Abstract
This study aimed to reveal the effect of selenium (Se) and heavy metals (chromium (Cr), cadmium (Cd), lead (Pb), and mercury (Hg)) on the quality, fatty acids, and 13 kinds of ions in the egg yolk and albumen. Four experimental groups were established, including a control group (control; basal diet), Se group (basal diet + Se), heavy metals group (basal diet + CdCl2 + Pb(NO3)2 + HgCl2 + CrCl3), and Se + heavy metal (HM) group (basal diet + Se + CdCl2 + Pb(NO3)2 + HgCl2 + CrCl3). Se supplementation significantly increased the experimental egg yolk percentage since Se accumulation mainly occurred in the yolks of the eggs. The Cr content in the yolks of the Se + heavy metal groups decreased at 28 days, while a significant reduction was evident in the Cd and Hg levels of the Se + heavy metal yolks compared to the heavy metal group at 84 days. The complex interactions between the elements were analyzed to determine the positive and negative correlations. Se displayed a high positive correlation with Cd and Pb in the yolk and albumen, while the heavy metals minimally affected the fatty acids in the egg yolk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential nutritional micro-element for many organisms and is indispensable for maintaining human health. Se displays antioxidant properties and participates in free radical removal and carcinogenic resistance (Pilarczyk et al. 2019). It is a vital component of glutathione peroxidase (GSH-Px), an antioxidant enzyme mainly responsible for preventing reactive oxygen species (ROS) formation while participating in the metabolic regulation of various hormones (such as thyroid hormones) (Muhammad et al. 2021). Se deficiency is associated with a compromised immune system and increased susceptibility to various disorders, including cancer, cardiovascular disease, diabetes, and immunodeficiency (Fisinin et al. 2009; Rayman 2012; Vinceti et al. 2018). The low dietary Se levels evident in regions of China, New Zealand, Europe, and Russia (Kipp et al. 2015) have necessitated the development of Se-enriched food, such as supplemented eggs, which is produced by adding Se to layer feed (Liu et al. 2022). These types of products may provide a solution for the widespread dietary Se deficiency.
Studies have shown that adding organic Se to the diets of chickens substantially increases Se accumulation in eggs (Payne et al. 2005; Skrivan et al. 2008). Liu et al. (2020) reported that adding Se to the diets of laying hens increased egg production, while Se yeast supplementation was more successful in raising the Se content in egg yolk than sodium selenite. Urso et al. (2015) indicated that the Se content in egg yolk increased when adding 0.15 to 0.3 mg/kg Se to the diet. Besides increasing the accumulation in eggs, Se also improves antioxidant ability while displaying higher levels in other tissues (Pappas et al. 2006a, 2006b; Liu et al. 2020). Qu et al. (2017) showed that adding Se nanoparticles to the feed of laying hens effectively increased the anti-oxidative protection against deoxynivalenol toxicity. Scheideler et al. (2010) indicated that supplementing the diets of laying hens with vitamin E and Se improved the vitelline membrane strength of fresh and aged eggs. Antioxidants, such as vitamin E and Se, provide crucial protection against lipid peroxidation (Pappas et al. 2005). As an anti-oxidative form of the active center of GPX, Se helps to lower cholesterol levels. Therefore, dietary Se supplementation significantly decreases the total egg yolk cholesterol (Muhammad et al. 2021).
Se is also vital in counteracting tagonizing metal toxicity. It restricts the oxidative stress induced by cadmium (Cd), protecting mammals against toxicity (Zhang et al. 2020a, b). Furthermore, Se supplementation reduces lead (Pb) toxicity and lipid peroxidation by increasing the GPX activity (Adel et al. 2016). It may act as an endogenous “stop signal” for the carcinogenic cell signals (such as AP-1 and NFκB activation) induced by arsenic (As), reducing its toxicity (Zeng et al. 2005). Moreover, Se and zinc (Zn) display a synergistic effect in resisting Cd and Pb toxicity. Luo et al. (2020) indicated that Se counteracted Cd, Pb, and chromium (Cr) while exhibiting a synergistic relationship with Zn and Copper (Cu). Cao et al. (2014) showed that Se, Zn, and silicon (Si) significantly decreased Cd, Cr, Cu, and Pb proliferation in heavy metal-contaminated grain with no effect on yield. The effect of Se against mercury (Hg) is complex and depends on several features, such as the form of Hg, the form of Se, the organ, and the dose (Spiller 2018). Se-enriched eggs are produced in over 25 countries (Fisinin et al. 2009). Since eggs represent a dietary staple food worldwide, the Se and heavy metals transferred to eggs via animal feed can easily reach humans. However, no studies are available regarding the combative effect of Se-enriched eggs against heavy metal toxicity. Therefore, this study explores the relationship between Se and other elements, providing a basis for producing eggs safe for consumption and Se-enriched eggs while discussing egg quality and fatty acid content.
Materials and methods
Experimental design and diets
The experimental procedures were approved by the Animal Care and Use Committee of Sichuan Agricultural University. A total of 160 56-week-old Lohmann laying hens were weighed and randomly divided into four groups (ten replicates per treatment, four hens per replicate), namely, the control group fed a basal diet, the Se group fed a basal diet supplemented with 0.4 mg/kg Se yeast, the heavy metal group fed a basal diet supplemented with heavy metals (5 mg/kg CdCl2 + 50 mg/kg Pb(NO3)2 + 3 mg/kg HgCl2 + 5 mg/kg CrCl3), and the Se + heavy metal group (Se + HM) fed a basal diet supplemented with Se and heavy metals (5 mg/kg CdCl2 + 50 mg/kg Pb(NO3)2 + 3 mg/kg HgCl2 + 5 mg/kg CrCl3 + 0.4 mg/kg Se yeast). The diets were formulated according to the published NRC (1994) recommendations and the Lohmann hen manual, while the feed was administered in mashed form. The entire feeding trial lasted 84 days. The composition and nutrient levels of the basal diet are presented in Table S1, while the metal ion content of presented in Table S2.
Egg quality determination
Egg samples were collected at the end of the supplementation period to analyze the egg quality. The eggshells were measured using the Egg Shell Force Gauge Model II, while shell strength was determined with an eggshell thickness gauge (Robotmation Co., Ltd, Tokyo, Japan). The color values (brightness (black/white) (L*), brightness (black/white) (a*) and chroma value (blue/yellow) (b*)) were measured using a Minolta colorimeter (Konica Minolta Sensing Inc., Osaka, Japan). The albumen height, yolk color, and Haugh units (HU) were analyzed via an Egg Multi-tester (EMT-7300, Robotmation Co., Ltd, Tokyo, Japan). The yolk index value was calculated as 100 × (yolk height (cm)/yolk diameter (cm)), while the yolk ratio was determined as yolk weight (g)/egg weight (g) × 100. The albumin ratio was computed as 100 × [albumin weight (g)/egg weight (g)].
Metal element analysis
Here, 1.5 mL nitric acid (HNO3) was added to 0.5 g of the sample, after which the digestion temperature was raised to 80 °C, where it was maintained for 8 h. The acid was then expelled via the open tank cover at 150 °C. After complete evaporation of the acid, 2 mL HNO3 was added and digested for a further 2 h at 150 °C. Following digestion, the sample was diluted to 50 mL with deionized water and analyzed using inductively coupled plasma mass spectrometry (ICP-MS). The instrumental parameters of the equipment used were described in previous studies (Zhao et al. 2021). The recovery via ICP-MS validation ranged from 91.6 to 98.3%, indicating the accuracy of the method.
Lipid profile evaluation
The fatty acids in the yolk were analyzed using a technique described by Batkowska et al. (2021). The fatty acid methyl esters were assessed via a standard gas chromatography method (Model 7890A, Agilent Technologies, Palo Alto, CA, USA) using an SP-2560 column (100 m × 0.25 mm ID Supelco Inc., Bellefonte, PA, USA) with a flame ionization detector. The initial column oven temperature was 100 °C, which was increased at 10 °C min−1 to 180 °C, where it was maintained for 6 min. Then, the temperature was increased at 1 °C min−1 to 200 °C, maintained for 20 min, and finally elevated to 230 °C at 4 °C min−1. The injector and detector temperatures were maintained at 270 °C and 280 °C, respectively.
Statistical analysis
The results were statistically analyzed using two-way and one-way analysis of variance (ANOVA), followed by post hoc Tukey’s test using SPSS version 19.0. The data were expressed as the means ± standard deviation and were assumed statistically significant at P < 0.05. The relationships between the metal elements were estimated via Pearson’s correlation coefficients.
Results and discussion
The effects of Cd, Pb, Hg, Cr, and Se co-treatment on the egg quality
The external and internal qualities of the fresh eggs from the hens provided with different treatment diets are shown in Table 1. The highest yolk percentage and lowest eggshell color were identified in the Se groups (P < 0.05). The absolute nutritional value of the egg yolk was relatively high, while its size directly affected the egg flavor (Zhang et al. 2021). Therefore, eggs with large yolks are more popular among consumers (Fernandez and Andersen 2015). Gao et al. (2021) found that Se supplementation significantly increased egg yolk percentage. Eggshell color is an important indicator affecting the price of eggs (Wei and Bitgood 1990). In addition to biliverdin, protoporphyrin IX has been identified as a main determinator of eggshell color (Miksik et al. 1996). Se accumulation may affect the expression of the synthetic pigment fund, increasing the color of the eggshell. The HUs of the control and Se groups were higher (P < 0.05) than the heavy metal and Se + HM treatment groups. The treatment did not significantly affect the weight and color of the yolk or the strength and thickness of the eggshell (P > 0.05). Mutlu et al. (2021) revealed a significant HU decrease in quails exposed to Pb. Since HUs are vital indicators of protein quality, higher content levels indicate superior internal egg quality. Therefore, Pb exposure may lead to liver cell toxicity and liver protein synthesis damage, reducing albumen protein production. Ovomucin represented the key protein responsible for egg albumen viscosity and elevated HU levels (Omana et al. 2010). However, Kim et al. (2019) indicated no statistical differences between the external and internal egg quality parameters measured in the laying hens exposed to feed containing heavy metals. This variation in results may be due to the different heavy metal doses.
The Se and heavy metals in the egg yolk and albumen
The Se and heavy metal content in the eggs during the laying period is presented in Fig. 1. The Se content in the egg yolks of the control group increased from 27.80 to 46.87 μg/g, followed by a decrease to 34.07 μg/g, while displaying higher levels in the Se and Se + HM groups at 6.38–14.88 μg/g and 5.43–12.21 μg/g, respectively. No significant differences (P > 0.05) were evident between the Se content of the heavy metal group at the beginning and end of the feeding period. The experimental results of this study are consistent with those of previous research. For example, Utterback et al. (2005) reported that the Se content in the eggs of hens fed a basal diet decreased as the feeding period was extended. Pan et al. (2011) indicated that the Se content in the eggs exposed to different Se treatments was significantly higher at 18 days than at 14 days and 21 days. The Se content in the albumen of the control, Se, heavy metal, and Se + HM treatment groups increased from 10.89–17.85 μg/g, 21.75–33.61 μg/g, 10.56–17.17 μg/g, and 20.44–31.11 μg/g, respectively. The mean Se content was higher in the yolk than the albumen in all the treatment groups, which was consistent with research by Pilarzyk et al. (2019), who found that the yolk displayed higher Se levels than the albumen, presenting a more potent Se source. After Se supplementation, the Se content was two-fold higher than the unsupplemented yolk and albumen, while the increase was statistically significant. Zhang et al. (2020a, b) showed that the Se content of the entire egg increased when adding 0.25 mg/kg Se to the diet.
The Cr content in the yolk of all the experimental groups was affected over time, while the Cd levels remained unaffected, and the Hg content decreased. However, although the Pb levels displayed no differences in the heavy metal and Se + HM groups, they increased in the control and Se groups. The Cd, Pb, and Hg content in the yolk was not affected by dietary Se addition, while the Cr levels in the yolk of the group supplemented with Se for 28 days were higher than in the control group. These findings were consistent with the results of previous studies (Zhao et al. 2021), showing a positive correlation between Cr and Se. Moreover, Se + HM group for 28 days was decreased than the heavy metal group; these could be due to the Se could alleviate the toxic effects of Cr (Liu et al. 2018). The Cr, Cd, and Pb levels in the albumen were affected over time. Compared to the Se group, the Cr content was higher in the Se + HM group at 84 days, while the Pb levels were higher at 28 days, 56 days, and 84 days (P < 0.05), respectively. Previous studies have revealed a correlation between Se and Pb in both humans and animals (Yoo et al. 2002; Zhao et al. 2021). Zhang et al. (2017) reported that Se supplementation increased the Cr, Mn, and Zn contents in the kidneys of chickens. Although the Hg content was not affected over time, differences were apparent between the batches since the Se + HM group differed (P < 0.05) from the others, presenting the lowest values at 28 days (0.09 μg/g), while it was no longer detectable at 56 days and 84 days. Methyl Hg represented the predominant form of Hg in the eggs (Ackerman et al. 2013) and was readily absorbed in the intestine and accumulated in the body. It is considered more toxic than inorganic Hg (Fant et al. 2001). Se minimized the hazardous effect of Hg(II) and MeHg (Luque-Garcia et al. 2013).
The elemental content in the egg yolk and albumen
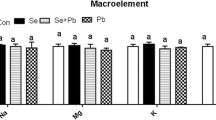
The content of four major elements (Na, Mg, K, and Ca), six minor elements (Mn, Fe, Ni, Cu, Zn, and Mo), and three trace elements (As, Co, and V) in the yolk (Table 2) and albumen (Table 3) were examined to determine the effect of Se, heavy metals, and their combined treatment on ion homeostasis and disturbance. The egg yolks of all the batches displayed a significant decrease (P < 0.05) in the levels of K and Fe over time and a significant increase (P < 0.05) in the Mn, Co, and V contents, while the Ca content was not affected. The Na, Cu, Zn, and As content in the Se group declined over time. The Mg content in the control group displayed a significant decrease (P < 0.05), while the Mg, Mn, Fe, and Co levels presented higher values (14.65 mg/g, 1.43 mg/g, 2.37 mg/g, and 17.92 μg/g) at 84 days. The Mg, Ca, Fe, Cu, and Co values in the albumen of all the batches were highest at 56 days, while the Na and K levels declined over time. No significant differences were evident in the Na, K, Ca, Mn, and Cu contents of each batch. Compared with the control group, the three minor elements (Fe, Zn, and Mo) decreased significantly in the other groups at 28 d (P < 0.01). The Mg and Mo levels were reduced in the heavy metal group, while the As content displayed significantly increased levels compared with the control group at 56 d (P < 0.05). The Ni content was significantly lower in the Se + HM group than in the heavy metal group (P < 0.01). Heavy metal poisoning affects the heavy metal content and other ion levels, disrupting the mineral elements (Bargellini et al. 2008). Although various in vivo and in vitro studies reported that the absorption, distribution, and retention of Zn, Fe, and Cu were affected by either Se deficiency or supplementation, the results are controversial and require confirmation (Klotz et al., 2003). Li et al. (2018) reported that treating the ovaries of laying hens with Cd reduced the Ca, Ti, Cu, and Zn levels, while significantly decreasing the Ba content and substantially increasing the Fe, Mo, and Cd levels.
Pearson’s correlation analysis was performed to investigate the correlation between the mineral elements. These correlations are presented in Fig. 2. Se was positively correlated with Na, Mg, Ca, Fe, Cu, Cd, and Pb (P < 0.05 for the Mg in the yolk, as well as the Na and Cd in the albumen, and P < 0.01 for the other elements). In the yolk, Se appeared to be correlated positively with Zn, As, and Hg (P < 0.01) and negatively with Mn (P < 0.01). In the albumen, Se was positively correlated with Cr, Mn (P < 0.01), and Mo (P < 0.05), and negatively with K. Pappas et al. (2011) investigated the interaction between Se and the various trace elements involved in the antioxidant system of chicken eggs. Cr was positively associated with the Pb in the albumen and negatively with Hg in the yolk (P < 0.01). Furthermore, Cd was correlated positively with Fe, Zn, and Pb in both the yolk and albumen. A positive correlation was evident between Hg and Cd or Cr in the egg yolk, while Cr was positively associated with Pb in the albumen. The Se displayed a high positive correlation with Cd and Pb in the yolk and albumen in the present study. Previous research involving commercially Se-enriched eggs also revealed that Se was positively correlated with the Pb in the eggs (Zhao et al. 2021). Overall, the complex interactions indicated that both synergistic and antagonistic effects persisted among these elements. The possible protective role of Se and the toxic effect of heavy metals may contribute to changes in the trace elements.
The effect of Cd, Pb, Hg, Cr, and Se co-treatment on fatty acids
The fatty acid profiles in the egg yolks of all the experimental groups are presented in Table 4. Nine saturated fatty acids (SFAs) were found (C4:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C21:0, and C24:0) in the analyzed eggs. Except for butyric acid (C4:0), no significant differences were evident between the other SFAs of the four treatment groups. Moreover, Se addition significantly increased the butyric acid (C4:0) content compared to the group without Se supplementation (Se group vs. the control group, and Se + HM group vs. the heavy metals group). These outcomes were consistent with data reported by Gangdadoo et al. (2018), who indicated that providing broilers with a 0.9-mg/kg Se nanoparticle diet increased the short-chain fatty acids, especially butyric acid. Of the monounsaturated fatty acids (MUFA), oleic acid (C18:1n9c) dominated in all the batches, ranging from 36.98 to 38.04%, with no significant differences between the four treatment groups. The nervonic acid (C24:1) level increased significantly after heavy metal supplementation. Furthermore, Se supplementation increased the linolelaidic acid (C18:2n6t) content, although the impact was lower. The α-linolenic acid (C18:3n3) level was considerably higher in the Se group than in the control group. However, this relationship was not observed in the Se + HM and heavy metal groups, consequently exhibiting a higher ω-3 polyunsaturated fatty acid (PUFA) level than in the control group eggs. These findings corresponded with data obtained by Zudunczyk et al. (2013), who noticed an increase in the C18:3n3 and ω-3 PUFA content of eggs after dietary Se supplementation. This may be connected to the interaction between the PUFAs and Se, possibly via GSH-Px action (Fasiangova & Borilova 2017).
Conclusion
This study investigates the effect of Se and heavy metal (Cr, Cd, Pb, and Hg) interaction on the quality, fatty acid levels, and content of 13 types of ions in eggs. Se supplementation increases the yolk percentage of the eggs, as well as the Se content, which mainly accumulates in the yolk. Moreover, Se reduces the Cr content in the yolk at 28 days and the Cd and Hg levels in the albumen at 84 days. The complex interactions between the elements are analyzed, and both positive and negative correlations between these elements are presented. Se displays a significant positive correlation with Cd and Pb in the yolk and albumen, while heavy metals minimally affect the fatty acids in the yolk.
Data availability
The datasets used or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Ackerman JT, Herzog MP, Schwarzbach SE (2013) Methylmercury is the predominant form of mercury in bird eggs: a synthesis. Environ Sci Technol 47:2052–2060
Adel M, Conti GO, Dadar M, Mahjoub M, Copat C, Ferrante M (2016) Heavy metal concentrations in edible muscle of whitecheek shark, Carcharhinus dussumieri (elasmobranchii, chondrichthyes) from the Persian Gulf: A food safety issue. Food Chem Toxicol 97:135–140
Bargellini A, Marchesi I, Rizzi L, Cauteruccio L, Masironi R, Simioli M, Borella P (2008) Selenium interactions with essential and toxic elements in egg yolk from commercial and fortified eggs. J Trace Elem Med Bio 22:234–241
Batkowska J, Drabik K, Brodacki A, Czech A, Adamczuk A (2021) Fatty acids profile, cholesterol level and quality of table eggs from hens fed with the addition of linseed and soybean oil. Food Chem 334:127612
Cao FB, Wang RF, Cheng WD, Zeng FR, Ahmed IM, Hu XN, Zhang GP, Wu FB (2014) Genotypic and environmental variation in cadmium, chromium, lead and copper in rice and approaches for reducing the accumulation. Sci Total Environ 496:275–281
Fant ML, Nyman M, Helle E, Rudback E (2001) Mercury, cadmium, lead and selenium in ringed seals (Phoca hirspida) from the Baltic Sea and from Svalbard. Environ Pollut 111(3):493–501
Fasiangova M, Borilova G (2017) Impact of Se supplementation on the oxidation stability of eggs. World Poultry Sci J 73:175–184
Fernandez ML, Andersen CJ (2015) The good egg, the forgotten benefits: protein, carotenoids, choline and glycemic index. Human Health Handbooks, 17–34
Fisinin VI, Papazyan TT, Surai PE (2009) Producing selenium-enriched eggs and meat to improve the selenium status of the general population. Crit Rev Biotechnol 29:18–28
Gao S, Heng N, Guo Y, Chen Y, Dong YC, Feng BQ, Qi XL, Lu XZ (2021) Effects of dietary supplementation of bioactive selenium on performance, egg quality and selenium content in the egg yolk of laying hens. J Zhejiang University (Agri Life Sci) 47:261–267
Kim E, Wickramasuriya SS, Shin TK, Cho HM, Macelline SP, Lee SD, Jung JH, Heo JM (2019) Bioaccumulation and toxicity studies of lead and mercury in laying hens: effects on laying performance, blood metabolites, egg quality and organ parameters. J Poult Sci 56:277–284
Kipp AP, Strohm D, Brigelius-Flohe R, Schomburg L, Bechthold A, Leschik-Bonnet E, Heseker H (2015) Revised reference values for selenium intake. J Trace Elem Med Bio 32:195–199
Klotz LO, Kroncke KD, Buchczyk DP, Sies H (2003) Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J Nutr 133:1448S-1451S
Li JH, Xing L, Zhang RX (2018) Effects of Se and Cd co-treatment on the morphology, oxidative stress, and ion concentrations in the ovaries of laying hens. Biol Trace Elem Res 183(1):156–163
Liu XT, Rehman MU, Mehmood K, Huang SC, Tian XX, Wu XX, Zhou DH (2018) Ameliorative effects of nano-elemental selenium against hexavalent chromium-induced apoptosis in broiler liver. Environ Sci Pollut 25:15609–15615
Liu H, Yu QF, Fang CK, Chen SJ, Tang XP, Ajuwon KM, Fang RJ (2020) Effect of selenium source and level on performance, egg quality, egg selenium content, and serum biochemical parameters in laying hens. Foods 9:68
Liu Q, Wang Q, He P, Zhang Y, Pan LM, Chen Y, Wu H, Zhang MM (2022) Heat-induced gel properties and gastrointestinal digestive properties of egg white produced by hens fed with selenium-enriched yeast. Food Chem 366:130712
Luo HT, Yang YF, Wang Q, Wu YJ, He ZL, Yu WB (2020) Protection of Siganus oramin, rabbitfish, from heavy metal toxicity by the selenium-enriched seaweed Gracilaria lemaneiformis. Ecotox Environ Safe 206:111183
Luque-Garcia JL, Cabezas-Sanchez P, Anunciacao DS, Camara C (2013) Analytical and bioanalytical approaches to unravel the selenium-mercury antagonism: a review. Anal Chim Acta 801:1–13
Miksik I, Holan V, Deyl Z (1996) Avian eggshell pigments and their variability. Comp Biochem Phys B 113:607–612
Muhammad AI, Mohamed DA, Chwen LT, Akit H, Samsudin AA (2021) Effect of selenium sources on laying performance, egg quality characteristics, intestinal morphology, microbial population and digesta volatile fatty acids in laying hens. Animals 11:1681
Mutlu SI, Seven I, Arkali G, Birben N, Arslan AS, Aksakal M, Seven PT (2021) Ellagic acid plays an important role in enhancing productive performance and alleviating oxidative stress, apoptosis in laying quail exposed to lead toxicity. Ecotox Environ Safe 208:111608
NRC - National Research Council (1994) Nutrient requirement of poultry. (9th reviseded) NationalAcademic Press. Washington, DC, USA.
Omana DA, Wang JP, Wu JP (2010) Ovomucin — a glycoprotein with promising potential. Trends Food Sci Tech 21(9):455–463
Pan CL, Zhao YX, Liao SFF, Chen F, Qin SY, Wu XS, Zhou H, Huang KH (2011) Effect of selenium-enriched probiotics on laying performance, egg quality, egg selenium content, and egg glutathione peroxidase activity. J Agr Food Chem 59:11424–11431
Pappas AC, Acamovic T, Sparks NHC, Surai PF, McDevitt RM (2005) Effects of supplementing broiler breeder diets with organic selenium and polyunsaturated fatty acids on egg quality during storage. Poultry Sci 84:865–874
Pappas AC, Acamovic T, Sparks NHC, Surai PF, McDevitt RM (2006a) Effects of supplementing broiler breeder diets with organoselenium compounds and polyunsaturated fatty acids on hatchability. Poultry Sci 85(9):1584–1593
Pappas AC, Acamovic T, Surai PF, McDevitt RM (2006b) Maternal organo-selenium compounds and polyunsaturated fatty acids affect progeny performance and levels of selenium and docosahexaenoic acid in the chick tissues. Poultry Sci 85:1610–1620
Pappas AC, Zoidis E, Georgiou CA, Demiris N, Surai PF, Fegeros K (2011) Influence of organic selenium supplementation on the accumulation of toxic and essential trace elements involved in the antioxidant system of chicken. Food Addit Contam 28(4):446–454
Payne RL, Lavergne TK, Southern LL (2005) Effect of inorganic versus organic selenium on hen production and egg selenium concentration. Poultry Sci 84:232–237
Pilarczyk B, Tomza-Marciniak A, Pilarczyk R, Kuba J, Hendzel D, Udala J, Tarasewicz Z (2019) Eggs as a source of selenium in the human diet. J Food Compos Anal 78:19–23
Qu WH, Yang JH, Sun ZZ, Zhang RH, Zhou F, Zhang KC, Xia Y, Huang KH, Mao DN (2017) Effect of selenium nanoparticles on anti-oxidative level, egg production and quality and blood parameter of laying hens exposed to deoxynivalenol. J Anim Res Nutrition 2:1–8
Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268
Scheideler SE, Weber P, Monsalve D (2010) Supplemental vitamin E and selenium effects on egg production, egg quality, and egg deposition of alpha-tocopherol and selenium. J Appl Poultry Res 19:354–360
Skrivan M, Marounek M, Dlouha G, Sevcikova S (2008) Dietary selenium increases vitamin E contents of egg yolk and chicken meat. Brit Poultry Sci 49:482–486
Spiller HA (2018) Rethinking mercury: the role of selenium in the pathophysiology of mercury toxicity. Clin Toxicol 56:313–326
Urso URA, Dahlke F, Maiorka A, Bueno IJM, Schneider AF, Surek D, Rocha C (2015) Vitamin E and selenium in broiler breeder diets: Effect on live performance, hatching process, and chick quality. Poultry Sci 94:976–983
Utterback PL, Parsons CM, Yoon I, Butler J (2005) Effect of supplementing selenium yeast in diets of laying hens on egg selenium content. Poultry Sci 84:1900–1901
Vinceti M, Filippini T, Wise LA (2018) Environmental selenium and human Health: an Update. Curr Env Hlth Rep 5:464–485
Wei R, Bitgood JJ (1990) A new objective measurement of eggshell color. 1. A test for potential usefulness of two color measuring devices. Poultry Sci 69:1775–1780
Yoo YC, Lee SK, Yang JY, Kim KW, Lee SY, Oh SM, Chung KH (2002) Interrelationship between the concentration of toxic and essential elements in Korean tissues. J Health Sci 48:195–200
Zeng HW, Uthus EO, Combs GF (2005) Mechanistic aspects of the interaction between selenium and arsenic. J Inorg Biochem 99:1269–1274
Zhang RX, Wang YN, Wang C, Zhao P, Liu H, Li JH, Bao J (2017) Ameliorative effects of dietary selenium against cadmium toxicity is related to changes in trace elements in chicken kidneys. Biol Trace Elem Res 176:391–400
Zhang C, Wang LL, Cao CY, Li N, Talukder M, Li JL (2020a) Selenium mitigates cadmium-induced crosstalk between autophagy and endoplasmic reticulum stress via regulating calcium homeostasis in avian leghorn male hepatoma (LMH) cells. Environ Pollut 265:114613
Zhang XF, Tian L, Zhai SS, Lin ZP, Yang HY, Chen JP, Ye H, Wang WC, Yang L, Zhu YW (2020b) Effects of selenium-enriched yeast on performance, egg quality, antioxidant balance, and egg selenium content in laying ducks. Front Vet Sci 7:591
Zhang JN, Duan ZY, Wang XQ, Li FN, Chen JJ, Lai XF, Qu L, Sun CJ, Xu GY (2021) Screening and validation of candidate genes involved in the regulation of egg yolk deposition in chicken. Poultry Sci 100:101077
Zhao X, Liang KH, Zhu H, Wang J (2021) Health risk assessment of heavy metals contamination in selenium-enriched eggs. Environ Sci Pollut R 28:27047–27055
Funding
This work was supported by the National Natural Science Foundation of China (no. 31801605).
Author information
Authors and Affiliations
Contributions
All the authors contributed significantly to the research article. Kehong Liang made substantive contributions to the experimental processing and data interpreting and wrote the paper; Shiping Bai and Hong Zhu performed part of the experiments, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, K., Bai, S. & Zhu, H. Effects of cadmium, lead, mercury, chromium, and selenium co-treatment on egg quality and fatty acids. Environ Sci Pollut Res 30, 73941–73951 (2023). https://doi.org/10.1007/s11356-023-27493-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27493-1