Abstract
Soil salinization adversely affects sustainable land use and limitation of greenhouse gas emission. Methane (CH4) uptake and the specific activity of methanotrophs in three saline–alkaline soils—S1, electrical conductivity (EC) 4.80 dS m−1; S2, EC 2.60 dS m−1; and S3, EC 0.74 dS m−1—were observed and measured across crop phenological development in the Hetao Irrigation District of Inner Mongolia, China. There were significant differences in CH4 uptake between the three soil types. The cumulative uptake of CH4 was 97.97 mg m−2, 109.49 mg m−2, and 150.0 mg m−2 in S1, S2, and S3, respectively. Cumulative CH4 uptake was 35%, 35%, and 53% lower in S1 than in S3, and was 27%, 28%, and 19% lower in S2 than in S3 in 2014, 2015, and 2016, respectively. Differences in CH4 uptake were driven by the different specific activities of the methanotrophs in the three soils, of which the key controlling factor was soil EC. The findings demonstrate that saline–alkaline soils with high EC led to low CH4 uptake and thereby significantly increased the total greenhouse effect of CH4.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric CH4 (methane) is recognized as one of the most important greenhouse gases (GHG) as a result of its high global warming potential [(GWP), 34 times greater than that of CO2 per molecule over a 100-year period] (Myhre et al. 2013). Further, this gas contributes to approximately 20% of total global warming (Shao et al. 2017). Well-ventilated and -drained forest, grassland, and cropland soils are important sinks for atmospheric methane (Van Zandvoort et al. 2017). Although the soil sink is relatively small, it is similar in magnitude to the annual CH4 increase in the atmosphere, and is strongly affected by human activities (Mosier et al. 1997). However, soil is in general studied far more as an atmospheric CH4 source than as a CH4 sink.
Saline–alkaline soil is one of the most widely distributed soil types worldwide and covers approximately 7% of the world land area (Ghassemi et al. 1995). There are about 99 million hectares of saline–alkaline soil in China. This area is mainly distributed over northern and coastal China (Wang et al. 1993). One of these areas is the Hetao Irrigation District in the northwestern inland, irrigated by water from the Yellow River. In this region, the ground water level is shallow and the level of return of salt to the surface of the soil is of concern. This accounts for about 60% of the acreage of saline–alkaline land in Inner Mongolia, China (Qin et al. 2005).
Excess salt concentration and alkalinity affect soil microbial activities, mineralization of carbon and nitrogen, and soil enzyme activities (Lozupone and Knight 2007). CH4 uptake in upland soils is highly sensitive to salt, with strong inhibition by even a small amount of salt (Whalen 2000). The salt-adding method, in which different concentrations of salt are added to a non-saline soil, has been used to study the effects of salinity on soil CH4 uptake (Whalen 2000). The methanotrophs, a specialized group of bacteria, are mostly responsible for CH4 oxidation (Trotsenko and Murrell 2008; Semrau et al. 2010) and play an important role in the oxidation of atmospheric CH4 (Cai et al. 2016). The key methanotroph enzyme is methane monooxygenase (MMO), which converts methane into methanol (Hakemian and Rosenzweig 2007; Trotsenko and Murrell 2008). Two types of methane monooxygenases have been described to date: a membrane-bound version (pMMO) and a cytoplasm-soluble version (sMMO). Nearly all aerobic methanotrophs described possess pMMO, with the exception of the genera Methylocella and Methyloferula, in which only sMMO has been detected (Dedysh et al. 2005; Vorobev et al. 2011). Thus, a gene encoding for pMMO polypeptides, pmoA, has been widely used as a molecular marker to characterize methanotrophic communities in environmental samples (Dumont and Murrell 2005). The specific activity of methanotrophs (Reim et al. 2012) is positively related to CH4 uptake rate in dryland farmland soil (Yang et al. 2010). Little information, however, has been published on the specific activity of methanotrophs or the microbial mechanisms affecting CH4 uptake in saline–alkaline soil.
Also lacking are field observations of the CH4 uptake flux in saline–alkaline soils. Despite the expansion of saline–alkaline soil area worldwide (Ho et al. 2018), CH4 consumption data and information regarding the total amount of CH4 uptake for saline–alkaline soils are very limited, and the impact of soil salinity on CH4 uptake has rarely been studied when assessing soil CH4 sinks (Zhang et al. 2011). CH4 uptake rates in salt-amended soils may not represent rates in native saline soils because microbial communities have insufficient time to adapt to the changing conditions in salt-affected soils (Conde et al. 2005). Therefore, it is very important that field observations of greenhouse gases in saline–alkaline soils be carried out to reduce the uncertainty in estimating total uptake in saline–alkaline soils.
To this end, in the present study, we investigated CH4 uptake by naturally saline–alkaline soils via field observations. The objectives of this study were to estimate the potential for CH4 uptake in native saline–alkaline soil, and to assess the control of CH4 uptake by methanotrophs as a result of increased soil salinity.
Materials and methods
Study site and plot selection
The study area was located in the Urad Front Banner in the Hetao Irrigation District in Inner Mongolia (108°11′–109°54′E, 40°28′–41°16′N) 1007 m above sea level. The land is located in arid and semiarid areas on the upper and middle reaches of the Yellow River in northwestern China. There are 3202 h of annual average sunshine. The annual average temperature is 3.6–7.3 °C. The highest and lowest temperature extremes are 38.9 °C and − 36.5 °C respectively. The frost-free period is 120 days per year. The average annual precipitation is 200–260 mm, and the annual average evaporation 1900–2300 mm (Yang et al. 2015).
To reduce soil heterogeneity, three adjacent farmlands with saline–alkaline soils of different salinity levels were selected as research plots. The electrical conductivity (EC) and saline content of the three types of soil were 4.80 dS m−1 and 1.69% (S1), 2.60 dS m−1 and 0.83% (S2), and 0.74 dS m−1 and 0.12% (S3), respectively (Tables 1, 3). The soils with the EC values of 0.74 and 2.6 dS m−1 could be considered saline–alkaline soil (Wang et al. 1993). Wang et al. (1993) confirmed that saline content is the basis for distinguishing saline–alkaline soil and non-saline soil. Non-alkaline soil is defined as soil with a saline content lower than 0.1%. The saline content in the soils with the EC values of 0.74 and 2.6 dS m−1 in this study is 0.12% and 0.83%, respectively, which are higher than 0.1% (Table 2). The plots tested were about 100 m apart and were all within an area of 5 ha. Three replicate sub-plots of 100 m × 100 m were defined in each study plot.
Field trials were conducted from April 2014 to October 2016. The physical and chemical properties of the various soils were determined (Tables 3, 4). The fields were planted with sunflowers in June and harvested in October in every year. The fields were machine-plowed before crops were planted each year. Diammonium phosphate (DAP) was applied as a seeding fertilizer, at a total nitrogen rate of 127.5 kg ha−1. Urea was applied at a total nitrogen rate of 345.0 kg ha−1 at the seedling stage. The chemical control of diseases, insect pests, weeds, as well as P and K fertilizer application amounts and other field management measures were uniform across treatment groups and consistent with typical management measures for local farmland production.
Collection and determination of gas samples
Three fixed sampling points were set within each replicate sub-plot of each study plot. Atmospheric air samples were collected using the static chamber. The chamber was placed on the ground when samples were taken. The chamber measured 0.5 m × 0.5 m × 0.5 m. Gas samples were collected between 07:00 and 10:00 once every 10 days from July to September and twice per month in April and October. About 100 mL of gas was drawn through a 100-mL injector connected to three sampling ports that passed through the chamber. The sampling time was 20 min per chamber. Samples were taken over 0, 5, 10, 15, and 20 min, with five samples per chamber and three replicate sets of samples per sampling. The collected gas samples, in cap-lock syringes, were quickly taken back to the laboratory, where they were analyzed using an Agilent 6820 gas chromatograph (Agilent 6820D, Agilent Technologies, Santa Clara, CA, USA). A linear regression was performed by comparing the CH4 mixing ratio and the corresponding sampling interval (0, 5, 10, 15, or 20 min) for each of the five samples. The soil CH4 uptake rate was estimated based on this regression. The CH4 uptake flux per unit area was calculated from the atmospheric pressure, temperature, universal gas constant, the effective height of the sample chamber, and CH4 molecular weight.
Collection and preparation of soil samples
While gas samples were being acquired, soil samples were collected using a soil drill with a diameter of 0.05 m, using the S-shaped sampling method. Samples were taken from 10 points per plot and mixed together, then packed into sealed bags and brought back to the laboratory. A sub-sample of 20 g of soil was taken from each sample and stored at − 80 °C until DNA extraction. The remaining soil was dried and passed through a 5-mm sieve.
Determination of soil physical and chemical properties
The soil temperature and water content was measured in situ at a depth of 0.075 m using a temperature analyzer and TDR moisture analyzer, respectively. In the laboratory, soil organic carbon (SOC) was determined using a TOC analyzer (Sievers 5310 C, GE Analytical Instruments, USA) (Lim and Choi 2014). Total nitrogen (TN) in the soil was determined via a dry combustion method using a C/N Analyzer (Vario Macro, Elementar, Germany) (Yan et al. 2012). Soil NH4+–N and NO3−–N were measured using a micro-Kjeldahl procedure (Aulakh et al. 2000). pH of the samples (1:1 soil:water) was determined using an AS-3000 Dual pH Analyser (Labfit Pty Ltd, Burswood, Western Australia) (Godsey et al. 2007). Soil electrical conductivity (EC) was measured using the composite electrode method at a soil: water ratio of 1:5 (He et al. 2012). Soil bulk density (ρb) was determined via ring shear testing. Soil texture was determined via the pipette method (Schlichting et al. 1995). The concentrations of Cl−, SO42−, Na+, K+, Mg2+, and Ca2+ were determined using a DIONEX ICS-3000 Ion Chromatography System (Pisinaras et al. 2010). Direct spectrophotometric measurements of (CO32−) were performed using procedures similar to those outlined by Easley et al. (2013). Bicarbonate ion (HCO3−) content was determined using potentiometric titration (Bocanegra-Garcia and Carrillo-Chavez 2003).
Assessment of abundance of methanotrophs
Soil methanomorph DNA was extracted using the CTAB/SDS method (Henckel et al. 2000). The abundance of methanotrophs was analyzed by the qPCR for methanogens (pmoA) (Ho et al. 2011) conducted using the primers A189f (5′-GGN GAC TGG GAC TTC TGG-3′) and A682r (5′-GAA SGC NGA GAA GAA SGC-3′) in the study. Holmes et al. (1995) designed degenerate oligonucleotide primers A189f/A682r used to amplify pmoA, which has been widely used to reveal the community characteristics of methanooxidas in various environments, and demonstrated that there is higher identity to pmoA sequences from the y-Proteobacteria (methanotrophs) than to amoA sequences. The amplification system (25 μL): 10 × PCR buffer, 2.5 μL; dNTP (2.5 mmol L−1), 1.6 μL; primers F (5 P), 1 μL; primers R (5 P), 1 μL; Taq (5 U μL−1), 0.125 μL; template DNA, 50 ng, and H2O 16.775 µL. The PCR amplification procedure was as follows: pre-denaturation at 95 °C for 4 min, denaturation at 95 °C for 30 s, renaturation at 60 °C for 30 s, and extension at 72 °C for 30 s. These steps were repeated for 30 cycles. Then, a final extension at 72 °C for 10 min was carried out.
PCR products were purified using a DNA Gel Extraction Kit (Axygen Scientific, Inc., Union City, CA, USA). Screening of positive clones and sequencing was performed on inserted bacterial DNA fragments. Plasmids were extracted using a needle provided in the kit and used as a standard for positive clones of the correct sequence. The prepared plasmid standard was subjected to a 10-fold gradient mass concentration dilution. A standard template of four dilutions was obtained. The number of cycles in the qPCR reaction was plotted versus the logarithm of the number of copies of different templates to create standard curves. qPCR was performed in a T gradient thermocycler (Biometra GmbH, Germany). The gel imager was a Gel-Doc2000 gel imaging system (BioRad, Hercules, CA, USA).
CH4 incubation
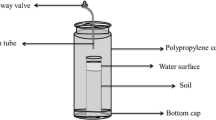
The CH4 incubation experiment was carried out to calculate the specific activity of methane-oxidizing bacteria. The S1, S2, and S3 soil samples were air-dried, sifted to 50 g, and loaded into a 250-mL culture bottle (Glasgeratebau OCHS GmbH). Then, 5 mL of sterilized and deionized water was added to this bottle, which was then pre-incubated for 7 days in order to activate soil microbes in the incubator. The culture bottle was taken out after a week and more water was added to achieve a water content of 25% after pre-incubation. The bottle was then sealed using a T type silica gel plug and placed in a thermostat incubator to be cultivated for 360 h at 25 °C. Each treatment was repeated three times. In order to maintain a constant pressure in the bottle, mixed air at the same volume as the bottle was pumped out by an injector before pure CH4 (2.64 mg L−1) was injected into the bottle. CH4 concentrations in the ambient were approximately 2.64 mg L−1. The gas was extracted once every 12 h the first 12 times, then once every 24 h. The concentration of CH4 was determined using a gas chromatograph (Agilent 6820, Agilent Technologies) equipped with a flame ionization detector (FID) detector and Porapak Q of stainless steel (80/100 mesh). The working conditions with regards to the column temperature, injector, and detector temperatures were 55 °C, 130 °C, 250 °C. The flow rate of carrier gas (N2), hydrogen and air in FID were set to 30 mL min−1, 30 mL min−1 and 400 mL min−1, respectively. The uptake rate of CH4 is represented as ng kg−1 h−1.
Calculations
The equations for calculation of the abundance of methanotrophs, CH4 uptake flux, CH4 uptake rate, and specific activity of methanotrophs are shown as Eqs. (1)–(4), respectively.
C, abundance of methanotrophs (number of copies g−1 soil); V, DNA volume (µL); n, amount of DNA (g mol−1); C, mass concentration of the target to be detected. The mass concentration of the sample fragments is obtained by data conversion from PCR based on a standard (ng µL−1); N, base number of the pEASY-T base. This is the standard base pair number constructed for the vector (bp); M, average molecular weight of DNA (607.4 g mol−1)
K, CH4 uptake flux (µg m−2 h−1); H, static camera height (10−2 m); Mc, molar mass of greenhouse gases (g mol−1); V0, standard-state CH4 molar volume (L); P0, standard-state atmospheric pressure (Pa); T0, standard-state temperature (°C); dc/dt, slope of CH4 gas concentration versus sampling time (ppm h−1)
P, uptake rate of CH4 per unit soil (ng kg−1 h−1); dc/dt, variation of CH4 mass concentration in culture flasks unit time; Vh, volume of interior space of culture bottle (mL); Ws, weight of soil sample (g); MW, molecular weight of CH4 (16.04); MV, volume of 1 mol gas at standard state (22.4 L); T, culture temperature (°C); Tst, standard temperature (273 °C)
η, specific activity (ng kg−1 copies−1 h−1); P, uptake rate of CH4 per unit (ng kg−1 h−1) is calculated using Eq. (3); C, pmoA gene abundance (number of copies g−1 soil).
Data analysis
Data and graphics were processed using SigmaPlot13, OriginPro8, and Excel2010 software. Single-factor analysis of variance (ANOVA) was performed in SPSS22.0. The significance of the differences in CH4 uptake was analyzed using ANOVA. Correlation analysis and stepwise regression analysis were performed to investigate the effects of soil properties on CH4 uptake.
Results
Seasonal variations CH4 uptake flux in soil with different salinity and alkalinity
The average annual CH4 uptake flux was 0.001–0.108 mg m−2 h−1 over the 3-year study period (Fig. 1). The CH4 flux was negative, indicating that saline soil acted as a sink for atmospheric CH4 in the study plots. The seasonal variation in soil CH4 uptake flux was significantly different across years for all three soil types. The obvious peak in CH4 uptake rate coincided with the flowering stage of crop phenological development in all three soils. These peaks in July and August were respectively 0.271 mg m−2 h−1 and 0.201 mg m−2 h−1 in 2014, 0.199 mg m−2 h−1 and 0.234 mg m−2 h−1 in 2015, and 0.292 mg m−2 h−1 and 0.261 mg m−2 h−1 in 2016. The trend in the fluctuation in S1 was less obvious than that for the other two soils, but it was not flat. The maximum CH4 uptake in S1 in 2014 was 0.058 mg m−2 h−1, that in 2015 was 0.118 mg m−2 h−1, and that in 2016 was 0.113 mg m−2 h−1. Cumulative CH4 uptake was lowest in S1 and highest in S3 throughout the growing season.
Effects of cumulative CH4 uptake on different saline and alkaline levels
There were significant differences in cumulative CH4 uptake across S1, S2, and S3 soils in 2014 (F = 18.0, p < 0.001), 2015 (F = 23.6, p < 0.001), and 2016 (F = 28.4, p < 0.001) (Fig. 2). The uptake of CH4 decreased with the increase in soil EC. The total amount of CH4 accumulated in S3 was 150.0 mg m−2, 119.6 mg m−2, and 99.9 mg m−2 in the 2014, 2015, and 2016 growth seasons, respectively. The amount of CH4 accumulated in S2 was 27%, 28%, and 19% lower than that in S3 in each year, respectively. The cumulative uptake amount of CH4 in S1 was 35%, 35%, and 53% lower than that of S3 in the 3 years. The cumulative CH4 uptake was greatest in 2014 and least in 2016, for all three soil types.
Cumulative uptake of CH4 by saline–alkaline soils during the growing seasons in 2014, 2015, and 2016. The vertical bars represent standard error. There was significant difference for different lowercase letters in the same column (p < 0.05). S1: EC 4.80 dS m−1, S2: EC 2.60 dS m−1, and S3: EC 0.74 dS m−1
Analysis of abundance and specific activity of methanotrophs in different saline–alkaline soils
There were significant differences in the abundance of methanotrophs between the three soil types in 2014 (F = 29.3, p < 0.001), 2015 (F = 28.4, p < 0.001), and 2016 (F = 26.6, p < 0.001), with the values increasing yearly (Fig. 3). The abundance of methanotrophs was greatest in S1 and least in S3 in all 3 years. The overall range of abundance of soil methanotrophs was 10,730–25,010 copies g−1 soil. The activity of individual methanotrophs may be characterized by the value of their specific activity. During the peak of methane uptake in July each year, the specific activity of methanotrophs was between 38.3 × 10−4 ng kg−1 copies−1 h−1 and 5.4 ng kg−1 copies−1 h−1, and there were significant differences (F = 15.51, p < 0.01) between the three soils types from 2014 to 2016. The specific activity of methanotrophs was greatest in S3 and least in S1—as EC increased, methanotroph activity decreased. The annual specific activity of methanotrophs was greatest in 2014 and decreased over time.
Abundance of methanotrophs in saline–alkaline soils during the growing seasons in 2014, 2015, and 2016. The vertical bars represent standard error. There was significant difference for different lowercase letters in the same column (p < 0.05). S1: EC 4.80 dS m−1, S2: EC 2.60 dS m−1, and S3: EC 0.74 dS m−1
Correlation analysis of soil CH4 uptake flux to specific activity of methanotrophs, EC, moisture, and temperature
The correlation analysis showed that EC of soil was negatively correlated with specific activity of soil methanotrophs (Fig. 4). There was a positive correlation between CH4 uptake flux and the specific activity of soil methanotrophs (Fig. 5). The higher the EC, the lower was the specific activity of methanotrophs and the rate of CH4 uptake. Single-factor correlation analysis showed that there was no significant correlation between CH4 uptake and soil temperature or moisture (p > 0.05).
Discussion
Potential links of CH4 uptake in different saline–alkaline soils
CH4 uptake flux was far greater in the summer (July and August), when temperatures and moisture levels were high. The seasonal variations in soil moisture and temperature in all three soil types were consistent with the seasonal variation of CH4 uptake, which was low in the periods from April to June and from August to November (Fig. 1).
The finding that CH4 uptake flux was largest when temperatures and moisture levels were highest was consistent with the seasonal variation of CH4 uptake flux measured in montane grasslands saline soils by Unteregelsbacher et al. (2013). The latter study reported that the flux was highest in summer, which is also consistent with the findings of other studies on arid and semi-arid saline alkali soil (Hart 2006; Sjogersten and Wookey 2002). An increase in soil temperature and moisture not only accelerates the enzyme catalytic activity involved in CH4 uptake, but also increases the porosity of soil gas exchange with the atmosphere, thus improving CH4 uptake flux in saline–alkaline soil.
CH4 uptake is altered by different ecosystems and regional environments (Wang et al. 2014). In this study, the annual CH4 uptake fluxes from 2014 to 2016 were 2.0–75.26 mg m−2 h−1, and were higher than the published CH4 flux of 0.013–0.279 mg m−2 h−1 in well-aerated non-saline arable soils in other studies (Geng et al. 2010). The saline–alkali soils in the Hetao Irrigation District of Inner Mongolia acted as a CH4 sink.
The total amount of CH4 accumulated was highest in the S3 soil and lowest in the S1 soil. The uptake rate of CH4 decreases with increase in soil EC (Fig. 2). salt inhibits methane oxidation (Saari et al. 2004; Zhang et al. 2011). When salt, especially chloride salt, was added to salt-free soils, CH4 uptake was strongly inhibited (King and Schnell 1998; Whalen 2000). There is a significant negative correlation between CH4 flux and soil salinity in salt marsh soil in the United States (Bartlett et al. 1987). The low saline–alkaline soil (3.2 dS m−1) exhibited enhanced CH4 uptake potential under the same texture conditions as soil with higher salt concentrations (Zhang et al. 2011). This inhibition of methane oxidation by salt is owing to the high ionic salt concentrations inhibiting oxidation process as a result of moisture stress (Dalal et al. 2008).
Abundance of methanotrophs in saline–alkaline soils
Methanotrophs play an important role in the oxidation of CH4 in the atmosphere. Their activity directly affects the rate of CH4 oxidation in the soil and the amount of CH4 moved from the soil into the atmosphere (Sitaula et al. 1995). High-salt soils (pH 10–10.5) have high abundance of methanotrophs, as some types of methanotrophs in high-salinity soils exhibit strong salt tolerance (Sorokin et al. 2000). Tsubota et al. (2005) showed that the thermophilic mesophilic methanotrophs in hot spring sediment soil tolerate NaCl with a mass concentration of 3%. Sorokin et al. (2000) isolated a new type I methanotroph that can survive in a high-salinity and high-alkalinity environment. In our study, pmoA was used to detect the abundance of methanotrophs in saline–alkaline soils. The methanotrophs harbouring only the mmoX gene in the methanotrophic community composition are not expected to thrive in environments with a high salinity (Dedysh 2011). The abundance of methanotrophs was the highest in S1 and lowest in S3. Generally, methanotrophs were most abundant in soils with high salinity (Fig. 3).
Specific activity of methanotrophs in saline–alkaline soils
The activity of individual methanotrophs can be characterized by measurement of specific activity determined by measuring the CH4 uptake rate and abundance of methanotrophs (Kolb et al. 2005). The abundance of methanotrophs during the peak of CH4 uptake in July was the highest, and their specific activity was the lowest in soil with the highest EC, in all 3 years (Figs. 3, 4). These results are consistent with those of Deng et al. (2017). The specific activity of methanotrophs in the high-salt soil was low, possibly because a pMMO enzyme in the methanotroph group is not expressed in saline soils (Deng et al. 2017).
Correlation between CH4 uptake flux and the specific activity of the methanotrophs
This study shows that the CH4 uptake flux was significantly positively correlated with the specific activity of the methanotrophs (Fig. 5). In another study, we set up a gradient of salt contents to study the effects of CH4 uptake on specific activity of methanotrophs in saline–alkaline soil, and obtained the same results as in this study (Yang et al. 2015). Yang et al. (2010) also showed that the specific activity of methane-oxidizing bacteria in dark brown soil was positively correlated with the CH4 uptake rate. The specific activity of methanotrophs had an effect on the CH4 uptake rate after the transformation of grassland into artificial forest in Russia (Menyailo et al. 2008). Saline soil (EC 9.0 dS m−1) and extremely saline soil (EC 84.8 dS m−1) restricted the pmoA gene diversity of methanotrophs, with lower bacterial specific activity and decreased CH4 uptake flux than that of aerobic upland soil (Serrano-Silva et al. 2014). Therefore, the community abundance of methanotrophs is not the key factor that affects CH4 uptake rates in the soil. It was found that the greater the specific activity of the methanotrophs, the greater the CH4 uptake flux (Fig. 5). This study indicates that modification of the oxidation capacity by regulation of the specific activity of methanotrophs represents a strategy to control CH4 uptake in saline–alkaline soil.
CH4 uptake is controlled by the specific activity of the methanotrophs driven by EC
Soil temperature and moisture are not important single factors affecting CH4 uptake. This may be because CH4 uptake is subject to the cumulative effect of methanotroph specific activity, EC, moisture, and temperature—the contribution of soil temperature and moisture to CH4 uptake is smaller and was obscured by the effects of specific activity of methanotrophs and EC to CH4 uptake. The oxidation capacity of methanotrophs from different upland soils varied by about one order of magnitude (Knief and Dunfield 2005). Soil ionic salt concentrations, such as EC and pH, are key factors in regulating bacterial diversity and community structure (Fierer and Jackson 2006). The high EC content in soil activated only a small subset of the overall methanotroph diversity. Methylobacter, belonging to type I methanotrophs, were favored by salt stress in the soil with an EC of 28 dS m−1 (Ho et al. 2018). In this study, the abundances of methanotrophs in the S1 soil with the highest EC was more than 1 times that in the S3 soil, with the lowest EC (Fig. 3). However, the CH4 uptake rate from S1 soil did not increase correspondingly (Fig. 1). A correlation analysis showed that there was a negative correlation between soil EC and the specific activity of methanotrophs (Fig. 4), but a positive correlation between CH4 uptake flux and the specific activity of soil methanotrophs (Fig. 5). As soil EC decreased, the specific activity of the methanotrophs increased, and the uptake rate of CH4 increased. The rate of methane oxidation decreased significantly with increasing salinity, indicating that some methanotrophs may not oxidize methane in soil with high EC. The exact reason for this observation remains to be elucidated. Further studies on the relationship between soil properties, methanotrophic community characteristics, and soil CH4 uptake rate are critical for an in-depth understanding of CH4 uptake by different saline–alkaline soil.
Conclusions
Field observation data for CH4 uptake and the results of analysis of specific activity of methanotrophs were integrated in saline–alkaline soils with different EC from 2014 to 2016. The community abundance of methanotrophs was determined not to be a key factor affecting soil CH4 uptake rates. However, the specific activity of methanotrophs represents a key microbial driver of CH4 uptake. Modification of the CH4 uptake capacity by regulation of the specific activity of the methanotrophs represents a control strategy for CH4 uptake by saline–alkaline soil. Soil EC influences the specific activity of methanotrophs in saline–alkaline soils. Changes in soil EC may alter the strength of the soil CH4 sink, altering the global CH4 budget. Soil remediation, the control of saline soil, and the reduction of ionic salt content in saline soil are common challenges for global scientists.
References
Aulakh MS, Khera TS, Doran JW (2000) Mineralization and denitrification in upland, nearly saturated and flooded subtropical soil I. Effect of nitrate and ammoniacal nitrogen. Biol Fertil Soils 31:162–167
Bartlett KB, Bartlett DS, Harriss RC, Sebacher DI (1987) Methane emissions along a salt marsh salinity gradient. Biogeochemistry 4:183–202
Bocanegra-Garcia G, Carrillo-Chavez A (2003) Hydro-geochemical behavior of bicarbonate and sulfate ions leaching from a sulfide-poor silver mine in Central Mexico: potential indicator of acid mine drainage. Bull Environ Contam Toxicol 71:1222–1229
Cai YF, Zheng Y, Bodelier PLE, Conrad R, Jia ZJ (2016) Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soils. Nat Commun 7:11728
Conde E, Cardenas M, Ponce-Mendoza A, Luna-Guido ML, Cruz-Mondragon C, Dendooven L (2005) The impacts of inorganic nitrogen application on mineralization of 14C-labelled maize and glucose, and on priming effect in saline–alkaline soil. Soil Biol Biochem 37:681–691
Dalal RC, Allen DE, Livesley SJ, Richards G (2008) Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: a review. Plant Soil 309:43–76
Dedysh SN (2011) Cultivating uncultured bacteria from northern wetlands: knowledge gained and remaining gaps. Front Microbiol 2(184):1–15
Dedysh SN, Knief C, Dunfield PF (2005) Methylocella species are facultatively methanotrophic. J Bacteriol 187:4665–4670
Deng YC, Liu YQ, Dumont M, Conrad R (2017) Salinity affects the composition of the aerobic methanotroph community in alkaline lake sediments from the Tibetan Plateau. Microb Ecol 73:101–110
Dumont MG, Murrell JC (2005) Community-level analysis: key genes of aerobic methane oxidation. Methods Enzymol 397:413–427
Easley RA, Patsavas MC, Byrne RH, Liu X, Feely RA (2013) Spectrophotometric measurement of calcium carbonate saturation states in seawater. Environ Sci Technol 47(3):1468–1477
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631
Geng YB, Luo GQ, Yuan GF (2010) CH4 uptake flux of Leymus chinensis steppe during rapid growth season in Inner Mongolia, China. Sci China Earth Sci 53(7):977–983
Ghassemi F, Jakeman AJ, Nix HA (1995) Salinisation of land and water resources: human causes, extent, management and case studies. University of New South Wales Press Ltd, Canberra
Godsey CB, Pierzynski GM, Mengel DB, Lamond RE (2007) Changes in soil pH, organic carbon, and extractable aluminum from crop rotation and tillage. Soil Sci Soc Am J 71:1038–1044
Hakemian AS, Rosenzweig AC (2007) The biochemistry of methane oxidation. Annu Rev Biochem 76:223–241
Hart SC (2006) Potential impacts of climate change on nitrogen transformations and greenhouse gas fluxes in forests: a soil transfer study. Glob Change Biol 12:1032–1046
He YB, DeSutter T, Prunty L, Hopkins D, Jia XH, Wysocki DA (2012) Evaluation of 1:5 soil to water extract electrical conductivity methods. Geoderma 185–186:12–17
Henckel T, Roslev P, Conrad R (2000) Effects of O2 and CH4 on presence and activity of the indigenous methanotrophic community in rice field soil. Environ Microbiol 2(6):666–679
Ho A, Lüke C, Frenzel P (2011) Recovery of methanotrophs from disturbance: population dynamics, evenness and functioning. ISME J 5(4):750–758
Ho A, Mo YL, Lee HJ, Sauheitl L, Jia ZJ, Horn MA (2018) Effect of salt stress on aerobic methane oxidation and associated methanotrophs; a microcosm study of a natural community from a non-saline environment. Soil Biol Biochem 125:210–214
Holmes AJ, Costello AM, Lidstrom ME, Murrell JC (1995) Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett 132(3):203–208
King GM, Schnell S (1998) Effects of ammonium and non-ammonium salt additions on methane oxidation by Methylosinus trichosporium OB3b and Maine forest soils. Appl Environ Microb 64:253–257
Knief C, Dunfield PF (2005) Response and adaptation of different methanotrophic bacteria to low methane mixing ratios. Environ Microbiol 7:1307–1317
Kolb S, Knief C, Dunfield PF, Conrad R (2005) Abundance and activity of uncultured methanotrophic bacteria involved in the consumption of atmospheric methane in two forest soils. Environ Microbiol 7:1150–1161
Lim SS, Choi WJ (2014) Changes in microbial biomass, CH4 and CO2 emissions, and soil carbon content by fly ash co-applied with organic inputs with contrasting substrate quality under changing water regimes. Soil Biol Biochem 68:494–502
Lozupone CA, Knight R (2007) Global patterns in bacterial diversity. Proc Natl Acad Sci USA 104:11436–11440
Menyailo OV, Hungate BA, Abraham WR, Conrad R (2008) Changing land use reduces soil CH4 uptake by altering biomass and activity but not composition of high-affinity methanotrophs. Glob Change Biol 14:2405–2419
Mosier AR, Parton WJ, Valentine DW, Ojima DS, Schimel DS, Heinemeyer O (1997) CH4 and N2O fluxes in Colorado shortgrass steppe. 2. Long-term impact of land use change. Glob Biogeochem Cycles 11:29–42
Myhre G, Shindell D, Bréon FM (2013) Anthropogenic and natural radiative forcing. In: Stocker TF, Qin D, Plattner GK et al (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 659–740
Pisinaras V, Tsihrintzis VA, Petalas C, Ouzounis K (2010) Soil salinization in the agricultural lands of Rhodope District, northeastern Greece. Environ Monit Assess 166:79–94
Qin R, Wang XF, Liu ST (2005) Advances in saline alkali soil improvement. Contemp Eco-Agric 1:32–34 (in Chinese)
Reim A, Lüke C, Krause S, Pratscher J, Frenzel P (2012) One millimetre makes the difference: high-resolution analysis of methane-oxidizing bacteria and their specific activity at the oxic–anoxic interface in a flooded paddy soil. ISME J 6:2128–2139
Saari A, Smolander A, Martikainen PJ (2004) Mathane consumption in a frequently nitrogen-fertilized and limed spruce forest soil after clear-cutting. Soil Use Manag 20:65–73
Schlichting E, Blume HP, Stahr K (1995) Bodenkundliches Praktikum. Blackwell, Berlin
Semrau JD, DiSpirito AA, Yoon S (2010) Methanotrophs and copper. FEMS Microbiol Rev 34:496–531
Serrano-Silva N, Valenzuela-Encinas C, Marsch R, Dendooven L, Alcántara-Hernández RJ (2014) Changes in methane oxidation activity and methanotrophic community composition in saline alkaline soils. Extremophiles 18:561–571
Shao R, Xu M, Li RQ, Dai XQ, Liu LX, Yuan Y, Wang HM, Yang FT (2017) Land use legacies and nitrogen fertilization affect methane emissions in the early years of rice field development. Nutr Cycl Agroecosyst 107:369–380
Sitaula BK, Bakken LRR, Abrahamsen G (1995) CH4 uptake by temperate forest soil effect of N input and soil acidification. Soil Biol Biochem 27:871–880
Sjogersten S, Wookey PA (2002) Spatio-temporal variability and environmental controls of methane fluxes at the forest-tundra ecotone in the fennoscandian mountains. Glob Change Biol 8:885–894
Sorokin DY, Jones BE, Kuenen JG (2000) An obligate methylotrophic, methane-oxidizing methylomicrobiura species from a highly alkaline environment. Extremophiles 4:145–155
Trotsenko YA, Murrell JC (2008) Metabolic aspects of aerobic obligate methanotrophy. Adv Appl Microbiol 63:183–229
Tsubota J, Eshinimaey BT, Khmelenina VN, Trotsenko YA (2005) Methylothermus thermalis gen. nov., sp. nov., a novel moderately thermophilic obligate methanotroph from a hot spring in Japan. Int J Syst Evolut Microbiol 55:1877–1884
Unteregelsbacher S, Gasche R, Lipp L, Sun W, Kreyling O, Geitlinger H, Kögel-Knabner I, Papen H, Kiese R, Schmid HP, Dannenmann M (2013) Increased methane uptake but unchanged nitrous oxide flux in montane grasslands under simulated climate change conditions. Eur J Soil Sci 64:586–596
Van Zandvoort A, Lapen DR, Clark LD, Flemming C, Craiovan E, Sunohara MD, Boutz R, Gottschall N (2017) Soil CO2, CH4, and N2O fluxes over and between tile drains on corn, soybean, and forage fields under tile drainage management. Nutr Cycl Agroecosyst 109:115–132
Vorobev AV, Baani M, Doronina NV, Brady AL, Liesack W, Dunfield PF, Dedysh SN (2011) Methyloferula stellata gen. nov., sp. nov., an acidophilic, obligately methanotrophic bacterium that possesses only a soluble methane monooxygenase. Int J Syst Evolut Microbiol 61:2456–2463
Wang ZQ, Zhu SQ, Yu RP (1993) Chinese salty soil. Science Press, Beijing (in Chinese)
Wang YF, Chen H, Zhu QA, Peng CH, Wu N, Yang G, Zhu D, Tian JQ, Tian LX, Kang XM, He YX, Gao YH, Zhao XQ (2014) Soil methane uptake by grasslands and forests in China. Soil Biol Biochem 74:70–81
Whalen SC (2000) Influence of N and non-N salts on atmospheric methane oxidation by upland boreal forest and tundra soils. Biol Fertil Soils 31:279–287
Yan Y, Tian J, Fan MS, Zhang FS, Li XL, Christie P, Chen HQ, Lee JW, Kuzyakov Y, Six J (2012) Soil organic carbon and total nitrogen in intensively managed arable soils. Agric Ecosyst Environ 150:110–120
Yang QB, Fan FL, Wang WX, Liang YC, Li ZJ, Cui XA, Wei D (2010) Effects of different long-term fertilizations on community properties and functions of methanotrophs in dark brown soil. Environ Sci 31:2756–2762 (in Chinese)
Yang MD, Jiao Y, Li X, Wen HY (2015) Specific activity of methanotrophs in saline–alkaline soils retrieved from a fluorescent quantitative real-time PCR technique. Ecol Environ Sci 24:797–803 (in Chinese)
Zhang JF, Li ZJ, Ning TY, Gu SB (2011) Methane uptake in salt-affected soils shows low sensitivity to salt addition. Soil Biol Biochem 43:1434–1439
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Nos. 41675140, 41565009, 41765010) and the Inner Mongolia Youth Innovative Talent Training Program of Prairie Excellence Project 2016.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, W., Jiao, Y., Yang, M. et al. Methane uptake by saline–alkaline soils with varying electrical conductivity in the Hetao Irrigation District of Inner Mongolia, China. Nutr Cycl Agroecosyst 112, 265–276 (2018). https://doi.org/10.1007/s10705-018-9943-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-018-9943-5