Abstract
Fungal pre-treatment using Pleurotus ostreatus (PO) was carried out on individual and combinations of agro-waste wheat straw (WS), rice straw (RS), and pearl millet straw (PMS) with the addition of biochar (5%,7.5% and 10%) to reduce the pre-treatment duration. Further remaining substrate known as spent mushroom substrate (SMS) was used in anaerobic digestor (AD) for estimation enhanced biomethane yield. Equal ratios of RS + WS, WS + PMS, PMS + RS, and RS + PMS + WS and biochar addition were taken for enhancing pre-treatment, PO growth and AD process. The extent of pre-treatment was recorded with the maximum lignin removal of 40.4% for RS + PMS + WS as compared to untreated counterparts and 0.5%, 2.2%, and 3.3% times more lignin removal from individual PMS, RS, and WS respectively. Addition of biochar to the substrates reduced the total pre-treatment duration by days as compared to the non-biochar substrates. Biological efficiency (BE) used for the analysis of mushroom growth varied from 51–92%. Further, the average bio-methane yield was 187 ml/gVS for SMS of PMS + WS + RS with 10% biochar indicating an increment of 83.33% from untreated SMS of PMS + WS + RS. This, higher biomethane yield was 9.35%, 22.22% and 57.14% times higher than individual SMS of PMS, RS, and WS respectively. The current study shows that biochar not only enhances the bio-methane yield but also reduces the biological pre-treatment duration and removes the dependency on one lignocellulosic biomass for energy (bio-methane) and food (mushroom) production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food crisis is one of the significant problems faced by the world at present whereas energy production from fossil fuels causes greenhouse gases emission (GHGs) around the world (Yuan et al. 2021). Currently, mushroom cultivation is quite gaining attention all over the world. As mushroom consists high amount of protein which is beneficial for the human body, and due to the global food crisis mushroom cultivation can be a sustainable solution for this crisis. On the other hand, a large amount of agro-waste and animal manure is generated annually with high organic content. To this, agro-waste is directly burned which causes GHGs emissions (Luskar et al. 2022). Therefore, the utilization of agro-waste for mushroom cultivation is a favorable ecological impact, and it not only reduces GHGs emissions but also provides food to tackle the food crisis faced by the world today. Mushroom cultivation also has a financial advantage and residue after cultivation is also utilized as cattle food or for energy generation, later as fertilizers. This process also helps in keeping the environment as it consumes agro-waste (Pérez-chávez et al. 2019).
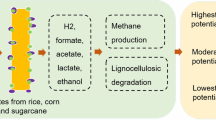
The world is facing an energy crisis as well as a climate crisis. The energy demand is increasing rapidly due to population growth, urbanization, and industrialization, while the world’s energy resources are finite and depleting. Simultaneously, energy fossil fuels are the largest contributor to emissions. Therefore, mushroom cultivation on agro-waste can act as a sustainable solution for energy production (bioenergy) and minimizing waste. The remaining residue after cultivating mushroom (PO) known as spent mushroom substrate (SMS), has great potential to generate bio-energy. With every kg of mushroom cultivated, approximately 5 kg of SMS is generated (Lin et al. 2014; Gao et al. 2021). This huge amount of SMS which has high organic content can be utilized to produce energy resulting in a cycle of food-energy nexus or sustainable reuse of resources as shown in Fig. 1. However, there are still challenges related to different properties of wastes and biomasses and other parameters such as AD parameters which may affect the mushroom growth as well as biogas yield.
As lignocellulosic biomass is consisting of three main components which are cellulose, hemicellulose, and lignin. While cellulose and hemicellulose are readily biodegradable, lignin is recalcitrant and highly resistant to microbial degradation. This makes the overall degradation of lignocellulosic biomass slow and difficult, resulting in low biogas yield during the AD process. The hydrolysis process in AD is crucial for the breakdown of complex polymers (organic matter) into modest molecules which can be utilized easily by microorganisms for biogas production (Kumar et al. 2019). However, cellulose and hemicellulose accessibility in lignocellulosic biomass is limited due to the presence of lignin, which creates a physical barrier. This limits the activity of cellulolytic microorganisms as well as their growth in the AD reactor, resulting in reduced biogas yield.
To overcome these challenges, numerous types of pre-treatment methods exist to enhance cellulose and hemicellulose accessibility to microorganisms. These methods include chemical treatments (like acid or alkaline hydrolysis), physical treatment (like sonication or steam explosion), thermal treatments (like autoclave or microwave), and biological treatments (like microbial or enzymatic) (Yadav et al. 2019). These pre-treatments in lignin breakdown in the biomass, increasing the surface area as well as cellulose and hemicellulose accessibility to microorganisms resulting in a significant increment in biogas production during the AD process (Paritosh et al. 2020). Biological pre-treatment takes longer duration but is an eco-friendly and inexpensive treatment method compared to thermal and chemical pre-treatment.
Mushroom cultivation is a biological (fungal) pre-treatment on agro-waste for the generation of biogas through AD. Agaricaceae, Polyporaceae, and Pluteaeceae families have commercial application for cultivation which comes under the Agaricales order which are edible fungi (Hoa and Wang 2015; Padri et al. 2022; Sharma et al. 2020). Moreover, the edible mushroom is an excellent source of vitamins, proteins, and minerals. Mushrooms include minerals such potassium, phosphorus, sodium, calcium, magnesium, copper, zinc, iron, molybdenum, selenium and, vitamins especially B vitamins are abundant in mushrooms. Mushrooms include thiamine (B1), riboflavin (B2), niacin (B3), and pantothenic acid (B5) Most of them have low starch content and act as ideal food for patients suffering from diabetes (Wang and Zhao 2023).
At the commercial level, cultivation of four major edible mushrooms is available, which are Volvariella spp (paddy straw mushroom or tropical mushroom), Pleurotus spp (Oyster mushroom), Lentinus edodes (Japanese mushroom) and Agaricus bisporus (white button mushroom) (Pérez-chávez et.al 2019; Chatterjee et al. 2017; Xiao et al; 2022). A low temperature and fermented substrates are needed for the button mushroom's growth, while the paddy straw mushroom needs a raised temperature of 35°C and thrives on unfermented substrates. In the range of 20°C to 30°C, the Japanese and oyster mushrooms grew well on non-fermented substrates (Silva et al. 2020; Song et al. 2021).
Biochar offers numerous advantages as it is porus in nature, provides microhabitat for microorganisms, provides direct inter-electron transfer, helps in purifying biogas, and reduce duration for biogas production (Chen et al. 2023). Therefore, to reduce biological pre-treatment duration biochar as additive can be used during pre-treatment process for enhanced mushroom growth as well as enhanced biogas genration. In this study, mushroom cultivation on agro-waste is adopted to utilize waste and fulfilling the need for food as the mushroom which is considered a substitute source of protein for vegetarian diets. After the harvesting of the mushroom, an important amount of organic residue known as spent mushroom substrate (SMS) (pre-treated agro-waste) remains, which will be utilized for the production of biogas. Individual agro-waste as well as different combinations were used for mushroom cultivation. The growth was evaluated based on the duration of the first pinhead formation, duration of the first harvest, BE, and weight of fresh mushrooms. Organic matter in the SMS was analyzed by compositional analysis. This organic matter was further utilized for biogas generation by the AD process to estimate the enhanced biomethane yield.
Materials and methods
Oyster mushroom culture and selection of feedstock
Growth and analysis of PO mushrooms were studied on different substrates and their combinations at the biofuel lab at Malaviya National Institute of Technology Jaipur located in Rajasthan, India. The MTCC 1801 strain (PO) was obtained from the Institute of Microbial Technology, Chandigarh, and was cultured on potato dextrose agar in Petri dishes. These dishes were incubated at 21℃ in the incubator until mycelium was fully spawned (6–8 days) and stored at 4 ℃ in the refrigerator until further use. In Fig. 2, is an overview of this study and methods to be used to evaluate the mushroom cultivation and pre-treatment by it, biomethane yield, and nutrients present in slurry for fertilizer purposes. Wheat straw (WS), rice straw (RS), and pearl millet straw (PMS) were considered as substrates individually as well as in the combination of 1:1, a total of 7 samples (RS, WS, PMS, RS + WS, WS + PMS, PMS + RS, and RS + PMS + WS). Prosopis wood biochar commonly known as babool was used in this study whose C/N ratio 74.13 which was available at Biofuel lab at Malaviya National Institute of Technology Jaipur. Fresh effluent was locally available from a biogas plant (Durgapura, Jaipur; 26.8 N, 75.7 E) was used as inoculum, and before use, it was incubated at 55℃ for 7 days for the survival of thermophilic organisms only. Thermophilic conditions were considered because state of Rajasthan, India, comes under a high-temperature state hence the thermophilic conditions were used to match and make this process more applied in such environmental conditions. Table 1 shows the physical and chemical properties of PMS, RS, WS, and inoculum.
Substrate preparation and inoculation
A 45gm of each substrate (dry weight) was mixed in 800 ml of deionized (DI) water comprising each 1.5gm of hydrated lime (Ca(OH)2) for 6 h for cleaning and to prevent micro-organisms contamination on substrates. Before placing the substrate in DI, water and (Ca (OH)2) were thoroughly mixed to achieve homogenization. Substrates were left in the open overnight to remove excess water and placed in beakers for spawning accordingly to their ratios as indicated in Table 2. To enhance mushroom growth additive biochar in different ratios (5%, 7.5%, and 10%) was mixed with the substrate.
The sets were prepared with different combinations of additives for the biological pre-treatment (PO) on different substrates. Substrates were inoculated with mycelium (approx. 20%) in a laminar hood apparatus for a sterilized environment. It was placed in a controlled environment of 18–21 °C and around 70 \(\pm\) 5% relative humidity for the spawn run. After completion of the spawn run, the reduction in temperature was about 16–20°C, with no change in humidity, and was watered daily to keep moisture content in check.
Harvesting and parameters
When the fruiting body starts curling up and the tops were completely developed, mushrooms were harvested from substrates and the smaller ones were left to develop. Clusters of mushrooms were weighed and parameters were evaluated like duration from the day of inoculation to the day of mycelium appearance. Duration from the day of inoculation to the day of first gathering and the percent of the yield of fresh mushrooms over the dry weight of substrates is known as biological efficiency.
Chemical compositional analysis
Remaining substrate after fungal pre-treatment known as SMS, a small amount (around 1gm) of SMS was checked for the delignification by chemical compositional analysis. SMS was dissolved in 75 mL water and boiled for 1 h to check the material that could be dissolved in hot water. After a break of 1 h, the water was drained and replaced with the new water to boil for another hour. SMS was boiled, then cooled in cold water, dried for 15 h at 60 °C, and finally weighed. The remaining SMS was diluted with 30 mL of DI water containing sodium chlorite (0.6 g) and 10% acetic acid (2 mL) and heated for 1 h at 75°C to determine the lignin content. After cooling the SMS for 2 h, the same amount of sodium chlorite and acetic acid was added and was heated for another 2 h. Five water washes, two from acetone, and 1 from ether wash were performed. After 90 min of drying at 105°C, the remaining SMS was weighed (Yadav et al. 2019).
Biomethane potential (BMP) test
AD of SMS took place in 610 mL batch serum bottles which are sealed with a 400 mL working capacity, and the collected biogas was held in the remaining 210 mL. Each substrate SMS was used in the batch AD to produce biogas. Positive control and negative control were done by non-pre-treated combinations and inoculum respectively. The inoculum bottles were combined with 1.55 g substrate VS per liter to start the experiment and the pH was about 7.1. The bottles were sealed with a rubber stopper and used cello tape to hold the rubber stopper, and then placed in an incubator (55°C, 90 revolutions per minute, 15 days) on a shaker (REMI CIS 24, India) (Kumar et al. 2019). A gas chromatograph (TRACE 1300, Thermo Fisher Scientific, India) equipped with a thermal conductivity detector and Helium as carrier gas was used for the composition analysis of biogas (Yadav et al. 2019).
Daily, by utilizing a digital pressure meter (Testo 512, Germany) pressure was measured and a further volume of biogas generated in the headspace was determined. Biogas volume was calculated using daily pressure difference under standard pressure and temperature conditions using the following equation: (Kumar et al. 2019)
where,
- Vbiogas:
-
Biogas volume measured daily (L)
- P:
-
Absolute pressure difference (mbar)
- Vhead:
-
Headspace volume (L)
- C:
-
Molar volume (22.4 L/mol)
- R:
-
Universal gas constant (83.14 L mbar/mol K)
- T:
-
Absolute temperature (K)
Results
Biological pre-treatment duration
Duration of pre-treatment of agro-waste used in this study was calculated in 28 days. Fungal (PO) treatment was identified by the development of mycelium and mycelium development duration varies for different agro-waste and their combinations. Table 3 shows the duration of pre-treatment which was considered when mushroom growth starts and also shows the first mushroom harvest duration. Biological pre-treatment (PO) carried out for the combination samples finished earlier by 2–5 days when compared to the individual RS and WS whereas for PMS pre-treatment finished earliest by 1 day compared to combinations of PMS as shown in Table 3.
PMS is a C4 plant that is robust in nature whereas RS and WS are C3 plant that has more dense topology structure (Wang et al. 2012) due to which fungi easily breaks the structural bonding of PMS whereas, in the case of RS and WS, it is tough to break structure bonding. WS showed less lignin removal from its combinations due to the presence of selenium in it which lays a negative impact on fungi growth decrease in the amount of biomass and leakage of protein which is an essential nutrient for fungi growth (Xu et al. 2021; Peng et al. 2020).
While with the use of biochar in the pre-treatment process, duration was reduced due to biochar being a porous material that helps in providing a microhabitat for microorganism growth. Due to its high specific surface area, which offers more fungi and organic matter contact hence completing the pre-treatment process rapidly as a similar observation made by (Luz et al. 2018; Wang et al. 2022).
The growth of fungi and lignin degradation of lignocellulosic biomass goes simultaneously, through which the efficiency of pre-treatment is determined. As shown in Fig. 3(a), lignin removal without biochar addition, combination (RS + PMS + WS) showed maximum lignin removal of 33.2% due to better nutritional growth compared to their substrate for fungi growth. Whereas individual PMS and combination (PMS + WS) also showed almost the same lignin removal of 33% and 32.9% respectively, and the least lignin removal of 30.9 was seen for WS compared to their untreated counterpart. With the addition of 5%,7.5%, and 10% biochar maximum removal of lignin was seen for combination (RS + PMS + WS) of 34.1%,37.1%, and 40.2% respectively. Also, almost the same amount of lignin removal was seen for PMS at 33.9%, 37.1%, and 40.4% respectively compared to their untreated counterpart. However, the lowest removal of lignin was for WS as shown in Fig. 3(b), (c), (d). A higher amount of selenium in WS and RS can be the reason for less pre-treatment efficiency in the case of a combination of WS + RS as that of PMS + RS and PMS + WS (Solovyev et al. 2018).
(Kainthola et al. 2019) worked on RS pre-treated by PO showed 21.85% lignin removal whereas in this study for RS lignin removal was found 39% with biochar addition. Whereas this study showed lignin removal of 40% for PMS and 37% for WS with biochar addition which was found higher than the study done by (Yadav et al. 2019) showing lignin removal of 30% and 36% for PMS and WS respectively. (Mamimin et al. 2021) showed lignin removal from fungal pre-treatment was about 18% which was less compared to this study.
As shown in Fig. 4, the addition of a biochar percentage of 10% compared to no biochar addition resulted in maximum lignin removal of about 27% for RS and a minimum of about 21% for PMS due to its electron transfer property which enhances the direct inter-electron transfer which enhances the microbial community growth (Lin et al. 2022).
Mushroom analysis
Duration of the first oyster mushroom harvesting was seen between 26–36 days for all samples with or without additives. With the addition of biochar, the mushroom harvesting process was enhanced as for combination (WS + RS + PMS) with 10% biochar showed a minimum duration of 26 days as well as PMS also showed the same duration of 26 days whereas the maximum duration was seen for WS of 36 days without biochar addition as shown in Table 4. Duration for mushroom harvesting on RS, WS, and RS + WS without biochar is 34, 38, and 34 days, similar findings on the same substrate of PO cultivation were observed by (Elattar et al. 2019).
The carbon to nitrogen (C/N) ratio is a very essential factor in mycelium and mushroom growth as the most suitable range is 32 to 150 (Hoa and Wang 2015) and carbon and nitrogen are important nutrients for the growth and development of PO, an edible mushroom. Carbon serves as a building block for structural compounds such as cellulose and lignin and is also required for energy whereas nitrogen is needed for the synthesis of amino acids, nucleic acids, and other nitrogen compounds including chitin which is an important component of the cell wall. Thus, for proper growth of PO appropriate amount of carbon and nitrogen should be present for optimal growth and development, or else the C/N ratio being too high or too low can lead to inhibition of fungi growth (Zakil et al. 2022).
With the addition of biochar, mushroom growth was enhanced hence reducing the duration of harvesting. The porous nature of biochar is an essential factor responsible for the growth of mushrooms as it absorbs water and nutrients from agro-waste and is made available to mycelium. The ability of biochar to translocate these nutrients and water through its porous network may further support the growth of mycelium by enabling it easily colonize the substrate (Zhu et al. 2017). Biochar likely acted as a place for retaining nutrients and water from RS, WS, and PMS to promote PO growth.
The amount of fresh mushroom harvested on agro-waste was recorded as maximum for the combination of PMS + WS + RS with a 10% biochar addition of 746mg whereas a minimum yield of 460mg was recorded for the WS sample without the addition of biochar. It showed that using a variety of agro-waste for mushroom growth, some amount of increment is seen in the number of mushrooms harvested. But mushrooms harvested on PMS and RS alone with 10% biochar addition also showed good yields of 676 and 624mg respectively as shown in Fig. 5. Using PMS and RS samples individually and in combinations showed more yield compared to a sample consisting of WS.
For the analysis of mushrooms, BE is an important factor as it represents the amount of mushroom yield based on the amount of dry substrate. The significance of BE is to identify the effectiveness of mushrooms and the combination of substrates used for mushroom growth. As shown in Fig. 6, the most effective mushroom growth was best for the combination of substrates (PMS + WS + RS) of 91.42%, 89.17%, 86.64%, and 84.31% with 10%, 7.5%, 5% biochar addition, and without biochar addition respectively whereas RS alone (89.46%) showed better combination effectiveness compared to PMS (75.73%) with 10% biochar addition. BE of WS was a minimum of 55.99% which was similar to findings on the same substrate by (Muswati et al. 2021). Overall, biochar is promising bio-fertilizer or supplement for enhanced mycelium growth and further oyster mushroom growth.
Bio-methane potential (BMP) test
AD of SMS (remains after fungal pre-treatment) was carried in batch mode for 15 days duration at 55°C (thermophilic condition). Biochar traces were still present in the substrate after pre-treatment which acts as an improvement source in biogas production. To investigate the potential synergistic effects of combining various agricultural waste materials on biomethane production, the mixture of substrates resulted in higher biomethane yields compared to individual substrates. Combinations were utilized as a means of exploring the potential benefits of utilizing mixed substrates for biomethane production.
The spent mushroom substrate (SMS) was employed after the pre-treatment of agro-waste materials with Pleurotus ostreatus and biochar. The objective of the study was to determine the potential increase in biomethane production resulting from the use of treated SMS and to compare this with the biomethane yield of untreated SMS. The laboratory-scale batch mode bottles were supplemented with SMS samples, both treated and untreated. The bottle and incubator were purposefully engineered to create an environment that would facilitate the ideal conditions for the microbial consortium responsible for the production of methane. The SMS samples underwent controlled conditions of retention time, temperature, and pH to promote the growth of anaerobic microorganisms during the testing process.
The findings suggest that the application of treatment to the SMS resulted in a greater production of biomethane in comparison to the SMS that was not treated. The findings indicate that the application of Pleurotus ostreatus and biochar as pre-treatment agents resulted in an increase in substrate digestibility and a corresponding enhancement in methane gas produced during anaerobic digestion.
From Fig. 7, it is clear that all the substrates with biochar addition examined in this study show better cumulative biogas yield compared to substrates with no biochar addition, and the substrate with more biochar content showed a higher amount of biogas yield. The highest cumulative biogas yield was seen with 10% biochar content of 867 ml/gmVS for SMS of combination PMS + RS + WS and SMS of PMS sample along with RS individual and in combination also show a high cumulative yield of 711 ml/gmVS and 763 ml/gmVS respectively. Whereas minimum yield was seen in WS for all pre-treatment conditions with or without biochar. The highest cumulative biogas yield obtained from SMS of combination PMS + RS + WS with 10% biochar was 18%,24.1%, and 31.37% that of individual SMS of PMS, RS, and WS respectively, whereas 28.83% compared to combination PMS + RS + WS with no additive and 40.48% compared to no additive as well as no fungal pre-treatment. Whereas the average biogas yield of 36 ml/gmVS was also found maximum for SMS of combination PMS + RS + WS with 10% biochar.
The result of our study was found to be comparable with many other studies using fungal pre-treatment and substrates RS, PMS, and WS. (Yadav et al. 2019) analyzed the fungal pre-treatment on WS showed 470 ml/gmVS which was comparable to fungal pre-treatment on WS (452 ml/gmVS) in our study, whereas they preferred coupled pre-treatment followed by a bacterium which resulted in 570 ml/gmVS which was found less to our study in which fungal pre-treatment enhanced with the addition of 7.5% biochar on WS (647 ml/gmVS). Similarly, they analyzed the same for the PMS in which with fungal pre-treatment they found a biogas yield of 450 ml/gmVS and which was comparable with our study for PMS (472 ml/gmVS) with fungal pre-treatment.
With the fungal pre-treatment and biochar addition about 52% increment was seen in biogas generation compared to no pre-treatment in this study which was higher than study done by using other pre-treatment methods (Kucuker et al. 2020; Kumar et al. 2021a, b; Yuhendra et al. 2021). (Zhang et al. 2021) used PO for pre-treatment process showed 51% increment in biogas yield which was lower than the biogas yield found in this study. The amount biogas produced from RS was 658 ml/gmVS which was found higher than the study made by (Kainthola et al. 2019).
Cumulative and daily biogas production shows dynamic behaviour in the process and for methane content in biogas also (Gao et al. 2021). Biogas generated from the AD process of lignocellulosic biomass is low due to the complex structure at the molecular level of lignocellulosic biomass which shows difficulties in degradation and whereas the pre-treatment process breaks it into a modest structure and degrades the lignin content, hence making things easier for micro-organisms (Pan et al. 2021; Gómez et al. 2018).
Biochar being a porous material facilitates biofilm formation, which provides a shield to microorganisms for selective enrichment during the AD process when acidic conditions are formed. The biochar absorbs nutrients from biomass as well as pores of biochar delivering a microhabitat for the microbial community which leads to enhanced growth of microorganisms (Luz et al. 2018; Kumar et al. 2021a, b). Biochar pH varies in the alkaline range owing to the presence of ash content, so under acidic conditions, biochar can facilitate the methanogenesis process effectively which leads to optimal operating conditions and increased total solids (Yin et al. 2016, 2019).
The average biogas yield for SMS of RS, WS, and PMS alone was less compared to that with the combinations of SMS of PMS as shown in Table 5 due to a balanced C/N ratio and PMS having a low C/N ratio compared to others (Yadav et al. 2019; Paritosh et al. 2020). SMS of combinations PMS + RS and WS + PMS showed better biogas yield compared to SMS of combination WS + RS due to the less dense structure of PMS than RS and WS results in better lignin removal further increasing biogas yield.
Biomethane content
Average methane content generally varied between a minimum of 3.59 ml/gmVS for WS with no pre-treatment to a maximum of 15.82 ml/gmVS for combination PMS + RS + WS with 10% biochar addition. Combinations showed a high amount of methane content compared to their counterpart.
Cumulative bio-methane content generally varied between a minimum of 92 ml/gVS for WS with no pre-treatment to a maximum of 383 ml/gVS for a combination PMS + RS + WS with 10% biochar addition. Combinations showed a high amount of methane content compared to their counterpart as shown in Fig. 8. Using co-substrate in thermophilic conditions results faster degradation of organic matter which reduce the duration of BMP test and also the amount of CO2 produced is less (Paritosh et al. 2020).
(Mustafa et al. 2016) examine the PO pre-treatment to enhance the methane content from RS and obtained about 42.5% methane yield whereas methane yield obtained in this study for RS was found 5–8% higher. (Kainthola et al. 2019) analyzed the fungal pre-treatment by PO on RS which showed 269.99ml/gVS methane yield whereas in this study 298ml/gVS methane yield was seen which is more.
With the use of biochar, methane content increased as biochar adsorbs the CO2 through physical adsorption due to its high specific surface area and also the presence of free radicals, metals, and metal oxide on the biochar surface which enhance the electron donating via the oxidation process (Chacon et al. 2020; Arenas et al. 2020). PMS individually and in combination showed better methane content due to its less dense topology structure and CO2 fixation compared to RS and WS (Wang et al. 2012).
Conclusion
Fungal pre-treatment (PO) was carried out on individual and combinations of agro-waste (RS, WS, and PMS) with the addition of biochar (5%, 7.5%, and 10%) during the pre-treatment phase to reduce the duration of pre-treatment and to enhance the biomethane yield. The extent of pre-treatment was recorded with the maximum lignin removal of 40.4% for RS + PMS + WS as compared to untreated counterparts and 0.5%, 2.2%, and 3.3% times more lignin removal from individual PMS, RS, and WS respectively. PMS showed better lignin removal than RS and WS due to its less dense structure. The addition of biochar to the substrates reduced the total pre-treatment duration by 2–5 days as compared to the non-biochar substrates. Duration of the first oyster mushroom harvesting for all the substrates with or without biochar took 26–36 days. Biological efficiency used for the analysis of mushroom growth varied from 51–92%. Effective mushroom growth denoted by BE was seen with 10% biochar in combination with PMS + WS + RS (91.42%) and RS (89.46%) alone whereas minimum BE was seen for WS (55.99%) without biochar. Therefore, biochar can be said an excellent bio-fertilizer for mycelium and further mushroom growth as biochar provides a microhabitat for better fungal growth. Biogas yield was found maximum for the combinations due to the balance C/N ratio. Further, the highest cumulative biogas yield was seen with a 10% biochar content of 867 ml/gmVS for SMS of combination PMS + RS + WS. Additionally, this higher biomethane yield was 18%, 24.1%, and 31.37% times higher than individual SMS of PMS, RS, and WS respectively as biochar also absorbs CO2 which yields enhance biomethane yield. The current study shows that biochar not only enhances the bio-methane yield but also reduces the biological pre-treatment duration and it also removes the dependency on one lignocellulosic biomass for energy (bio-methane) and food (mushroom) production and also reduce the dependency on one substrate as combination showed better results.
Further this slurry obtained after the AD process can be tested for fertilizer purpose and hence creating a circular economy which can be studied in future. As the mushroom cultivation was used in this study, a simultaneous study including microalgae in AD process with SMS can be studied in future as well as also to examine simultaneously the bio-oil production from microalgae and biogas production from fungal pre-treatment.
Abbreviations
- AD:
-
Anaerobic digestion
- BE:
-
Biological efficiency
- C/N:
-
Carbon to nitrogen
- CO2 :
-
Carbon dioxide
- GHG:
-
Green-house gas
- PMS:
-
Pearl Millet Straw
- PO:
-
Pleurotus ostreatus
- RS:
-
Rice Straw
- SMS:
-
Spent mushroom substrate
- WS:
-
Wheat Straw
References
Arenas CB, Meredith W, Snape CE, Gómez X, González JF, Martinez EJ (2020) Effect of char addition on anaerobic digestion of animal by-products: Evaluating biogas production and process performance. Environ Sci and Pol Res 27:24387–24399. https://doi.org/10.1007/s11356-020-08828-8
Chacon FJ, Sanchez-Monedero MA, Lezama L, Cayuela ML (2020) Enhancing biochar redox properties through feedstock selection, metal preloading and post-pyrolysis treatments. Chem Eng J 395:125100. https://doi.org/10.1016/j.cej.2020.125100
Chatterjee S, Sarma MK, Deb U, Steinhauser G, Walther C, Gupta DK (2017) Mushrooms: from nutrition to mycoremediation. Environ Sci and Pol Res 24:19480–19493. https://doi.org/10.1007/s11356-017-9826-3
Chen L, Fang W, Liang J, Nabi M, Cai Y, Wang Q, Zhang P, Zhang G (2023) Biochar application in anaerobic digestion: Performances, mechanisms, environment assessment and circular economy. Resou Conserv Recycl 188:106720. https://doi.org/10.1016/j.resconrec.2022.106720
Elattar AM, Hassan S, Awd-Allah SF (2019) Evaluation of oyster mushroom (Pleurotus ostreatus) cultivation using different organic substrates. Alex sci exc J 427–40. https://doi.org/10.21608/asejaiqjsae.2019.49370
Gao X, Tang X, Zhao K, Balan V, Zhu Q (2021) Biogas production from anaerobic co-digestion of spent mushroom substrate with different livestock manure. Energies 14(3):570. https://doi.org/10.3390/en14030570
Gómez X, Meredith W, Fernández C, Sánchez-García M, Díez-Antolínez R, Garzón-Santos J, Snape CE (2018) Evaluating the effect of biochar addition on the anaerobic digestion of swine manure: application of Py-GC/MS. Environ Sci and Pol Res 25:2560011. https://doi.org/10.1007/s113560182644-4
Hoa HT, Wang CL (2015) The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycob 43(1):14–23. https://doi.org/10.5941/MYCO.2015.43.1.14
Kainthola J, Kalamdhad AS, Goud VV, Goel R (2019) Fungal pretreatment and associated kinetics of rice straw hydrolysis to accelerate methane yield from anaerobic digestion. Bioresou Tech 286:121368. https://doi.org/10.1016/j.biortech.2019.121368
Kucuker MA, Demirel B, Onay TT (2020) Enhanced biogas production from chicken manure via enzymatic pretreatment. J Mat Cyc and Waste Manag 22:1521–1528. https://doi.org/10.1007/s10163-020-01039-w
Kumar S, Gandhi P, Yadav M, Paritosh K, Pareek N, Vivekanand V (2019) Weak alkaline treatment of wheat and pearl millet straw for enhanced biogas production and its economic analysis. Renew Energy 1(139):753–764. https://doi.org/10.1016/j.renene.2019.02.133
Kumar M, Dutta S, You S, Luo G, Zhang S, Show PL, Sawarkar AD, Singh L, Tsang DC (2021) A critical review on biochar for enhancing biogas production from anaerobic digestion of food waste and sludge. J Clean Prod 305:127143. https://doi.org/10.1016/j.jclepro.2021.127143
Kumar V, Ahluwalia V, Saran S, Kumar J, Patel AK, Singhania RR (2021) Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresou Tech 323:124566. https://doi.org/10.1016/j.biortech.2020.124566
Lin Y, Ge X, Li Y (2014) Solid-state anaerobic co-digestion of spent mushroom substrate with yard trimmings and wheat straw for biogas production. Bioresou Tech 1(169):468–474. https://doi.org/10.1016/j.biortech.2014.07.020
Lin X, Wang N, Li F, Yan B, Pan J, Jiang S, Peng H, Chen A, Wu G, Zhang J, Zhang L (2022) Evaluation of the synergistic effects of biochar and biogas residue on CO2 and CH4 emission, functional genes, and enzyme activity during straw composting. Bioresou Tech 360:127608. https://doi.org/10.1016/j.biortech.2022.127608
Luskar L, Polanšek J, Hladnik A, Čeh B (2022) On-Farm Composting of Hop Plant Green Waste—Chemical and Biological Value of Compost. App Sci 12(9):4190. https://doi.org/10.3390/app12094190
Luz FC, Cordiner S, Manni A, Mulone V, Rocco V (2018) Biochar characteristics and early applications in anaerobic digestion-a review. J Environ Chem Eng 6(2):2892909. https://doi.org/10.1016/j.jece.2018.04.015
Mamimin C, Chanthong S, Leamdum C, Sompong O, Prasertsan P (2021) Improvement of empty palm fruit bunches biodegradability and biogas production by integrating the straw mushroom cultivation as a pretreatment in the solid-state anaerobic digestion. Bioresou Tech 319:124227. https://doi.org/10.1016/j.biortech.2020.124227
Mustafa AM, Poulsen TG, Sheng K (2016) Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. App Energy 15(180):661–671. https://doi.org/10.1016/j.apenergy.2016.07.135
Muswati C, Simango K, Tapfumaneyi L, Mutetwa M, Ngezimana W (2021) The effects of different substrate combinations on growth and yield of oyster mushroom (Pleurotus ostreatus). Int J Agro 22(2021):1. https://doi.org/10.1155/2021/9962285
Padri M, Boontian N, Teaumroong N, Piromyou P, Piasai C (2022) Application of Aspergillus niger F5 as an alternative technique to harvest microalgae and as a phosphorous removal treatment for cassava biogas effluent wastewater. J Water Pro Eng 46:102524. https://doi.org/10.1016/j.jwpe.2021.102524
Pan J, Xie Y, Ao N, Sun J, Zhang A, Yang Y, Shi C, Zhang H (2021) Synergistic enhancement of methane production from anaerobic digestion of spent mushroom substrate via addition of biochar and cerium chloride following compost pretreatment. Biomass Bioenergy 150:106128. https://doi.org/10.1016/j.biombioe.2021.106128
Paritosh K, Balan V, Vijay VK, Vivekanand V (2020) Simultaneous alkaline treatment of pearl millet straw for enhanced solid state anaerobic digestion: experimental investigation and energy analysis. J Clean Prod 252:119798. https://doi.org/10.1016/j.jclepro.2019.119798
Peng W, Pivato A, Garbo F, Wang T (2020) Effects of char from biomass gasification on carbon retention and nitrogen conversion in landfill simulation bioreactors. Environ Sci Pol Res 27:6401–6410. https://doi.org/10.1007/s11356-019-07391-1
Pérez-Chávez AM, Mayer L, Albertó E (2019) Mushroom cultivation and biogas production: A sustainable reuse of organic resources. Energy Sustain Dev 1(50):50–60. https://doi.org/10.1016/j.esd.2019.03.002
Sharma VP, Kumar A, Kumar S, Barh A, Kamal S (2020) Substrate sterilization with thiophanate-methyl and its biodegradation to carbendazim in oyster mushroom (Pleurotus ostreatus var. florida). Environ Sci Pol Res 27(1):899–906. https://doi.org/10.1007/s11356-019-07050-5
Silva JS, Ortiz DW, Garcia LG, Asquieri ER, Becker FS, Damiani C (2020) Effect of drying on nutritional composition, antioxidant capacity and bioactive compounds of fruits co-products. Food Sci Tech 40:810–6. https://doi.org/10.1590/fst.21419
Solovyev N, Prakash NT, Bhatia P, Prakash R, Drobyshev E, Michalke B (2018) Selenium-rich mushrooms cultivation on a wheat straw substrate from seleniferous area in Punjab, India. J Trace Ele Med Bio 1(50):362–366. https://doi.org/10.1016/j.jtemb.2018.07.027
Song T, Shen Y, Jin Q, Feng W, Fan L, Cao G, Cai W (2021) Bacterial community diversity, lignocellulose components, and histological changes in composting using agricultural straws for Agaricus bisporus production. PeerJ 9:e10452. https://doi.org/10.7717/peerj.10452
Wang C, Guo L, Li Y, Wang Z (2012) Systematic comparison of C3 and C4 plants based on metabolic network analysis. InBMC systems biology (Vol. 6, pp. 1–14). BioMed Central https://doi.org/10.1186/1752-0509-6-S2-S9
Wang Q, Shao J, Shen L, Xiu J, Shan S, Ma K (2022) Pretreatment of straw using filamentous fungi improves the remediation effect of straw biochar on bivalent cadmium contaminated soil. Environ Sci Pol Res 60933–44. https://doi.org/10.1007/s11356-022-20177-2
Wang M, Zhao R (2023) A review on nutritional advantages of edible mushrooms and its industrialization development situation in protein meat analogues. J Future Food 3(1):1–7. https://doi.org/10.1016/j.jfutfo.2022.09.001
Xiao P, Wu D, Wang J (2022) Bibliometric analysis of global research on white rot fungi biotechnology for environmental application. Environ Sci Pol Res 1491–507. https://doi.org/10.1007/s11356-021-15787-1
Xu M, Zhu S, Wang L, Wei Z, Zhao L, Shi G, Ding Z (2021) Influence of selenium biofortification on the growth and bioactive metabolites of Ganoderma lucidum. Foods 10(8):1860. https://doi.org/10.3390/foods10081860
Yadav M, Paritosh K, Pareek N, Vivekanand V (2019) Coupled treatment of lignocellulosic agricultural residues for augmented biomethanation. J Clean Prod 10(213):75–88. https://doi.org/10.1016/j.jclepro.2018.12.142
Yin Y, Liu YJ, Meng SJ, Kiran EU, Liu Y (2016) Enzymatic pretreatment of activated sludge, food waste and their mixture for enhanced bioenergy recovery and waste volume reduction via anaerobic digestion. App Energy 1(179):1131–1137. https://doi.org/10.1016/j.apenergy.2016.07.083
Yin C, Shen Y, Yuan R, Zhu N, Yuan H, Lou Z (2019) Sludge-based biochar-assisted thermophilic anaerobic digestion of waste-activated sludge in microbial electrolysis cell for methane production. Bioresou Tech 1(284):315–324. https://doi.org/10.1016/j.biortech.2019.03.146
Yuan T, Shi X, Sun R, Ko JH, Xu Q (2021) Simultaneous addition of biochar and zero-valent iron to improve food waste anaerobic digestion. J Clean Prod 278:123627. https://doi.org/10.1016/j.jclepro.2020.123627
Yuhendra AP, Farghali M, Mohamed IM, Iwasaki M, Tangtaweewipat S, Ihara I, Sakai R, Umetsu K (2021) Potential of biogas production from the anaerobic digestion of Sargassum fulvellum macroalgae: Influences of mechanical, chemical, and biologicalpretreatments. Biochem Eng J 175:108140. https://doi.org/10.1016/j.jece.2021.106405
Zakil FA, Isa RM, Sueb MS, Isha R (2022) Growth performance and mineral analysis of Pleurotus ostreatus (oyster mushroom) cultivated on spent mushroom medium mixed with rubber tree sawdust. Mat T Pro 1(57):1329–1337. https://doi.org/10.1016/j.matpr.2022.01.112
Zhang J, Wang L, Ni H, Shi Q, Zhang X, Yu H, Ma F (2021) Selective fungal pretreatment favored pyrolysis products of wheat straw based on pyrolytic polygeneration system. Fuel Proc Tech 215:106749. https://doi.org/10.1016/j.fuproc.2021.106749
Zhu X, Chen B, Zhu L, Xing B (2017) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pol 1(227):98–115. https://doi.org/10.1016/j.enviornpol.2017.04.032
Acknowledgements
The authors would like to extend their appreciation to Biofuel Lab, Centre for Energy and Environment, Malaviya National Institute of Technology, Jaipur, India for providing research and laboratory facilities for conducting the experiments.
Funding
The research was supported by Department of Science & Technology (DST), Ministry of Science and Technology, New Delhi, Government of India within the framework of the Indo-Russian Project DST/INT/RUS/RSF/P-62/2021 (VV) and by the Russian Science Foundation (RSF, Grant No. 22–49-02002 (AAK), https://rscf.ru/en/project/22-49-02002/ and also the Grant (File No: F. No: 1-9/2020(IC)) from University Grants Commission, Government of India under the framework of the Indo-German Project.
Author information
Authors and Affiliations
Contributions
Conceptualization was performed by Prakhar Talwar and Vivekanand Vivekanand; Guidance was performed by Vivekanand Vivekanand and Christoph Lindenberger; Methodology was performed by Prakhar Talwar and Apoorva Upadhyay; Data curation, Formal analysis, and Writing—original draft preparation was performed by Prakhar Talwar; Review, and Editing were performed by Vivekanand Vivekanand, Christoph Lindenberger, Andrey A. Kovalev, Nidhi Pareek and Rickwinder Singh.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interest.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Talwar, P., Upadhyay, A., Verma, N. et al. Utilization of agricultural residues for energy and resource recovery towards a sustainable environment. Environ Sci Pollut Res (2023). https://doi.org/10.1007/s11356-023-29500-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-023-29500-x