Abstract
In the present research work, artificial neural network (ANN) is used to model the performance and emission parameters in a four-stroke, single-cylinder diesel engine combusting a blended fuel of diesel and catalytic co-pyrolysis oil produced from seeds of Pongamia pinnata, waste LDPE, and calcium oxide catalyst. The optimum yield of oil obtained was 92.5% at 500 °C temperature. Physical properties of the obtained oil, such as calorific value (44.85 MJ/kg) and density (797 kg/m3), level it by that of diesel while the flash point and fire point were found to be lower than that of pure diesel. An ANN model was then generated for the prediction of performance characteristics (BTE and BSFC) and emission characteristics (NOx and smoke) under varying loads, braking power, brake mean effective pressure, and torque as inputs using the Levenberg-Marquardt back-propagation training technique. The regression coefficients (R2) for BTE, BSFC, smoke, and NOx predictions were determined to be close to unity at 0.99859, 0.99814, 0.96129, and 0.92505, respectively (all values being close to unity). It has been discovered that ANN makes an effective simulation and prediction tool for blended fuels in CI engines. It is also suggested to predict the mechanical efficiency, volumetric efficiency, and CO, CO2, HC emissions using ANN in its future work.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are 380 million trucks and 1.1 billion light-duty cars on the road globally. These figures will probably exceed the 1.7 to 1.9 billion figures by 2040, with India expected to experience a major enhancement in the increase. Future technology is unpredictable for the vehicle industry, which is now reliant on internal combustion engines (Bhatt and Shrivastava 2022). Almost 11 billion liters of liquid fuels (diesel, gasoline, and jet fuel) are being used each day globally (Leach et al. 2020). Simply switching to electric vehicles would not solve the environmental issues. Given the socio-technical constraints and high investment costs, it is also challenging to completely replace internal combustion engines with new technology (Vandepaer et al. 2017; Huang 2018). As a way to fulfil this increment in demand for energy besides reduction in the environmental damage caused by fossil fuels, renewable fuel sources are the demand of the hour. Less viscous fuels are therefore thought to be potential replacements for diesel engines (Zhu and Andersson 2020). In such a scenario, one of the choices for blending with diesel to be used in CI engines for transportation would be fuels obtained through the co-pyrolysis of waste plastics and biomass. This will not only make use of readily available waste biomass seeds but also assist in reducing the environmental problems caused by plastic waste. The method of co-pyrolysis has been discovered to be more reliable than the method of mixing various oils to produce homogenous pyrolysis oil. Owing to the interaction of radicals during the co-pyrolysis event, phase separation could be prevented by producing a stable pyrolysis fuel (Martínez et al. 2014). However, it has also been discovered that the addition of catalysts during co-pyrolysis enhances the yield quantity of the liquid oil and decreases the quantity of solid char and non-condensable gas emissions in the pyrolysis process in addition to improving the quality of the oil obtained to that of fuels derived from petroleum (Simell et al. 1995; Kumar et al. 2015, Ryu et al. 2020).
Every change made to the speed, load, blend ratios, or other factors when using alternative fuel blends in a CI engine requires re-conducting the entire experimental process. Both time and money are required for this. For a specific objective to anticipate and evaluate the outputs in reaction to variations in the inputs, a theoretical model can be developed. For this theoretical study, artificial neural networks (ANN) can be utilized for the prediction of the outcomes of the performance and emission characteristics. ANN is superior to traditional polynomial approaches since it requires less complexity, money, and time. ANN was used successfully for predicting the heat transfer performance of a fabricated heat exchanger (Verma et al. 2017). The ANN approach is applied to fit the complicated nonlinear relationship. The ANN is generating a lot of curiosity because of its capability to map non-linear data utilizing a range of approximation functions in predictive modeling for non-linear complex systems (Mohan et al. 2023a). The integration of empirical modeling via artificial neural networks (ANN) was successfully incorporated into numerical modeling through the utilization of the Diesel-RK software (Salam and Verma 2019). To adhere to rigorous emission regulations for diesel engines, the current trend is to utilize eco-friendly fuel blends for diesel engines that generate very few amounts of dangerous pollutants while performing at par with pure diesel. Numerous emission factors, including NOx and CO emissions for diesel in blend with crude palm oil (Yusaf et al. 2011), modifying engine settings to reduce emissions (Yap et al. 2012), emissions from biodiesel (Ismail et al. 2012), and piston bowl optimization for soot and NOx emissions (Costa et al. 2014) have all been predicted using ANN. With a prediction accuracy of 0.85 ± 0.12 across 19 response variables, an ANN was used to create a rough empirical model of the experimental data; empirical redundancy exists in the functioning of the IC engine due to the high number of strong relationships between different system variables (Salam and Verma 2020).
Additionally, prediction of performance characteristics such as efficiency or specific fuel consumption and emission parameters such as NOx have also been conducted for different types of engines. The use of butanol and ethanol (Rezaei et al. 2015) or vegetable oil (Krishnamoorthi et al. 2019) are only a few to name. In one study, artificial neural network (ANN) technique was used to predict the most significant operating conditions for CI engine when a mixture of pyrolytic plastic oil, ethanol, and diesel was tested (Das 2021). In a recent study, the waste plastic oil (WPO) obtained by the catalytic pyrolysis of medical plastic wastes was tested in a diesel engine, ANN modeling is applied to predict the performance and emission characteristics (Panda et al. 2023). However, not much research works have been performed on the pyrolytic oil obtained from biomass being blended with pure diesel in a CI engine. Therefore, in the current research work, three blends of diesel and catalytic co-pyrolytic fuel (PB2) produced from the waste seeds of Pongamia pinnata and waste LDPE (low-density polyethylene) in the presence of calcium oxide (CaO) catalyst are being tested for the modeling of CI engines using artificial neural networks (ANN). Synthesized calcium oxide as a heterogeneous catalyst has already been utilized previously in extraction of methyl ester from waste cooking oil (Singh and Verma 2019). It is worth noting that this research marks the first instance of such experimentation using ANN. The performance and emission parameters of the blended fuels were predicted through the neural network using experimentally obtained engine-specific inputs and a trained neural network.
Materials and methods
Preparation of catalytic co-pyrolysis oil and engine set-up
Seeds of Pongamia pinnata, usually referred to as Karanja, were gathered from a neighboring market of the Central University of Jharkhand, Ranchi, extracted from their shells, and then, dried in the sun for 24 to 48 h. The seeds were ground into powder after drying. The LDPE, on the other hand, was gathered as used curd and milk pouches from the neighborhood restaurants and hotels. The pouches were then thoroughly rinsed with water, allowed to dry in the sun for 24–48 h, and finally cut into little (1–2 mm) pieces. To get rid of any remaining moisture, the seeds and waste LDPE were dried for 24 h at 50 °C temperature in a hot-air oven. Locally purchased calcium oxide (CaO) catalyst was utilized in its original form for the catalytic co-pyrolysis procedure in a batch reactor. The catalytic co-pyrolysis was carried out in a furnace-reactor setup to obtain the liquid fuel as described in our previous work (Mohan et al. 2023b). The resulting liquid fuel was designated as PB2, and three blends made for use in engines were designated as PB2@10 (10% PB2 + 90% diesel), PB2@20 (20% PB2 + 80% diesel), and PB2@30 (30% PB2 + 70% diesel), with D100 code being used for pure diesel in this study.
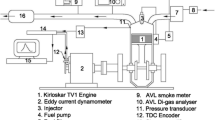
Figure 1 depicts the experimental configuration of the CI engine using diesel and mixed fuels. An eddy current dynamometer that was synchronized with the engine was used to manually change the load from no load (0%) to the highest load (80%). The speed was set at 1500 revolutions per minute, and measurements of variables including frictional power, brake mean effective pressure, and brake power were made to be used in calculations for the current study. Table 1 displays the engine’s specifications that were used for this study. For this study, a single-cylinder, 4-stroke diesel engine with a fixed speed of 1500 rpm was employed.
Uncertainty analysis
According to the relationship demonstrated below in Eq. (1), errors and uncertainty about observations from experiments were examined (Habib 2020). The calculation errors were considered as per the values provided below in Table 2.
Artificial Neural Network (ANN)
Models called artificial neural networks (ANN) resemble how the human brain operates. ANNs are composed of multiple layers of neurons comprehending that the relationships between the input, hidden, and output layers can assist with future problem-solving techniques and use real and expected data. Once the response is aligned, the network self-adjusts utilizing a feedback mechanism using input from the dynamic network as a whole. Because it can map non-linear data using a variety of approximation functions, the ANN has generated a lot of attention in predictive modeling for complicated, non-linear systems. The complex nonlinear connection is fitted using this method (Mohan et al. 2023c). The load, brake mean effective pressure (BMEP), brake power (BP), and torque were chosen as the associated input factors for the current research to forecast the performance in the form of brake thermal efficiency (BTE) and brake specific fuel consumption (BSFC), as well as NOx and smoke emission characteristics. One hundred layers of neurons (optional) were chosen for the current prediction modeling, whereby one hidden layer of neurons mimics one human brain. Data distribution was another factor that was taken into account in which the developed model was trained using 70% of the whole data, while the applied model was validated and tested using 15% of the total data each. The ANN model was created utilizing MATLAB® 2022a, version (9.12.0.2039608), while the Levenberg-Marquardt algorithm was trained to forecast the results.
Equation (1) displays the error function used to assess the phase performance as a function of mean square error (MSE).
where n is the number of data points. λi and βi, respectively, represent the experimental and expected values. The optimization of a network model based on the input (t) and output (o) is displayed in Eq. (2) below.
Results and discussion
Catalytic co-pyrolysis
The thermo-catalytic pyrolysis of Pongamia pinnata seeds and waste LDPE along with CaO catalyst was performed in a temperature range of 375–550 °C. The maximum liquid oil yield of 92.5 wt% was obtained at the temperature of 500 °C when the ratio of seeds and LDPE was kept at 1:2 while the CaO catalyst was kept at 10% of the total feed, forming the ratio of 3:6:1 for biomass seeds, waste plastic, and catalyst. At this temperature, the yield of non-condensable gases (NC Gases) was also found to be minimum at about 1.5 wt%, and the residual char was found to be about 6 wt% at the optimum condition implying that almost all the feed was able to get converted to liquid fuel with minimum residue. The amount of NC gases and the solid char increased with temperature beyond and before 500 °C temperature while the maximum NC gas formation was observed at the highest temperature and the maximum char was observed to be formed at the lowest temperature under consideration. It has also been found that calcium oxide, when used as a catalyst during the pyrolysis process, helps in reducing the yield of tar in addition to the reduction in the formation of CO2. Its low cost also promotes it as a feasible option to be used as a catalyst (Simell et al. 1995). The liquid oil was then processed through fractional distillation to remove any water content or sediments for obtaining the purest form of fuel to be characterized and blended with diesel to be further used in the CI engine. The ratio of 3:6:1 was selected for the biomass seed, waste plastic, and CaO as the other ratios did not provide the optimum amount of liquid fuel as desired. After checking the output through various ratios, the current ratio was selected.
Properties of obtained pyrolytic fuel
At a temperature of 500 °C, the Pongamia pinnata seeds and waste LDPE were catalytically co-pyrolyzed with CaO catalyst in a 3:6:1 ratio. The highest amount of 92.5% liquid fuel yield was attained at this temperature and ratio. Table 3 displays the physical characteristics of the produced fuel in comparison to diesel. Although it was discovered that PB2 has a lower density at room temperature than diesel, the difference between the density of the two fuels is too small, making the fuel blending practical. It was also discovered that the calorific values (LCV and HCV) were very close to those of diesel. It was also found that PB2 had a lower flash point and fire point than D100, which may help with more rapid and thorough combustion. Additionally, it was discovered that the kinematic and dynamic viscosity was only slightly higher than D100, which would not impede the flow of fuel inside the combustion cylinder. When compared to D100, PB2 was determined to have a smoky smell and was darker in color in comparison to diesel.
Performance and emission of obtained liquid oil in CI engine
Brake-specific fuel consumption and brake thermal efficiency
At 20% load, the BSFC was 0.49, 0.5, 0.51, and 0.48 kg/kWh respectively for D100, PB2@10, PB2@20, and PB2@30. There was found a decrement in the BSFC as the load increased. The enhanced fuel atomization, better mixing, and high in-cylinder temperature at increased loads contribute to the combustion process and result in low specific fuel consumption. Fuel combustion is improved at higher engine speeds due to improved fuel and air mixing. As the load increased, the BSFC decreased for all blends as at 40% load, the BSFC was found to be 0.35, 0.34, 0.31, and 0.35 kg/kWh for D100, PB2@10, PB2@20, and PB2@30, respectively. As the load increased to 60%, the BSFC was found to be 0.29, 0.3, 0.3, and 0.3 kg/kWh while at the highest load (80%), minimum BSFC of 0.3, 0.28, 0.3, and 0.3 kg/kWh was found for D100, PB2@10, PB2@20, and PB2@30, respectively.
A pattern of increase in the BTE with an increase in load was observed for all fuel blends and D100. At 20% load, the BTE observed for D100 was 17.4% while for the blends PB2@10, PB2@20, and PB2@30, it was found to be 16.11%, 17.46%, and 17.06%, respectively. At 40% load, the BTE was found to be 24.77%, 24.32%, 26.53%, and 23.11% respectively for D100, PB2@10, PB2@20, and PB2@30. However, with an increase in load to 60%, the BTE was found to be 29.16%, 27.11%, 28.79%, and 27.38% for D100, PB2@10, PB2@20, and PB2@30, respectively. At the highest load of 80%, the BTE for D100, PB2@10, PB2@20, and PB2@30 were found to be 28.69%, 28.66%, 28.8%, and 28.5%. It was observed that there is a small difference between the BTE of D100 and three blends (PB2@10, PB2@20, and PB2@30), however, the closest efficiency to the D100 was observed when PB2@20 was used at all loads.
NOx and smoke emissions
At 0% load, the NOx emission of PB2@20 was found to be 80% less than D100, while for PB2@30, it decreased by 25%, and PB2@10 had a similar emission to that of D100. Within the load range of 20–60%, the NOx emission of all blends increased compared to D100; however, the least increment was observed with PB2@20 compared to the other two blends. At the highest load (80%), the NOx emission of PB2@10 was found to decrease by 3.8%, PB2@30 observed a 19.5% decrement, while PB2@30 was found to have 13% lesser NOx emission than D100.
The smoke formation was the least for PB2@30 with a decrement of 10.37% at 20% load, 31.51% at 40% load, and 39.57% at 60% load, while at 80% load, PB2@10 had the maximum decrement of 22.5% in comparison to D100. At 0% load, the PB2@30 blend had the lowest smoke formation at 21.53% less than that of D100. The presence of oxygenated fuels in the form of PB2 blends resulted in a complete combustion of the blended fuel which might be the reason for the decrement of smoke at higher loads with higher blends assisting in complete and efficient combustion with a better air-fuel ratio maintained with blends in comparison to D100.
ANN prediction modeling
The CI engine was tested with three blends of pure diesel (PB2@10, PB2@20, and PB2@30), and the data was successfully utilized to fit the ANN prediction model. The development of a highly effective ANN model was dependent on the performance and emissions data gathered through the trials. The next sections go into further depth about the performance (BTE and BSFC) and emissions (NOx and smoke) results from the ANN modeling.
Brake-specific fuel consumption (BSFC) and brake thermal efficiency (BTE)
The MSE for the training of the created ANN model was successful because the MSE for the applied algorithm training with BSFC results was quite low at 5.2915E−05 for training, 2.7716E−04 for validation, and 7.0508E−06 for testing, with value of R being 0.99 for training and 1 each for validation and testing. The MSE results found for prediction of BTE prediction were also very low at 0.00 for training, 2.2513 for the validation, and 0.8568 for testing the applied algorithm in the dataset. As displayed in Fig. 2a, for BSFC prediction, the validation performance was the best at 2 epochs with the minimum possible MSE value of 0.00027716, while the error histogram was found to be evenly distributed signifying fewer errors while computing, as shown in Fig. 2b. Similarly, the training for BTE prediction of PB2 at all blends considered was effectively implemented at 3 epochs each with the lowest feasible MSE value of 2.2513, as displayed in Fig. 3a. Furthermore, the resulting dataset appeared to be generally evenly distributed, as indicated by the distribution curve of the error histogram in Fig. 3b. Obtaining lower MSE values and R close to 1 in all phases of application of the neural network has been the prime objective since it leads to better prediction results. A similar value of MSE (< 4%) was obtained while predicting BTE and other performance and emissions parameters with inputs of fuel blend and equivalence ratio (Rezaei et al. 2015). The value of MSE was found to be 0.01 for performance prediction using a diesel-ethanol blend as fuel and different pressures as input parameters (Bahri et al. 2017).
R values close to 1 have also been found at 0.999 while predicting the performance (BTE, BSFC) and emission characteristics using waste frying oil as fuel blend and injection timings as input parameters (Babu et al. 2020). The R value of 0.99 was also found using castor oil as a fuel blend to predict the brake power, BSFC, and emission characteristics with blend percentage and engine speed as inputs (Etghani et al. 2013) while similar results were obtained using compression ratio and exhaust gas as inputs (Krishnamoorthi et al. 2019).
The training, testing, and validation test regression plots for BSFC and BTE are displayed in Figs. 4 and 5, respectively, which illustrate how closely the targeted and output values corresponded at the specified input, yielding an R2 value of 0.99859 for BSFC prediction and 0.99814 for BTE prediction for all ANN implementation phases. The validation and testing regressions also achieved a value of 1 in the case of BSFC; however, the training regression for BSFC was found to be at 0.99889. For BTE prediction, the training, testing, and validation phases of implementation gained the R2 value of 1. A regression coefficient (R2) value close to 1 has been the target for all predictions done in previous works as well. An R2 value of 0.9915 was found using Karanja and Rosell oil as blends for the prediction of BSFC, BTE, and other performance parameters with engine load, blend percentage, and compression ratio as inputs (Shrivastava et al. 2020) while a value of 0.99 for R2 was obtained using waste cooking oil and methyl ester biodiesel as blends to obtain the same performance characteristics (Muralidharan and Vasudevan 2014). In all the discussed cases, the Levenberg-Marquardt algorithm with the back-propagation technique was applied as in the current study. The little variation in the value of regression coefficient might be due to the BSFC along with BTE performance of PB2@10 and PB2@30, found to have a difference with that of PB2@20 and D100.
Smoke and NOx emissions
The ANN model utilized for the prediction of smoke and NOx emissions was found to be successful. Figure 6a, b displays the validation performance curve and error histogram respectively for smoke emissions prediction. The best validation was found at 2 epochs with an MSE value of 2.3601, while for training, validation, and testing of smoke emission prediction, the MSE was found to be 45.2901 for testing, 2.3601 for validation, and 16.3130 for testing while the values of R were determined to be 0.9726 for training and 1 for both testing and validation phases. The MSE for NOx emission prediction was found to be at 27.1447 for training, 1.3343E+04 for validation, and 6.5241E+04 for testing with best validation at 8 epochs while R was found to be 0.9998 for training and − 1 both for validation and testing of the applied model. Lower values of R during the prediction of emission parameters were also found using vegetable oil as a fuel blend with compression ratio and engine speeds as inputs (Krishnamoorthi et al. 2019) or while using hydrogen and lemongrass oil as fuel blends with load and hydrogen percentage as input parameters (Hariharan et al. 2020). Error histograms for smoke and NOx emissions prediction are displayed in Figs. 6b and 7b. The plots show an even distribution of the patterns with the minimum error possible determining the best fit possible for the applied model.
The regression plots are displayed in Figs. 8 and 9 for the prediction of smoke and NOx emissions, respectively. The value of the regression coefficient (R2) for all cases combined was found to be 0.96 for smoke emission prediction, while R2 was found to be 0.92 for NOx emission prediction. However, the R2 for training, validation, and test for smoke emissions were determined to be 0.97, 1, and 1, respectively, while for NOx emission prediction, R2 was found to be 0.99 for training and − 1 each for validation and test phases of the implemented ANN model. The R2 for all cases needs to be close to 1 for a successful implementation of the applied model. R2 has also been found to be 0.96 using butanol as a blend with diesel having fuel mixtures and engine speed as input (Gürgen et al. 2018) while an R2 value of 0.93 was found for the prediction of emission using engine speed, load, and blends as input parameters (Liu et al. 2020). The deviation in the regression coefficient might be due to the more NOx and smoke formation by PB2@10 and PB2@30 at lower loads; however, the emission of PB2@20 was found to be in line with that of pure diesel, resulting in the overall better performance of obtained catalytic oil.
Conclusions
The following conclusions could be derived from the above study:
-
The seeds of Pongamia pinnata, waste LDPE, and CaO catalyst were utilized for catalytic co-pyrolysis in the ratio of 3:6:1 in a batch reactor yielding the optimum yield of oil (92.5 wt%) at 500 °C.
-
The obtained fuel (PB2) was then tested for its physical properties in which the calorific value of PB2 was observed to be 44.85 MJ/kg while the density was also observed to be in line with D100.
-
The load, torque, BP, and BMEP were chosen as the inputs to the artificial neural network to predict BTE and BSFC (performance characteristics) and NOx and smoke emissions (emission characteristics).
-
The value of R2 was found to be 0.99 each for BTE and BSFC while it was 0.96 and 0.92 for smoke and NOx emission prediction, respectively.
-
The error histogram showed an even distribution in all cases while the MSE was found to be as minimum as possible.
-
The values of MSE, R, and regression coefficient were found to be in line with the existing literature.
-
The current study also validates that the aforementioned feed mixture can definitely be used for usage as transportation fuels as the implemented ANN model was successful in forecasting the performance and emission characteristics of the PB2 and diesel blends.
The future work might include the prediction modeling of other performance and emission parameters such as mechanical and volumetric efficiencies and the CO, CO2, or HC emissions. The ANN modeling could also be implemented to further assert the combustion parameters for the selected fuel blends.
Data availability
All data generated or analyzed during this study are included in this article.
References
Babu D, Thangarasu V, Ramanathan A (2020) Artificial neural network approach on forecasting diesel engine characteristics fuelled with waste frying oil biodiesel. Appl Energy. https://doi.org/10.1016/j.apenergy.2020.114612
Bahri B, Shahbakhti M, Aziz AA (2017) Real-time modeling of ringing in HCCI engines using artificial neural networks. Energy 125:509–518. https://doi.org/10.1016/j.energy.2017.02.137
Bhatt AN, Shrivastava N (2022) Application of artificial neural network for internal combustion engines: a state of the art review. Arch Comput Methods Eng 29:897–919. https://doi.org/10.1007/s11831-021-09596-5
Costa M, Bianchi GM, Forte C, Cazzoli G (2014) A numerical methodology for the multi-objective optimization of the DI diesel engine combustion. Energy Proceedia 45:711–720. https://doi.org/10.1016/j.egypro.2014.01.076
Das AK (2021) Prediction of engine performance in a single-cylinder diesel engine fueled with waste plastic oil, ethanol, and diesel blend by artificial neural network. SAE Technical Paper 2021-01-5072. https://doi.org/10.4271/2021-01-5072
Etghani MM, Shojaeefard MH, Khalkhali A, Akbari M (2013) A hybrid method of modified NSGA-II and TOPSIS to optimize performance and emissions of a diesel engine using biodiesel. Appl Therm Eng 59:309–315. https://doi.org/10.1016/j.applthermaleng.2013.05.041
Gürgen S, Ünver B, Altın İ (2018) Prediction of cyclic variability in a diesel engine fueled with n-butanol and diesel fuel blends using artificial neural network. Renew Energy 117:538–544. https://doi.org/10.1016/j.renene.2017.10.101
Habib G (2020) The effect of H2 purity on the combustion, performance, emissions and energy costs in an SI engine. Therm Sci 24:37–49. https://doi.org/10.2298/TSCI180705315G
Hariharan N, Senthil V, Krishnamoorthi M, Karthic SV (2020) Application of artificial neural network and response surface methodology for predicting and optimizing dual-fuel CI engine characteristics using hydrogen and bio fuel with water injection. Fuel 270:117576. https://doi.org/10.1016/j.fuel.2020.117576
Huang B (2018) Recycling of lithium-ion batteries: recent advances and perspectives. J Power Sources 399:274–286. https://doi.org/10.1016/j.jpowsour.2018.07.116
Ismail HM, Ng HK, Queck CW, Gan S (2012) Artificial neural networks modelling of engine-out responses for a light-duty diesel engine fuelled with biodiesel blends. Appl Energy 92:769–777. https://doi.org/10.1016/j.apenergy.2011.08.027
Krishnamoorthi M, Malayalamurthi R, Sakthivel R (2019) Optimization of compression ignition engine fueled with diesel—chaulmoogra oil—diethyl ether blend with engine parameters and exhaust gas recirculation. Renew Energy 134:579–602. https://doi.org/10.1016/j.renene.2018.11.062
Leach F, Kalghatgi G, Stone R, Miles P (2020) The scope for improving the efficiency and environmental impact of internal combustion engines. Transp Eng Aust 1:100005. https://doi.org/10.1016/j.treng.2020.100005
Liu W, Safdari SM, Tlili I, Maleki A, Bach QV (2020) The effect of alcohol–gasoline fuel blends on the engines’ performances and emissions. Fuel 276:117977. https://doi.org/10.1016/j.fuel.2020.117977
Martínez JD, Veses A, Mastral AM, Murillo R, Navarro MV, Puy N, Artigues A, Bartoli J, Garcia T (2014) Co-pyrolysis of biomass with waste tyres: upgrading of liquid bio-fuel. Fuel Process Technol 119:263–271. https://doi.org/10.1016/j.fuproc.2013.11.015
Mohan I, Arya A, Singh R, Kumar S (2023a) Pyrolysis of Phoenix Dactylifera and Phyllanthus Emblica seeds to produce biofuel. Mater Today: Proceed 72:713–718. https://doi.org/10.1016/j.matpr.2022.08.490
Mohan I, Panda AK, Mandal S, Kumar S (2023b) Co-pyrolysis of Azadirachta indica non-edible seed and waste LDPE: analysis of kinetic models using thermogravimetric analyser and prediction modeling with Artificial Neural Network (ANN). Fuel 350:128765. https://doi.org/10.1016/j.fuel.2023.128765
Mohan I, Sahoo A, Mandal S, Kumar S (2023c) Kinetic modeling and thermogravimetric investigation of Phoenix dactylifera and Phyllanthus emblica non-edible oil seeds: artificial neural network (ANN) prediction modeling. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-023-04094-z
Muralidharan K, Vasudevan D (2014) Applications of artificial neural networks in prediction of performance, emission and combustion characteristics of variable compression ratio engine fuelled with waste cooking oil biodiesel. J Braz Soc Mech Sci Eng 37:915–928. https://doi.org/10.1007/s40430-014-0213-4
Panda AK, Rout SK, Das AK (2023) Optimization of diesel engine performance and emission using waste plastic pyrolytic oil by ANN and its thermo-economic assessment. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-26891-9
Rezaei J, Shahbakhti M, Bahri B, Aziz AA (2015) Performance prediction of HCCI engines with oxygenated fuels using artificial neural networks. Appl Energy 138:460–473. https://doi.org/10.1016/j.apenergy.2014.10.088
Ryu HW, Kim DH, Jae J, Lam SS, Park ED, Park YK (2020) Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Bioresour Technol 310:123473. https://doi.org/10.1016/j.biortech.2020.123473
Salam S, Verma TN (2019) Appending empirical modelling to numerical solution for behaviour characterisation of microalgae biodiesel. Energy Convers Manag 180:496–510. https://doi.org/10.1016/j.enconman.2018.11.014
Salam S, Verma TN (2020) Analysis of significance of variables in IC engine operation: an empirical methodology. Energy Convers Manag 207:112520. https://doi.org/10.1016/j.enconman.2020.112520
Shrivastava P, Salam S, Verma TN, Samuel OD (2020) Experimental and empirical analysis of an IC engine operating with ternary blends of diesel, karanja and roselle biodiesel. Fuel 262:116608. https://doi.org/10.1016/j.fuel.2019.116608
Kumar S, Nayan NK, Singh RK (2015) Kinetics of the pyrolysis and combustion characteristics of non-edible oilseeds (Karanja and Neem Seed) using thermogravimetric analysis. Energy Sources A Recovery Util Environ Eff 37(21):2352–2359. https://doi.org/10.1080/15567036.2012.748106
Simell PA, Leppälahtia JK, Kurkela EA (1995) Tar-decomposing activity of carbonate rocks under high CO2 partial pressure. Fuel 74(6):938–945. https://doi.org/10.1016/0016-2361(95)00012-T
Singh TS, Verma TN (2019) Taguchi design approach for extraction of methyl ester from waste cooking oil using synthesized CaO as heterogeneous catalyst: response surface methodology optimization. Energy Convers Manag 182:383–397. https://doi.org/10.1016/j.enconman.2018.12.077
Vandepaer L, Cloutier J, Amor B (2017) Environmental impacts of lithium metal polymer and lithium-ion stationary batteries. Renew Sust Energ Rev 78:46–60. https://doi.org/10.1016/j.rser.2017.04.057
Verma TN, Nashine P, Singh DV, Singh TS, Panwar D (2017) ANN: prediction of an experimental heat transfer analysis of concentric tube heat exchanger with corrugated inner tubes. Appl Ther Eng 120:219–227. https://doi.org/10.1016/j.applthermaleng.2017.03.126
Yap WK, Ho T, Karri V (2012) Exhaust emissions control and engine parameters optimization using artificial neural network virtual sensors for a hydrogen-powered vehicle. Int J Hydrog Energy 37:8704–8715. https://doi.org/10.1016/j.ijhydene.2012.02.153
Yusaf TF, Yousif BF, Elwad MM (2011) Crude palm oil fuel for diesel-engines: experimental and ANN simulation approaches. Energy 36:4871–4878. https://doi.org/10.1016/j.energy.2011.05.032
Zhu X, Andersson O (2020) Performance of new and aged injectors with and without fuel additives in a light duty diesel engine. Transp Eng Aust 1:100007. https://doi.org/10.1016/j.treng.2020.100007
Author information
Authors and Affiliations
Contributions
IM: conceptualization, formal analysis, investigation, methodology, software, visualization, data curation, and writing—original draft. AKD: data curation, supervision, and writing—review and editing. RP: data curation, supervision, and writing—review and editing. SK: project administration, supervision, validation, resources, and writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Co-pyrolysis of Pongamia pinnata seeds and waste low-density polyethylene.
Calcium oxide (CaO) is used as a catalyst in the pyrolysis process.
Characterization of liquid fuel obtained by catalytic co-pyrolysis.
ANN model is applied for the prediction of performance and emission characteristics in a CI engine.
Feasibility of obtained catalytic co-pyrolysis oil as a renewable fuel as an alternative to fossil fuels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohan, I., Das, A.K., Prakash, R. et al. Prediction modeling using artificial neural network (ANN) for the performance and emission characteristics of catalytic co-pyrolytic fuel blended with diesel in a CI engine. Environ Sci Pollut Res (2023). https://doi.org/10.1007/s11356-023-30209-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-023-30209-0