zheng X (2018) The inactivation kinetics of polyphenol oxidase and peroxidase in bayberry juice during thermal and ultrasound treatments. Innovative Food Sci Emerg Technol 45:169–178. https://doi.org/10.1016/j.ifset.2017.09.018
Cao X, Ma R, Zhang Q, Wang W, Liao Q, Sun S et al (2020) The factors influencing sludge incineration residue (SIR)-based magnesium potassium phosphate cement and the solidification/stabilization characteristics and mechanisms of heavy metals. Chemosphere 261:127789. https://doi.org/10.1016/j.chemosphere.2020.127789
Chachina SB, Voronkova NA, Baklanova ON (2015) Biological remediation of the engine lubricant oil-contaminated soil with three kinds of earthworms, Eisenia fetida, Eisenia andrei, Dendrobenaveneta, and a mixture of microorganisms. Procedia Eng 113:113–123. https://doi.org/10.1016/j.proeng.2015.07.302
Cheng Q, Lu C, Shen H, Yang Y, Chen H (2021) The dual beneficial effects of vermiremediation: reducing soil bioavailability of cadmium (Cd) and improving soil fertility by earthworm (Eisenia fetida) modified by seasonality. Sci Total Environ 755:142631. https://doi.org/10.1016/j.scitotenv.2020.142631
Cleophas FN, Zahari NZ, Murugayah P, Rahim SA, Mohd Yatim AN (2022) Phytoremediation: a novel approach of bast fiber plants (hemp, kenaf, jute and flax) for heavy metals decontamination in soil. Toxics 11(1):5. https://doi.org/10.3390/toxics11010005
Cui J, Cui J, Li J, Wang W, Xu B, Yang J et al (2023) Improving earthworm quality and complex metal removal from water by adding aquatic plant residues to cattle manure. J Hazard Mater 443:130145. https://doi.org/10.1016/j.jhazmat.2022.130145
Ekperusi OA, Aigbodion IF, Iloba BN, Okorefe S (2016) Assessment and bioremediation of heavy metals from crude oil contaminated soil by earthworms. Ethiop J Environ Stud Manag 9(2):1036-1046.ISSN:1998-0507. https://doi.org/10.4314/ejesm.v9i2.9S
Feria-Cáceres PF, Penagos-Velez L, Moreno-Herrera CX (2022) Tolerance and cadmium (Cd) immobilization by native bacteria isolated in cocoa soils with increased metal content. Microbiol Res 13(3):556–573. https://doi.org/10.3390/microbiolres13030039
Ghazal H, Koumaki E, Hoslett J, Malamis S, Katsou E, Barcelo D, Jouhara H (2022) Insights into current physical, chemical and hybrid technologies used for the treatment of wastewater contaminated with pharmaceuticals. J Clean Prod 361:132079. https://doi.org/10.1016/j.jclepro.2022.132079
Gusain R, Suthar S (2020) Vermicomposting of duckweed (Spirodelapolyrhiza) by employing Eisenia fetida: changes in nutrient contents, microbial enzyme activities and earthworm biodynamics. Bioresour Technol 311:123585. https://doi.org/10.1016/j.biortech.2020.123585
Hussain Qaiser MS, Ahmad I, Ahmad SR, Afzal M, Qayyum A (2019) Assessing heavy metal contamination in oil and gas well drilling waste and soil in Pakistan. Pol J Environ Stud 28(2). https://doi.org/10.15244/pjoes/85301
Jahirul MI, Rasul MG, Brown RJ, Senadeera W, Hosen MA, Haque R, Saha SC, Mahlia TMI (2021) Investigation of correlation between chemical composition and properties of biodiesel using principal component analysis (PCA) and artificial neural network (ANN). Renew Energy 168(632–646):483. https://doi.org/10.1016/j.renene.2020.12.078
Johnson JI, Temple KL (1964) Some variables affecting the measurement of catalase activity in soil. Soil Sci Soc Am Proc 28:207–216. https://doi.org/10.2136/sssaj1964.03615995002800020024x
Koolivand A, Saeedi R, Coulon F, Kumar V, Villaseñor J, Asghari F, Hesampoor F (2020) Bioremediation of petroleum hydrocarbons by vermicomposting process bioaugmentated with indigenous bacterial consortium isolated from petroleum oily sludge. Ecotoxicol Environ Saf 198:110645
Kotoky P, Bora BJ, Baruah NK, Baruah J, Baruah P, Borah GC (2003) Chemical fractionation of heavy metals in soils around oil installations, Assam. Chem Speciat Bioavailab 15(4):115–126. https://doi.org/10.3184/095422903782775181
Lai W, Wu Y, Zhang C, Dilinuer Y, Pasang L, Lu Y et al (2022) Combination of biochar and phosphorus solubilizing bacteria to improve the stable form of toxic metal minerals and microbial abundance in Lead/Cadmium-contaminated soil. Agronomy 12(5):1003. https://doi.org/10.3390/agronomy12051003
Lemtiri A, Liénard A, Alabi T, Brostaux Y, Cluzeau D, Francis F, Colinet G (2016) Earthworms Eisenia fetida affect the uptake of heavy metals by plants Vicia faba and Zea mays in metal-contaminated soils. Appl Soil Ecol 104:67–78. https://doi.org/10.1016/j.apsoil.2015.11.021
Mallampati SR, Mitoma Y, Okuda T, Sakita S, Kakeda M (2013) Total immobilization of soil heavy metals with nano-Fe/Ca/CaO dispersion mixtures. Environ Chem Lett 11:119–125. https://doi.org/10.1007/s10311-012-0384-0
Medina-Sauza RM, Álvarez-Jiménez M, Delhal A, Reverchon F, Blouin M, Guerrero-Analco JA et al (2019) Earthworms building up soil microbiota, a review. Front Environ Sci 7:81. https://doi.org/10.3389/fenvs.2019.00081
Munir MAM, Yousaf B, Ali MU, Dan C, Abbas Q, Arif M, Yang X (2021) In situ synthesis of micro-plastics embedded sewage-sludge co-pyrolyzed biochar: implications for the remediation of Cr and Pb availability and enzymatic activities from the contaminated soil. J Clean Prod 302:127005. https://doi.org/10.1016/j.jclepro.2021.127005
Ordoñez-Arévalo B, Guillén-Navarro K, Huerta E, Cuevas R, Calixto-Romo MA (2018) Enzymatic dynamics into the Eisenia fetida (Savigny, 1826) gut during vermicomposting of coffee husk and market waste in a tropical environment. Environ Sci Pollut Res 25:1576–1586
Pancholy SK, Rice EL, Turner JA (1975) Soil factors preventing revegetation of a denuded area near an abandoned zinc smelter in Oklahoma. J Appl Ecol 12(1):337–342 https://www.jstor.org/stable/2401736
Parelho C, Rodrigues A, do Carmo Barreto M, Cruz JV, Rasche F, Silva L, Garcia P (2021) Bioaccumulation and potential ecotoxicological effects of trace metals along a management intensity gradient in volcanic pasturelands. Chemosphere 273:128601. https://doi.org/10.1016/j.chemosphere.2020.128601
Patel AB, Shaikh S, Jain KR, Desai C, Madamwar D (2020) Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Front Microbiol 11:562813. https://doi.org/10.3389/fmicb.2020.562813
Paul S, Das S, Raul P, Bhattacharya SS (2018) Vermi-sanitization of toxic silk industry waste employing Eisenia fetida and Eudrilus eugeniae: substrate compatibility, nutrient enrichment and metal accumulation dynamics. Bioresour Technol 266:267–274. https://doi.org/10.1016/j.biortech.2018.06.092
Paz-Ferreiro J, Fu S (2016) Biological indices for soil quality evaluation: perspectives and limitations. Land Degrad Dev 27(1):14–25. https://doi.org/10.1002/ldr.2262
Rajadurai M, Karmegam N, Kannan S, Yuvaraj A, Thangaraj R (2022) Vermiremediation of engine oil contaminated soil employing indigenous earthworms, Drawidamodesta and Lampitomauritii. J Environ Manag 301:113849. https://doi.org/10.1016/j.jenvman.2021.113849
Rashmi I, Roy T, Kartika KS, Pal R, Coumar V, Kala S, Shinoji KC (2020) Organic and inorganic fertilizer contaminants in agriculture: impact on soil and water resources. Contaminants in Agriculture:3–41. https://doi.org/10.1007/978-3-030-41552-5_1
Rich N, Bharti A, Kumar S (2018) Effect of bulking agents and cow dung as inoculant on vegetable waste compost quality. Bioresour Technol 252:83–90. https://doi.org/10.1016/j.biortech.2017.12.080
Roberge MR (1978) Methodology of enzymes determination and extraction. In: Burns RG (ed) Soil enzymes. Academic Press, New York, pp 341–373. https://doi.org/10.1016/B978-012513840-6/50022-7
Sanchez-Hernandez JC, Del Pino JN, Capowiez Y, Mazzia C, Rault M (2018) Soil enzyme dynamics in chlorpyrifos-treated soils under the influence of earthworms. Sci Total Environ 612:1407–1416. https://doi.org/10.1016/j.scitotenv.2017.09.043
Shameema KP, Chinnamma MA (2018) Vermiremediation of heavy metal contaminated soil. Int J Adv Inf Eng Technol 5(4):32–38
Singh BM, Singh D, Dhal NK (2022) Enhanced phytoremediation strategy for sustainable management of heavy metals and radionuclides. Case Stud Chem Environ Eng 5:100176. https://doi.org/10.1016/j.cscee.2021.100176
Sohal B, Bhat SA, Vig AP (2021) Vermiremediation and comparative exploration of physicochemical, growth parameters, nutrients and heavy metals content of biomedical waste ash via ecosystem engineers Eisenia fetida. Ecotoxicol Environ Saf 227:112891. https://doi.org/10.1016/j.ecoenv.2021.112891
Sun W, Zhu B, Yang F, Dai M, Sehar S, Peng C et al (2021) Optimization of biosurfactant production from Pseudomonas sp. CQ2 and its application for remediation of heavy metal contaminated soil. Chemosphere 265:129090. https://doi.org/10.1016/j.chemosphere.2020.129090
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1(4):301–307. https://doi.org/10.1016/0038-0717(69)90012-1
Tang J, Zhang L, Zhang J, Ren L, Zhou Y, Zheng Y et al (2020) Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci Total Environ 701:134751. https://doi.org/10.1016/j.scitotenv.2019.134751
Ukalska-Jaruga A, Siebielec G, Siebielec S, Pecio M (2022) The effect of soil amendments on trace elements’ bioavailability and toxicity to earthworms in contaminated soils. Appl Sci 12(12):6280. https://doi.org/10.3390/app12126280
Van Elsas JD, Pratama AA, de Araujo WL, Trevors JT (2019) Microbial interactions in soil. In: Modern soil microbiology, third edition. CRC Press, pp 141–161
Wang G, Pan X, Zhang S, Zhong Q, Zhou W, Zhang X et al (2020) Remediation of heavy metal contaminated soil by biodegradable chelator–induced washing: efficiencies and mechanisms. Environ Res 186:109554. https://doi.org/10.1016/j.envres.2020.109554
Wang G, Wang L, Ma F, Yang D, You Y (2021) Earthworm and arbuscular mycorrhiza interactions: strategies to motivate antioxidant responses and improve soil functionality. Environ Pollut 272:115980. https://doi.org/10.1016/j.envpol.2020.115980
Wang Q, Ma M, Jiang X, Guan D, Wei D, Zhao B et al (2019a) Impact of 36 years of nitrogen fertilization on microbial community composition and soil carbon cycling-related enzyme activities in rhizospheres and bulk soils in northeast China. Appl Soil Ecol 136:148–157. https://doi.org/10.1016/j.apsoil.2018.12.019
Wang Y, Wang HS, Tang CS, Gu K, Shi B (2019b) Remediation of heavy-metal-contaminated soils by biochar: a review. Environ Geotech 9(3):135–148. https://doi.org/10.1680/jenge.18.00091
Warman PR (1999) Evaluation of seed germination and growth tests for assessing compost maturity. Compost Sci Util 7(3):33–37. https://doi.org/10.1080/1065657X.1999.10701972
Xiao R, Ali A, Xu Y, Abdelrahman H, Li R, Lin Y et al (2022) Earthworms as candidates for remediation of potentially toxic elements contaminated soils and mitigating the environmental and human health risks: a review. Environ Int 158:106924. https://doi.org/10.1016/j.envint.2021.106924
Xu K, Liu YX, Wang XF, Li SW, Cheng JM (2020) Combined toxicity of functionalized nano-carbon black and cadmium on Eisenia fetida coelomocytes: the role of adsorption. J Hazard Mater 398:122815. https://doi.org/10.1016/j.jhazmat.2020.122815
Xu Z, Yang Z, Zhu T, Shu W, Geng L (2021) Ecological improvement of antimony and cadmium contaminated soil by earthworm Eisenia fetida: Soil enzyme and microorganism diversity. Chemosphere 273:129496. https://doi.org/10.1016/j.chemosphere.2020.129496
Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci 11:359. https://doi.org/10.3389/fpls.2020.00359
Yano K, Morinaka Y, Wang F, Huang P, Takehara S, Hirai T, Ito A, Koketsu E, Kawamura M, Kotake K, Yoshida S, Endo M, Tamiya G, Kitano H, Ueguchi-Tanaka M, Hirano K, Matsuoka M (2019) GWAS with principal component analysis identifies a gene comprehensively controlling rice architecture. Proc Natl Acad Sci 116:2162–21267. https://doi.org/10.1073/pnas.1904964116
Yuvaraj A, Govarthanan M, Karmegam N, Biruntha M, Kumar DS, Arthanari M et al (2021) Metallothionein dependent-detoxification of heavy metals in the agricultural field soil of industrial area: earthworm as field experimental model system. Chemosphere 267:129240. https://doi.org/10.1016/j.chemosphere.2020.129240
Zeng XY, Li SW, Leng Y, Kang XH (2020) Structural and functional responses of bacterial and fungal communities to multiple heavy metal exposure in arid loess. Sci Total Environ 723:138081. https://doi.org/10.1016/j.scitotenv.2020.138081
Zhang D, Yin C, Abbas N, Mao Z, Zhang Y (2020) Multiple heavy metal tolerance and removal by an earthworm gut fungus Trichodermabrevicompactum QYCD-6. Sci Rep 10(1):1–9. https://doi.org/10.1038/s41598-020-63813-y
Zhou Y, Li H, Guo W, Liu H, Cai M (2022) The synergistic effect between biofertility properties and biological activities in vermicomposting: a comparable study of pig manure. J Environ Manag 324:116280. https://doi.org/10.1016/j.jenvman.2022.116280
Acknowledgements
The authors are thankful to the Department of Botany, Gauhati University, Assam, India, for providing the basic laboratory facilities to carry out the work smoothly.
Code availability
Not applicable
Author information
Authors and Affiliations
Contributions
HD provided laboratory facilities and guided GB for PhD. GB carried out the experimental works, analysis and statistical work, and wrote the MS under direct supervision of HD.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Chris Lowe
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 57 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borah, G., Deka, H. Vermiremediation of heavy metals (HMs)-contaminated agricultural land: synergistic changes in soil enzyme activities and earthworm’s growth parameters. Environ Sci Pollut Res 30, 115266–115278 (2023). https://doi.org/10.1007/s11356-023-30500-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30500-0