Abstract
Considerable advances have been made in the substrate design and operation strategies of constructed wetlands to facilitate nitrogen elimination. However, few studies examined the complicated interaction between solid organic substrates and limited aeration on nitrogen removal. A vertical flow constructed wetlands in gradient distribution of inorganic and solid organic substrates (polycaprolactone/PCL) (P-VFCW) and a controlled vertical flow constructed wetland without PCL (C-VFCW) were developed for the tertiary treatment of municipal tailwater. Results indicated that ammonia was nearly converted to nitrate, while the total nitrogen removal efficiencies (TNREs) in C-VFCW were negligible. In P-VFCW, however, optimal TNREs approached 95% with an aeration rate of 0.06 mL·min−1 and hydraulic retention time (HRT) of 24 h, and simultaneous nitrification and denitrification process (SND) in aerobic conditions was confirmed. As for the spatial microbial community structure evolution, Comamonas, which is associated with heterotrophic nitrification and anoxic/aerobic denitrification, was enriched along the vertical profiles of P-VFCW. Autotrophic nitrifier (Nitrospira), aerobic denitrifier (Bradyrhizobium and Azospira), and anoxic denitrifier (Ignavibacterium and Methyloversatilis) were dominated in different depths of P-VFCW, respectively. Besides, Canna indica biomass in P-VFCW was significantly larger than that in C-VFCW, which was attributed to the plant adaption response to diverse nitrogen. The P-VFCW in gradient distribution of inorganic and solid carbon sources under limited aeration is a promising technology for advanced nitrogen removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen is a prevalent pollutant present in surface water and groundwater, which would result in water environment quality’s deterioration. Diverse procedures were performed to remove nitrogen from wastewater, including adsorption, ion exchange, reverse osmosis, air stripping, and electrochemical and biological process (Dehghani et al. 2018; Hossini et al. 2015; Jonoush et al. 2020). Among them, constructed wetland (CW) is widely applied for nitrogen removal due to its low cost, easy maintenance requirements, and eco-friendliness (Gunes et al. 2021; Parde et al. 2021; Vymazal et al. 2021 Wallace and Waltham 2021). The nitrogen removal mechanisms in CWs include plants’ uptake, substrates’ adsorption, and biotransformation, with the microbial nitrification and denitrification being the most dominant pathway (Tang et al. 2020; Zhou et al. 2020). In general, oxygen deficiency is always present in the CWs (Nguyen et al. 2019), and the scarcity of electron donors further restricts the denitrification process. Due to the limited oxygen and electron donor, CWs consistently exhibit suboptimal total nitrogen removal efficiency (TNRE) when treating wastewater with a low C/N ratio.
Previous studies have demonstrated that operation mode and substrate design of CWs are effective strategies to improve its nitrogen removal. Aeration was frequently used to improve the overall treatment efficacy of CWs (Boog et al. 2019; Wang et al. 2020), and it was found that aeration can significantly boost the total nitrogen removal of CWs (Jehawi et al. 2020; John et al. 2020; Li et al. 2021). Compared to the CW without aeration, the aerated CW presented superior nitrogen removal capability, with a NH4+-N removal efficiency of 94.3% and TNRE of 91.5% (Li et al. 2021). Meanwhile, nitrate arising from the nitrification process cannot be eliminated completely owing to the insufficient electron donor for denitrification, resulting in nitrate accumulation accompanied by a low TNRE in CWs. From this, how to eliminate the nitrate produced by the nitrification process is a crucial issue. Several researchers have recently attempted to tackle the insufficient electron supply for denitrification in CWs by employing solid organic substrates that served as both substrates and the carbon source (Si et al. 2018). It has been demonstrated that biodegradable polymers with a steady and robust electron supply capacity considerably improve the TNREs of CWs (Jia et al. 2021; Shen et al. 2014). These biodegradable polymers, mainly including polycaprolactone (PCL) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), have been investigated (Li et al. 2016; Xu et al. 2019; Yang et al. 2020). However, the cost of PHBV (21.0–37.2 €/kg NO3−-N) is significantly higher than that of PCL (5.4–8.9 €/kg NO3−-N) (Zhang et al. 2016). In light of this, PCL is more economically viable.
As such, newly, some research has attempted to combine the benefits of aeration with biodegradable polymers to promote the TNREs of CWs. In a PHBV/PLA- and ceramsite-supported CW, Yang (Yang et al. 2018) discovered that under the limited aeration, higher ammonia removal efficiency (91%) and TNRE (97%) were higher than in the absence of aeration. Similarly, the NH4+-N and TN removal efficiencies in an aerated PHBV-supported CW were much greater than in non-aerated PHBV-supported CW, reaching 90.4% and 92.1%, respectively (Sun et al. 2018). However, in the experiments described above, the biodegradable polymers were placed at the bottom of CWs, which would result in two distinct types of issues. On the one hand, substrates inextricably link with the other key operation parameters and significantly influence the performance of CWs (Ji et al. 2021). In the presence of abundant organic matter, the competition of aerobic heterotrophic microorganisms for oxygen would bring adverse effects on nitrifiers (Tran et al. 2018, Zhu and Chen 2001). On the other hand, aeration would increase the consumption of biopolymers by aerobic heterotrophic microorganisms, resulting in the clogging of CWs, secondary effluent pollution, and a decline in the in polymers’ efficient utilization. The distribution pattern of biopolymers imposed considerable influences on nitrogen removal in aerated CWs. In the case of aeration, it was critical to develop a proper distribution of biopolymer and other inorganic substrates in CWs. Additionally, the underlying mechanism about simultaneous ammonia and nitrate removal in aerated polymer-supported CW was still unclear.
In this study, two VFCWs with limited aeration were developed: a VFCW without PCL polymer (C-VFCW) and PCL-supported VFCW (P-VFCW). This study aims to (i) investigate whether P-VFCW would show a good treatment performance during a long-term continuous operation and (ii) explore the spatial evolution of microbial community structure, and thereby elucidating the underlying mechanism about simultaneous ammonia and nitrate removal.
Materials and methods
Materials
In the present study, two VFCWs were built with multiple inorganic/organic media: volcanic rock, ceramsite, and PCL polymer. Volcanic rock and ceramsite granules were purchased from GongYi Songxin Filter Material Industrial Enterprise, and the brand of PCL granules was American Solvay Advanced polymers 6800. The sizes of volcanic rock and ceramsite granules ranged from 4 to 6 mm and from 2 to 3 mm, respectively. The physical properties of PCL granules were cylindrical shape, a diameter of 3.0 mm, and a height of 1.00 mm.
Two VFCWs were intended for the advanced treatment of municipal tailwater. According to the “municipal wastewater treatment plant pollutants discharge standard” class A (GB18918-2002), synthetic wastewater containing 8 mg·L−1 NH4+-N and 7 mg·L−1 NO3−-N was prepared by adding NaNO3, NH4Cl, and KH2PO4 into a 50 L container, and the characteristics of influent during different phases are shown in Table 1. In phase I, due to a lower operating temperature, the influent DO concentrations were higher, and the influent nitrite concentrations were lower. There was no chemical oxygen demand in the influent to determine whether PCL biopolymer posed a risk of secondary pollution of the effluent.
Experimental apparatus and procedures
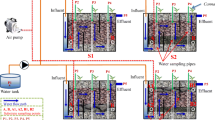
The structure of each VFCW is shown in Fig. 1. Two VFCWs were both performed using a Plexiglas cylinder with 20-cm diameter and 80-cm length. The substrates were supported by a Plexiglas mesh disc with a 2-mm pore size. A titanium aeration disc was at the end of the experimental devices, which connected with an air pump. Six sampling ports were set at 10-cm intervals from 0 to 60 cm along the reactors. Multi-dimensional gradation of substrates was adopted in this study: a 10-cm ceramsite in the top layer to promote the plant growth, followed by 30-cm PCL polymer (750.00 g)/ceramsite mixture or ceramsite and 20-cm inorganic substrate-volcanic rock in the bottom layer to facilitate the nitrification process. Meanwhile, Canna indica (average height of 48.36 ± 1.42 cm, average weight of 262.62 ± 6.02 g) was chosen and planted in each VFCW.
Activated sludge taken from the secondary sediment tank of the Quyang municipal wastewater treatment plant in Shanghai, China, was used as an inoculation source with a final biomass concentration of 800 mg MLSS·L−1 for each VFCW. After inoculation, synthetic wastewater was pumped into these systems from the bottom of the VFCWs at a flow rate of 13.08 mL·min−1. A rotameter was to adjust aeration rates of air pump (Hailea, ACO-5504) between 0.06 mL·min−1 and 0.15 mL·min−1. The experiment was divided into three phases to explore the effects of aeration rate and HRT on the effluent water quality. Two VFCWs were exposed to natural surroundings (such as air, temperature, light). The experiment operated from June 1 to August 30 and lasted for 3 months. The influent characteristics and operation parameters are listed in Table 1.
Analytical methods
Influent and effluent samples were collected daily. All the samples were filtrated through a 0.45 μm filter membrane to remove suspended solids. Total organic carbon (TOC) was determined by a TOC analyzer (TOC V-CPN, Shimadzu, Japan). NO3−-N, NO2−-N, and NH4+-N concentrations were measured according to the standard methods by UV spectrophotometer (UV 1900i, Shimadzu). Dissolved oxygen (DO) and pH were performed by a handled multi-parameter water quality analyzer (Hach, USA). All the tests were conducted in triplicate, and the measurement data were presented as mean values.
The TNRE (%) in this study was defined by the following equation:
where Cinammonia and Cinnitrate were influent NH4+-N and NO3−-N concentrations (mg·L−1); Cefammonia, Cefnitrate and Cefnitrite were effluent NH4+-N, NO3−-N, and NO2−-N concentrations (mg·L−1), respectively.
Microbial community analysis
To reveal explore the underlying microbial mechanism for SND performance, five biofilm samples were collected at the vertical intervals of 0–10 cm, 10–20 cm, 20–30 cm, 30–40 cm, and 40–50 cm in P-VFCW and marked as 0_10A, 10_20B, 20_30C, 30_40D, and 40_50E, respectively. The DNA extraction was extracted using a microbial DNA extraction kit (Biocolors, Shanghai). The V4-V5 regions of 16SrRNA genes were amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). To obtain the microbial community, Illumina high-throughput sequencing was performed at the Majorbio Bio-pharm Biotechnology Company (Shanghai, China). Operational taxonomic units (OTUs) clustering was performed at 97% sequence identity. The phylotype coverage, richness, and diversity were calculated by Mothur analysis (http://www.mothur.org) at a 3% distance level.
Results and discussion
Effluent characteristics in two different VFCWs
The difference in treatment performance between the two VFCWs could be disclosed from the effluent quality. Figures 2, 3, and 4 depict the physical characteristics of pH and DO, nitrogen removal performance, and TOC variations for each VFCW during a long-term operation.
Effluent pH and DO characteristics
Figure 2 depicts the variations of pH and DO in the influent and effluent. During the whole operation process, the influent and effluent pH were 6.73–7.56 and 6.63–7.70 in P-VFCW and C-VFCW, respectively. On the whole, the difference in pH between influent and effluent in each VFCW was negligible. In general, the effluent pH always decreased in the biodegradable polymer-supported denitrification system, which was ascribed to the acidic intermediate products resulting from the hydrolysis of the polymers (Xu et al. 2018a). It is known that H+ and OH− are produced during the nitrification and denitrification processes, respectively; hence, SND could alleviate pH fluctuations (Hu et al. 2021). Meanwhile, the influent DO in phase I remained reasonably stable, ranging from 6.09 to 7.16 mg·L−1. The DO concentrations in the influent tank were 0.17–5.97 mg·L−1 and 0.32–5.92 mg·L−1, respectively, which fluctuated with the exchange frequency of fresh wastewater in the tank. Whereas, the effluent DO in three stages slightly fluctuated. The effluent DO in C-VFCW varied between 5.00 and 8.46 mg·L−1 with an average of 7.61 ± 0.55 mg·L−1. The effluent DO in P-VFCW ranged from 7.48 to 8.41 mg·L−1, 4.35 to 7.32 mg·L−1, and 3.72 to 7.55 mg·L−1, with averages of 8.07 ± 0.28, 6.03 ± 0.93, and 5.44 ± 0.90 mg·L−1 for phases I, II, and III, respectively. Compared to the C-VFCW, the lower effluent DO in P-VFCW may be attributed to the DO consumption by aerobic heterotrophic bacteria. Additionally, there was no substantial suppression of the nitrification when DO exceeded 1.7–2.0 mg·L−1; nevertheless, concerning the denitrification process, the DO should be maintained below 0.3–0.5 mg·L−1 (Lin et al. 2020). Thus, high DO concentrations in this CW may inhibit denitrification, thereby decreasing the TNREs.
Effluent nitrogen characteristics
Figure 3 depicts the variations of nitrogen in different forms in each VFCW. The influent consisted of ammonia and nitrate, with a TN of approximately 14.07 ± 0.78 mg·L−1. During the whole operation, the influent NH4+-N varied from 3.82 to 8.38 mg·L−1 with an average of 6.71 ± 1.10 mg·L−1, while the effluent NH4+-N concentrations was negligible in each VFCW (Fig. 3a). Clearly, the NH4+-N removal efficiencies in all phases exceeded 95%, and it revealed that aeration rates of 0.06–0.15 mL·min−1 resulted in excellent nitrification efficiency. Compared to the non-aerated CW, the intermittent-aerated CW had a higher NH4+-N removal efficiency of 94.3% (Li et al. 2021), which further indicated that the DO supply was advantageous for nitrogen removal in CW.
However, in phase I, the effluent nitrogen was mostly nitrate in each VFCW (Fig. 3b and d). Due to the residual organic carbon source in the inoculated activated sludge or the substrates’ adsorption, the TN slightly decreased in each VFCW in the first few days. The adsorption capacity of the substrates for NO3−-N was extremely low, and its adsorption capacity for NH4+-N was about 0.01 mg NH4+-N/g substrates, indicating the substrates have a weak adsorption capacity for nitrogen. Effluent NO3−-N concentrations averaged at 12.54 ± 1.84 mg·L−1 and 7.91 ± 0.56 mg·L−1 with low TNREs of 8% and 40% in C-VFCW and P-VFCW, respectively. Meanwhile, an excessive DO would create a high redox potential and further seriously hinder the denitrification process. When the aeration rate decreased from 0.15 to 0.06 mL·min−1 (phase II), effluent nitrogen primarily in the form of NO3−-N remained high in C-VFCW, but it sharply declined in P-VFCW. After a 16-day adaption period, the effluent NO3−-N concentrations were extremely low, and the effluent TN averaged at 1.50 ± 1.34 mg·L−1 with a remarkable TNRE of 90% in P-VFCW. Similarly, in an intermittent-aeration CW, a lower DO of 3 mg·L−1 and shorter HRT of 2 days resulted in a larger TNRE than a DO of 4 mg·L−1 and HRT of 3 days (Li et al. 2021), suggesting that a lower DO was closely related to a higher TNRE. It was observed that the nitrate reduction enzyme-Nap locating at periplasm is not sensitive to the inhibition of DO; thus, Nap can still drive the reduction of NO3−-N (Chen et al. 2018). Nevertheless, DO was still a crucial factor influencing the denitrification performance under aerobic conditions. In general, the optimal DO concentration for aerobic denitrification was generally found in the range of 3.0–5.0 mg·L−1 (Hu et al. 2021). Collectively, it was inferred that the SND process and aerobic denitrification contributed to a considerable TNRE in P-VFCW.
When the HRT decreased from 24 h (phase II) to 12 h (phase III), there was no noticeable difference in the effluent of C-VFCW. However, the effluent NO3−-N and the TN concentrations both increased and averaged at 2.63 ± 1.01 mg·L−1 and 3.28 ± 1.08 mg·L−1 in P-VFCW, respectively; thus, the TNRE decreased from 90 to 76%. It indicated that TNRE decreased with a decline of HRT in P-VFCW. Despite this, the TNRE was unexpectedly high under a shorter HRT and aerobic condition (DO > 4 mg·L−1). As an intermediate product of nitrification and denitrification processes, effluent nitrite accumulation was both negligible in each VFCW (Fig. 3c), revealing that these processes were largely complete. In addition, the cost of PCL was about 2–7 times that of using methanol and ethanol, but the same as that of using acetic acid (Wang and Chu 2016). However, there are no safety concerns in terms of toxicity and inflammability for PCL during transport and operation. If the price of PCL can be reduced further, it is reasonable to assume that an aerated P-VFCW for advanced nitrogen treatment will become more economically viable.
Effluent TOC characteristics
The effluent TOC concentrations are illustrated in Fig. 4, which could determine whether TOC accumulation occurred in the effluent of VFCWs. In general, TOC accumulation occurred in biodegradable polymer-supported denitrification systems for nitrate removal in previous studies (Xu et al. 2019, 2018b). During the whole experiment, the effluent TOC was negligible in C-VFCW, and the effluent TOC concentrations were in the range of 1.11–5.95 mg·L−1 in P-VFCW. The average effluent TOC concentrations for phases I, II, and III were 2.53 ± 0.66, 2.71 ± 1.22, and 2.13 ± 0.68 mg·L−1,respectively. According to the environmental quality standards for surface water (GB3838-2002), the effluent residual TOC concentrations in the P-VFCW were too low to pose a threat of secondary pollution to the surface water. However, in an aerated PHBV/PLA-supported SND system, effluent TOC rapidly increased up to a maximal concentration of 36.40 mg·L−1 (Sun et al. 2020). Meanwhile, in an aerated PHBV and ceramsite-supported VFCW where PHBV polymer deposited at the bottom of systems, the COD concentrations were about 19.44 ± 7.91 mg·L−1, which was still higher than that in the present study (Sun et al. 2018). It indicated that the gradient structure of biopolymer and other inorganic substrates in this study may have an obvious advantage in reducing effluent organics. Additionally, a lower effluent TOC in P-VFCW was attributed to the proper distribution pattern of organic and inorganic substrates under aerobic conditions.
Growth characteristic of Canna indica
Plants require substantial amounts of nitrogen to support growth, and hence, nitrogen acquisition is typically a growth-limiting factor. It is believed that plants in CWs make an important contribution in terms of contaminant uptake (Schierano et al. 2020; Zhang et al. 2021). In general, approximately 60–70% of TN is removed via denitrification, while 20–30% of TN can be consumed by plant uptake in CWs (Spieles and Mitsch 2000). After 90 days of cultivation, the average height of Canna indica increased from 37.55 ± 5.53 to 75.20 ± 4.08 cm and 105.97 ± 15.36 cm for C-VFCW and P-VFCW, respectively, and its wet weight rose from 64 ± 6.10 to 173.13 ± 2.16 g and 733.96 ± 4.04 g in C-VFCW and P-VFCW, respectively. Their dry weights were about 31.16 g and 132.06 g, respectively, and it contained 15 g N /Kg Canna indica. The nitrogen absorption amount of Canna indica was about 300.84 mg and 1848.09 mg, respectively. This result indicated that some of influent NH4+-N and NO3−-N were removed by Canna indica for its growth in each VFCW, and the contribution ratio of Canna indica for nitrogen removal was about 13.53% in P-VFCW.
Nitrogen response mainly refers to the plant adaptation responses to diverse nitrogen conditions or fluctuations in the nitrogen supply, which is a critical component of the regulatory network controlling plant growth (Ueda et al. 2017). NO3−-N and NH4+-N are the primary forms of nitrogen accessible to plants for assimilation. The relative growth rate of Canna indica exceeded 0.01 g·day−1 in the absence of L NH4+-N (Wang et al. 2016), indicating that a low effluent NH4+-N concentration would not inhibit the growth of Canna indica in either C-VFCW or P-VFCW. Meanwhile, it was found that when plants were supplied with both NO3−-N and NH4+-N, they preferred NH4+-N uptake more than NO3−-N. For example, when a candidate plant for wetland Arundo donax was fed with NO3−-N, it had significantly less above-ground biomass than when it was fed with NH4+-N (Tho et al. 2017). Besides, high nitrate concentrations (> 5 mM) also negatively affected axial root growth in maize (Tian et al. 2008). Consequently, a higher NO3−-N concentration likely inhibited the growth of Canna indica, resulting in a lower biomass growth in C-VFCW than in P-VFCW.
Microbial community structures’ spatial evolution
Due to a negligible TNRE in C-VFCW, the microbial community structures’ spatial evolution in P-VFCW was deeply explored, which was closely associated with its good TNRE under an aerated condition. Illumina high-throughput sequencing for 16S rRNA genes was to reveal the microbial community structures of the biofilm samples collected along the vertical regions of P-VFCW. Table 2 summarizes the sequencing analysis results and the diversity of microbial communities. The sequences without sampling obtained from five samples were respectively 72,251, 60,581, 69,620, 71,239, and 72,995 with a length range of 414–420 bp. They were resampled to 54,460 for the subsequent analysis. The rarefaction curves indicated that the sequencing depth was adequate for the microbial community analysis. The Chao and Shannon indices (Xu et al. 2021) can be used to estimate the richness and diversity of the microbial community, respectively. The richness and diversity among these five samples differed significantly, according to the different microbial community structures.
The microbial communities were further identified at phylum and genus levels (Fig. 5). The relative abundances of major phyla (sequence percentage > 1% in at least one sample) are shown in Fig. 5a. The main phyla among all samples were divided into 12 phyla, but their relative abundances differed. Proteobacteria was the predominant phylum in all samples, with significantly higher relative abundances in samples 20_30C (60%), 30_40D (60%), and 40_50E (52%) than in samples 0_10A (32%) and 10_20B (40%) (Fig. 5a). In terms of the biological nitrogen removal processes, ammonia-oxidizing bacteria and denitrifying bacteria in PCL-supported system both lay in Proteobacteria (Bucci et al. 2021; Zhang et al. 2016). The difference in the relative abundances of Proteobacteria among five samples might be associated with distinct nitrogen biological processes in vertical regions of P-VFCW.
Top 30 genera are depicted in Fig. 5b, and the microbial community structures significantly differed with the vertical profile of P-VFCW. On the whole, Comamonas predominated in all samples, with relative abundances of 6.22%, 14.35%, 28.17%, 26.87%, and 13.79% in samples 0_10A, 10_20B, 20_30C, 30_40D, and 40_50E, respectively. Comamonas has been observed in a SND bioreactor under oxygen-rich conditions (Lai et al. 2020). Some strains of Comamonas were identified based on the denitrifying gene coding for nirS in a PHBV-supported denitrification system (Shams Tabrez Khan et al. 2007), while some of which were also capable of heterotrophic nitrification and aerobic heterotrophic denitrification (Cao et al. 2021; Liu et al. 2021; Moura et al. 2018). These results revealed that Comamonas played a crucial role in nitrification and denitrification processes in this system.
Aeration intensity regulates the bacteria growth and the distribution of bacteria species with depth in CWs (John et al. 2020), which further confirmed the genera variations with the vertical profile of P-VFCW. Nitrospira was found to be much more abundant in the samples 0_10A (2.06%) and 10_20B (1.21%) than in other samples. Similarly, Nitrospira, as a kind of autotrophic nitrifying microorganism (Xiang et al. 2020), played a critical role in the nitrification process in an aerated PHBV-supported denitrification system (Sun et al. 2019). As stated, Nitrospira also contributed to the nitrification process in the 0–20-cm zone of the system. Meanwhile, denitrification-associated microbes including Bradyrhizobium and Azospira (Horn et al. 2006; Vymazal et al. 2021) were observed in each sample, while their relative abundances in samples 0_10 A, 10_20B, and 20_30C were higher than in other samples. In particular, the relative abundance of Bradyrhizobium in sample 10_20 B was much higher in other samples and reached about 4.76%. This result indicated that aerobic denitrification occurred in the 10–20-cm section of the system. Furthermore, with the vertical depth in P-VFCW, Syntrophomonas, Ignavibacterium, Bdellovibrio, Methyloversatilis, and Desulfovibrio all became much more abundant in samples 20_30 C, 30_40D, and 40_50E samples. Among them, Ignavibacterium and Methyloversatilis were associated with anoxic denitrification (Ali et al. 2016, Liu et al. 2020, Shams Tabrez Khan et al. 2007). Bdellovibrio was heterotrophic aerobic bacteria involving in the degradation of organic matters (Iannacone et al. 2021), which was related to the organic substrates-PCL polymers in this study. Collectively, it was concluded that a spatial microbial community structure evolution mainly including the nitrifiers and aerobic/anoxic denitrifiers was responsible for a considerable TNRE in P-VFCW.
Additionally, a PCA analysis was performed and is presented in Fig. 5c. The PCA analysis revealed the resemblance of community structures among these five samples. Environmental factors such as DO concentration, nitrogen concentrations and form (mainly including NH4+-N and NO3−-N), and substrate (inorganic or organic substances) were believed to be responsible for the microbial community structures. There was no visible similarity between 0_10A and 10_20B samples despite the fact that both samples were volcanic. This could be attributed to different concentrations and form of nitrogen. However, samples 20_30C, 30_40D, and 40_50E (the blue oval) were in close proximity, indicating that they shared a partial portion of microbial structure. The similarity among these three samples was contributed to the same substrates (PCL polymer and ceramsite), while the longitudinally decreasing NO3−-N concentrations brought subtle differences. Overall, the difference in the microbial structure could be a result of the distinct nitrogen metabolic capacities with the vertical profile of P-VFCW.
Simultaneous nitrification and denitrification mechanism
Aeration and organic substrate-PCL polymers were discovered to be critical factors for microbial community structures’ spatial evolution. The association of aeration and PCL polymers resulted in a spatial microbial distribution involving in nitrification and denitrification processes (Fig. 5). It was reported that in an aerated PHBV/PLA-supported biofilter, simultaneous nitrification and denitrification occurred in the first 5 cm of the column, but denitrification happens exclusively in higher sections of the column (Sun et al. 2020). Due to the DO consumption by the nitrification process, the DO concentration was highest at the bottom of P-VFCW and reduced with the increasing depth of the system. Nitrospira and Comamonas, both autotrophic/heterotrophic microbes, were responsible for the nitrification process in the 0–20-cm region of the system. Denitrification also occurred simultaneously in the 10–20 cm zone due to the presence of abundant aerobic denitrification bacteria such as Comamonas, Azospira, and Stenotrophobacter. PCL granules as a high-molecular-weight biopolymer were hydrolyzed and degraded into some low-molecular–weight substances (Fang et al. 2020). These dissolved intermediate degradation products of PCL granules would diffuse into the adjacent region of 10–20 cm, resulting in an aerobic heterotrophic denitrification process. Meanwhile, there is no doubt that PCL granules facilitated the denitrification process in 20–50-cm zone, which were consistent with the abundant aerobic denitrifying microbes and anoxic heterotrophic microbes. Besides, the mixture pattern of PCL and ceramsite would increase the effective utilization ratio of PCL polymers for denitrification, given that both the biofilm on the PCL polymer and ceramsite played an essential role in nitrate reduction. As a result, the underlying mechanism about SND in an aerated PCL-supported CW was deduced and is illustrated in Fig. 6.
Conclusion
Results showed that SND occurred in the P-VFCW; both aeration rate and HRT were crucial factors for the TNREs. The TNRE of P-VFCW still reached 76% even at a shorter HRT of 12 h and a higher aeration rate of 0.15 mL·min−1. Illumina sequencing analysis implied that the dominant microbes (mainly including ammonia-oxidizing bacteria, aerobic or anoxic denitrification bacteria) differed with the vertical profile of P-VFCW, which was associated with nitrification or denitrification processes. After the whole operation, a bigger biomass growth of Canna indica (increasing by 58.41 cm and 471.97 g) occurred in P-VFCW than that in C-VFCW, which was ascribed to a plant response to diverse nitrogen. This study developed a novel VFCW for advanced nitrogen removal from municipal tailwater.
Data availability
The manuscript has not been published elsewhere. The article is not a consideration in any other journal in full or in part.
References
Ali M, Chai L-Y, Min X-B, Tang C-J, Afrin S, Liao Q, Wang H-Y, Peng C, Song Y-X, Zheng P (2016) Performance and characteristics of a nitritation air-lift reactor under HRT shortening. Int Biodeterior Biodegrad 111:45–53
Boog J, Kalbacher T, Nivala J, Forquet N, van Afferden M, Muller RA (2019) Modeling the relationship of aeration, oxygen transfer and treatment performance in aerated horizontal flow treatment wetlands. Water Res 157:321–334
Bucci P, Coppotelli B, Morelli I, Zaritzky N, Caravelli A (2021) Heterotrophic nitrification-aerobic denitrification performance in a granular sequencing batch reactor supported by next generation sequencing. Int Biodeterior Biodegrad 160:105210
Cao Q, Li X, Jiang H, Wu H, Xie Z, Zhang X, Li N, Huang X, Li Z, Liu X, Li D (2021) Ammonia removal through combined methane oxidation and nitrification-denitrification and the interactions among functional microorganisms. Water Res 188:116555
Chen H, Zhao X, Cheng Y, Jiang M, Li X, Xue G (2018) Iron robustly stimulates simultaneous nitrification and denitrification under aerobic conditions. Environ Sci Technol 52:1404–1412
Dehghani S, Rezaee A, Hosseinkhani S (2018) Effect of alternating electrical current on denitrifying bacteria in a microbial electrochemical system: biofilm viability and ATP assessment. Environ Sci Pollut Res Int 25:33591–33598
Fang D, Wu A, Huang L, Shen Q, Zhang Q, Jiang L, Ji F (2020) Polymer substrate reshapes the microbial assemblage and metabolic patterns within a biofilm denitrification system. Chem Eng J 387:124128
Gunes K, Masi F, Ayaz S, Tuncsiper B, Besiktas M (2021) Domestic wastewater and surface runoff treatment implementations by constructed wetlands for Turkey: 25 years of experience. Ecol Eng 170:106369
Horn MA, Drake HL, Schramm A (2006) Nitrous oxide reductase genes (nosZ) of denitrifying microbial populations in soil and the earthworm gut are phylogenetically similar. Appl Environ Microbiol 72:1019–1026
Hossini H, Rezaee A, Ayati B, Mahvi AH (2015) Simultaneous nitrification and denitrification using a polypyrrole/microbial cellulose electrode in a membraneless bio-electrochemical system. RSC Adv 5:72699–72708
Hu B, Quan J, Huang K, Zhao J, Xing G, Wu P, Chen Y, Ding X, Hu Y (2021) Effects of C/N ratio and dissolved oxygen on aerobic denitrification process: a mathematical modeling study. Chemosphere 272:129521
Iannacone F, Di Capua F, Granata F, Gargano R, Esposito G (2021) Shortcut nitrification-denitrification and biological phosphorus removal in acetate- and ethanol-fed moving bed biofilm reactors under microaerobic/aerobic conditions. Bioresour Technol 330:124958
Jehawi OH, Abdullah SRS, Kurniawan SB, Ismail NI, Idris M, Al Sbani NH, Muhamad MH, Hasan HA (2020) Performance of pilot Hybrid Reed Bed constructed wetland with aeration system on nutrient removal for domestic wastewater treatment. Environ Technol Innov 19:100891
Ji Z, Tang W, Pei Y (2021) Constructed wetland substrates: a review on development, function mechanisms, and application in contaminants removal. Chemosphere 286:131564
Jia L, Sun H, Zhou Q, Zhao L, Wu W (2021) Pilot-scale two-stage constructed wetlands based on novel solid carbon for rural wastewater treatment in southern China: Enhanced nitrogen removal and mechanism. J Environ Manage 292:112750
John Y, Langergraber G, Adyel TM, Emery David V Jr (2020) Aeration intensity simulation in a saturated vertical up-flow constructed wetland. Sci Total Environ 708:134793
Jonoush ZA, Rezaee A, Ghaffarinejad A (2020) Electrocatalytic nitrate reduction using Fe0/Fe3O4 nanoparticles immobilized on nickel foam: selectivity and energy consumption studies. J Clean Prod 242:118569
Lai C, Guo Y, Cai Q, Yang P (2020) Enhanced nitrogen removal by simultaneous nitrification-denitrification and further denitrification (SND-DN) in a moving bed and constructed wetland (MBCW) integrated bioreactor. Chemosphere 261:127744
Li P, Zuo J, Wang Y, Zhao J, Tang L, Li Z (2016) Tertiary nitrogen removal for municipal wastewater using a solid-phase denitrifying biofilter with polycaprolactone as the carbon source and filtration medium. Water Res 93:74–83
Li J, Zheng L, Ye C, Ni B, Wang X, Liu H (2021) Evaluation of an intermittent-aeration constructed wetland for removing residual organics and nutrients from secondary effluent: performance and microbial analysis. Bioresour Technol 329:124897
Lin CJ, Chyan JM, Zhuang WX, Vega FA, Mendoza RMO, Senoro DB, Shiu RF, Liao CH, Huang DJ (2020) Application of an innovative front aeration and internal recirculation strategy to improve the removal of pollutants in subsurface flow constructed wetlands. J Environ Manage 256:109873
Liu T, He X, Jia G, Xu J, Quan X, You S (2020) Simultaneous nitri fi cation and denitri fi cation process using novel surface-modified suspended carriers for the treatment of real domestic wastewater. Chemosphere 247:125831
Liu T, Xu J, Tian R, Quan X (2021) Enhanced simultaneous nitrification and denitrification via adding N-acyl-homoserine lactones (AHLs) in integrated floating fixed-film activated sludge process. Biochem Eng J 166:107884
Moura RB, Santos CED, Okada DY, Martins TH, Ferraz Junior ADN, Damianovic M, Foresti E (2018) Carbon-nitrogen removal in a structured-bed reactor (SBRRIA) treating sewage: operating conditions and metabolic perspectives. J Environ Manage 224:19–28
Nguyen TT, Bui XT, Dang BT, Ngo HH, Jahng D, Fujioka T, Chen SS, Dinh QT, Nguyen CN, Nguyen PT (2019) Effect of ciprofloxacin dosages on the performance of sponge membrane bioreactor treating hospital wastewater. Bioresour Technol 273:573–580
Parde D, Patwa A, Shukla A, Vijay R, Killedar DJ, Kumar R (2021) A review of constructed wetland on type, treatment and technology of wastewater. Environ Technol Innov 21:101261
Schierano MC, Panigatti MC, Maine MA, Griffa CA, Boglione R (2020) Horizontal subsurface flow constructed wetland for tertiary treatment of dairy wastewater: removal efficiencies and plant uptake. J Environ Manage 272:111094
Shams Tabrez Khan YH, Takahashi N, Hiraishi A (2007) Activity and community composition of the denitrification bacteria in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) using solid-phase denitrification process. Microbes Environ 22:20–31
Shen Z, Zhou Y, Liu J, Xiao Y, Cao R, Wu F (2014) Enhanced removal of nitrate using starch/PCL blends as solid carbon source in a constructed wetland. Bioresour Technol 175C:239–244
Si Z, Song X, Wang Y, Cao X, Zhao Y, Wang B, Chen Y, Arefe A (2018) Intensified heterotrophic denitrification in constructed wetlands using four solid carbon sources: denitrification efficiency and bacterial community structure. Bioresour Technol 267:416–425
Spieles DJ, Mitsch WJ (2000) The effects of season and hydrologic and chemical loading on nitrate retention in constructed wetlands: a comparison of low- and high-nutrient riverine systems. Ecol Eng 14:77–91
Sun H, Yang Z, Wei C, Wu W (2018) Nitrogen removal performance and functional genes distribution patterns in solid-phase denitrification sub-surface constructed wetland with micro aeration. Bioresour Technol 263:223–231
Sun H, Wang T, Yang Z, Yu C, Wu W (2019) Simultaneous removal of nitrogen and pharmaceutical and personal care products from the effluent of waste water treatment plants using aerated solid-phase denitrification system. Bioresour Technol 287:121389
Sun H, Yang Z, Yang F, Wu W, Wang J (2020) Enhanced simultaneous nitrification and denitrification performance in a fixed-bed system packed with PHBV/PLA blends. Int Biodeterior Biodegrad 146:104810
Tang S, Liao Y, Xu Y, Dang Z, Zhu X, Ji G (2020) Microbial coupling mechanisms of nitrogen removal in constructed wetlands: a review. Bioresour Technol 314:123759
Tho BT, Lambertini C, Eller F, Brix H, Sorrell BK (2017) Ammonium and nitrate are both suitable inorganic nitrogen forms for the highly productive wetland grass Arundo donax, a candidate species for wetland paludiculture. Ecol Eng 105:379–386
Tian Q, Chen F, Liu J, Zhang F, Mi G (2008) Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J Plant Physiol 165:942–951
Tran NH, Reinhard M, Gin KY (2018) Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res 133:182–207
Ueda Y, Konishi M, Yanagisawa S (2017) Molecular basis of the nitrogen response in plants. Soil Sci Plant Nutr 63:329–341
Vymazal J, Zhao Y, Mander Ü (2021) Recent research challenges in constructed wetlands for wastewater treatment: a review. Ecol Eng 169:106318
Wallace J, Waltham NJ (2021) On the potential for improving water quality entering the Great Barrier Reef lagoon using constructed wetlands. Mar Pollut Bull 170:112627
Wang J, Chu L (2016) Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol Adv 34:1103–1112
Wang Y, Wang J, Zhao X, Song X, Gong J (2016) The inhibition and adaptability of four wetland plant species to high concentration of ammonia wastewater and nitrogen removal efficiency in constructed wetlands. Bioresour Technol 202:198–205
Wang W, Song X, Li F, Ji X, Hou M (2020) Intensified nitrogen removal in constructed wetlands by novel spray aeration system and different influent COD/N ratios. Bioresour Technol 306:123008
Xiang Y, Shao Z, Chai H, Ji F, He Q (2020) Functional microorganisms and enzymes related nitrogen cycle in the biofilm performing simultaneous nitrification and denitrification. Bioresour Technol 314:123697
Xu Z, Dai X, Chai X (2018a) Effect of influent pH on biological denitrification using biodegradable PHBV/PLA blends as electron donor. Biochem Eng J 131:24–30
Xu Z, Song L, Dai X, Chai X (2018b) PHBV polymer supported denitrification system efficiently treated high nitrate concentration wastewater: denitrification performance, microbial community structure evolution and key denitrifying bacteria. Chemosphere 197:96–104
Xu Z, Dai X, Chai X (2019) Biological denitrification using PHBV polymer as solid carbon source and biofilm carrier. Biochem Eng J 146:186–193
Xu Z, Qiao W, Song X, Wang Y (2021) Pathways regulating the enhanced nitrogen removal in a pyrite based vertical-flow constructed wetland. Bioresour Technol 325:124705
Yang Z, Yang L, Wei C, Wu W, Zhao X, Lu T (2018) Enhanced nitrogen removal using solid carbon source in constructed wetland with limited aeration. Bioresour Technol 248:98–103
Yang Z, Sun H, Zhou Q, Zhao L, Wu W (2020) Nitrogen removal performance in pilot-scale solid-phase denitrification systems using novel biodegradable blends for treatment of waste water treatment plants effluent. Bioresour Technol 305:122994
Zhang Q, Ji F, Xu X (2016) Effects of physicochemical properties of poly-ε-caprolactone on nitrate removal efficiency during solid-phase denitrification. Chem Eng J 283:604–613
Zhang H, Tang W, Wang W, Yin W, Liu H, Ma X, Zhou Y, Lei P, Wei D, Zhang L, Liu C, Zha J (2021) A review on China’s constructed wetlands in recent three decades: application and practice. J Environ Sci (china) 104:53–68
Zhou X, Chen Z, Li Z, Wu H (2020) Impacts of aeration and biochar addition on extracellular polymeric substances and microbial communities in constructed wetlands for low C/N wastewater treatment: Implications for clogging. Chem Eng J 396:125349
Zhu S, Chen S (2001) Effects of organic carbon on nitrification rate in fixed film biofilter. Aquacult Eng 25:1–11
Funding
This work was supported by Shanghai Sailing Program (20YF1400200), the Fundamental Research Funds for the Center Universities (20D111324), and the National key research and development project (2019YFC0408603).
Author information
Authors and Affiliations
Contributions
Yuhui Wang: writing-review and editing. Panpan Zhou: data curation and analysis. Xinshan Song: writing-review and editing. Zhongshuo Xu: conceptualization, investigation, draft, writing-review and editing, supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Alexandros Stefanakis
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Zhou, P., Song, X. et al. Simultaneous nitrification and denitrification in a PCL-supported constructed wetland with limited aeration. Environ Sci Pollut Res 30, 22606–22616 (2023). https://doi.org/10.1007/s11356-022-23748-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23748-5