Abstract
Experiments were conducted under lead (Pb), cadmium (Cd), and copper (Cu) exposure to observe germination and seedling growth of wheat (Triticum aestivum L), pea (Pisum sativum), and tomato (Solanum lycopersicum L.). Metals were applied in five concentrations (20, 65, 110, 175, and 220 ppm) and Hoagland solution was used to feed the seedlings. Irrespective of the tested crop seeds, copper revealed maximum effect (51.2%) on germination followed by lead (47.5%) and cadmium (35.3%). Tomato seeds were most sensitive in germination stage followed by pea and wheat. In seedling stage, tomato also showed highest sensitivity to both Cd and Cu. However, pea seedlings showed higher tolerance to Pb and wheat seedlings had the highest tolerance to both Cu and Cd. Toxicity and tolerance of metals was found to vary with crops and growth stages. Higher transfer of metals (Pb, Cd, and Cu) in wheat seedling indicates higher risk of food chain contamination when grown in polluted soil. Higher mobility and uptake of Cd in tomato and wheat seedlings even under lower concentration of exposure needs further study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Abolition of metal pollution is a challenging task due to its non-degradable nature (Azimi et al. 2017) allowing it to persist in soil for much longer period than the other components of biosphere (Lasat 2002). Rapid industrialization and poor management of industrial effluents lead to increased metal pollution in all components of the environment. Some potentially toxic heavy metals such as chromium (158.7 mg/kg), lead (73.6 mg/kg), nickel (58.97 mg/kg), and cadmium (15.5 mg/kg) were reported in surface soils of Asia’s oldest oil and gas drilling sites in Assam, northeast India (Sarma et al. 2016). Bora et al. (2012) reported higher concentration of zinc (176.2–222.1 mg/kg), lead (67.8–125.1 mg/kg), copper (68.2–89.9 mg/kg), and cadmium (6.86–10.1 mg/kg) in soils of Silghat region of Assam due to solid wastes from jute mills. Chahal et al. (2014) reported higher concentration of iron (5629 ppm), zinc (320 ppm), manganese (194 ppm), lead (118 ppm), copper (24 ppm), and cadmium (1.4 ppm) in an Indian agricultural soil from Amritsar, Panjab. Central pollution control board (Marg 2011) designated Gujarat, Maharashtra, and Andhra Pradesh as most polluted states of India, contributing 80% hazardous waste (including heavy metals). Sharma et al. (2009) reported higher level of cadmium, above the Indian standard in wastewater-irrigated sites of Uttar Pradesh. Significant contamination of lead, chromium, copper, zinc, strontium, and vanadium in Pali Industrial areas of Rajasthan, India, was also reported by Krishna and Govil (2004). Thus, these earlier studies reveal the prevalence of various heavy metals in higher concentrations in different sites of India. Among the heavy metals, lead (Pb) is reported to bio-magnify in animal body through contaminated crops apart from morpho-physiological and oxidative damage in plants (Rahman et al. 2013). With higher transfer factor of cadmium from soil to plants, cadmium concentration is found predominantly high in fruits and vegetables (Satarug et al. 2009). Although copper is an essential micronutrient and act as co-factor for enzymes, it become toxic at higher concentration creating metal stress to plants and animals (Küpper et al. 2009). At cellular level, copper binds to sulfhydral groups in proteins causing inhibition of enzyme activity and protein function, alter the availability of other essential ions and impaired cell transport processes (Meharg 1994).
Terrestrial biomagnification of metals in organism is more prevalent through food crops (Atafar et al. 2010). Crops demonstrated diverse tolerance capacity to metal stress based on its biochemical and genetic makeup (Dhankher et al. 2002) which enables them to reduce metal uptake or increase internal sequestration (Hong-Bo et al. 2010). Three major crops of Indian subcontinent, i.e., cereal (Triticum aestivum L), legume (Pisum sativum), and fruit/vegetable (Solanum lycopersicum L.) were chosen for this experiment to compare the tolerance ability for the tested metals.
Seed germination and seedling growth are important stages of growth in plant life cycle (Liu et al. 2011) as the number of healthy seedlings ensures greater crop stand in the field and thus higher production. Though literatures on effects of heavy metals (Pb, Cd, and Cu) on crops are plenty, but very few works have been done during germination stage of the crops. The objective of the study was to find out whether both seed germination and seedling growth are equally affected under the exposure of metals and thereby to screen the vulnerable stage of the crops. We also wanted to detect the bioaccumulation ability of the tested crops for the studied metals. We hypothesized that applied levels of metal toxicity will have varied effect on growth stages of crops.
2 Materials and Methods
2.1 Plant Materials and Treatment Details
Seeds of pea (Pisum sativum L.) cv. GS 10, wheat (Triticum aestivum L.) cv. PBW 343, and tomato (Solanum lycopersicum L.) cv. Durgesh navodya were collected from the Krishi Vigyan Kendra, Sonitpur, Assam, India. Experiments were carried out in two sets during the months of November to December 2016 at the Department of Environmental Science, Tezpur University (26° 69′ N and 92° 82′), Tezpur, Assam, India. First set of the experiment was conducted in Petri plates for germination test. For the second set of the experiments, sand culture medium was used to test the seedling growth under metal exposure. Hydroponic nutrient solution (developed by Hoagland and Arnon 1950) was prepared using KNO3, Ca(NO3)2.4H2O, MgSO4.7H2O, KH2PO4, Fe-EDTA as sources of macronutrients and H3BO3, ZnSO4.7H2O, CuSO4.5H2O, MnSO4.H2O, and NaMoO4 for micronutrients. Metal stress were created in both sets of experiments by applying three different metals cadmium (Cd), copper (Cu), and lead (Pb) in the form of CdCl2.H2O, CuSO4.5H2O, and Pb(NO3)2 respectively. Five concentrations (20 ppm, 65 ppm, 110 ppm, 175 ppm, and 220 ppm) of each metal solution were prepared using distilled water with a control (without metal).
2.2 Germination Test
Germination test was performed in sterilized Petri plates using Whatman No. 1 filter paper. Petri plates were tagged for the treatments and made ready by placing filter paper on it. The papers were moistened with respective concentration of metal solutions. One treatment was kept as control using deionized water. Distilled water washed seeds were dripped in 2% sodium hypochlorite (NaClO) solution for 30 min to make it disinfected and washed it repeatedly to remove the sodium hypochlorite traces. Twelve sterilized seeds were placed in each Petri plates and the filter papers were kept moisten with respective treatment solutions throughout the experimental period by adding the solutions periodically. Each treatment was replicated for four times. BOD incubator (IK 120) was set at a temperature of 22 ± 2 °C and kept the Petri plates for 7 days.
2.3 Germination Parameters
Number of seeds germinated in each day (24 h) was recorded and the lengths of plumules and radicals were noted at the end of the germination test (7th day). Total protein and carbohydrate content, and the activity of enzyme catalase (CAT, EC 1.11.1.6) were estimated on the samples (drawn from the seedlings) collected on completion of germination test. Percent germination, germination index, percent inhibition of germination, and vigor index was calculated to evaluate the germination performance under the applied treatments following Benech Arnold et al. (1991), Sarma et al. (2014) and Abdul-Baki and Anderson (1973) as below:
where, n1, n2….n7 are number of germinated seeds on first, second, and subsequent days until 7th day.
2.4 Biochemical Parameters
Germinated seeds were washed with distilled water and pat dry with tissue paper to remove the treatment solutions. Composite sample was prepared by taking equal proportion of plumule and radical and used for assay. Total soluble protein was estimated by the method of Lowry et al. (1951) using bovine serum albumin (BSA) as a standard. Amount of soluble protein was measured with an ultra-spec double beam spectrophotometer (UV-1700 series, Pharma Spec, Japan). Total carbohydrate was estimated following the phenol sulfuric acid method of Sadasivam and Manickam (1991). CAT activity was estimated spectrophotometrically following the method of Aebi (1984).
2.5 Sand Culture Experiment
River sand was collected and after removing the foreign particles, the sand was washed under tap water to discard mud and soil. It was then kept submerged in 0.1% sulfuric acid (H2SO4) for 3 days to remove the nutrients and repeatedly washed with distilled water to eliminate the acid. Litmus paper was used to confirm the presence of any acid. Pots of size 11.5 cm height and 12.5 cm diameter were then filled with 1 kg of nutrient free sand. Total 144 pots were arranged in completely randomized design to accommodate all the treatments with three replications. In each pot, ten seeds were sown on the sand beds and after germination, six healthy seedlings were allowed to retain. Later, 10-day-old seedlings were treated for 3 weeks with nutrient solutions containing the respective metals in each treatment. Nutrient solutions (5 ml) were applied to pot at 2 days interval until the end of the experiment.
2.6 Metal Concentration in Plant Tissue and Sand Media
On completion of third week, total chlorophyll and chlorophyll stability index (CSI) of leaves were determined by the method of Anderson and Boardman (1964) and Sairam et al. (1997) respectively. Treated seedlings were uprooted carefully from the sand media on third week, washed with distilled water, and the fresh weight was taken. Dry biomass of the seedlings was measured after oven drying at 70 °C for 24 h. Dried sample (100 mg each of root and shoot) was ground and digested in 10 mL of di-acid mixture containing HNO3 and HClO4 at (9:4) ratio (AOAC 1990). Total metal content (each of lead, cadmium, and copper) in the initial sand sample were also estimated after digesting in tri-acid mixture (HNO3-HF-HClO4) at a ratio of 5:1:1 as described by Shentu et al. (2008). The digested samples (sand, root, and shoot) were then analyzed for metal concentration using an inductively coupled plasma optical emission spectrophotometer (ICP-OES) (Perkin Elmer, USA, Optima 2100 DV).
Bioconcentration factor (BCF), translocation factor (TF), and tolerance index (TI) were calculated to know the metal concentration, its translocation in plant, and crop’s tolerance to metals as given by Li et al. (2009) and Ng et al. (2016). The formulas used to calculate BCF, TF, and TI are
2.7 Statistical Analysis
Statistical analysis was carried out using SPSS (version 16.0; SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test (DMRT) at p ˂ 0.5 was used to estimate significant difference between means. Three-way analysis of variance (ANOVA) was performed to determine significance between metals (M), metal levels (ML), crops (C), and their respective interactions.
3 Results and Discussion
3.1 Influence of Metals on Seed Germination
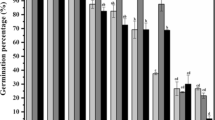
Metal exposure posed significant toxicity to the tested crops during germination. Highest reduction (51.2%) in percent germination was recorded in wheat seeds under copper treatment (Cu175) followed by lead (47.5%) and cadmium (35.3%) at an exposure of 220 ppm in both pea and tomato respectively (Table 1). Unlike the non-significant interaction between crops and metal levels (C × ML), other interactions between crops with metals (C × M) and metals with metal levels (M × ML) were highly significant (p ˂ 0.01) for percent germination (Table 1). Higher toxicity to copper in germination of the tested seeds might be due to greater permeability of the embryo cover to it or due to negative impact of the metals on the activity of enzyme such as amylase and protease causing inhibition of food supply to the growing radicle and plumule. Earlier studies also suggest reduction of seed germination under heavy metal effect on selected permeability behavior of cell membrane and osmotic alteration (Shafiq et al. 2008; Shaukat et al. 1999). Lowered activity of digestive enzymes such as amylase and protease was recorded by Singh et al. (2007) and Gopal et al. (2008) due to the ability of copper to substitute co-factors of various enzymes (Quartacci et al. 2001). Similar results of copper toxicity in germination of wheat was also reported by Wang et al. (2010), while seed germination was tested under copper, zinc, and lead. An approximate concentration dependent percent inhibition of germination was recorded for all the metals in the test crops. For wheat, percent inhibition of germination was in the order Cu > Cd > Pb, for pea and tomato it was Cu > Pb > Cd (Fig. 1). Maximum percent inhibition (61.8%) in wheat seed was documented under exposure of copper at 175 ppm (Cu175) followed by (47.2%) 220 ppm cadmium (Cd220) and (45%) 65 ppm lead (Pb65). While in pea, the highest inhibition was noted (69.4%) under lead exposure at 220 ppm (Pb220) followed by (62.7%) 175 ppm and 220 ppm of copper (55.2%). For tomato, the maximum inhibition (68.7%) was also recorded under copper treatment at 175 ppm (Cu175) following lead (67.9%) at 220 ppm (Pb220) and copper (57.1%) at 220 ppm (Cu220) (Fig. 1). The observed higher tolerance of wheat seeds for germination compared to tomato and pea might be due to differences in permeability of their embryo cover for the tested metals as suggested by Li et al. (2005). Variation in metal tolerance is also documented in mustard genotypes when exposed to higher metal toxicity (Bauddh and Singh 2011). Highest reduction (83.4%) in vigor index was noted under 175 ppm of copper treatment (Cu175) in wheat followed by 220 ppm of lead (Pb220) in pea (79.2%) and 220 ppm (Cd220) of cadmium in wheat (78.8%) (Table 2). However, overall toxicity on vigor was found in the order of Cu ˃ Cd ˃ Pb. The documented higher vigor index under lead exposure among the tested metals indicates minor influence of Pb on meristematic cells of the tested crops in the region of cell division. Contrarily, the documented higher impact of copper and cadmium reveals their influence on hydrolytic enzymes making lesser availability of food to growing radicle and plumule. Metal stress induced diminish meristematic cell growth (Kabir et al. 2010) and reduced activity of hydrolytic enzymes amylase and protease leading to lower plumule and radicle length as described by earlier researchers (Ashraf et al. 2011). Treatment with cadmium revealed lesser effect on GI compared to lead and copper in both tomato and pea (Table 2). Interactions between crops with metals (C × M) and crops and metal levels (C × ML) (Table 2) documented a significant difference (p ˂ 0.01) for both vigor index and germination index.
Percent inhibition of germination as effected in wheat, pea, and tomato seeds under metal exposure. Data shown are mean ± S.E. (n = 4). Treatments Pb indicate lead, Cd indicate cadmium, and Cu indicate copper. Subscript numbers 20, 65, 110, 175, and 220 are levels of applied metal concentration in ppm. Mean values for same crop sharing same letter are not significantly different according to the Duncan’s multiple range test at p ˂ 0.05
3.2 Influence of Metals on Total Protein, Carbohydrate, and CAT Activity
On exposure to cadmium, higher synthesis of total protein content was recorded up to 65 ppm concentration with a maximum value of 124.33 mg g−1 DW in wheat and 86.26 mg g−1 DW in tomato under 20 ppm (Cd20) treatment (Table 3). This might be due to increased activity of antioxidant defense, which is supported by the documented higher CAT activity under these treatments (Fig. 2). Contrastingly, maximum inhibition of total protein synthesis in pea was noted under cadmium treatments with a highest decrease (49.4%) at 110 ppm (Cd110) (Table 3). Similarly, a reverse relation of metal concentration on carbohydrate content was noted. Reduced carbohydrate content of the emerging seedlings under metal exposure might be due to inhibition of chlorophyll biosynthesis as observed from the lower chlorophyll content in the present study (Fig. 3). Irrespective of tested crops, highest reduction in carbohydrate content was noted at 220 ppm (Cd220) of cadmium exposure compared to lead and copper. Gubrelay et al. (2013) also documented inhibition of chlorophyll biosynthesis under cadmium exposure leading to massive reduction in carbohydrate content. Total carbohydrate content of tomato was affected most, followed by wheat and pea. Interactions among crops, metals, and metal levels (C × M, C × ML, and M × ML) on carbohydrate and protein content were significantly different (p ˂ 0.01) (Table 3). Regardless of the crop families, a concentration dependent increase of catalase (CAT) activity was observed under metal treatments (Fig. 2). This could be due to elevated production of H2O2 under metal stress, triggering the synthesis of catalase production and its activity to subsequent degradation of H2O2. Catalase is a unique antioxidant enzyme having the capability to convert H2O2 to H2O and O2 in an energy efficient way unlike other antioxidant enzyme, which use reducing equivalents (Das and Roychoudhury 2014; Gechev et al. 2006). However, a minor decrease of the same was noted from copper and cadmium exposure of 175 ppm in pea and 220 ppm in tomato (Fig. 2). This indicates the weakening of H2O2 breakdown process due to the binding of the metals to thiol groups of enzymes (Ouzounidou et al. 1997). Highest (256.1%) CAT activity was noted in pea at 220 ppm of lead exposure followed by tomato under same concentration of both cadmium (230.3%) and copper (185%). Catalase activity showed a positive correlation with plants metal uptake (r = 0.634) (Table 6).
Effect of different metal concentrations on catalase (CAT) activity of wheat, pea, and tomato. Data shown are mean ± S.E. (n = 4). Treatments Pb indicate lead, Cd indicate cadmium, and Cu indicate copper. Subscript numbers 20, 65, 110, 175, and 220 are levels of applied metal concentration in ppm. Mean values for same crop sharing same lowercase letter are not significantly different according to the Duncan’s multiple range test at p ˂ 0.05
Effect of different metal concentrations on chlorophyll stability index (CSI) of wheat, pea, and tomato seedlings. Data shown are mean ± S.E. (n = 4). Treatments Pb indicate lead, Cd indicate cadmium, and Cu indicate copper. Subscript numbers 20, 65, 110, 175, and 220 are levels of applied metal concentration in ppm. Mean values for same crop sharing same lowercase letter are not significantly different according to the Duncan’s multiple range test at p ˂ 0.05
3.3 Accumulation of Metals and Tolerance of Seedlings to Metals
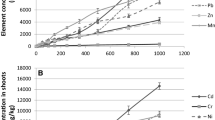
With increased concentration of the applied metals, tolerance index (TI) decreased gradually in all the crops (Table 4). Among the tested crops, pea showed higher tolerance to lead with maximum TI (0.97) at 110 ppm of exposure (Pb110). While, wheat documented higher tolerance to both copper (Cu) and cadmium (Cd) with maximum TI value of 0.97 and 0.96 respectively at an exposure of 65 ppm. Despite its lowest CAT activity, the higher tolerance of wheat crop to copper and cadmium indicates the existence of avoidance and homeostatic mechanism leading to lower metal buildup at sensitive sites. As a mechanism to avoid metal stress, building up of toxic metals at sensitive site is prevented with enhanced activation of oxidative defense by synthesis of protein (Dietz et al. 1999). Least tolerance observed in tomato seedlings for both cadmium and copper (at 200 ppm) with lowest TI value of 0.25 and 0.38 respectively. The lower TI value of cadmium compared to copper indicates higher toxicity of cadmium than copper to tomato seedlings that contradict the findings of Mediouni et al. (2006), where greater toxicity of copper than cadmium at higher concentrations was documented. Highly significant difference (p ˂ 0.01) of interactions between crops, metals, and metal levels (C × M and C × ML) on tolerance index was observed (Table 4). Recorded lower dry matter yield in all the treatments compared to control in our experiment reveals the toxic effect of applied concentrations of metals on the tested crops. Highest decline in dry matter yield was recorded in tomato under the exposure of 220 ppm of Cd (74.9%) followed by copper (62.4%) at the same concentration. A significant difference was noted for dry matter yield (p ˂ 0.01) between crops × metals and crops × metal levels (Table 4). Plant metal uptake at harvest showed a significant negative correlation with dry matter yield (r = 0.555) and tolerance index (r = 0.597) (Table 6). Roots recorded higher metal concentration irrespective of crops except in wheat at 20 ppm of lead exposure (Fig. 4). This could be due to the complex root system in higher plants that prevent metal translocation to shoot while maintaining several physiological and metabolic processes in aerial parts (Krupa et al. 1993) or could be due to direct exposure of roots to metals. Metal uptake was noted to rise in plant parts in lower concentration of metal in culture media, while at higher concentration of metal, it was noted to decrease metal uptake. BCF value was highest (15.1) in tomato at 20 ppm of cadmium exposure followed by pea (14.9) under the same treatment. Exposure to copper (Cu175) also showed higher BCF value (14.2) in tomato roots. The calculated greater BCF values (> 1) under the exposure of lower to moderate concentrations of both the metals suggested higher accumulation. Irrespective of the tested metals, highest TF noted in wheat compared to other crops (Table 5) reveals higher metal translocation from root to shoot. Similarly, higher TF and TI value of wheat under Cd and Cu treatments indicated the existence of internal metal detoxification mechanism for both the metals. Higher accumulation of cadmium in wheat and tomato shoots from the present experiment shows its greater mobility and hence the risk of food chain contamination even at lower concentration. Lower affinity or higher mobility of cadmium in soil was reported by Fifi et al. (2013) and Ahmadipour et al. (2014). A significant difference (p ˂ 0.01) of interactions between crops × metals and crops × metal levels was recorded for bioconcentration factor and transfer factor (Table 5).
Lead, cadmium, and copper uptake in different plant parts (root and shoot) of wheat, pea, and tomato plants when grown under their different concentrations. Data shown are mean ± S.E. (n = 4). Treatments Pb indicate lead, Cd indicate cadmium, and Cu indicate copper. Subscript numbers 20, 65, 110, 175, and 220 are levels of applied metal concentration in ppm. Mean values for same crop followed by the same lowercase letter are not significantly different according to the Duncan’s multiple range test (p ˂ 0.05)
3.4 Influence of Metals on Crop Morphology
The most prominent morphological alteration caused by metal stress is reduction of plant growth. In the current study, visible symptoms in the form of tips dieback in roots of tomato at 110 ppm and above concentrations of cadmium exposure were noted along with leaf chlorosis and finally death of the seedlings towards the end of third week. Mohanpuria et al. (2007) also recorded similar result of chlorosis, growth inhibition, browning of root tips, and finally death of plants under high levels of Cd exposure. Plumule blackening and interveinal foliar chlorosis under 65 ppm and above concentrations of copper was also recorded in tomato in this study as the initial symptoms of copper toxicity (Zhu and Alva 1993). Similar results of darkening of plumule and radicle in lettuce, spinach, and swiss chard under Cd and Cr exposure were reported by Bautista et al. (2013). Leaf chlorosis was observed in pea at 110 ppm and above concentrations of copper whereas in tomato at 220 ppm of copper exposure confirming their least tolerance for copper toxicity. The chlorosis under excess copper exposure could be due decreased uptake of iron (Ouzounidou 1995) depleting photosystem II (PSII) action centers leading to photo-inhibition and disruption of its repair cycle (Pätsikkä et al. 1998).
4 Conclusion
This study revealed that copper is the most toxic metal for seed germination of tested crops. Tomato seedlings from family Solanaceae had the highest sensitivity followed by pea (Fabaceae) and wheat (Poaceae) for the tested metals. Higher transfer of metals (Pb, Cd, and Cu) in wheat seedling indicates higher risk of food chain contamination when grown in contaminated soil. Further study is needed to understand the mechanism of higher uptake and mobility of cadmium in tomato and wheat seedlings.
References
Abdul-Baki, A. A., & Anderson, J. D. (1973). Vigor determination in soybean seed by multiple criteria 1. Crop Science, 13(6), 630–633.
Aebi, H. (1984). [13] Catalase in vitro. In Methods in enzymology (Vol. 105, pp. 121–126). Academic Press.
Ahmadipour, F., Bahramifar, N., & Mahmood Ghasempouri, S. (2014). Fractionation and mobility of cadmium and lead in soils of Amol area in Iran, using the modified BCR sequential extraction method. Chemical Speciation & Bioavailability, 26(1), 31–36.
Anderson, J. M., & Boardman, N. K. (1964). Studies on the greening of dark-grown bean plants II. Development of photochemical activity. Australian Journal of Biological Sciences, 17(1), 93–101.
Ashraf, M. Y., Sadiq, R., Hussain, M., Ashraf, M., & Ahmad, M. S. A. (2011). Toxic effect of nickel (Ni) on growth and metabolism in germinating seeds of sunflower (Helianthus annuus L.). Biological Trace Element Research, 143(3), 1695–1703.
Association of Official Analytical Chemists. (1990). Official methods of analysis of the association of official analytical chemists (15th ed.). Washington DC: Association of Official Analytical Chemists.
Atafar, Z., Mesdaghinia, A., Nouri, J., Homaee, M., Yunesian, M., Ahmadimoghaddam, M., & Mahvi, A. H. (2010). Effect of fertilizer application on soil heavy metal concentration. Environmental Monitoring and Assessment, 160(1–4), 83.
Azimi, A., Azari, A., Rezakazemi, M., & Ansarpour, M. (2017). Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Reviews, 4(1), 37–59.
Bauddh, K., & Singh, R. P. (2011). Differential toxicity of cadmium to mustard (Brassica juncia L.) genotypes under higher metal levels. Journal of environmental biology, 32(3), 355.
Bautista, O. V., Fischer, G., & Cárdenas, J. F. (2013). Cadmium and chromium effects on seed germination and root elongation in lettuce, spinach and Swiss chard. Agronomía Colombiana, 31(1), 48–57.
Benech Arnold, R. L., Fenner, M., & Edwards, P. J. (1991). Changes in germinability, ABA content and ABA embryonic sensitivity in developing seeds of Sorghum bicolor (L.) Moench. induced by water stress during grain filling. New Phytologist, 118(2), 339–347.
Bora, P. K., Chetry, S., Sharma, D. K., & Saikia, P. M. (2012). Distribution pattern of some heavy metals in the soil of Silghat region of Assam (India), influenced by jute mill solid waste. Journal of Chemistry, 2013.
Chahal, V., Chand, P., Nagpal, A., Kaur, K. J., & Pakade, Y. B. (2014). Evaluation of heavy metals contamination and its genotoxicity in agricultural soil of Amritsar, Punjab, India. International Journal of Research in Chemistry and Environment (IJRCE), 4(4), 20–28.
Das, K., & Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science, 2, 53.
Dhankher, O. P., Li, Y., Rosen, B. P., Shi, J., Salt, D., Senecoff, J. F., Sashti, N. A., & Meagher, R. B. (2002). Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and γ-glutamylcysteine synthetase expression. Nature Biotechnology, 20(11), 1140.
Dietz, K. J., Baier, M., & Krämer, U. (1999). Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In Heavy metal stress in plants (pp. 73–97). Berlin, Heidelberg: Springer.
Fifi, U., Winiarski, T., & Emmanuel, E. (2013). Assessing the mobility of lead, copper and cadmium in a calcareous soil of Port-au-Prince, Haiti. International Journal of Environmental Research and Public Health, 10(11), 5830–5843.
Gechev, T. S., Van Breusegem, F., Stone, J. M., Denev, I., & Laloi, C. (2006). Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays, 28(11), 1091–1101.
Gopal, R., Giri, V., & Nautiyal, N. (2008). Excess copper and manganese alters the growth and vigour of maize seedlings in solution culture. Indian Journal of Plant Physiology, 13(1), 44–49.
Gubrelay, U., Agnihotri, R. K., Singh, G., Kaur, R., & Sharma, R. (2013). Effect of heavy metal Cd on some physiological and biochemical parameters of barley (Hordeum vulgare L.). International Journal of Agriculture and Crop Sciences, 5(22), 2743.
Hoagland, D. R., & Arnon, D. I. (1950). The water-culture method for growing plants without soil. Circular. California Agricultural Experiment Station, 347, 1–32.
Hong-Bo, S., Li-Ye, C., Cheng-Jiang, R., Hua, L., Dong-Gang, G., & Wei-Xiang, L. (2010). Understanding molecular mechanisms for improving phytoremediation of heavy metal-contaminated soils. Critical Reviews in Biotechnology, 30(1), 23–30.
Kabir, M., Iqbal, M. Z., Shafiq, M., & Farooqi, Z. R. (2010). Effects of lead on seedling growth of Thespesia populnea L. Plant, Soil and Environment, 56, 194–199.
Krishna, A. K., & Govil, P. K. (2004). Heavy metal contamination of soil around Pali industrial area, Rajasthan, India. Environmental Geology, 47(1), 38–44.
Krupa, Z., Öquist, G., & Huner, N. P. A. (1993). The effects of cadmium on photosynthesis of Phaseolus vulgaris-a fluorescence analysis. Physiologia Plantarum, 88, 626–630.
Küpper, H., Götz, B., Mijovilovich, A., Küpper, F. C., & Meyer-Klaucke, W. (2009). Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiology, 151(2), 702–714.
Lasat, M. M. (2002). Phytoextraction of toxic metals. Journal of Environmental Quality, 31(1), 109–120.
Li, W., Khan, M. A., Yamaguchi, S., & Kamiya, Y. (2005). Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regulation, 46(1), 45–50.
Li, N. Y., Li, Z. A., Zhuang, P., Zou, B., & McBride, M. (2009). Cadmium uptake from soil by maize with intercrops. Water, Air, and Soil Pollution, 199(1–4), 45–56.
Liu, T. T., Wu, P., Wang, L. H., & Zhou, Q. (2011). Response of soybean seed germination to cadmium and acid rain. Biological Trace Element Research, 144(1–3), 1186–1196.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Marg, B. Z. (2011). Hazardous metals and minerals pollution in India: Sources, toxicity and management. A Position Paper. New Delhi: Indian National Science Academy.
Mediouni, C., Benzarti, O., Tray, B., Ghorbel, M. H., & Jemal, F. (2006). Cadmium and copper toxicity for tomato seedlings. Agronomy for Sustainable Development, 26, 227–232.
Meharg, A. A. (1994). Integrated tolerance mechanisms: constitutive and adaptive plant responses to elevated metal concentrations in the environment. Plant, Cell & Environment, 17(9), 989–993.
Mohanpuria, P., Rana, N. K., & Yadav, S. K. (2007). Cadmium induced oxidative stress influence on glutathione metabolic genes of Camellia sinensis (L.) O. Kuntze. Environmental Toxicology: An International Journal, 22(4), 368–374.
Ng, C. C., Rahman, M. M., Boyce, A. N., & Abas, M. R. (2016). Heavy metals phyto-assessment in commonly grown vegetables: water spinach (I. aquatica) and okra (A. esculentus). SpringerPlus, 5(1), 469.
Ouzounidou, G. (1995). Cu-ions mediated changes in growth, chlorophyll and other ion contents in a Cu-tolerantKoeleria splendens. Biologia Plantarum, 37(1), 71–78.
Ouzounidou, G., Moustakas, M., & Eleftheriou, E. P. (1997). Physiological and ultrastructural effects of cadmium on wheat (Triticum aestivum L.) leaves. Archives of Environmental Contamination and Toxicology, 32(2), 154–160.
Pätsikkä, E., Aro, E. M., & Tyystjärvi, E. (1998). Increase in the quantum yield of photoinhibition contributes to copper toxicity in vivo. Plant Physiology, 117(2), 619–627.
Quartacci, M. F., Cosi, E., & Navari-Izzo, F. (2001). Lipids and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. Journal of Experimental Botany, 52(354), 77–84.
Rahman, M. M., Azirun, S. M., & Boyce, A. N. (2013). Enhanced accumulation of copper and lead in amaranth (Amaranthus paniculatus), Indian mustard (Brassica juncea) and sunflower (Helianthus annuus). PLoS One, 8(5), e62941.
Sadasivam, S., & Manickam, A. C. (1991). Biochemical methods for agricultural sciences-Phenolics (3rd ed.). New Delhi: New age international publishers.
Sairam, R. K., Deshmukh, P. S., & Shukla, D. S. (1997). Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. Journal of Agronomy and Crop Science, 178(3), 171–178.
Sarma, B., Devi, P., Gogoi, N., & Devi, Y. M. (2014). Effects of cobalt induced stress on triticum aestivum L. crop. Asian Journal of Agriculture and Biology 2(2), 137–147.
Sarma, H., Islam, N. F., Borgohain, P., Sarma, A., & Prasad, M. N. V. (2016). Localization of polycyclic aromatic hydrocarbons and heavy metals in surface soil of Asia’s oldest oil and gas drilling site in Assam, north-east India: implications for the bio-economy. Emerging Contaminants, 2(3), 119–127.
Satarug, S., Garrett, S. H., Sens, M. A., & Sens, D. A. (2009). Cadmium, environmental exposure, and health outcomes. Environmental Health Perspectives, 118(2), 182–190.
Shafiq, M., Iqbal, M. Z., & Mohammad, A. (2008). Effect of lead and cadmium on germination and seedling growth of Leucaena leucocephala. Journal of Applied Sciences and Environmental Management, 12(3), 61–66.
Sharma, R. K., Agrawal, M., & Marshall, F. M. (2009). Heavy metals in vegetables collected from production and market sites of a tropical urban area of India. Food and Chemical Toxicology, 47(3), 583–591.
Shaukat, S. S., Mushtaq, M., & Siddiqui, Z. S. (1999). Effect of cadmium, chromium and lead on seed germination, early seedling growth and phenolic contents of Parkinsonia aculeata L. and Pennisetum americanum (L.) Schumann. Pakistan Journal of Biological Sciences, 2(4), 1307–1313.
Shentu, J., He, Z., Yang, X. E., & Li, T. (2008). Accumulation properties of cadmium in a selected vegetable-rotation system of southeastern China. Journal of Agricultural and Food Chemistry, 56(15), 6382–6388.
Singh, D., Nath, K., & Sharma, Y. K. (2007). Response of wheat seed germination and seedling growth under copper stress. Journal of Environmental Biology, 28(2), 409.
Wang, H., Zhong, G., Shi, G., & Pan, F. (2010, October). Toxicity of Cu, Pb, and Zn on seed germination and young seedlings of wheat (Triticum aestivum L.). In International Conference on Computer and Computing Technologies in Agriculture (pp. 231–240). Berlin, Heidelber: Springer.
Zhu, B., & Alva, A. K. (1993). Effect of pH on growth and uptake of copper by Swingle citrumelo seedlings. Journal of Plant Nutrition, 16(9), 1837–1845.
Acknowledgements
We are gratified to the Department of Environmental Science and Sophisticated Analytical Instrument Centre (SAIC), Tezpur University, Assam, India, for providing the required instrumentation facilities for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baruah, N., Mondal, S.C., Farooq, M. et al. Influence of Heavy Metals on Seed Germination and Seedling Growth of Wheat, Pea, and Tomato. Water Air Soil Pollut 230, 273 (2019). https://doi.org/10.1007/s11270-019-4329-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4329-0