Abstract
The increase in temperature caused by global climate change has promoted the salinization of wetlands. Inland saline-alkaline wetlands have an environment of over-humidity and shallow water and are hot spots for CH4 emissions. However, there are few reports on the effect of salinity on CH4 emissions in inland saline-alkaline wetlands. This study conducted simulation experiments of increased salinity to investigate the impact of salinity, habitat, and their interactions on CH4 emissions, as well as to examine the response of methanogenic archaea to salinity. Overall, salinity inhibited CH4 emissions. But there were different responses in the three habitat soils. Salinity decreased the relative abundance of methanogenic archaea and changed the community structure. In addition, salinity changed soil pH and dissolved organic carbon (DOC) and ammonium (NH4+) concentrations, which were significantly correlated with methanogenic archaea. Our study showed that salinity changed the soil physicochemical properties and characteristics of the methanogenic archaeal community, affecting CH4 emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the second largest greenhouse gas after CO2, CH4 contributed about 22% to the greenhouse effect (Wang et al. 2018). Atmospheric CH4 contents have increased dramatically since the Industrial Revolution, already rising from 719 ppb in 1750 to 1895 ppb in 2021 (IPCC 2021; Lan et al. 2022). Sources of CH4 include wetland systems (including swamps, sediments, rice fields, etc.), ruminant digestive systems, landfills, leakage during energy production and utilization, and sewage treatment systems (Kirschke et al. 2013). Among them, wetlands produce about 164 Tg CH4 per year, contributing about 1/3 of global CH4 emissions, and are the most important source of CH4 emissions (Bridgham et al. 2013).
CH4 is produced by archaea-dominated anaerobic decomposition of organic matter (Hofmann et al. 2016; Gütlein et al. 2018). The known methanogenic archaea are divided into seven orders (Borrel et al. 2014). The community and diversity of methanogenic archaea are influenced by various environmental factors. For example, the abundance of methanogenic mcrA genes decreased with increasing pH in acidic rice fields (Luo et al. 2022). In studies with a pH range of 4.0~10.0, extreme pH reduces the relative abundance of acetoclastic methanogens responsible for acetic acid breakdown, while increased those of hydrogenotrophic and hydrogen- or acetic acid-utilizing methanogens (Qiu et al. 2023). In addition, soil organic matter is an important factor affecting methanogenic archaea and CH4 emissions (Zhang et al. 2018; Wu et al. 2022). Soil dissolved organic carbon (DOC) is an important unstable carbon substrate that provides carbon sources for methanogenic archaea and has a positive effect on CH4 emissions (Kong et al. 2019; Wang et al. 2021). Nitrate (NO3−) and Fe3+ can be used as electron acceptors to participate in the methane oxidation process and affect CH4 emissions (Fan et al. 2021; Chen et al. 2022). Cover plant is also an important factor affecting the methanogen community and CH4 emissions (Duan et al. 2022; Venturini et al. 2022). The peatlands where vascular plants grow are dominated by acetoclastic methanogens (Ström et al. 2003). In peatlands with non-vascular plants, hydrogenotrophic methanogens are mostly present (Nakagawa et al. 2002).
Methanogenic archaea are sensitive to salinity. In general, increased salinity can inhibit methanogenic archaeal activity, and the number of methanogenic archaea will decrease accordingly, thus reducing the CH4 emissions in wetlands (Sun et al. 2013). In addition, increased salinity alters microbial community structure (Pattnaik et al. 2000; Feng et al. 2023). In a salinity study of mangrove peat soil, the abundance of microorganisms did not change, but the community structure changed significantly (Chambers et al. 2016). When a large amount of NaCl was input into coastal wetlands, CH4 emissions from the soil surface was significantly inhibited (Chambers et al. 2011). However, lower concentrations of salt input had some promotion or no significant effect on CH4 emissions in wetlands (Weston et al. 2011; Krauss and Whitbeck 2012; Konnerup et al. 2014). In the Mobile Bay freshwater swamp, CH4 emissions did not change significantly in different salinity areas (Wilson et al. 2015). Under high salinity, species with high salinity tolerance can replace species with low salinity tolerance and become the dominant microorganisms in the environment (Rath et al. 2019; Zhang et al. 2019). In conclusion, the effects of salinity on CH4 emissions and methanogenic archaeal communities were spatially variable. At present, most studies on the influence of salinity on CH4 emissions and methanogenic archaea focus on coastal wetlands (Dang et al. 2019; Chen et al. 2020b). However, little research has been done in inland saline-alkaline wetlands.
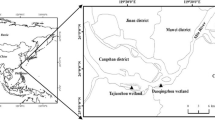
In recent years, global warming has accelerated the evaporation of water from wetlands, resulting in wetland salinization (Jeppesen et al. 2020). Saline groundwater conducts upwards and surface water evaporates, which leads to an increase in wetland salinity (Herbert et al. 2015). Zhalong wetland is located in the Songnen Plain of China, which is an area where inland saline-alkali wetlands are concentrated. The wetland is low-lying and flat, with many swamps and a large amount of water evaporation, which form an ecological environment with a slightly higher salinity. The slightly alkaline soil is suitable for the growth of methanogens, making this wetland a hot spot for CH4 emissions (Liu et al. 2019). In addition to reed (Phragmites australis), the dominant vegetation in Zhalong wetland also includes star grass (Puccinellia tenuiflora) and guinea grass (Leymus chinensis).
Due to the severe salinization of the Zhalong wetland, we collected soil from three habitats in the wetland to simulate the increase in salinity (Liu et al. 2019; Luo et al. 2022). The CH4 emission process and soil physicochemical characteristics were measured. The community composition and relative abundance of archaea were studied by using high-throughput sequencing and quantitative PCR technology. The objectives of this study were to reveal the effects of increased salinity on CH4 emissions and associated microbes in inland saline-alkaline wetlands and to explain key environmental drivers. This study helps to understand the response of CH4 emissions and methanogenic archaea to the salinization of wetlands, which will provide a theoretical basis for subsequent research on CH4 emissions in salinized soils.
Materials and methods
Soil sampling and experimental design
The soil used in this study was collected in July 2022 from the surface (0~20 cm) of the Zhalong wetland (46° 52′–47° 32′ N, 123° 47′–124° 37′ E) in Heilongjiang Province, China. Zhalong wetland has a mid-temperate climate, with an average annual precipitation of 420 mm, an annual average temperature of 3.9 °C, and a freezing period of 7 months (Gao et al. 2018). The specific sampling process was described before (Liu et al. 2019). Each sample site was divided into 3 plots. Homogenous mixing was performed after collecting at least 3 soil samples per plot. The soil samples were stored at low temperatures and transported to the laboratory and were divided into three parts: a part of fresh soil was extracted with 1 mol L−1 KCl to determine the content of inorganic nitrogen (Wang et al. 2023), a part of the soil was air-dried for soil physicochemical analysis, and another part was stored at −80 °C for later experiments. The details of the soil are shown in Table 1. The dominant vegetation in sites was Puccinellia tenuiflora (H1), hygrophyte Phragmites australis (H2), and aquatic Phragmites australis (H3), respectively. The total organic carbon (TOC) and total nitrogen (TN) contents in H2 soils were significantly higher than those in H1 and H3 soils (P < 0.05) (Table 1). The soil salinity in the H1 site was significantly higher than that in the other two sites, which were 0.04% (H1), 0.01% (H2), and 0.01% (H3), respectively.
We added 50 mL of sterile anaerobic saline with different NaCl concentrations to 120 mL serum bottles. Fresh soil (equivalent to 10 g of dry soil) was added to the serum bottle in an anaerobic glove box (Coy, USA) to establish anaerobic microcosms. Five treatments were set up for each habitat soil: (i) control without NaCl addition (CK), (ii) 1.0% salinity (S1), (iii) 2.5% salinity (S2), (iv) 3.5% salinity (S3), and (v) 5.0% salinity (S4), with three replicates. The serum bottles were sealed with sterile neoprene septa and secured with aluminum caps to maintain an anaerobic environment. All serum bottles were incubated for 68 days at 25 °C in the dark without shaking.
Measurement of soil CH4 emissions and physicochemical characteristics
The 2 mL of gas samples was taken from the headspace of each bottle, and the concentration of CH4 was measured by a gas chromatograph (Agilent 8890A, Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector (FID). 80/100 mesh HayeSep Q column with an inner diameter of 2 mm was used, and the carrier gas was high-purity N2. The maximum CH4 emission rate was calculated in the linear range of CH4 emission increase. The rate of CH4 emission was calculated using the following formula (Luo et al. 2022):
where F is CH4 emission rate (mg kg−1 d−1), ρ is the density of CH4 at standard temperature and pressure, V (m3) is the headspace volume of the serum bottle, m (kg) is the dry soil weight, \(\frac{dc}{dt}\) (ppm d−1) is the changed concentration of CH4 in the unit time (d), and T is the incubation temperature.
After the incubation, soil physicochemical characteristics were measured for data analysis. Soil pH was measured with a pH meter. Soil electrical conductivity (EC) was measured with a conductivity meter (DDS-307, Leici, Shanghai, China). Soil dissolved organic carbon (DOC) was measured with a total organic carbon analyzer (Multi-N/C 3100, Analytik Jena, Germany). Ammonium (NH4+) and nitrate (NO3−) concentrations were measured using a continuous flow analyzer (Seal Analytical AA3, Norderstedt, Germany). The concentrations of Fe3+ and Fe2+ were determined by colorimetry (Wallmann et al. 1993; Haese et al. 1997). Active iron was extracted from the soil with an HCl solution. Active iron and Fe2+ concentrations were determined with a 1, 10-phenanthroline and hydroxylamine hydrochloride. Then, Fe3+ content was obtained by calculating the difference between the two.
DNA extraction and high-throughput amplicon sequencing of archaeal 16S rRNA gene
To explore the effect of salinity on the archaeal community, high-throughput sequencing of archaeal 16S rRNA genes was performed on the CK and S4 treated samples of each habitat soil after the incubation. First, DNA in soil (0.5 g) was extracted according to the instruction manual of SPINeasy DNA Kit for Soil (MP Biomedicals, Santa Ana, CA, USA). The concentration of DNA was determined with a NanoDrop (NanoDrop OneC, Thermo Scientific, USA). The DNA samples were stored in a −20 °C refrigerator. PCR amplification on V4–V5 regions of archaeal 16S rRNA gene used 524F10extF (TGYCAGCCGCCGCGGTAA) and Arch958RmodR (YCCGGCGTTGAVTCCAATT) primer pair (Liu et al. 2016). The amplification reaction system (20 μL) included 10 μL 2×Pro Taq, 0.8 μL each for upstream and downstream primers (5 μM), and 10 ng μL−1 DNA template. PCR reaction parameters were 95 °C for 3 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s, and then 72 °C for 10 min. PCR amplification was performed by a thermocycler (GeneAmp 9700, ABI, USA). PCR products were detected by 2% agarose gel electrophoresis and recovered using the DNA Gel Extraction Kit (AxyPrep, USA). The sequencing was performed on the Illumina MiSeq PE300 platform (Shanghai Majorbio Bio-pharm Technology Co., Ltd.).
Statistical analysis
Statistical analysis of data was performed using SPSS 26.0 (SPSS, Inc., Chicago, IL) (Morgan et al. 2019). One-way analysis of variance (ANOVA) was used to explore the differences in soil physicochemical properties and CH4 emission rate in response to salinity. The influence of salinity, soil habitat, and their interaction on CH4 emissions and soil physicochemical properties was analyzed by multifactor analysis of variance. Correlations between CH4 emissions, archaeal genus composition, and environmental factors were assessed using Pearson’s correlation analysis. The quality filtering of raw reads was conducted by Fastp software (Chen et al. 2018). FLASH was used to merge paired-end reads (Magoč and Salzberg 2011). Then, the data was processed by using sequence denoising method (DADA2) to obtain amplicon sequence variant (ASVs) representative sequence and abundance information. Sequences with a similarity higher than 97% were classified as operational taxonomic units (OTUs) using Usearch (version 7.1) software (Edgar 2013). A classification was assigned to each sequence using the Ribosome Database Project (RDP) classifier (version 2.2, 70% confidence threshold) based on the SILVA 128 reference database (Quast et al. 2012). Alpha diversity indices were calculated using mothur (version 1.30) software. Canoco 5 was used to perform redundancy analysis (RDA) to explore the relationship between soil physicochemical properties and archaeal communities (Šmilauer and Lepš 2014). Draw various line charts, histograms, and heat maps through Origin 2021 (Moberly et al. 2018). The data were means ± standard deviations (Mean ± SD).
Results
CH4 emissions and soil physicochemical properties
In H1 soil, there was no significant difference in CH4 emissions between S1 and CK treatments in the first 25 days. After 25 days, CH4 emissions of S1 treatment were significantly reduced (Fig. 1a). After 68 days of incubation, compared with CK treatment (80.70 ± 0.54 mg kg−1), cumulative CH4 emissions with S1 (53.53 ± 0.59 mg kg−1) significantly decreased by 33.67% (Fig. 1a). The maximum CH4 emission rate of S1 treatment (3.31 ± 0.06 mg kg−1 d−1) was also significantly lower than that of CK (4.23 ± 0.21 mg kg−1 d−1) (Fig. 1d). In the early stage of incubation (the first 40 days), there was no significant increase in CH4 emissions of S2 treatment. CH4 emissions of S2 treatment occurred only in the middle of the incubation (40–50 d). S3 and S4 treatments considerably limited CH4 emissions, which remained at low levels throughout. After anaerobic cultivation, CH4 accumulations of S2, S3, and S4 treatments were 12.62 ± 0.30, 4.70 ± 0.13, and 1.76 ± 0.05 mg kg−1, respectively (Fig. 1a).
In H2 soil, CH4 emissions of S1 treatment were delayed, but there was no significant difference in the final CH4 accumulations compared with CK treatment. At the end of incubation, CH4 accumulations of S1 and CK treatments were 194.92 ± 5.87 and 195.86 ± 3.14 mg kg−1, respectively (Fig. 1b). There was also no significant difference in the maximum CH4 emission rates of the two treatments, which were 15.88 ± 0.39 mg kg−1 d−1 (CK) and 15.68 ± 0.14 mg kg−1 d−1 (S1), respectively (Fig. 1d). Different from H1 soil, the CH4 accumulations in H2 soil treated with S2 were less affected by the increased salinity (174.06 ± 4.23 mg kg−1). Cumulative CH4 emissions of S3 and S4 treatments were much lower compared to the other treatments with lower salinity, reaching up to 27.52 ± 4.54 and 21.04 ± 0.90 mg kg−1, respectively.
Similarly, increased salinity delayed CH4 emissions in H3 soil. At the initial stage of incubation, CH4 emission of the treatments with increased salinity was significantly lower than that of CK treatment (Fig. 1c). It mainly occurred in the middle and late stages of incubation (after 30 days). Furthermore, CH4 emissions decreased gradually with the increase of salinity gradient (Fig. 1c). At the end of incubation, CH4 accumulations in each treatment were 100.32 ± 1.24 (CK), 81.58 ± 2.14 (S1), 47.62 ± 1.79 (S2), 25.18 ± 0.64 (S3), and 12.07 ± 1.12 mg kg−1 (S4). The treatments with increased salinity reduced CH4 emissions by 18.68%, 52.53%, 74.90%, and 87.97%, respectively. The maximum CH4 emission rate also gradually and significantly decreased with the increase of salinity (Fig. 1d).
Salinity, habitat, and the interaction of these two variables significantly affected cumulative CH4 emissions (P < 0.001) (Table 2). Salinity and habitat had significant effects on soil pH, EC, DOC, NH4+, Fe3+, and Fe2+ contents (P < 0.05). Among them, DOC, Fe3+, and Fe2+ contents were also affected by the interaction between salinity and habitat (P < 0.05). Pearson’s correlation analysis showed that CH4 emissions were significantly affected by pH, EC, DOC, and NH4+ contents (P < 0.05) (Table 3). DOC and NH4+ contents were significantly positively and negatively correlated with EC, respectively (P < 0.01).
Analysis of archaeal communities
Archaeal community analysis was performed by high-throughput sequencing of 16S rRNA genes, and 7 major lineages were found in samples at the order level of archaea (Fig. 2). As salinity increased, the summed relative abundance of methanogenic orders decreased in all three sites, by 35.25% (H1), 29.31% (H2), and 14.34% (H3), respectively. There were mainly 6 orders of methanogenic archaea: Methanobacteriales (12.64~41.79%), Methanosarciniales (0.54~41.93%), norank_c_Bathyarchaeia (1.25~42.31%), Methanomicrobiales (0~4.23%), Methanocellales (0.04~2.53%), and Methanomassiliicoccales (0.04~2.54%). In H1 and H3 soils, the relative abundance of Methanosarciniales was most affected by increasing salinity, decreasing by 41.39% and 20.00%, respectively. In H2 soil, the relative abundance of Methanosarciniales was reduced by only 7.30%, while that of Bathyarchaeia was reduced by 23.78%.
We calculated the total archaeal richness and diversity of each sample using alpha-diversity analysis (coverage, Chao1 diversity, Shannon index, and Simpson index) (Fig. 3). The community coverage was >99%, indicating that the sequencing depth was sufficient to cover most species information in the sample. For soils in the three habitats, the Chao1 diversity was significantly reduced by increasing salinity (P < 0.05) (Fig. 3a), indicating that elevated salinity reduced the richness of soil archaeal communities. Especially in the H2 soil with the most drastic change, the Chao1 index decreased from 350.27 to 229.26. It is worth noting that the richness of archaeal communities in H1 soils was significantly lower than that in H2 and H3 soils, regardless of the addition of salt. Comparing the Shannon and Simpson indices, we found that increased salinity reduced the diversity of archaeal communities in soil (Fig. 3b and c).
Community structure of methanogenic archaea at the genus level
As salinity increased, the relative abundance of methanogenic archaea was significantly reduced and the dominant archaea were changed (Fig. 4). The relative abundance of Methanobacterium increased by 8.71% (H1), 2.03% (H2), and 13.90% (H3), respectively, with the increase of salinity. Affected by salinity, the relative abundance of methanogenic archaea decreased the most in H1 soil (from 65.65 to 30.87%). Among them, the genus of Methanosarcina changed most drastically, and its relative abundance decreased from 41.88 to 0.31%. In H2 soil, salinity reduced the relative abundance of methanogenic archaea from 75.25 to 46.93%. Among them, the relative abundance changes of Bathyarchaeia (decreased from 42.31 to 18.53%) and Methanosaeta (decreased from 10.43 to 0.48%) were the most significant (Fig. 4). However, salinity had the least effect on the relative abundance of methanogenic archaea in H3 soil, reducing the relative abundance of methanogenic archaea from 76.09 to 62.60% (Fig. 4). The relative abundance of Methanosaeta decreased most obviously, with a decrease of 16.62%.
The relationship between archaea and soil physicochemical properties and CH4 emissions
Redundancy analysis (RDA) was used to evaluate the influences of soil physicochemical factors on archaeal communities at the order level (Fig. 5). The first and second axes explained 58.53% and 28.38% of the variance in archaeal community composition, respectively. The first axis was positively correlated with NH4+ and NO3− concentrations and negatively correlated with pH, DOC, and Fe2+ contents. The second axis was positively correlated with pH and NO3− and negatively correlated with NH4+, DOC, and Fe2+ concentrations (Fig. 5). DOC and NH4+ contents had a significant effect on the archaeal community composition (P < 0.05). We observed the positive correlation between Methanosarciniales and NO3− concentrations and Bathyarchaeia and NH4+ concentrations. This suggests that archaeal community was influenced by soil inorganic nitrogen.
To reveal the impact of environmental variables on genus-level archaea, we plotted a heatmap of correlations between archaeal at the genera level (top 15) and physicochemical properties (Fig. 6). Bathyarchaeia, Methanomassiliicoccus, Candidatus_Methanoperedens, and NH4+ concentrations were significantly positively correlated (P < 0.05). Methanosarcina and NO3− concentrations had a significant positive correlation (P < 0.05). Methanocella was negatively correlated with EC (P < 0.05) and DOC (P < 0.01) significantly. Rice_Cluster_II had a significant negative correlation with soil pH (P < 0.01). Methanobacteriaceae (P < 0.01) and Rice_Cluster_I (P < 0.05) were significantly negatively correlated with Fe2+ concentrations. There was a significant negative correlation between Fe3+ concentration and Shannon index (P < 0.05). Methanomassiliicoccus was significantly positively correlated with CH4 emission (P < 0.05).
Discussion
Salinity reduced CH4 emissions
Our study has shown that salinity suppressed CH4 emissions, which is consistent with the findings in Cumberland Marsh Preserve (Dang et al. 2019). However, the inhibitory effects of salinity were different in the three habitat soils. Salinity reduced CH4 emissions by 33.67~97.82% (H1), 0.48~89.26% (H2), and 18.68~87.97% (H3), respectively. This may be influenced by multiple effects of wetland habitat, soil characteristics, and microbial community (Alves et al. 2022; Luo et al. 2022; Zhang et al. 2023). It is worth noting that a low concentration of salinity (1%) did not have a significant effect on CH4 emissions in H2 soil, which is consistent with the results in mangrove wetlands (Konnerup et al. 2014). In H1 soil, the four salinity treatments all significantly inhibited CH4 emissions. This may be related to lower microbial community richness and diversity in H1 soils (Table 1). In this study, CH4 emission was significantly correlated with soil DOC and NH4+ contents (P < 0.01). This is because CH4 emission is controlled by substrate availability (Yuan et al. 2018a). In addition, CH4 flux was mainly produced by methanogenic archaea and consumed by methanotrophs. The metabolic activity of these microorganisms is an important factor affecting CH4 emission (Lai 2009).
The effect of salinity on archaeal community
The alpha diversity of methanogenic archaea decreased significantly with the increase in salinity (Zhang et al. 2019). Similarly, salinity reduced the richness and diversity of soil archaeal communities in this study. Characterization of archaeal communities using high-throughput analysis revealed that elevated salinity significantly reduced the relative abundance of Methanosarciniales and Bathyarchaeia, which indicated that these two methanogens were less resistant to high salinity. They were found and dominated in paddy soils and marine sediments (Yuan et al. 2018b; Romano et al. 2021).
The relative abundance of Methanobacterium belonging to the order of Methanobacteriales increased under the salinity treatment. Methanobacterium is a salt-tolerant methanogen that is found to live in estuarine wetlands even in areas of high salinity (Mori and Harayama 2011; Chen et al. 2020b). The genus of Methanosarcina can use H2/CO2, acetic acid, and methyl substances as substrates to produce CH4 (Youngblut et al. 2015; Lyu et al. 2018). The inhibitory response of this genus to salinity was most evident in H1 soil. However, the genus of Methanosaeta belonging to the same order of Methanosarciniales was not detected in H1 soil. In H2 and H3 soils, salinity decreased the relative abundance of Methanosaeta. Different from Methanosarcina, Methanosaeta can only use acetate to produce CH4 (Mori et al. 2012). Previous research results have shown that the relative abundance of Bathyarchaeia was positively correlated with CH4 emissions (Cui et al. 2019). The relative abundance of Bathyarchaeia in H2 soil (18.53~42.31%) was higher than that in H3 soil (7.68~13.44%) and H1 soil (1.25~3.52%). This could partly explain why CH4 emissions were higher in H2 soil than those in H3 and H1 soils. Bathyarchaeia is widespread in deep-sea sediments and peatlands (Cui et al. 2019; L. Bräuer et al. 2020). However, high salinity inhibits the growth of Bathyarchaeia (Kallistova et al. 2020). The same result was obtained in this study. This may be because salinity affects the energy costs (associated with osmoregulation) and metabolic pathways of Bathyarchaeia, acting as an evolutionary barrier leading to the diversification of Bathyarchaeota (Fillol et al. 2016). Methanomassiliicoccus is a methylotrophic methanogen that is widely distributed in rice fields and wetlands (Söllinger et al. 2016; Lyu et al. 2018; Lu et al. 2022). In the present study, Methanomassiliicoccus was significantly positively correlated with CH4 emissions (P < 0.05), which was consistent with the results in rice fields in Hunan Province, China (Lu et al. 2022). However, there were inconsistent results in other regions (Jiang et al. 2022; Luo et al. 2022).
The relationship between archaea and soil characteristics
In this study, archaeal community characteristics were affected by soil pH, DOC, NH4+, NO3−, and Fe2+ concentrations. In general, a slightly alkaline environment is suitable for the growth of methane archaea (Malyan et al. 2016). The relative abundance of Methanobacteriales was positively correlated with pH, which was consistent with studies at pH 9.0 and 9.5 (Rao et al. 2018). However, Rice_Cluster_II, which prefers low hydrogen, was significantly negatively correlated with pH (P < 0.01). Rice_Cluster_II is widely present in acidic rice fields, and the change of pH could affect its metabolic pathways (Luo et al. 2022). In this study, Methanocella was significantly negatively correlated with DOC concentration and some other methanogenic archaea had weaker negative correlations with DOC, which might be due to the development of methanogenic archaea in tight association with the consumption of DOC. After the incubation, soil DOC content decreased by 20.87~97.71%. In addition, DOC can promote the utilization of CH4 by methanotrophs (Lew and Glińska-Lewczuk 2018). The same result was also found in rice fields (Luo et al. 2022). NH4+ was significantly positively correlated with Bathyarchaeia, Methanomassiliiccus, and Candidatus_Methanoperedens (P < 0.05). Among them, Candidatus_Methanoperedens was only detected in H2 soil. This genus is an anaerobic methanotrophic archaeon that oxidizes CH4 to CO2 and reduces NOX− to N2 and NH4+ (Ettwig et al. 2016; Chen et al. 2021). In this study, Fe3+ decreased the archaeal community diversity. Besides, Fe2+ concentration was significantly negatively correlated with the relative abundance of Methanobacteriaceae and Rice_Cluster_II (P < 0.05). This revealed a correlation between iron and CH4 emissions. Under anaerobic conditions, iron can promote decomposition of soil organic matter (Chen et al. 2020a). Methanogenic archaea can anaerobically degrade microscopic organic matter into CH4 (Cai et al. 2019).
Conclusion
High salinity (5%) significantly suppressed CH4 emission, reducing by 97.82% (H1), 89.20% (H2), and 87.97% (H3), respectively. Low salinity (1%) showed a significant inhibitory effect on CH4 emission in H1 and H3 soils, but had no significant effect in H2 soils. In addition, salinity decreased the relative abundance of methanogenic archaea and changed the community structure. Specifically, salinity had a promoting effect on Methanobacterium, while an inhibitory effect on Bathyarchaeia and Methanosaeta. The response of Methanosarcina to salinity was different in the three habitat soils. Soil pH, EC, DOC, and NH4+ concentrations were significantly correlated with the characteristics of the methanogenic archaeal community, thereby affecting CH4 emissions.
Data availability
All data are mentioned in the body of manuscript, tables, and figure.
References
Alves KJ, Pylro VS, Nakayama CR, Vital VG, Taketani RG, Santos DG, Rodrigues JLM, Tsai SM, Andreote FD (2022) Methanogenic communities and methane emissions from enrichments of Brazilian Amazonia soils under land-use change. Microbiol Res 265:127178. https://doi.org/10.1016/j.micres.2022.127178
Borrel G, Parisot N, Harris H, Peyretaillade E, Gaci N, Tottey W, Bardot O, Raymann K, Gribaldo S, Peyret P (2014) Comparative genomics highlights the unique biology of Methanomassiliicoccales, a Thermoplasmatales-related seventh order of methanogenic archaea that encodes pyrrolysine. BMC genomics 15(1):1–24. https://doi.org/10.1186/1471-2164-15-679
Bräuer SL, Basiliko N, Siljanen HMP, Zinder SH (2020) Methanogenic archaea in peatlands. FEMS Microbiol Lett 367(20):fnaa172. https://doi.org/10.1093/femsle/fnaa172
Bridgham SD, Cadillo Quiroz H, Keller JK, Zhuang Q (2013) Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob Change Biol 19(5):1325–1346. https://doi.org/10.1111/gcb.12131
Cai P, Ning Z, Zhang N, Zhang M, Guo C, Niu M, Shi J (2019) Insights into biodegradation related metabolism in an abnormally low dissolved inorganic carbon (DIC) petroleum-contaminated aquifer by metagenomics analysis. Microorganisms 7(10):412. https://doi.org/10.3390/microorganisms7100412
Chambers LG, Guevara R, Boyer JN, Troxler TG, Davis SE (2016) Effects of salinity and inundation on microbial community structure and function in a mangrove peat soil. Wetlands 36:361–371. https://doi.org/10.1007/s13157-016-0745-8
Chambers LG, Reddy KR, Osborne TZ (2011) Short-term response of carbon cycling to salinity pulses in a freshwater wetland. Soil Sci Soc Am J 75(5):2000–2007. https://doi.org/10.2136/sssaj2011.0026
Chen C, Hall SJ, Coward E, Thompson A (2020a) Iron-mediated organic matter decomposition in humid soils can counteract protection. Nat Commun 11(1):2255. https://doi.org/10.1038/s41467-020-16071-5
Chen F, Zheng Y, Hou L, Niu Y, Gao D, An Z, Zhou J, Yin G, Dong H, Han P (2021) Microbial abundance and activity of nitrite/nitrate-dependent anaerobic methane oxidizers in estuarine and intertidal wetlands: heterogeneity and driving factors. Water Res 190:116737. https://doi.org/10.1016/j.watres.2020.116737
Chen L, Li L, Zhang S, Zhang W, Xue K, Wang Y, Dong X (2022) Anaerobic methane oxidation linked to Fe(III) reduction in a Candidatus Methanoperedens-enriched consortium from the cold Zoige wetland at Tibetan Plateau. Environ Microbiol 24(2):614–625. https://doi.org/10.1111/1462-2920.15848
Chen S, Wang P, Liu H, Xie W, Wan XS, Kao SJ, Phelps TJ, Zhang C (2020b) Population dynamics of methanogens and methanotrophs along the salinity gradient in Pearl River Estuary: implications for methane metabolism. Appl Microbiol Biotechnol 104:1331–1346. https://doi.org/10.1007/s00253-019-10221-6
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34(17):i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Cui H, Su X, Chen F, Holland M, Yang S, Liang J, Su P, Dong H, Hou W (2019) Microbial diversity of two cold seep systems in gas hydrate-bearing sediments in the South China Sea. Mar Environ Res 144:230–239. https://doi.org/10.1016/j.marenvres.2019.01.009
Dang C, Morrissey EM, Neubauer SC, Franklin RB (2019) Novel microbial community composition and carbon biogeochemistry emerge over time following saltwater intrusion in wetlands. Glob Change Biol 25(2):549–561. https://doi.org/10.1111/gcb.14486
Duan B, Cai T, Man X, Xiao R, Gao M, Ge Z, Mencuccini M (2022) Different variations in soil CO2, CH4, and N2O fluxes and their responses to edaphic factors along a boreal secondary forest successional trajectory. Sci Total Environ 838:155983. https://doi.org/10.1016/j.scitotenv.2022.155983
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998. https://doi.org/10.1038/nmeth.2604
Ettwig KF, Zhu B, Speth D, Keltjens JT, Jetten MSM, Kartal B (2016) Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc Natl Acad Sci 113(45):12792–12796. https://doi.org/10.1073/pnas.1609534113
Fan L, Schneider D, Dippold MA, Poehlein A, Wu W, Gui H, Ge T, Wu J, Thiel V, Kuzyakov Y (2021) Active metabolic pathways of anaerobic methane oxidation in paddy soils. Soil Biol Biochem 156:108215. https://doi.org/10.1016/j.soilbio.2021.108215
Feng L, Zhang Z, Yang G, Wu G, Yang Q, Chen Q (2023) Microbial communities and sediment nitrogen cycle in a coastal eutrophic lake with salinity and nutrients shifted by seawater intrusion. Environ Res 225:115590. https://doi.org/10.1016/j.envres.2023.115590
Fillol M, Auguet JC, Casamayor EO, Borrego CM (2016) Insights in the ecology and evolutionary history of the Miscellaneous Crenarchaeotic Group lineage. ISME J 10(3):665–677. https://doi.org/10.1038/ismej.2015.143
Gao D, Liu F, Xie Y, Liang H (2018) Temporal and spatial distribution of ammonia-oxidizing organisms of two types of wetlands in Northeast China. Appl Microbiol Biotechnol 102(16):7195–7205. https://doi.org/10.1007/s00253-018-9152-9
Gütlein A, Gerschlauer F, Kikoti I, Kiese R (2018) Impacts of climate and land use on N2O and CH4 fluxes from tropical ecosystems in the Mt. Kilimanjaro region, Tanzania. Glob Change Biol 24(3):1239–1255. https://doi.org/10.1111/gcb.13944
Haese RR, Wallmann K, Dahmke A, Kretzmann U, Müller PJ, Schulz HD (1997) Iron species determination to investigate early diagenetic reactivity in marine sediments. Geochim Cosmochim Acta 61(1):63–72. https://doi.org/10.1016/S0016-7037(96)00312-2
Herbert ER, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardón M, Hopfensperger KN, Lamers LPM, Gell P (2015) A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6(10):1–43. https://doi.org/10.1890/es14-00534.1
Hofmann K, Praeg N, Mutschlechner M, Wagner AO, Illmer P (2016) Abundance and potential metabolic activity of methanogens in well-aerated forest and grassland soils of an alpine region. FEMS Microbiol Ecol 92(2):fiv171. https://doi.org/10.1093/femsec/fiv171
IPCC (2021) Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL (eds) The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press. https://www.ipcc.ch/report/sixth-assessment-report-working-group-i/. Accessed 20 March 2023
Jeppesen E, Beklioğlu M, Özkan K, Akyürek Z (2020) Salinization increase due to climate change will have substantial negative effects on inland waters: a call for multifaceted research at the local and global scale. The Innovation 1(2):100030. https://doi.org/10.1016/j.xinn.2020.100030
Jiang M, Xu P, Wu L, Zhao J, Wu H, Lin S, Yang T, Tu J, Hu R (2022) Methane emission, methanogenic and methanotrophic communities during rice-growing seasons differ in diversified rice rotation systems. Sci Total Environ 842:156781. https://doi.org/10.1016/j.scitotenv.2022.156781
Kallistova A, Merkel A, Kanapatskiy T, Boltyanskaya Y, Tarnovetskii I, Perevalova A, Kevbrin V, Samylina O, Pimenov N (2020) Methanogenesis in the Lake Elton saline aquatic system. Extremophiles 24:657–672. https://doi.org/10.1007/s00792-020-01185-x
Kirschke S, Bousquet P, Ciais P, Saunois M, Canadell JG, Dlugokencky EJ, Bergamaschi P, Bergmann D, Blake DR, Bruhwiler L (2013) Three decades of global methane sources and sinks. Nat Geosci 6(10):813–823. https://doi.org/10.1038/ngeo1955
Kong D, Li S, Jin Y, Wu S, Chen J, Hu T, Wang H, Liu S, Zou J (2019) Linking methane emissions to methanogenic and methanotrophic communities under different fertilization strategies in rice paddies. Geoderma 347:233–243. https://doi.org/10.1016/j.geoderma.2019.04.008
Konnerup D, Betancourt Portela JM, Villamil C, Parra JP (2014) Nitrous oxide and methane emissions from the restored mangrove ecosystem of the Ciénaga Grande de Santa Marta, Colombia. Estuar Coastal Shelf Sci 140:43–51. https://doi.org/10.1016/j.ecss.2014.01.006
Krauss KW, Whitbeck JL (2012) Soil greenhouse gas fluxes during wetland forest retreat along the lower Savannah River, Georgia (USA). Wetlands 32:73–81. https://doi.org/10.1007/s13157-011-0246-8
Lai DYF (2009) Methane dynamics in northern peatlands: a review. Pedosphere 19(4):409–421. https://doi.org/10.1016/s1002-0160(09)00003-4
Lan X, KW Thoning, EJ Dlugokencky (2022) Trends in globally-averaged CH4, N2O, and SF6 determined from NOAA Global Monitoring Laboratory measurements. Version 2023-03. Global Monitoring Laboratory. https://doi.org/10.15138/P8XG-AA10
Lew S, Glińska Lewczuk K (2018) Environmental controls on the abundance of methanotrophs and methanogens in peat bog lakes. Sci Total Environ 645:1201–1211. https://doi.org/10.1016/j.scitotenv.2018.07.141
Liu C, Li H, Zhang Y, Si D, Chen Q (2016) Evolution of microbial community along with increasing solid concentration during high-solids anaerobic digestion of sewage sludge. Bioresource Technol 216:87–94. https://doi.org/10.1016/j.biortech.2016.05.048
Liu F, Zhang Y, Liang H, Gao D (2019) Long-term harvesting of reeds affects greenhouse gas emissions and microbial functional genes in alkaline wetlands. Water Res 164:114936. https://doi.org/10.1016/j.watres.2019.114936
Lu Y, Liu Q, Fu L, Hu Y, Zhong L, Zhang S, Liu Q, Xie Q (2022) The effect of modified biochar on methane emission and succession of methanogenic archaeal community in paddy soil. Chemosphere 304:135288. https://doi.org/10.1016/j.chemosphere.2022.135288
Luo D, Li Y, Yao H, Chapman SJ (2022) Effects of different carbon sources on methane production and the methanogenic communities in iron rich flooded paddy soil. Sci Total Environ 823:153636. https://doi.org/10.1016/j.scitotenv.2022.153636
Lyu Z, Shao N, Akinyemi T, Whitman WB (2018) Methanogenesis. Curr Biol 28(13):R727–R732. https://doi.org/10.1016/j.cub.2018.05.021
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Malyan SK, Bhatia A, Kumar A, Gupta DK, Singh R, Kumar SS, Tomer R, Kumar O, Jain N (2016) Methane production, oxidation and mitigation: a mechanistic understanding and comprehensive evaluation of influencing factors. Sci Total Environ 572:874–896. https://doi.org/10.1016/j.scitotenv.2016.07.182
Moberly JG, Bernards MT, Waynant KV (2018) Key features and updates for origin 2018. J Cheminfom 10:1–2. https://doi.org/10.1186/s13321-018-0259-x
Morgan GA, Barrett KC, Leech NL, Gloeckner GW (2019) IBM SPSS for introductory statistics: use and interpretation. Routledge Press, UK
Mori K, Harayama S (2011) Methanobacterium petrolearium sp. nov. and Methanobacterium ferruginis sp. nov., mesophilic methanogens isolated from salty environments. International Journal of Systematic and Evolutionary Microbiology 61(1):138–143. https://doi.org/10.1099/ijs.0.022723-0
Mori K, Iino T, Suzuki KI, Yamaguchi K, Kamagata Y (2012) Aceticlastic and NaCl-requiring methanogen “Methanosaeta pelagica” sp. nov., isolated from marine tidal flat sediment. Appl Environ Microb 78(9):3416–3423. https://doi.org/10.1128/aem.07484-11
Nakagawa F, Yoshida N, Nojiri Y, Makarov V (2002) Production of methane from alasses in eastern Siberia: implications from its 14C and stable isotopic compositions. Glob Biogeochem Cycles 16(3):14–11. https://doi.org/10.1029/2000gb001384
Pattnaik P, Mishra SR, Bharati K, Mohanty SR, Sethunathan N, Adhya TK (2000) Influence of salinity on methanogenesis and associated microflora in tropical rice soils. Microbiol Res 155(3):215–220. https://doi.org/10.1016/s0944-5013(00)80035-x
Qiu S, Zhang X, Xia W, Li Z, Wang L, Chen Z, Ge S (2023) Effect of extreme pH conditions on methanogenesis: methanogen metabolism and community structure. Sci Total Environ 877:162702. https://doi.org/10.1016/j.scitotenv.2023.162702
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41(D1):D590–D596. https://doi.org/10.1093/nar/gks1219
Rao Y, Wan J, Liu Y, Angelidaki I, Zhang S, Zhang Y, Luo G (2018) A novel process for volatile fatty acids production from syngas by integrating with mesophilic alkaline fermentation of waste activated sludge. Water Res 139:372–380. https://doi.org/10.1016/j.watres.2018.04.026
Rath KM, Fierer N, Murphy DV, Rousk J (2019) Linking bacterial community composition to soil salinity along environmental gradients. ISME J 13(3):836–846. https://doi.org/10.1038/s41396-018-0313-8
Romano RG, Bendia AG, Moreira JCF, Franco DC, Signori CN, Yu T, Wang F, Jovane L, Pellizari VH (2021) Bathyarchaeia occurrence in rich methane sediments from a Brazilian ría. Estuar Coastal Shelf Sci 263:107631. https://doi.org/10.1016/j.ecss.2021.107631
Šmilauer P, Lepš J (2014) Multivariate analysis of ecological data using CANOCO 5. Cambridge university press, UK
Söllinger A, Schwab C, Weinmaier T, Loy A, Tveit AT, Schleper C, Urich T (2016) Phylogenetic and genomic analysis of Methanomassiliicoccales in wetlands and animal intestinal tracts reveals clade-specific habitat preferences. FEMS Microbiol Ecol 92(1):fiv149. https://doi.org/10.1093/femsec/fiv149
Ström L, Ekberg A, Mastepanov M, Røjle Christensen T (2003) The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Glob Change Biol 9(8):1185–1192. https://doi.org/10.1046/j.1365-2486.2003.00655.x
Sun Z, Jiang H, Wang L, Mou X, Sun W (2013) Seasonal and spatial variations of methane emissions from coastal marshes in the northern Yellow River estuary, China. Plant Soil 369:317–333. https://doi.org/10.1007/s11104-012-1564-1
Venturini AM, Dias NMS, Gontijo JB, Yoshiura CA, Paula FS, Meyer KM, Nakamura FM, da França AG, Borges CD, Barlow J (2022) Increased soil moisture intensifies the impacts of forest-to-pasture conversion on methane emissions and methane-cycling communities in the Eastern Amazon. Environ Res 212:113139. https://doi.org/10.1016/j.envres.2022.113139
Wallmann K, Hennies K, König I, Petersen Wand Knauth HD (1993) New procedure for determining reactive Fe(III) and Fe(II) minerals in sediments. Limnol Oceanogr 38(8):1803–1812. https://doi.org/10.4319/lo.1993.38.8.1803
Wang J, Cai C, Li Y, Hua M, Wang J, Yang H, Zheng P, Hu B (2018) Denitrifying anaerobic methane oxidation: a previously overlooked methane sink in intertidal zone. Environ Sci Technol 53(1):203–212. https://doi.org/10.1021/acs.est.8b05742
Wang W, Liang H, Li F, Su H, Li H, Gao D (2023) Water level of inland saline wetlands with implications for CO2 and CH4 fluxes during the autumn freeze–thaw period in Northeast China. Environ Sci Pollut Res 30(17):50125–50133. https://doi.org/10.1007/s11356-023-25862-4
Wang Y, Hu Z, Shen L, Liu C, Islam ARMT, Wu Z, Dang H, Chen S (2021) The process of methanogenesis in paddy fields under different elevated CO2 concentrations. Sci Total Environ 773:145629. https://doi.org/10.1016/j.scitotenv.2021.145629
Weston NB, Vile MA, Neubauer SC, Velinsky DJ (2011) Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102:135–151. https://doi.org/10.1007/s10533-010-9427-4
Wilson BJ, Mortazavi B, Kiene RP (2015) Spatial and temporal variability in carbon dioxide and methane exchange at three coastal marshes along a salinity gradient in a northern Gulf of Mexico estuary. Biogeochemistry 123:329–347. https://doi.org/10.1007/s10533-015-0085-4
Wu J, Wang M, Li P, Shen L, Ma M, Xu B, Zhang S, Sha C, Ye C, Xiong L (2022) Effects of pig manure and its organic fertilizer application on archaea and methane emission in paddy fields. Land 11(4):499. https://doi.org/10.3390/land11040499
Youngblut ND, Wirth JS, Henriksen JR, Smith M, Simon H, Metcalf WW, Whitaker RJ (2015) Genomic and phenotypic differentiation among Methanosarcina mazei populations from Columbia River sediment. ISME J 9(10):2191–2205. https://doi.org/10.1038/ismej.2015.31
Yuan J, Yuan Y, Zhu Y, Cao L (2018a) Effects of different fertilizers on methane emissions and methanogenic community structures in paddy rhizosphere soil. Sci Total Environ 627:770–781. https://doi.org/10.1016/j.scitotenv.2018.01.233
Yuan Q, Hernández M, Dumont MG, Rui J, Scavino AF, Conrad R (2018b) Soil bacterial community mediates the effect of plant material on methanogenic decomposition of soil organic matter. Soil Biol Biochem 116:99–109. https://doi.org/10.1016/j.soilbio.2017.10.004
Zhang K, Shi Y, Cui X, Yue P, Li K, Liu X, Tripathi Binu M, Chu H (2019) Salinity is a key determinant for soil microbial communities in a desert ecosystem. mSystems 4(1):e00225. https://doi.org/10.1128/mSystems.00225-18
Zhang W, Sheng R, Zhang M, Xiong G, Hou H, Li S, Wei W (2018) Effects of continuous manure application on methanogenic and methanotrophic communities and methane production potentials in rice paddy soil. Agric Ecosyst Environ 258:121–128. https://doi.org/10.1016/j.agee.2018.02.018
Zhang Z, Yang Z, Yue H, Xiao M, Ge T, Li Y, Yu Y, Yao H (2023) Discrepant impact of polyethylene microplastics on methane emissions from different paddy soils. Appl Soil Ecol 181:104650. https://doi.org/10.1016/j.apsoil.2022.104650
Acknowledgements
The authors gratefully acknowledge the financial supports by the National Natural Science Foundation of China (No. 31971468).
Author contributions
Feng Li: investigation, data analysis, and writing original draft. Huiju Li: data curation and investigation. Huihui Su: data curation and investigation. Wei Du: investigation and technical support. Zhongyan Gao: investigation and technical support. Huajun Liu: investigation and technical support. Hong Liang: supervision, draft revision, funding resources, and conceptualization. Dawen Gao: conceptualization, supervision, and draft revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors have read and approved the manuscript and accorded the consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Robert Duran
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, ., Li, H., Su, H. et al. Effects of salinity on methane emissions and methanogenic archaeal communities in different habitat of saline-alkali wetlands. Environ Sci Pollut Res 30, 106378–106389 (2023). https://doi.org/10.1007/s11356-023-29922-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29922-7