Abstract
Residue analysis to detect thiophanate-methyl and its primary metabolite (carbendazim) during oyster mushroom (Pleurotus ostreatus var. florida) cultivation was done for two consecutive years 2017 and 2018. Wheat straw substrate was chemically treated with different treatments of thiophate-methyl, viz, thiophanate-methyl 30 ppm + formalin 500 ppm (T1), thiophanate-methyl 40 ppm + formalin 500 ppm (T2), thiophanate-methyl 50 ppm + formalin 500 ppm (T3), thiophanate-methyl 60 ppm + formalin 500 ppm (T4), and formalin 500 ppm (T5 as control and recommended concentration), and utilized for cultivation of oyster mushroom. Treatments T3 and T4 exhibited significant difference in pH levels during both the trials. Minimum spawn run, pinhead formation, and fruit body formation time were recorded in treatments T3 and T4. Significantly higher biological efficiency (%) was recorded in treatments T3 and T4 as compared with all other treatments. No incidence of competitor molds was recorded in T3 and T4. Pesticide residue analysis for detection of thiophanate-methyl and its metabolite (carbendazim) was done in the fruit body produced in T3 and T4 treatments using liquid chromatography with tandem mass spectrometry method. No residue of thiophanate-methyl and carbendazim was detected at 50 ppm concentration of thiophanate-methyl during both the trials. However, in trial II, residue of carbendazim (5.39 μg/kg) was detected at 60 ppm. Based on the findings of the trials I and II, T3 (thiophanate-methyl 50 ppm + formalin 500 ppm) may be utilized for substrate sterilization for oyster mushroom cultivation and Pleurotus ostreatus var. florida could be recognized as microorganism which could play a role in degradation of thiophanate-methyl.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oyster mushroom (Pleurotus ostreatus var. florida) is a lignocellulolytic fungus that can be cultivated on varieties of agricultural wastes including banana leaves, sugarcane bagasse, tea wastes, pine needles, coconut leaves, wheat straw, rice straw, etc. (Mandeel et al. 2005). Cultivation of edible mushrooms represents one of the most efficient biotechnological processes for lignocellulosic organic waste recycling (da Luz et al. 2012). Oyster mushrooms are rich in protein, plentiful in B vitamins, have no cholesterol, and have significant levels of the cholesterol-lowering molecule lovastatin. Because of their native lovastatin content, oyster mushrooms have been studied for their benefits in modulating blood cholesterol levels (Caz et al. 2015). However, the mycelial growth of Pleurotus spp. can take place on a simple water-treated straw but cellulolytic molds present on straw can compete with its mycelium during spawn run and may release toxic metabolites affecting growth of the mushroom. The major competitor molds of oyster mushroom are cobweb (Cladoboyrum spp.) and green mold (Trichoderma spp.) (Kredics et al. 2009). Various methods have been employed to treat the substrate for cultivation of oyster mushroom such as steam pasteurization, hot water treatment, chemical sterilization, sterile technique, and fermentation or composting to kill undesirable microorganism present in the straw to favor the growth of Pleurotus mycelium (Saritha and Pandey 2010). Among all, chemical sterilization technique is very popular due to low input cost (Dinesh and Babu 2013) and is being adopted by many farmers (Potocnik et al. 2015). However, in India, none of the fungicides have the label claim for mushroom cultivation. All the fungicides used in European countries or elsewhere are used on mushrooms in India. Benzimidazole fungicides, viz, benomyl, carbendazim, and thiophanate-methyl, are the most utilized fungicide for substrate sterilization of mushroom. Benzimidazoles are broad spectrum systemic fungicides, commonly used under mushroom cultivation to prevent competitor molds; although, their residue is detected in the mushrooms intermittently, which is a serious matter of concern for human health and environmental safety (Grogan and Jukes 2003). The present study was aimed to detect thiophanate-methyl and its metabolite carbendazim in oyster mushroom fruit bodies after sterilizing the substrate with thiophanate-methyl (Nakamura et al. 2011) and to identify safe optimum concentration of thiophanate-methyl that can be used in oyster mushroom cultivation without any harmful effect on human health.
Materials and methods
Culture and spawn preparation
Authenticated culture of the oyster mushroom (Pleurotus florida) having accession number DMRP-136 was procured from the culture bank of ICAR-Directorate of Mushroom Research, Solan. Spawn was prepared by using healthy wheat grain following standard package of practice. Freshly grown culture on malt extract agar medium was utilized for mass multiplication. Mycelial growth on grains was completed in 10–15 days.
Chemical sterilization of substrate
Five different treatments of thiophanate-methyl, viz, thiophanate-methyl 30 ppm + formalin 500 ppm (T1), thiophanate-methyl 40 ppm + formalin 500 ppm (T2), thiophanate-methyl 50 ppm + formalin 500 ppm (T3), thiophanate-methyl 60 ppm + formalin 500 ppm (T4), and formalin 500 ppm (T5 as control and recommended concentration), were used for substrate sterilization for oyster mushroom cultivation. Thiophanate-methyl 70% WP (dimethyl-4, 4-0-phenylenebis-3 thioallophanate) with trade name Ditto, manufactured by Coromandel Agrochemicals Private Limited, India, was used in the present investigations. One hundred liter solution of each treatment was prepared and 100 kg of wheat straw was soaked into it (1:1). The substrate was left as such for 12 h. The straw was then sieved to drain off the excess water and air dried till the substrate attained the desired moisture level (65%).
Spawning and cultivation of oyster mushroom
The spawning was done in pre-fumigated room. A total of 30 g spawn was mixed in 1 kg wet-treated substrate. The spawn was mixed thoroughly and spawned substrate was filled in polythene bags of 10 kg capacity. Ten to fifteen small holes (0.5–1.0 cm dia) were made on all sides especially in the bottom for leaching of excess water and maintain desirable level of CO2 (15000–20000 ppm). The spawned bags were kept on the shelves for mycelial colonization of substrate. During spawn run, the temperature of growing room was maintained in the range of 22–26 °C. During spawn run, bags were neither opened and nor any ventilation was given. After spawn run, the room temperature was reduced to 16–20 °C, and relative humidity was maintained between 75 and 85%. Light of 200 lux intensity for 8–12 h was given in the room for fruit body initiation.
Experiment was conducted in randomized block design (RBD) with 10 replications. Ten bags were kept under each replication. Data were subjected to statistical analysis using the SPSS software for Duncan’s multiple range test. Under present investigations, two trials were conducted. Trial I was conducted during October–November (autumn) and trial II was conducted during December–January (winter). Environmental conditions, materials, and methods were kept same under both the trials. Observations were recorded on number of days required for spawn run, pin head formation, fruit body formation, presence/absence of green mold, and biological efficiency (%). The fresh mushroom yield obtained was converted into percent biological efficiency (kg q−1 dry substrate) using the equation:
Pesticide residue sample of dried samples of mushroom was done through Punjab Biotechnology Incubator (PBTI), SAS Nagar (Mohali), Punjab. PBTI is a State Government Undertaking registered as a “Society for Biotechnology Incubator” under the Society Registration Act 1961 and is professionally governed by the Governing Council of the Society under the Government of Punjab (India).
Pesticide residue analysis
Samples for pesticide residue analysis were harvested at crop maturity stage. Samples were air dried at room temperature and the dried samples were packed in polybags. Then, the samples were taken to the PBTI laboratory for further analysis (www.pbtilabs.com). Liquid chromatography with tandem mass spectrometry (LC-MS/MS) method was utilized for pesticide residue analysis.
A finely grounded sample weighing 5.0 ± 0.1 gm was taken in a 50-mL centrifuge tube. Water, MgSO4, and NaCl was added with continuous stirring to avoid the lump formation and finally vortexed. Ten milliliter acetonitrile was added to each tube and centrifuged. Supernatant was taken and evaporated completely under gentle stream of nitrogen, reconstituted with water:acetonitrile (80:20) and injected into LC-MS/MS (Q-Trap 4000 (AB Sciex)). The unknown concentration of the sample is calculated from the following formula:
Results
Effect of thiophanate-methyl treatment on pH of the substrate
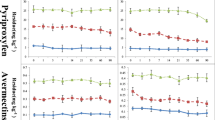
Chemical sterilization of substrate significantly affected the pH level in both the trials and consequently affected the growth and development of oyster mushroom crop (Bellettini et al. 2016). In trial I, only T3 and T4 showed significant differences, whereas, in trial II all the treatments showed significant difference in pH level as compared with control (T5) (Fig. 1). In the treatment T3, pH level increased to 7.30 (trial I) and 7.35 (trial II), whereas, in T4, pH level increased to 7.45 in both trial I and trial II. In control treatment (T5), the pH levels were recorded as 6.42 and 5.80 in trial I and II, respectively. In trial II, all the treatment combinations exhibited significant difference over control in respect of change in pH level of the substrate.
Effect of thiophanate-methyl treatment on spawn run
Oyster mushroom (Pleurotus ostreatus var. florida) took minimum time of 11.70 days and 12.10 days to complete the spawn run under the T4 in trial I and trial II, respectively. Whereas, maximum time of 23 days and 24.05 days for spawn run was taken by test strain with T5. The trend observed in both the trials I and II was similar (Fig. 2). All the treatments exhibited significant difference with the check (T5) in both the experimental trials. However, non-significant difference in respect of spawn run was recorded between the treatments T2 and T3. The quick spawn run in the treatment T4 may be attributed to the fact that the pH levels of the substrate was near neutral or slightly basic.
Effect of thiophanate-methyl treatment on pinhead formation
After spawning, earliest pinhead formation was recorded after 17.10 days in trial I and 17.30 days in trial II, respectively. Maximum time for pinhead formation, i.e., 27.00 and 28.50 days, was recorded with control treatment (T5). All the treatments exhibited significant difference with control during both the trials (Fig. 3).
Effect of thiophanate-methyl treatment on fruit body formation
All the treatments showed significant difference in time taken for fruit body formation after spawning (Fig. 4). Maximum time of 32.20 days (trial I) and 35.50 days (trial II) was observed for fruit body formation in control (T5). Minimum time for fruit body formation was observed in T4, i.e., 21.30 days (trial I) and 25.80 days (trial II). It was followed by T3 by showing fruit body formation after 22.80 days (trial I) and 27.60 days (trial II).
Effect of thiophanate-methyl treatment on biological efficiency (%)
Maximum biological efficiency was obtained under T4 (31.68–63.68%) and minimum was recorded under T5 (6.61–12.09%) during both the experimental trials. T4 treatment was followed by T3 exhibiting 25.81 to 50.32% biological efficiency of oyster mushroom. In trial II, significant difference in biological efficiency was recorded between T3 and T4; however, in trial I, no significant difference in biological efficiency was recorded (Fig. 5).
Pesticide residue detection
Mushroom samples for the best two treatments (T3 and T4) were subjected to residue analysis. The dried samples of oyster mushroom were also given the registration numbers, viz, T3-trial I (PBTI/FAO/030318/004529), T4-trial I (PBTI/FAO/030318/004530), T3-trial II (PBTI/FAO/030318/004531), and T4-trial II (PBTI/FAO/030318/004532) by PBTI (www.pbtilabs.com) for reference. Figure 6 shows the acquisition spectra of standards while. Figure 7 shows the spectra, run time, and peak height; the peak area of the sample showed the detectable residue of thiophanate-methyl. The report of analysis (Table 1) indicated that at 50 ppm concentration of thiophanate-methyl, no residue of thiophanate-methyl was detected while residue of its primary metabolite (carbendazim) was detected below minimum quantification limit (2.5 μg/kg). However, at 60 ppm concentration, thiophanate-methyl was not traced in both the experimental trials but residue of carbendazim (5.39 μg/kg) was detected in the samples harvested from trial II.
Discussion
Generally, oyster mushroom require pH near to neutral or slightly basic (Wajid Khan et al. 2013). In our studies, under effective treatments of thiophanate-methyl (50 and 60 ppm), pH of the substrate ranged from 7.3 to 7.5. However at this pH, oyster mushroom grew well, and positive effect on mycelial growth, pinhead formation, fruit body formation, and biological efficiency was recorded. Under chemical sterilization of the substrate, it is important that the optimum concentration of fungicides should reach each and every corner of the substrate because method of substrate sterilization could also affect the final crop yield (Kalita 2015). It was also recorded that in the end of process of biodegradation of thiophanate-methyl, abundant carbon dioxide gas is liberated (Cycon et al. 2011) which positively increased the spawn run rate under the treated substrate as evidenced by our data also. Oyster mushroom requires high CO2 concentration during spawn run for their growth and development. No colony of competitor molds like Trichoderma sp. (green mold) was observed in the bags treated with effective concentrations of thiophanate-methyl. Spores of Trichoderma might not germinate under such conditions and crop was found completely free from competitor mold (Trichoderma sp.) in the treatments T3 and T4. Undoubtedly, thiophanate-methyl (60 ppm) under T4 showed best results in all respect; however, the residue of its primary metabolite (carbendazim) was detected at this concentration. Previously also, it has been reported that thiophanate-methyl encouraged the mycelial growth of button mushroom (Agaricus bisporus) at 12 mgl−1 but suppressed the growth when applied at higher concentration, i.e., 25 mgl−1 (Potocnik et al. 2009). Formerly, carbendazim-treated oyster mushroom samples have also been analyzed for pesticide residue after processing operations like washing, drying, and cooking, and significant decrease in carbendazim residue has been reported. A total of 70.30% loss in carbendazim residue has been reported when rinsing the mushroom under tap water and when dried under sunlight (Xia et al. 2016). Excellent control of many fungal diseases of mushrooms was secured when fungicides from the group of methyl benzimidazole carbamates (MBC) were introduced in the late 1960s (Delp 1987). Benzimidazole fungicides like carbendazim, benomyl, thiophanate-methyl, etc. have also been recommended previously for the management of destructive disease of oyster crop like cobweb (Cladobotryum mycophilum) (Kim et al. 2014). Rinker and Alm (2008) evaluated in vitro and in vivo toxicity of fungicides against Trichoderma sp. in A. bisporus. Thiabendazole was the most effective, followed by benomyl and thiophanate-methyl.
It can be concluded from the present studies that chemical sterilization of oyster mushroom substrate with thiophanate-methyl at 50 ppm concentration is safe to use because no residue of either thiophanate-methyl or its primary residue carbendazim was detected at this concentration. This treatment may be used by the mushroom growers while they grow the crop for drying purpose. Pesticide degradation through microbial activities is considered an important mode of their dissipation (Cycon et al. 2011). In our studies, it is also evident that during the course of growth and development of Pleurotus ostreatus var. florida, it transformed thiophanate-methyl to carbendazim. Therefore, it would be unbiased to recognize Pleurotus ostreatus var. florida as microorganism which could participate in biodegradation of thiophanate-methyl to carbendazim. It might be utilized for bioremediation of thiophanate-methyl and fungicides with similar chemistry. Kaushik et al. (2009) reviewed the common food processing operations along with the degree of residue removal in each process, which include washing, cooking, drying, thermal processing, and freezing, and most of the reports say that the common food processing operation removes the residual toxicity in the food. In India, carbendazim and thiophanate-methyl are generally utilized for substrate sterilization under oyster mushroom (Shah et al. 2013) and the importance of fungicides of the benzimidazole group in oyster mushroom could be sensed in our findings from their role in mycoparasitism management and crop yield enhancement. Therefore, present studies will be very helpful for oyster mushroom growers and for the researchers in utilizing pesticides under safer optimum concentrations.
References
Bellettini MB, Fiorda FA, Maieves HA, Teixeira GL, Ávila S, Hornung PS, Júnior AM, Ribani RH (2016) Factors affecting mushroom Pleurotus spp. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2016.12.005
Caz V, Gil-Ramírez A, Largo C, Tabernero M, Santamaría M, Martín-Hernández R, Marín FR, Reglero G, Soler-Rivas C (2015) Modulation of cholesterol-related gene expression by dietary fiber fractions from edible mushrooms. J Agric Food Chem 63:7371–7380. https://doi.org/10.1021/acs.jafc.5b02942
Cycon M, Zmijowska A, Piotrowska-Seget Z (2011) Biodegradation kinetics of 2,4-D by bacterial strains isolated from soil. Cent. Eur. J. Biol. 6(2): 188–198. https://doi.org/10.2478/s11535-011-0005-0
da Luz JMR, Nunes MD, Paes SA, Torres DP, da Silva M d CS, Kasuya MCM (2012) Lignocellulolytic enzyme production of Pleurotus ostreatus growth in agroindustrial wastes. Braz J Microbiol 43:1508–1515. https://doi.org/10.1590/S1517-83822012000400035
Delp CJ (1987) Benzimidazole and releted fungicides. In: H. Lyr (ed) Modern selective fungicides: Properties, applications, mechanisms of action, Longman Scientific and Technical, John Wiley and Sons, New York
Dinesh CR, Babu GRN (2013) Comparative study of oyster mushroom (Pleurotus ostreatus) cultivation by physical and chemical method of sterilization using two different substrates. Int J Sci Eng Res 4:898–902
Grogan HM, Jukes AA (2003) Persistence of the fungicides thiabendazole, carbendazim and prochloraz-Mn in mushroom casing soil. Pest Manag Sci 59:1225–1231. https://doi.org/10.1002/ps.759
Kalita MK (2015) Impact of various sterilization methods on growth and yield of oyster mushroom (Pleurotus florida). Int J Agricult Sci 11:104–107. https://doi.org/10.15740/HAS/IJAS/11.1/104-107
Kaushik G, Santosh SS, Naik SN (2009) Food processing a tool to pesticide residue dissipation – A review. Food Research International. 42:26–40
Kim MK, Seuk SW, Lee YH, Kim HR, Cho KM (2014) Fungicide sensitivity and characterization of cobweb disease on a Pleurotus eryngii mushroom crop caused by Cladobotryum mycophilum. Plant Pathol J 30:82–89. https://doi.org/10.5423/PPJ.OA.09.2013.0098
Kredics L, Kocsubé S, Nagy L, Komoń-Zelazowska M, Manczinger L, Sajben E, Nagy A, Vágvölgyi C, Kubicek CP, Druzhinina IS, Hatvani L (2009) Molecular identification of Trichoderma species associated with Pleurotus ostreatus and natural substrates of the oyster mushroom. FEMS Microbiol Lett 300:58–67. https://doi.org/10.1111/j.1574-6968.2009.01765.x
Mandeel QA, Al-Laith AA, Mohamed SA (2005) Cultivation of oyster mushrooms (Pleurotus spp.) on various lignocellulosic wastes. World J Microbiol Biotechnol 21:601–607. https://doi.org/10.1007/s11274-004-3494-4
Nakamura M, Furumi Y, Watanabe F, Mizukoshi K, Taniguchi M, Nemoto S (2011) Determination of carbendazim, thiophanate, thiophanate-methyl and benomyl residues in agricultural products by liquid chromatography-tandem mass spectrometry. Shokuhin eiseigaku zasshi. J Food Hyg Soc Jpn 52:148–155. https://doi.org/10.3358/shokueishi.52.148
Potocnik I, Vukojevic J, Stajic M, Rekanovic E, Milijasevic S, Todorovic B, Stepanovic M (2009) In vitro toxicity of selected fungicides from the groups of benzimidazoles and demethylation inhibitors to Cladobotryum dendroides and Agaricus bisporus. J Environ Sci Health B 44(4):365–370
Potocnik I, Stepanovic M, Rekanovic E, Todorovic B, Milijasevic-Marcic S (2015) Disease control by chemical and biological fungicides in cultivated mushrooms: button mushroom, oyster mushroom and shiitake. Pesticidi i Fitomedicina 30:201–208. https://doi.org/10.2298/PIF1504201P
Rinker DL, Alm G (2008) Management of casing trichoderma using fungicides. Int. Soc. Mushroom Sci 17:496–509
Saritha B, Pandey M (2010) Evaluation of alternate substrate pasteurization techniques for culinary-medicinal white oyster mushroom, Pleurotus ostreatus var. florida (Agaricomycetideae) cultivation. Int J Med Mushrooms 12:309–316. https://doi.org/10.1615/IntJMedMushr.v12.i3.100
Shah S, Nasreen S, Kousar S (2013) Efficacy of fungicides against Trichoderma spp. causing green mould disease of oyster mushroom (Pleurotus sajor-caju). Res J Microbiol 8(1):13–24
Wajid Khan M, Asif Ali M, Ahmad Khan N, Aslam Khan M, Rehman A, Javed N (2013) Effect of different levels of lime and pH on mycelial growth and production efficiency of oyster mushroom (Pleurotus SPP.). Pak J Bot 45:297–302
Xia E, Tao W, Yao X, Wang J, Tang F (2016) Effects of processing on carbendazim residue in Pleurotus ostreatus. Food Sci Nutr 4:645–650. https://doi.org/10.1002/fsn3.328
Acknowledgments
Authors are thankful to the Director, ICAR-Directorate of Mushroom Research, Solan (India) for providing necessary laboratory facilities for conducting the study.
Author information
Authors and Affiliations
Contributions
VPS prepared its design and helped to draft the manuscript. AK (corresponding author) carried out all experiments and drafted the manuscript. ShK discussed and suggested with the manuscript. SK planned its design and helped to draft the manuscript. AB analyzed the experimental data. All authors read and approved the final manuscript.
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, V.P., Kumar, A., Kumar, S. et al. Substrate sterilization with thiophanate-methyl and its biodegradation to carbendazim in oyster mushroom (Pleurotus ostreatus var. florida). Environ Sci Pollut Res 27, 899–906 (2020). https://doi.org/10.1007/s11356-019-07050-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07050-5