Abstract
Total coliforms, E. coli, and fecal streptococci are the important indicators linked to the human health. This study investigated presence of these indicator bacteria in the Himalayan springs at various locations in the district Kulgam of Kashmir valley. A total of 30 spring water samples were collected from rural, urban, and forest areas during post-melting season 2021 and pre-melting season 2022. The springs in the area originate from the alluvium deposit, Karewa, and hard rock formations. The physicochemical parameters were found within the acceptable limits. However, nitrate and phosphate were found above the permissible limit at few sites, thus indicating the presence of anthropogenic activities in the area. Majority of the samples during both the seasons were found highly loaded with total coliforms with maximum limit of greater than 180 MPN/100 ml. E. coli and fecal streptococci were found in the range of less than 1 to more than 180 MPN/100 ml. The results of Pearson correlation of physicochemical parameters with indicator bacteria showed that chemical oxygen demand, rainfall, spring discharge, nitrate, and phosphate are the main factors affecting the concentration of indicator bacteria in the spring water at each site. Principal component analysis showed the most influencing factors of water quality in most of the spring sites are total coliforms, E. coli, fecal streptococci, rainfall, discharge, and chemical oxygen demand. The results of this study showed that the spring water is unfit for drinking purpose because of high concentration of fecal indicator bacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Demand for drinking water has raised over time due to poor quality or lack of freshwater, particularly in highly populated, arid, and semi-arid parts of the world (Chen et al., 2019; Singh et al., 2019). Due to this, 40% and 20% of the global population is presently under critical and high water strain respectively (Guppy & Anderson, 2017). Also, the availability of safe water resources is crucial to human health and growth (WHO, 2017). Water is also a highly effective means of disease transmission (Wen et al., 2020). According to the WHO (2019) 4.6% of global disability-adjusted life-years (DALYs), that is the sum total of years of life lost because of mortality (YLLs) and number of years lived with a disability (YLDs) and 3.3% of global deaths is due to water quality. Globally, contaminated water causes deaths due to gastrointestinal illnesses like cholera, typhoid, dysentery, and diarrhea (Singh et al., 2019). People in the developing countries bear a prominent burden of diarrheal disease and other crippling, life-threatening illnesses as a result of lack of access to potable water (Singh et al., 2019; Sobsey et al., 2008). Due to inadequate access to drinking water and inadequate health care systems, developing countries are particularly adversely affected (WHO, 2018). The quality of raw water at the source, the method used to purify it, and the distribution system are the three key factors that have substantial impact on the quality of drinking water in the distribution network (Ikonen et al., 2017). These three components influence the physicochemical properties and microbiological makeup of drinking water (Singh et al., 2019). Many of the pathogens in water bodies come from animal and human wastes. The public is exposed to these pathogens through oral contact. The goal of evaluating the microbiological quality of drinking water resources should be to safeguard people from diseases because of its use that may comprise pathogens such as viruses, bacteria, and protozoa that cause water-borne diseases (Wen et al., 2020). Microbiological indicators of drinking water quality are typically one of the particular group or species of bacteria that can pass into water through feces but are simpler to assess than the entire range of microorganisms that are harmful to human health (Bosch, 2007; Saxena et al., 2015; Wen et al., 2020). The majorly used indicators are total coliforms (TCs), fecal coliforms, Escherichia coli (E. coli), enterococci (intestinal enterococci or fecal streptococci) and bacteriophages. The microbiological investigation of drinking water quality is established on the correlation between indicator microorganisms and pathogens (Saxena et al., 2015).
Spring is one of the important freshwater resource which have gained more significance and attention in recent years as a result of their crucial role in supplying the expanding need for drinking water (Bhat et al., 2020; India Water Portal, 2022). In Kashmir, which is a NW-Himalayan union territory of India, spring serves as one of the important freshwater source for drinking in urban and mainly in rural areas (Bhat et al., 2020; Jeelani et al., 2011). Most of the potable water used for drinking and domestic purposes in rural areas of Kashmir comes from springs without the required filtration or treatment. A number of publications are present on hydrochemistry, isotope hydrology, limnochemistry of spring water in Kashmir valley (Jeelani & Bhat, 2012; Jeelani et al., 2011, 2015, 2017; Bhat et al., 2014; Bhat & Jeelani, 2018; Bhat & Pandit, 2010, 2018, 2020; Bhat et al., 2010, 2020, 2021, 2022; Lone et al., 2021). However, little data is present on bacteriological quality of spring water systems in the valley.
Taking into consideration the aforementioned scenario, the present study investigates microbiological and physicochemical characteristics of springs in SW district Kulgam of Kashmir valley. The main goals of this study are to (a) to evaluate the physicochemical and microbiological parameters of spring water, (b) to identify the correlation between microbiological and physicochemical parameters, and (c) to identify the fundamental parameters affecting the water quality at each spring site.
Study area
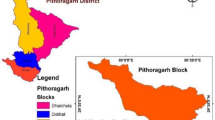
The study area is a part of Kulgam district, SW part of Kashmir valley, India. It lies between 33° 36′ 30''N to 33° 49′ 30''N latitudes and 74° 55′ 0'' to75° 10′ 0'' E longitudes. It covers an area about 317 Sq km (Fig. 1). The area is drained by Vishaw River which is the main tributary of Jhelum River. The elevation of the study area varies from 1544 to 2700 m above mean sea level comprising alluvial plains, Karewa hills, and Pir Panjal Traps (Mir & Bhat, 2017).
The average annual temperature and rainfall in Kulgam is 13.7°C and 922 mm respectively. Precipitation is typically lowest in the month of November, with a long-term average of 30 mm, while the wettest month is March, with a long-term average of 145 mm. The district has an average temperature of 23.2°C, with January being the coldest month and July being the hottest (Mir & Bhat, 2017). The location wise total rainfall data during the sampling period, i.e., post-melting 2021 and pre-melting 2022 was downloaded from Center for Hydrometeorology and remote sensing (CHRS, 2022).
Springs in the Kulgam
Kashmir valley, the Indian Western Himalayan region due to the undulating landscape and high relief have aquifers that are smaller, both in thickness and dimensions. High magnitude of deformation in the Himalaya resulting in profound folding, faulting and development of fracture zones that causes loss of aquifer interconnection in the mountain belts (Mahamuni & Kulkarni, 2012). Under the existing conditions a great number of springs are created in the Himalaya mountain ranges (Mahamuni & Kulkarni, 2012). Springs represents the transition from groundwater to surface water (Brune, 1975). The district Kulgam is the SW Himalayan part of the Kashmir valley possess numerous springs that serve as the vital source for drinking water and other uses. In Kulgam, the alluvium deposits and Karewa Formation dominate the spring water systems (Jeelani & Bhat, 2012; Bhat et al., 2010, 2020, 2022). During the field survey, a spring from hard rock Formation of Pir Panjal Traps was also sampled (Table 1).
The location of springs and their sample identity (ID) is shown in the Table 1. The springs from alluvial deposits, Karewa and hard rock Formations were sampled from the rural, urban and from forest blocks. The spring site S27 was sampled from hard rock, however, spring sites S26 and S30 were sampled from Karewas in the forest blocks and rest of the alluvial and Karewa springs were sampled from rural and urban areas. The discharge of each location was measured by the volumetric method during post-melting 2021 and pre-melting 2022 field works (Stevens et al., 2011). On the basis of flow rate, springs in the study area falls in the fifth order (1–10 l/s) and sixth order (0.1–1 l/s) classifications (Meinzer, 1923). Discharge of springs measured during post-melting and pre-melting seasons is shown in Table 1. The flow rate was high during post-melting season due to the high temperature that melts the snow and hence increase the discharge as snow melt is the main source of recharge (Jeelani & Bhat, 2012; Bhat et al., 2014; Jeelani et al., 2017; Bhat & Jeelani, 2018). However, in the district Kulgam during the pre-melting season (2022) rainfall increases the discharge instantly at few locations. The results of discharge measurements of springs of different stratigraphic units in the study area showed no such prominent difference (Table 1). On the basis of temperature, the ranges are given in Table 2, the springs in the study area are classified in the cold (< 12°C), normal (within 12.2°C of the mean ambient temperature) and warm spring systems(> 12°C of ambient temperature but < 37.8°C) (Alfaro & Wallace, 1994). The temperature of springs that emanates from shallow aquifers is within the 12.2°C of the mean ambient temperature and may fluctuate seasonally (Springer et al., 2008). Moreover, warm spring waters of temperature > 12.2°C are linked to very large aquifers with prolonged flow paths (Springer et al., 2008). On the basis of spheres of discharge, the spring systems in the study area falls in the category of limnocrene originating from lentic pools as shown in the field photograph of study area in (Fig. 2a) and rheocrene lotic channel floor, flowing spring emanating directly into one or more stream channels as shown in Fig. 2e, f (Hynes, 1970; Meinzer, 1923; Springer et al., 2008; Stevens et al., 2021). The springs in the study area are flowing consistently and falls in the class of perennial springs (Meinzer, 1923). The surrounding ecosystems of the spring systems in the study area are terrestrial like forest and woodlands and freshwater (lentic and lotic). On the basis of pollution indicators, the springs in the study area falls in the polluted–biological classification (fecal coliforms) (Springer et al., 2008).
Most of the Kulgam’s population is dependent on surface water resources, mainly spring water for their domestic use. The district has different types of water resources including rivers, streams, waterfalls, springs, wetlands, ponds, and glaciers. The springs are locally called as “nag” and are open to the environment flowing from alluvial deposits like the limnocrene spring at site S2 (Fig. 2a). Many of these springs are regarded as blessed for example the springs at sites S16 and S30, and are protected from any biotic interferences. The S16 is an alluvial and S30 is a Karewa spring which is located in the middle of forest (Fig. 2b). Some springs are maintained by Rural Development Panchayat Raj through the Integrated Watershed Management Programme for example spring site S1 flowing through alluvial deposit and fall into the category of community reservoirs (Fig. 2c). Pipes are inserted in the spring orifices at several locations like in the spring site S8 and water is then delivered to household for domestic use and to a certain extent, for the irrigation purposes (Fig. 2d). The rheocrene springs in the study area flowing through the alluvial deposits, serves the important source of drinking water at the sites S9 and S25 (Fig. 2e and f).
Materials and methods
Sampling procedure
Water samples were collected from each site in 200-ml pre-sterilized bottles for microbial analysis. A separate set of samples for DO, BOD, COD and phosphate was also collected in 1L high density polyethylene(HDPE) bottles. The samples were collected by completely immersed bottles into water and were opened at a depth of 30 cm with its mouth facing the current in order to avoid any type of contamination. The bottles were filled by holding them diagonally immersed. The two set of samples were delivered to the laboratory within 6 h, in ice cooled conditions and protected from light for further investigation. Another set of samples was collected for major ion (nitrate) analysis following the standard procedures.
Physicochemical analysis
The temperature of spring samples was measured on site using a calibrated alcoholic thermometer. The pH, electric conductivity (EC), total dissolved solids (TDS), and salinity were also measured on site. A potable pH meter (Hanna HI2002-02) was used for pH measurement and a potable conductivity meter (Hach SensION) was used for the measurements of EC, TDS, and salinity. Nitrate was determined by spectrophotometer in the Geochemical Laboratory at the Department of Geology, Aligarh Muslim University (AMU) following the standard methods of (APHA, 2017). The analysis of dissolved oxygen (DO), biochemical oxygen demand (BOD), chemical oxygen demand (COD), and phosphate was carried out in the Mycology laboratory at the Department of Microbiology, Sher-i-Kashmir Institute of Medical Sciences, Kashmir Srinagar (SKIMS). Winklers method was used for the estimation of dissolved oxygen (DO), 2 ml of manganous sulfate and standard potassium iodide solution (0.0125N) were added to the water samples at the time of collection in order to allow the formation of precipitate. The DO in all water samples was later determined in the laboratory as per the standard methodologies of (APHA, 2017). BOD in the water samples was calculated as the difference between initial DO of sample and blank and final DO of sample and blank measured after 5 days of incubation at 20°C in BOD incubator. The COD in the water samples was determined by adding 0.4 g of mercuric sulfate in reflux tubes marked as sample and blank, 10 ml of sample and distilled water was added in sample and blank tubes respectively and then 15 ml of COD acid was added to both types of reflux tubes. The reflux tubes were connected to condenser and were allowed to reflux for 2 h at 150°C. The standard method of (APHA, 2017) was followed to calculate the COD of all samples. Phosphate in the water samples was determined by chlorimeter following the standard methods of (APHA, 2017).
Microbiological analysis
For microbial analysis multiple tube fermentation technique was followed (Senior, 1989). The analysis was done in the Mycology Laboratory at the Department of Microbiology (SKIMS). Total coliform (presumptive coliform) count was done by using the double strength and single strength MacConkey broth as an indicator medium. The inoculated medium was incubated aerobically at 37°C for 24 h. The most probable number (MPN) of positive and negative reactions of indicator bacteria in 100 ml of the water sample was estimated by the MPN statistical tubes (Senior, 1989). Eijkman test was performed to investigate the presence of E. coli in water samples by using the brilliant-green bile broth as an indicator medium (Senior, 1989). In this test 5-ml sterilized tubes of brilliant-green bile broth with inverted Durham tubes and 5 ml tubes of tryptone water were inoculated with a drop of presumptive positive culture and were incubated in thermostatically controlled water-baths at 44.0 ± 0.25°C. Kovac’s reagent was added to the tryptone water cultures for the indole ring formation. The E. coli count in the water samples was confirmed and the combination of positive and negative results for gas and indole formation at 44°C was read off from the MPN statistical table (Senior, 1989). The presence of fecal streptococci was determined by heavy inoculation of positive presumptive cultures into 5 ml sterile glucose azide broth incubated at 44-45°C (Senior, 1989). The presence of fecal streptococci was confirmed by subculturing of each positive glucose azide culture on bile aesculin azide agar plates and incubated at 44-45°C for few hours. The results of confirmed tubes of fecal streptococci were estimated by reference of the MPN statistical tables (Senior, 1989).
Data treatment
Chemical and microbiological parameters were statistically analyzed. The statistical evaluation of these data can help to clarify the key elements influencing the water quality. To comprehend the vast amount of data, multivariate statistical methods have been used. Using a large number of inter-correlated variables that have been transformed into independent variables, principal component analysis (PCA), a pattern recognition approach, may explain variance within a vast volume of variables. PCA reduces the data to the key factors affecting the water quality by screening the most essential parameters representing the complete data set.
PCA was performed using the Scikit-learn library of Python programming ecosystem with the goal of reducing and interpreting a multivariate data set into its primary components. A correlation study was carried out to determine how the various physicochemical factors are related to indicator bacteria. A significant correlation between the parameters was developed using Pandas library of Python which has in build option for calculating Pearson correlation. At a 5% level, the significance of the correlation of physicochemical parameters with bacterial parameters was examined. Pearson's correlation analysis was used to determine the degree of linearity between two variables. The correlation coefficient varied from − 1 to + 1 and the linearity was disregarded when the correlation coefficient was between − 0.1 and + 0.1. When the correlation coefficient was between ± 0.1 and ± 0.3, there was a mild or weak positive/negative linear correlation, there was a moderate positive/negative linear correlation when it was between ± 0.3 and ± 0.7 and there was a strong positive/negative linear correlation when it was between ± 0.7 and ± 1.0. Finally, for plotting graphs, figures etc. matplotlib library of Python programming, Arc GIS version 10.3 and CorelDRAW technical suite 2021 was used.
Results and discussion
Springs in Kulgam
The springs in the study region are emanating mainly through alluvial deposits and Karewa Formation and one of them flowing through the hard rock at location S27. The discharge measurements of springs showed that the flow rate was high during the post-melting season (2021) due to the high temperature that melts the snow and thus accelerates the flow rate. In the pre-melting season (2022), the rainfall increases discharges instantly at few sites as shown in (Table 1). The results of discharge measurements of alluvial, Karewa and hard rock springs showed no prominent difference in flow rate. On the basis of flow rate, the springs in the study area falls in the fifth order (1-10 l/s) and sixth order (0.1-1 l/s) classes. The temperature measurements showed that the springs are cold, normal and warm types (Table 2). The springs with temperature of 12.2°C and > 12.2°C are emanating from shallow and large aquifers respectively. On the basis of spheres of discharge, the spring types are limnocrene and rheocrene types, flowing perennially. The surrounding ecosystems of springs are terrestrial, lentic and lotic. On the basis of pollution indicators like fecal coliforms the springs are polluted biological types.
Physicochemical parameters
The range of physicochemical parameters during post-melting (2021) and pre-melting (2022) is shown in Table 2. The results of physicochemical evaluation of springs in the study area showed prominent seasonal variation (Table 2). The values of pH varied from 6.8 to 8.1 and 6.4 to 8 in post-melting and pre-meting seasons respectively and lie within the permissible limits of 8.5 (BIS, 2012). The EC in the post-melting was found in the ranges of 78.7μS/cm and 656 μS/cm however in the pre-melting season the values varied between 59.3μS/cm and 435μS/cm. Salinity in spring showed variation from 36.7 to 317 mg/l and 27.7 to 209 mg/l during post-melting and pre-melting seasons respectively. The TDS values ranged from 47.9 to 401 mg/l in post-melting season and from 36.2 to 226 mg/l during pre-melting season and are within permissible limits of 2000 mg/l and 1500 mg/l of BIS (2012) and WHO (2017) respectively. The DO in springs lie within permissible limits of 8 mg/l (BIS, 1991; WHO, 2011) and varied between 6.8 to 8.4 mg/l and 7 to 8.9 mg/l during post-melting and pre-melting seasons respectively. DO values > 6 mg/l indicates that the springs are not contaminated with organic wastes (Kumar & Kumar, 2013; Rawat & Joshi, 2019). BOD and COD lie within the permissible of 4 mg/l and 200 mg/l respectively (BIS, 1991; WHO, 2011). In post-melting season, BOD was found in the range of 2.6 to 3.6 mg/l and in pre-melting season the values varied from 2.5 to 3.4 mg/l. The concentration of COD varied from 25 to 34 mg/l and 24 to 33 mg/l during post-melting and pre-melting seasons respectively. However, nitrate and phosphate were found above the permissible limit of 45 mg/l (BIS, 2012; WHO, 2017) and 1 mg/l (WHO, 1999) respectively at few spring sites. In the post-melting season high concentration of nitrate was found at S14, S13, and S17 with values of 75.6 mg/l, 52 mg/l, and 47 mg/l respectively and that of phosphate concentration was 1.5 mg/l at S14, 1.2 mg/l at S13 and 1.1 mg/l at S17.The values of nitrate and phosphate concentration in the pre-melting season was 57.1 mg/l and 1.2 mg/l respectively at spring site (S14). The strong positive correlation between nitrate and phosphate (Fig. 5a and b) during both the seasons indicates the same source of nitrate and phosphate in the spring water. The possible sources of nitrates and phosphates in the study area indicate surface runoff and profound leaching from agricultural fields and soils, surface drains, and domestic sewage (Bhat et al., 2022).
Microbiological spring water quality
The coliforms are members of the Enterobacteriaceae which comprised Enterobacter and E. coli, the typical intestinal organisms and incidental pathogens viz Citrobacter, Klebsiella, Leclercia genera and Kluyvera and few members of the genus Serratia (Figueras & Borrego, 2010). Majority of the water samples during post-melting season (2021) contained total coliform count > 180 MPN/100 ml except Mishpora spring (S9), Chumgund spring (S23), Dardgund spring (S26), and Akhal pring (S27) with total coliform count of 161 MPN/100 ml, 43 MPN/100 ml and 3 MPN /100 ml respectively (Fig. 3a). In the pre-melting season (2022) the total coliform count ranges from 13 MPN/100 ml to > 180 MPN /100 ml (Table 2, Fig. 3b).
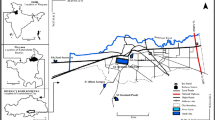
However, there are few limitations in the common use of coliforms as indicator microorganisms as these indicators can also be found in the natural environment (Verhille, 2013). Hence, contamination of spring water by presumptive bacteria is not an absolute evidence that contamination is through fecal matter. In order to confirm the spring water contamination is due to the fecal material, differential coliform test (Eijkman test) was performed for the detection of E. coli. As it is present in animal and human feces, usually not present elsewhere in the environment. Moreover, it is easily accessible, specific, affordable, fast, and sensitive to detect and is the best and familiar indicator microorganism to report the public health risks related to the consumption of contaminated drinking water (Odonkor & Ampofo, 2013). The results showed that the E. coli falls in the range of < 1 to 161 MPN/100 ml and < 1 to > 180 MPN/100 ml during post- melting (2021) and pre-melting (2022) respectively (Table 2). The results showed that all the water samples were contaminated with E. coli (Fig. 3a and b), except Akhal spring (S27) with E. coli count < 1MPN /100 ml during both the seasons. The Akhal spring is present in the middle of forest and far away from many of the contamination sources that are responsible for polluting springs in the study area. Figure 4 shows the various pathways and sources that are existing in the study area for contamination of springs. The Matalhama spring (S6) and Kadder spring (S15) are highly contaminated with E. coli count of 161 MPN/ 100 ml during post-melting (2021). The highest E. coli values of > 180 MPN /100 ml and 161 MPN /100 ml was found in Redwani Spring (S8) and Matibugh spring (S14) respectively during pre-melting season (2022) as depicted in Fig. 3a and b. The Matalhama spring and Redwani spring are located close to the contaminated streams, the overflow of these streams during rainfall contaminate the springs and leads high contamination loading. The high contamination of E. coli in these spring sites is due to the various conditions prevailing in the surroundings of sprigs as shown in the Fig. 4.
The existence of fecal streptococci in the water confirms that the contamination is due to the human and animal feces (Senior, 1989; Saxena et al., 2015; Rochelle-Newall et al., 2015). Fecal streptococci have sanitary significance. They exhibit a close correlation with the health risks (gastrointestinal symptoms) because of using contaminated drinking water. They are always existing in the feces of warm-blooded animals, unable to increase in number in water resources contaminated by sewage and are not present predominantly as coliforms. These organisms have slower die-off rate in water than that of coliforms and their persistent pattern is identical to that of possible waterborne bacterial pathogens (Layton et al., 2010; Saxena et al., 2015). The results showed that the fecal streptococci was present in the ranges of < 1 to > 180 MPN/ 100 ml and < 1 to 24 MPN / 100 ml during post-melting (2021) and pre-melting (2022) respectively as shown in the Table 2. In the post-melting season (2021) Jadiporsa spring (S2), Qouimuh spring (S7), Redwani spring (S8), Mishpora spring (S9), Kathpora spring (S11), Yaripora spring (S12), Shgenpora spring (S17), and Aakhal spring (S27) showed lowest fecal streptococci count with < 1MPN/100 ml and Matalhama spring (S6) with highest limit of > 180 MPN /100 ml because close to the spring Matalhama there is flowing a stream that contaminates it and leads the highest total coliform count in the spring. During pre-melting season (2022) < 1 MPN/100 ml fecal streptococci count was shown by the Srandoo spring (S1), Jadipora spring (S 2), Kulpora spring (S3), Nanibugh spring (S4), Ashmuji Spring (S5), Mishpora spring (S9), Such spring (S24) and Aakhal spring (S27) however fecal streptococci count of 24 MPN/100 ml was found in the Qouimuh spring (S7) as shown in the bar diagram (Fig. 3b).
From the results, it can be concluded that there is no prominent seasonal change in the total coliform count of spring water in the study area. However, E. coli and fecal streptococci showed slight dynamic seasonal variations in the count. The rainfall increases the contamination level of indicator microorganisms in the water resources due to the mixing of runoff water with the fecal matter with water sources at some level (Byeon et al., 2011; Seo et al., 2019; Singh et al., 2019). The study area received rainfall in both the seasons and hence gives dynamic variations in the E. coli and fecal streptococci count (Table 2). The evaluation of indicator microorganism exhibited that the springs in the study area are contaminated with indicator microorganisms (Table 2). The presence of indicator microorganisms depicts the fecal contamination of potable water with pathogens and hence its quality degradation (Saxena et al., 2015).
Overall, it is concluded that the spring water in the study area is not safe for drinking purpose. The typical water borne disease includes; cholera, typhoid, hepatitis A and E, giardiasis, amoebiasis, dracunculiasis, bacillary dysentery, acute diarrhea (Gastro Enteritis), E. coli diarrhea, enteric fever, paratyphoid fever, hookworm, roundworm, and whipworm (Nwabor et al., 2016). The causing agents of these water borne diseases are bacteria, parasite, virus, and protozoa. The mode of transmission is through feces/urine, cutaneous, and anal and that of infection is oral (Nwabor et al., 2016). Among the above-mentioned diseases hepatitis A and E (Viral), acute diarrhoeal (Gastro Enteritis) and enteric fever (bacteria) were reported in the district Kulgam and their mode of transmission and infection is feces and oral respectively (Table 3). The data of different diseases has been collected from the Chief Medical Office (CMO), Kulgam, Kashmir and from a study published by (Kadri et al., 2018). The water borne disease data (Table 3) of the district Kulgam depicted that the drinking water resources are microbiologically contaminated. This data also supported the results of present study that the water resources are contaminated in the study area. According to the sanitary inspection risk score by WHO (2017) if the indicator microorganisms exist in the range of < 1 it indicates low risk and no action is needed. If the ranges are between 1 and 10, then there is intermediate risk and low action priority is required. The ranges of 11–100 and > 100 indicates high risk: higher priority and very high risk: urgent action required respectively. The springs in the study area falls in the intermediate risk to urgent risk score and needs urgent action. The residents of the district protested several times and appealed local government for safe potable water access (Daily Excelsior, 2019; Greater Kashmir, 2020; Kashmir Reader, 2020).
Correlation analysis
Correlation between microbial indicators and physicochemical parameters of the spring water during both the seasons can be seen in the correlation plots (Fig. 5a and b). The total coliform, E. coli and fecal streptococci showed weak to moderate positive correlation with each other as can be seen in the Pearson correlation plots (Fig. 5a and b) during both the seasons and indicates same possible source of fecal waste of these bacteria in the water. The three indicator bacteria in the post-melting and pre-melting season showed weak to moderate positive correlation with COD and rainfall expect E. coli which showed weak negative correlation with rainfall during the post-melting season (2021). Weak to moderate negative correlations of total coliform was found with pH, temperature, discharge, DO, and BOD in both post-melting and pre-melting seasons. Total coliform recorded weak positive and negative correlation with salinity during the post-melting season and pre-melting season respectively. The weak positive correlation of total coliform was found with nitrate and phosphate during both the sampling seasons and this can be observed in the Fig. 5a and b. E. coli and fecal streptococci exhibited weak negative correlation with the DO, BOD, nitrate and phosphate in the post-melting season and weak positive correlation with discharge of spring water in both the seasons. The weak positive correlation of E. coli with pH and salinity was found in both the post-melting and pre-melting seasons. Fecal streptococci shows weak negative correlation with salinity and pH, and weak positive correlation with temperature in the post melting season. However, it shows weak to moderate positive correlation with salinity, pH, nitrate and phosphate during the pre-melting season (Fig. 5a and b). The E. coli showed weak negative correlation with temperature in both the sampling seasons but fecal streptococci exhibited weak positive and negative correlation with temperature during post-melting season and pre-melting season respectively.
The positive correlation of fecal indicator bacteria with COD and rainfall was also reported by Byeon et al. (2011). Singh et al. (2019), Seo et al. (2019). Rainfall washout different wastes from agricultural fields, soils, domestic waste dumps etc. and the leached material has ability to adsorb in water resources and cause positive correlation with the concentration of indicator bacteria (Seo et al. 2019). The rainfall increases the discharge rate of spring systems and also carried various wastes from different places thus by this way discharge showed positive correlation with the bacterial concentration. The negative correlation of BOD with total coliforms and fecal coliforms was also reported by Seo et al. 2019. The results of correlation showed the predominant physicochemical factors that affects the bacteriological quality of spring water in the study area are COD, rainfall, nutrients, and discharge (Fig. 5a and b).
Principal component analysis (PCA)
Principal component analysis is one of the multivariate statistical methods that is most commonly used presently. It is a statistical technique that generally goes with the common name of factor analysis and has been largely used in the fields like pattern recognition which includes analysis of multivariate hydrogeological data as well (Rawat & Joshi, 2019). Using a smaller number of “summary indices” that are easy to present and understand, PCA is a statistical technique that condenses the information of huge data tables (Rawat & Joshi, 2019; Yang et al., 2020). The fundamental information can, for example, be measurements presenting the characteristics of spring samples as is the case in our study. By turning a set of values for correlated variables like spring water characteristics into a set of values for linearly uncorrelated variables, PCA is used to reduce the number of parameters. Old parameters are changed into new ones. These new parameters indicate that since the majority of the information is included in the first few new parameters, it is acceptable to eliminate less important ones and instead choose the most significant ones, which results in dimensionality reduction. In conclusion, PCA's basic principle is to minimize the number of variables in a data set while maintaining as much accuracy as possible (Rawat & Joshi, 2019).
PCA was employed to ascertain the physicochemical and microbiological parameter profile of springs during post-melting and pre-melting seasons. During post-melting (2021), PC1, PC2, PC3, and PC4 accounts for about 24%, 18%, 14%, and 11% variation respectively. All parameters show considerable participation in both PC1 and PC2. PC1 includes high positive loading of DO and BOD followed by moderate positive loading of pH and rainfall, and have weak positive loading of temperature and discharge. Moreover, PC1 also showed high negative loading of COD and total coliform followed by moderate negative loading of E. coli, fecal streptococci, nitrate and phosphate, followed by weak negative loading of salinity. PC2, on the other hand, showed high positive loading of parameters like salinity, phosphate and nitrate, followed by medium positive loading of pH and BOD then mild positive loading of temperature and DO. In addition to this, PC2 also showed high negative loading of fecal streptococci followed by medium negative loading of rainfall and E. coli. PC2 is found to have mild negative loading of variables like COD, total coliform and discharge. Parameters like DO, BOD, pH and temperature control the water quality of sites S8, S11, S26, S27, S29, and S30. The water quality of S12, S13, S14, S16, and S17 is chiefly influenced by parameters like salinity, phosphate and nitrate. Parameters like COD, total coliform, E. coli and fecal streptococci showed influence on water quality of sites S1, S2, S3, S5, S7, S18, S19, S20, and S28 while the water quality of sites S4, S9, S15, S21, S22, S23, S24, and S25 depicted dependence mostly on parameters like rainfall and discharge (Fig. 6a1, a2 and Table 4).
During pre-melting (2022), PC1, PC2, PC3, and PC4 account for about 26%, 21% 11%, and 9% variation respectively. All parameters show considerable participation in both PC1 and PC2. PC1 includes high positive loading of variables like nitrate, COD, total coliform, and phosphate followed by moderate positive loading of E. coli and fecal streptococci, and weak positive loading of salinity and rainfall. Moreover, PC1 also showed high negative loading of BOD followed by moderate negative loading of DO, temperature and pH, followed by weak negative loading of discharge. PC2, on the other hand, exhibited high positive loading of parameters like salinity and pH followed by medium positive loading of phosphate, temperature, nitrate, fecal streptococci, BOD and E. coli and mild positive loading of DO. In addition to this, PC2 also showed high negative loading of total coliform followed by medium negative loading of rainfall and mild negative loading of variables like COD and discharge. Parameters like nitrate, phosphate, E. coli, and fecal streptococci control the water quality of sites S8, S12, S13, S14 and S28. The water quality of S11, S15, S17, S21, S26, S27, S29, and S30 is mainly influenced by parameters like BOD, DO, temperature and pH. Only discharge showed influence on water quality of sites S4, S5, S9, S22, S24, and S25 while the water quality of sites S1, S2, S3, S6, S7, S10, S16, S18, S19, S20, and S23 depicted dependence mostly on parameters like total coliform, rainfall, and COD (Fig. 6b1, b2 and Table 5).
The PCA analysis showed that the water quality of most of spring sites in the study area are influenced largely by the indicator bacteria, COD, rainfall, and nutrients. Rainfall is the important parameter as it causes the change in pH, increase the flow rate of spring and wastes from various sources to the springs. Rainfall influences the water quality directly and indirectly and hence is the important parameter (Byeon et al., 2011; Singh et al., 2019; Seo et al., 2019).
Conclusions
The present study assessed the presence of total coliform, E. coli and fecal streptococci bacteria in the Himalayan springs during the post-melting season 2021 and pre-melting season 2022. The springs were sampled from the rural, urban and forest areas. The spring systems in the district kulgam are flowing mainly from the alluvial deposits, Karewa Formation, and hard rock Formation of Pir Panjal Traps. The evaluation of physicochemical characteristics showed that all the parameters are within the permissible limits except nitrate and phosphate at sites S13, S14, and S17, which is related to the agricultural and domestic activities in the spring catchment. The springs are found largely contaminated with total coliforms and highly to moderately contaminated with E. coli and fecal streptococci during both the seasons this is because of the various sources of contamination like contaminated streams, polluted surroundings, latrine trenches etc. that are present in the vicinity of spring water systems. The correlation between physicochemical parameters and indicator microorganisms was derived by Pearson correlation analysis and the major controlling physicochemical parameters that controls the concentration of fecal indicator microbes were determined. The results of correlation analysis demonstrated that the rainfall, discharge, COD, nitrate and phosphate are the main influential parameters for the concentration of total coliform, E. coli and fecal streptococci. The rainfall is found one of the important factor in controlling the microbiological quality of springs as it influences the water quality by accelerating the problem of fecal contamination in springs both directly and indirectly by increasing the discharge. The PCA analysis showed that the water quality of most of the spring sites is influenced largely by the indicator bacteria, COD, rainfall, nitrate and phosphate. The results obtained revealed that the spring water in the study area is not safe for drinking water purpose. The water borne diseases data such as hepatitis A and E, jaundice, acute diarrhoeal (gastro enteritis), and enteric fever of the district collected from existing literature of (Kadri et al., 2018) and CMO Kulgam, Kashmir also supported the results of present investigation that the water resources are contaminated microbiologically in the research area. The springs in the study area falls in the intermediate to urgent risk score and needs urgent action like keeping surrounding ecosystem of spring water systems healthy, fencing and roofing around spring water in order to avoid contamination by animal feces, continuous monitoring and data collection of microbiological parameters should be definitely regulated for better water quality management. Chlorination, combined flocculation/disinfection and filtration should be practiced before consumption. In order to ensure the safe potable spring water supplies, the sand gravel filters should be installed at source supplies as they can sieve out microbes like protozoa and bacteria. The coagulation process by using aluminum sulfate (alum) can be employed to purify the water by removing the non-settling particles through the floc formation and the flocs formed can be removed through filtration. Proper boiling of spring water at household level before consumption should be done. At the local level springs should be maintained by preventing the possible animal and human wastes. Public awareness programs should be executed by government organizations. Persistent research can present valuable information on the appropriate possible management plans of coliform occurrence not only in spring water but also in other water resources. This study may help in prospecting the future water quality management of Himalayan springs.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Alfaro, C., & Wallace, M. (1994). Origin and classification of springs and historical review with current applications. Environmental Geology, 24(2), 112–124.
APHA. (2017). Standard Methods for the Examination of Water and Waste Water. (23rd ed.). Washington DC, USA, (2017).
Bhat, N. A., & Jeelani, G. (2018). Quantification of groundwater–surface water interactions using environmental isotopes: A case study of Bringi Watershed Kashmir Himalayas India. Journal of Earth System Science, 127(5), 1–11. https://doi.org/10.1007/s12040-018-0964-x
Bhat, N. A., Jeelani, G., & Bhat, M. Y. (2014). Hydrogeochemical assessment of groundwater in karst environments, Bringi watershed Kashmir Himalayas India. Current Science, 106(7), 1000–1007. http://www.jstor.org/stable/24102386
Bhat, S. U., & Pandit, A. K. (2010). Limnochemistry of three freshwater springs of Kashmir Himalaya. Hydro Nepal Journal of Water Energy and Environment, 7, 54–59. https://doi.org/10.3126/hn.v7i0.4237
Bhat, S. U., & Pandit, A. K. (2018). Hydrochemical characteristics of some typical freshwater springs: A case study of Kashmir Valley Springs. International Journal Water Resources Arid Environments, 7(1), 90–100.
Bhat, S. U., Dar, S. A., & Hamid, A. (2022). A critical appraisal of the status and hydrogeochemical characteristics of freshwater springs in Kashmir Valley. Scientific Reports, 12(1), 1–13.
Bhat, S. U., Dar, S. A., & Sabha, I. (2021). Assessment of threats to freshwater spring ecosystems. Reference Module in Earth Systems and Environmental Sciences, 1–6.
Bhat, S. U., Mushtaq, S., Qayoom, U., & Sabha, I. (2020). Water Quality Scenario of Kashmir Himalayan Springs: A Case Study of Baramulla District Kashmir Valley. Water Air Soil Pollution, 231(9), 1–17. https://doi.org/10.1007/s11270-020-04796-4
Bhat, S. U., & Pandit, A. K. (2020). Water quality assessment and monitoring of Kashmir Himalayan freshwater springs: A case study. Journal Aquatic Ecosystem Management Health, 23(3), 274–287. https://doi.org/10.1080/14634988.2020.1816771
Bhat, S. U., Pandit, A. K., & Mudathir, R. (2010). Limnological investigation of three freshwater springs of Pulwama district Kashmir valley. Recent Research Science Technology, 2(2), 88–94.
BIS. (1991). Indian standard specifications for Drinking Water IS:10500, 1991. Bureau of Indian Standards, New Delhi, India.
BIS. (2012). Drinking Water Specification, Second Revision IS:10500, 2012. Bureau of Indian Standards, New Delhi, India.
Bosch, A. (2007). Human viruses in water: Perspectives in medical virology. Elsevier.
Brune, G. (1975). Major and Historical Springs of Texas. Texas Water Development Board, Austin, Report 189.
Byeon, S. J., Choi, G. W., & Jo, H. G. (2011). A study on discharged coliform into coastal bathing water after rainfall event. Journal of the Korean Society of Hazard Mitigation, 11(5), 337–344.
Center for Hydrometeorology and remote sensing (CHRS). (2022). Retrieved September 23, 2022, from https://chrs.web.uci.edu/
Chen, W., Tsangaratos, P., Ilia, I., Duan, Z., & Chen, X. (2019). Groundwater spring potential mapping using population-based evolutionary algorithms and data mining methods. Science of the Total Environment, 684, 31–49. https://doi.org/10.1016/j.scitotenv.2019.05.312
Daily excelsior. (2019). Kulgam better developed; portable water, pollution major issues. Retrieved September 27, 2022 from https://www.dailyexcelsior.com/kulgam-better-developed-portable-water-pollution-major-issues/
Figueras, M. J., & Borrego, J. J. (2010). New perspectives in monitoring drinking water microbial quality. International Journal of Environmental Research and Public Health, 7(12), 4179–4202. https://doi.org/10.3390/ijerph7124179
Greater Kashmir. (2020). Kulgam: Most filtration plants defunct, waterborne diseases on the rise. Retrieved September 27, 2022, from https://www.greaterkashmir.com/editorial/kulgam-most-filtration-plants-defunct-waterborne-diseases-on-the-rise
Guppy, L., & Anderson, K. (2017). Water Crisis Report. United Nations University Institute for Water, Environment and Health. Hamilton, Canada, 1–16.
Hynes, H. B. N. (1970). The Ecology of Running Waters (p. 555). Liverpool University Press.
Ikonen, J. M., Hokajärvi, A. M., Heikkinen, J., Pitkänen, T., Kolehmainen, M., Ciszek, R., Pursiainen, A., Kauppinena, A., Kusnetsova, J., Torvinen, E., & Miettinen I. T. (2017). Drinking water quality in distribution systems of surface and ground waterworks in Finland. Journal Water Security, 3, 1–10. https://doi.org/10.15544/jws.2017.004
India Water Portal. (2022). Springs in India. Retrieved September 15, 2022, from https://www.indiawaterportal.org/topics/springs/springs-india
Jeelani, G., & Bhat, N. A. (2012). Application of environmental isotopes for identification of possible recharge areas of alluvial springs of Vishaw catchment in Kashmir Himalaya. Journal Himalayan Ecology Sustainable Development, 7.
Jeelani, G., Bhat, N. A., Shivanna, K., & Bhat, M. Y. (2011). Geochemical characterization of surface water and spring water in SE Kashmir Valley, western Himalaya: Implications to water–rock interaction. Journal of Earth System Science, 120(5), 921–932. https://doi.org/10.1007/s12040-011-0107-0
Jeelani, G., Kumar, U. S., Bhat, N. A., Sharma, S., & Kumar, B. (2015). Variation of δ18O, δD and 3H in karst springs of south Kashmir, western Himalayas (India). Hydrol Processes, 29(4), 522–530. https://onlinelibrary.wiley.com/journal/10991085
Jeelani, G., Shah, R. A., Deshpande, R. D., Fryar, A. E., Perrin, J., & Mukherjee, A. (2017). Distinguishing and estimating recharge to karst springs in snow and glacier dominated mountainous basins of the western Himalaya, India. Journal Hydrology, 550, 239–252. https://doi.org/10.1016/j.jhydrol.2017.05.001
Kadri, S. M., Rehana, K., Benetou, D. R., Ahmad, D. F., Abdullah, A., & Chattu, V. K. (2018). Hepatitis A and E outbreak surveillance during 2015–2017 in Kashmir, India: Is the water to blame? Journal of Epidemiology and Global Health, 8(3–4), 203–207. https://doi.org/10.2991/j.jegh.2018.04.101
Kashmir Reader. (2020). Amid pandemic, Kulgam residents fear outbreak of waterborne diseases. Retrieved September 27, 2022, from https://kashmirreader.com/2020/05/11/amid-pandemic-kulgam-residents-fear-outbreak-of-waterborne-diseases/
Kumar, M., & Kumar, R. (2013). Assessment of physico-chemical properties of ground water in granite mining areas in Goramachia, Jhansi, UP India. International Research Journal Environmental Science, 2(1), 19–24.
Layton, B. A., Walters, S. P., Lam, L. H., & Boehm, A. B. (2010). Enterococcus species distribution among human and animal hosts using multiplex PCR. Journal of Applied Microbiology, 109(2), 539–547. https://doi.org/10.1111/j.1365-2672.2010.04675.x
Lone, S. A., Bhat, S. U., Hamid, A., Bhat, F. A., & Kumar, A. (2021). Quality assessment of springs for drinking water in the Himalaya of South Kashmir. Environmental Science Pollution Research, 28(2), 2279–2300. https://doi.org/10.1007/s11356-020-10513-9
Mahamuni, K., & Kulkarni, H. (2012). Groundwater resources and spring hydrogeology in South Sikkim, with special reference to climate change. In: Climate change in Sikkim- Patterns, impacts and initiatives, Arrawatia, M.L., & Tambe, S. (eds) Gangtok, India; Information and Public Relations Department, Government of Sikkim. Pp, 261–274.
Meinzer, O. E. (1923). Outline of ground-water hydrology, with definitions. United States Geological Survey Water Supply Paper, 494(48–56), 71. https://doi.org/10.3133/wsp494
Mir, K. A., & Bhat, F. A. (2017). Directorate of Geology and Mining, District Survey Report Kulgoam. Geological Division, DGM, Srinagar.
Nwabor, O. F., Nnamonu, E. I., Martins, P. E., & Ani, O. C. (2016). Water and waterborne diseases: A review. International Journal Tropical Disease Health, 12(4), 1–14. https://doi.org/10.9734/IJTDH/2016/21895
Odonkor, S. T., & Ampofo, J. K. (2013). Escherichia coli as an indicator of bacteriological quality of water: an overview. Microbiological Research, 4(1), e2, 5–11. https://doi.org/10.4081/mr.2013.e2
Rawat, A., & Joshi, G. K. (2019). Physicochemical and microbiological assessment of spring water in central Himalayan region. Environmental Monitoring and Assessment, 191(4), 1–16. https://doi.org/10.1007/s10661-019-7369-4
Rochelle-Newall, E., Nguyen, T. M. H., Le, T. P. Q., Sengtaheuanghoung, O., & Ribolzi, O. (2015). A short review of fecal indicator bacteria in tropical aquatic ecosystems: Knowledge gaps and future directions. Frontiers in Microbiology, 6, 308. https://doi.org/10.3389/fmicb.2015.00308
Saxena, G., Bharagava, R. N., Kaithwas, G., & Raj, A. (2015). Microbial indicators, pathogens and methods for their monitoring in water environment. Journal Water and Health, 13(2), 319–339. https://doi.org/10.2166/wh.2014.275
Senior, B. W. (1989). Examination of water, milk. food and air In: Mackie and McCartney Practical Medical Microbiology, 14th edn, Collee, J.G., Duguid, J.P., Fraser, A.G & Marinion, B.P. (eds). Churchill Livingstone, Edinburgh, New York, pp,883–918.
Seo, M., Lee, H., & Kim, Y. (2019). Relationship between coliform bacteria and water quality factors at weir stations in the Nakdong River South Korea. Water, 11(6), 1171. https://doi.org/10.3390/w11061171
Singh, A. K., Das, S., Singh, S., Pradhan, N., Gajamer, V. R., Kumar, S., & Tiwari, H. K. (2019). Physicochemical parameters and alarming coliform count of the potable water of Eastern Himalayan state Sikkim: An indication of severe fecal contamination and immediate health risk. Frontiers Public Health, 7, 174. https://doi.org/10.3389/fpubh.2019.00174
Sobsey, M. D., Stauber, C. E., Casanova, L. M., Brown, J. M., & Elliott, M. A. (2008). Point of use household drinking water filtration: A practical, effective solution for providing sustained access to safe drinking water in the developing world. Environmental Science and Technology, 42(12), 4261–4267.
Springer, A. E., Stevens, L. E., Anderson, D. E., Parnell, R. A., Kreamer, D. K., Levin, L. A., & Flora, S. P. (2008). A comprehensive springs classification system. Aridland springs in North America: ecology and conservation; University of Arizona Press and Arizona–Sonora Desert Museum, Tucson, 49–75.
Stevens, L. E., Springer, A. E., & Ledbetter, J. D. (2011). Inventory and monitoring protocols for springs ecosystems. http://docs.springstewardship.org/PDF/SpringsInventoryProtocols110602.pdf
Stevens, L. E., Schenk, E. R., & Springer, A. E. (2021). Springs ecosystem classification. Ecological Applications, 31(1), e2218.
Verhille, S. (2013). Understanding microbial indicators for drinkingwater assessment: interpretation of test results and public health significance. National collaborating centre for environmental health. 1–12. Available from: https://www.ncceh.ca/sites/default/files/Microbial_Indicators_Jan_electroniclink2013_0.pdf
Wen, X., Chen, F., Lin, Y., Zhu, H., Yuan, F., & Kuang, D., & Yuan, Z. (2020). Microbial indicators and their use for monitoring drinking water quality: A review. Sustainability, 12(6), 2249. https://doi.org/10.3390/su12062249
WHO. (2017). Guidelines for drinking-water quality. World Health Organization.
WHO. (2018). Water Safety and Quality. World Health Organization.
WHO. (1999). Guidelines for drinking water quality. World Health Organization.
WHO. (2011). Guidelines for drinking-water quality. World health organization.
WHO. (2019). Water, sanitation, hygiene and health: a primer for health professionals (No. WHO/CED/PHE/WSH/19.149). World Health Organization.
Yang, W., Zhao, Y., Wang, D., Wu, H., Lin, A., & He, L. (2020). Using principal components analysis and IDW interpolation to determine spatial and temporal changes of surface water quality of Xin’anjiang river in Huangshan, China. International Journal of Environmental Research and Public Health, 17(8), 2942. https://doi.org/10.3390/ijerph17082942
Acknowledgements
The authors gratefully acknowledge the editors and reviewers for their valuable suggestions and comments which have improved the manuscript substantially. The authors appreciate the encouragement given by the Chairperson of the Department of Geology, Aligarh Muslim University, Aligarh, India. The authors would also like to appreciate Prof. Gulnaz Bashir at the Department of Microbiology Sher-i- Kashmir Institute of Medical Sciences (SKIMS), Srinagar Kashmir, India under the supervision of her the microbiological analysis was done. The authors also acknowledge Mr. Muzaffar, laboratory assistant at the Department of Microbiology (SKIMS) in Mycology Laboratory for helping in microbiological analysis.
Funding
The first author would like to acknowledge University Grant Commission (UGC) Government of India for providing PhD fellowship during this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, microbiological, and physicochemical analysis are performed by Fahim Un Nisa. The first draft of the manuscript is written by Fahim Un Nisa and Rashid Umar. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nisa, F.U., Umar, R. Evaluation of physicochemical and microbiological parameters, and their correlation in Himalayan Spring Water Systems: A case study of District Kulgam of Kashmir Valley, India, Western Himalaya. Environ Monit Assess 195, 441 (2023). https://doi.org/10.1007/s10661-023-11025-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11025-y