Abstract
The present study considers the impact of the alternating electric current on the viability and biological activity of denitrifying bacteria in a microbial electrochemical system (MES). The bio-stimulation using low-frequency low-voltage alternating current (AC) was studied in terms of the adenosine triphosphate (ATP) level of bacteria, viability, morphological characteristics, cell size, and complexity. Apoptosis assays by flow cytometry revealed that 81–95% of the cells were non-apoptotic, and cell membrane damage occurred < 18%. The applied AC could affect the bacterial metabolic activity and ATP content in the denitrifying bacteria depending on characteristics of the alternating electric current. Scanning electron microscopy (SEM) analysis of cell morphology illustrated low cell deformations under AC stimulation. The obtained results revealed that the applied alternating electrical current could increase the metabolic activity of denitrifying bacteria, leading to a better denitrification.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial electrochemical systems (MESs) are proposed as an attracting system in the wastewater treatment processes, because of their high efficiency and minimized use of external chemicals (Wang and Ren 2013; Hoseinzadeh et al. 2018). Utilizing electrical stimulation to stimulate bacterial processes has been considered for wastewater treatment (Wei et al. 2011). Applying direct current (DC) in MES has been reported as a feasible way to improve the biodegradation efficiency, since it affects the bacterial growth and metabolic functions of microorganisms (Feng et al. 2015). It has been reported that direct electrical current applied on a biofilm system with propylene packing increased the phenol removal efficiency (Li et al. 2017). The influences of electrical stimulation rely on the carrier type, applied electrical modes, and microbial community (Rezaee et al. 2015). In recent years, bio-electrochemical systems have been presented as an applicable technology for biological denitrification and wastewater treatments (Hossini et al. 2015; Wang et al. 2016a, b). The biological denitrification process is a biological method for nitrate removal which applies a specified bacteria known as denitrifiers. Denitrifying bacteria are commonly facultative bacteria, which under anoxic conditions could utilize nitrate (NO3−) as an electron acceptor (Rezaee et al. 2008). In denitrification process, nitrate conversion is caused by a series of enzymatic reactions. The reactions lead to the reduction of nitrate (NO3−) to nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O), and finally nitrogen gas (N2) production, respectively (Hoseinzadeh et al. 2017a). According to some previous scientific reports, bacterial reduction of nitrate is enhanced using electro-stimulation (Safari et al. 2014; Nguyen et al. 2016). In wastewater treatment based on biological processes, the influence of electrical current on microbial activity or viability has been raised as one of the major concerns in the application of these electro-technologies. The bacterial cell’s metabolic activity, shape, physiology, and movement are affected by stress or a strong electrical current. Generally, there is a linear relationship between the increase in the applied electrical current and decrease in process efficiency. An investigation of the effect of electro-stimulation on an aerobic culture showed that a small “window” of DC fields in the range of 0.57 and 1.14 V/cm makes an improvement in the biological removal of chemical oxygen demand (COD) (Alshawabkeh et al. 2004). Although the applied electrical current can affect microorganism species, a limited number of studies are available on the bio-electrostimulation of aquatic microbial species using alternative current (Hoseinzadeh et al. 2018). Therefore, there is a pressing necessity for quantifying viability of microbial populations in aquatic environments and biological treatment process based on bio-stimulation (Velasco-Alvarez et al. 2011). In this regard, some researchers have reported the needs for working on accurate quantification of microbial viability in a bio-electrochemical systems and treatment processes (Velasco-Alvarez et al. 2011). Microbial ATP content is a bioindicator for evaluation of the microbial metabolic activity during the biological treatments using electrostimulation (Wang and Ren 2013). The ATP as the “energy currency” of all biological living cells could be used as an independent, complementary, and a beneficial indicator for viability assessment (Velasco-Alvarez et al. 2011). Also, flow cytometric analysis has been proposed for evaluation of biochemical/cytotoxic effects on metabolic activities and other functions of the microbial community. Apoptosis and necrotic assays have been developed to serve as an alternative or supplementary assay for bio-stimulation (Hammes et al. 2010). Although bio-stimulation is considered as an efficient technique in biological treatment, to the best of our knowledge, there are no references in the scientific literature that specifically examine the viability and activity of denitrifying bacteria in MESs using ATP and flow cytometric analysis. Hence, this work was done to study the related effects of using electrical current particularly on the viability of denitrifying bacteria, apoptosis, and necrotic cells in wastewater treatment processes based on microbial stimulation. On the other hand, unlike the previous researchers that focus on the effect of electrostimulation on microorganisms using DC, we utilized the AC to enhance anoxic denitrification. The objective of this study is to improve information on the electro-stimulation effects of low-frequency low-voltage AC on the nitrate reducer’s bacteria. To better understand the effect of AC on the activity and viability of denitrifying bacteria, we studied several frequencies, voltages, and waveforms during the experiments. It is expected that experimental results could be used as a reference for the evaluation of bio-electrostimulation in the various fields of biological treatments.
Materials and methods
Bio-electro reactor configuration and operation
In this study, all chemicals were of analytical reagent grade and were used without further purification. A glass cylindrical bioreactor vessel with an effective volume of 5 L was made with dimensions of 18 cm × 20 cm. A cylindrical carbon cloth and a stainless steel mesh with 3-cm inter-electrode distance were mounted in the wall of the bioreactor. The electrodes were connected to an AC supplier as the function generator (AFG-2000 function generator; GW INSTEK; 0–10 peak-to-peak voltage (Vpp), 0.056 A, 50 Ω). The bioreactor was stirred manually to ensure complete mixing. The bioreactor was inoculated with an activated sludge from a wastewater treatment plant, Tehran, Iran. The concentration of the suspended biomass added to the bioreactor was 2000 mg/L as a mixed liquor suspended solid (MLSS). The reactor was operated in batch mode with a contact time of 24 h, an influent nitrate of 100 mg/L, sodium acetate as organic carbon source, an AC of 2 Vpp with a frequency of 10 Hz, a sinusoidal waveform, and an ambient temperature at a C/N ratio of 1.5:1. The composition of synthetic wastewater was 0.192 gL−1 C2H3NaO2, 0.5 gL−1 NaHCO3, 0.163 gL−1 KNO3, 0.45 gL−1 Na2HPO4, 0.15 gL−1 KH2PO4, and 0.4 gL−1 MgSO4 dissolved in tap water. The steady-state condition was considered when the biofilm formed on the surface of electrodes and denitrification efficiency was stable (variation of three sequential retention times was less than 5%). The bioreactor was electro-stimulated with the low-frequency low-voltage AC. Metabolic activity and viability of the bacteria were measured at different applied Vpp (2–10 V), frequencies (10–50 Hz), and sinusoidal, square, and triangle wave waveforms. The bacterial community was identified using polymerase chain reaction (PCR). The obtained results showed that the identified denitrifying bacteria in bioreactor belong to Pseudomonas spp., Nesterenkonia spp., Bacillus spp., and Brevibacillus spp. The experimentals were described in our previous study (Dehghani et al. 2018a). To evaluate the effect of electrostimulation in the microbial electrochemical system, a control reactor (without electrical stimulation) was operated in the same structure and similar experimental conditions.
Bacterial viability assays using flow cytometry analysis
The apoptotic and necrotic cells were analyzed by annexin V-fluorescein isothiocyanate (FITC) and propidiumiodide (PI) staining assay using an apoptosis detection kit (BioVision Inc., USA). The electrostimulated bacteria with AC were harvested and labeled with annexin V-FITC and PI and examined by flow cytometry (The FACSCalibur flow cytometer, USA). The viable and non-apoptotic cells (Annexin V− PI−), necrotic cells (Annexin V− PI+), early (Annexin V+ PI−), and late (annexin V+ PI+) apoptotic cells were analyzed. The Perttu Terho Software version 2.4.1 (Turku Centre for Biotechnology, Finland) was applied for data analysis.
Assessment of metabolic activity of microorganisms
The ATP bioluminescence assay was applied to quantify metabolic activity in the biofilm. Briefly, a sample of biofilm was taken at the end of the batch test and was transferred to 1.5-ml tubes. The samples were centrifuged at 5000 rpm for 10 min, and lysis reagent was added. The activity value was determined by the luminometric method, which is based on the luciferin-luciferase reaction using a Sirius Single Tube luminometer (Berthold Detection Systems, Germany). Reactions were performed by adding the purified luciferase enzyme to the standard solution (2 mM luciferin, and 10 mM MgSO4 in 50 mM Tris-HCl (pH 7.8) at 25 °C. The luminescence was subsequently measured and total light units were expressed in relative light units (RLU). The Bradford assay was used to determine protein contents of the sample ATP content estimation.
Results and discussions
The apoptosis assay and metabolic activity at different applied voltages
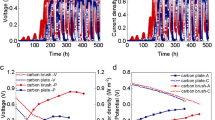
In this study, the denitrifying bacteria were exposed to various ranges of peak-to-peak voltages during denitrification process. The apoptosis assessment of the bacteria is shown in Fig. 1. The apoptosis assay using flow cytometry analysis illustrated that the content of viable and non-apoptotic cells (annexin V− PI−) in the control sample is 98.13%, which exceeds that of the same parameter in case of voltage application. Under different applied Vpps (2–10 V), the percentages of necrotic cells (Annexin V− PI+), early (Annexin V+ PI−), and late (annexin V+ PI+) apoptotic cells were changed in a limited range and a great number of cells (92.25–94.98%). According to the literature, the structure of the biological membranes, the morphology of cells, cell behavior, the metabolism, and vital processes in living organisms can be impacted by applying electrical currents (Zeyoudi et al. 2015). The disruption of cell performance and, subsequently, cell death can occur by any the change of the external or internal cell membrane. It was demonstrated that electrostimulation using low-voltage AC had no significant adverse effect on the viability of denitrifying. Figure 2 shows the microbial metabolic activity at different supplied Vpps. The obtained results show that increasing the Vpp from 2 to 4 V AC can cause a drop in bacterial activity. Higher values of Vpp have not a significant effect on the cell metabolism, suggesting that increasing the Vpp has no lethal effects on the microorganisms but it could affect the microbial metabolic activities. In bio-electrostimulation technique, microorganisms and cellular structures are exposed to electrical current. In this condition, the activity of enzymes, synthesis of cell biopolymers, membrane exchanges, and proliferation are influenced by these stimulants and could have positively or negatively effects (Hones et al. 1998; Dehghani et al. 2018b). It was reported that induction of low electrical current quantities could enhance the microbial activity (Liu et al. 2017; Loloi et al. 2017). Applying an electric field to the microbial system, the permeability of cytoplasmic membrane could be increased, and the nutrient transfer through the membrane improves. On the other hand, this increase in the permeability can cause leakage and excretion of cellular content and extracellular glycoproteins from bacterial metabolism, which in turn affects the synthesis and production of ATP (Zhang et al. 2014). It has been reported that appropriate electric current conditions enhances hexadecane biodegradation using Aspergilus niger attached on perlite, leading to a simultaneous increase in the total ATP content (Velasco-Alvarez et al. 2011). The results showed that ATP content is a key indicator of biological activity (Wang and Huang 2006). To study the role of applied electrode materials on electrostimulation in the bioreactor, the viability and metabolic activity of biofilm formed on electrodes was investigated at different Vpps. The results obtained from apoptosis assay indicated that the viable and non-apoptotic cells (annexin V− PI−) on the carbon cloth electrode were slightly (1.26–6.58%) higher than that of stainless steel mesh. The metabolic activities of biofilms on stainless steel mesh and carbon cloth were not significantly different. In some cases, the metabolic activity of biofilms was slightly higher in stainless steel mesh due to higher conductivity of stainless steel mesh than that of carbon cloth, as the former transfers more electrons to electroactive bacteria on bioelectrodes (Maadi et al. 2010). The effect of different AC voltages on Pseudomonas aeruginosa was investigated and reported that AC current has a low inhibitive effect on P. aeruginosa’s growth (Asadi and Torkaman 2014). These results, in line with our findings, revealed no considerable difference between the anode and cathode.
Effects of applied frequency on the viability and metabolic activity
The induction of apoptosis and necrosis by different frequencies were studied using the applied AC (Fig. 3). The results indicated that different frequencies have not a significant effect for enhancement of apoptotic and necrotic cells. The majority of cells (81.36–95.54%) were negative for annexin V/FITC binding (annexin V−), except PI (PI−) that showed small differences with the control samples with 98.5% non-necrotic cells. During the experiment, the microbial metabolic activity had an incremental trend, especially at higher frequencies (Fig. 4). It was reported that if the frequency is higher than the required amount for electrode polarization, the repulsive force can be reduced (Hoseinzadeh et al. 2017b). During the high frequency, electron transfer can also make the periodical ion movement. Although the intensity of these forces related to the applied frequency and solution properties, it can act on bacterial by electroosmotic flow (EOF) and electrohydrodynamic flow (EHF). The EHF forms fluid convection on the electrode surface and affects the biofilm structure. At a higher frequency (≥ 1 kHz), the imbalance of EHF and EOF leads to any fluid convection and so its effect reduces (Wang et al. 2016b). The amounts of early and late apoptosis cells were risen slightly by increasing frequency to 50 Hz. The stainless steel mesh bioelectrode was not significantly different with the carbon cloth electrode in terms of cell apoptosis and metabolic analysis. However, in some cases, the non-apoptotic/viable cells in carbon cloth bioelectrodes were slightly higher than stainless steel mesh bioelectrode. Similarly, it was reported that applying AC could be effected on anaerobic wastewater treatment.
Detection of microbial apoptosis and metabolic activity under different waveforms
To investigate whether AC waveforms induce apoptosis and influence on metabolic activity, sinusoidal, square, and triangle waveforms were evaluated. Detection of cell membrane changes using the annexin V and PI double staining is shown in Fig. 5. Applied waveforms had no remarkable impact on cell viability and the majority of cells were identified as non-apoptotic. A higher value of viable cells and lower number of cells expressing necrosis (annexin V− PI+), the early (annexin V+ PI−), and late (annexin V+ PI+) apoptosis were observed in triangle waveform. This observation can be explained by the characteristics of the various AC waveforms, including rest time, pulse width, and peak voltage, which create a different response to bioelectrostimulation. Our last study conducted on induced anoxic granulation by AC demonstrated that AC of 8 Vpp with a sinusoidal waveform and frequency of 10 Hz in biofilm-electrode reactor led to the production of dense and fast-settling granules and raised as an efficient way for sludge granulation (Hoseinzadeh et al. 2017a, b). Apoptosis assay-illustrated granules were viable and non-apoptotic in the majority. Figure 6 presents the microbial metabolic activity following applying various AC waveforms. As shown, the minimum and maximum levels of microbial activity were observed in square and triangular waveforms, respectively. Cell viability observed by triangle waveform was further confirmed by bacterial metabolism during changes in various wave shapes. The AC waveform can affect on the enzyme activity in the biological denitrification process. It can improve the metabolic activity of the denitrifying bacteria or cause an inhibitory effect on the process. Similarly, researchers have shown that with the proper application of electrical current, the total content of ATP has increased (Ailijiang et al. 2016). The AC waveform could also affect heat generation within ohmic heating. It was reported that the heating rate for the sinusoidal and triangle waves was remarkably more than those of the square wave. These observations were consistent with the researches that illustrated square wave was less effective than the sinusoidal wave (at 60 Hz) (Lee et al. 2013). Another research subjected Escherichia coli, Staphylococcus aureus, and P. aeruginosa to a sine wave, 5 or 20 mA, for 30 min in vitro (Asadi and Torkaman 2014; Wang et al. 2016b). The total ATP contents of biofilms on both bioelectrodes were measured at an AC of 2 Vpp with a triangle waveform and frequency of 50 Hz. The results showed about 1.32 ± 0.22-fold increase in ATP compared to that of the control sample; moreover, the total ATP content of biofilm on the carbon cloth-electrode was slightly higher than that of stain steel mesh electrode. This result indicates that the biofilm on the SSM electrodes was more sensitive to the LFLVAC stimulation than that on the CC electrode. Our results were similar to those presented by Ailijiang et al. (2016), who indicated that under appropriate electrical current situations, the total ATP content was enhanced.
Cell size and complexity under electrical stimulation
To consider the effect of electric current as an external factor on cell size and complexity, the flow cytometry technique was applied on the basis of cell scattering properties (Hammes et al. 2010). Generally, it is assumed that the forward scatter light (FSC) can be correlated with the size of a cell, while the side scatter light is representative of the internal granularity or complexity of a cell (Neumeyer et al. 2013). Clarifying the relationships between biostimulation and cell size is essential for understanding both normal and abnormal cell changes. Recent investigations in yeasts and bacteria provide some evidence for cell size regulation mechanisms (Tzur et al. 2011). In this study, regarding the presence of bacterial consortium in the bioreactor, the majority of the microbial population with the analogous size distribution in control sample was used as a changing pattern of bacterial communities. In Fig. 7a, b, c, FSC versus SSC distribution illustrates the representative dot plots of denitrifying cells under stimulation with several different LFLVAC values. As observed, the changes in the overall size distribution of bacterial populations in all three characteristics of AC including frequency, voltage, and waveform were about 4.5–14%. The variation of the complexity of the bacterial studied following the electro-stimulation was about 1–14.26%. Most of the denitrifying population (77–86.55%) had the lower signals of FSC and SSC and indicated small size and complexity. An explanation for this observation is that in the early logarithmic or stationary phase, bacterial cells give the smallest FSC signal. This signal remarkably increased during the mid-log phase. Furthermore, FSC and SSC results presented almost a similar variation in stainless steel mesh and carbon cloth bioelectrodes with respect to cell size distribution. Thus, it is inferred that applied electrode material induces the same electro-stimulation. Several studies revealed that simple parameters such as the cell size and granularity give valuable information about the metabolic condition and the uniformity of the whole population (Mesquita et al. 2013). For example, in the microscopy-based studies on Corynebacterium glutamicum, the authors reported a heterogeneity in cell size distribution and a dissimilarity in the dividing properties of large and small cells of C. glutamicum species (Neumeyer et al. 2013).
Morphology of the denitrifying bacteria
To survey the morphology of denitrifying bacteria, field emission-scanning electron microscopy (FE-SEM) was applied. As shown in Fig. 8, the most of denitrifying bacteria cells show normal morphology. However, some irregular-shaped and some damaged cells are observed during the electrostimulation, which are consistent with the above apoptosis assay observations by flow cytometry. According to FE-SEM micrographs, cocci bacteria are dominant in the microbial community and are presented in forms of monococcus, diplococcus, and a dense population of cocci-shaped bacteria attached to each other. We have reported similar results on the oily wastewater treatment using an electrostimulation applying DC (Adibzadeh et al. 2016). In our study, both cocci and rod-shaped bacteria were observed. This dissimilarity is attributed to the different contents and contaminants present in the wastewater. It is also stated that the dominance of cocci bacteria in the bioelectroreactor can be due to their structures that exhibit a good resistance to the electrical current. The existence of channels and empty spaces inside the biofilm could facilitate the material transfer between solution and biofilm.
Conclusions
The present study showed that AC biostimulation induces different effects on denitrifying bacteria depending on the type and amount of applied electric characteristics including voltage, frequency, and waveforms. The observations of the mechanism of electrical stimulation effect on bacteria in terms of viability/cell death, metabolic activity, morphology, cell membrane destruction, and effect on the size and complicity confirmed that electrostimulation in bioelectrode systems with low-frequency low-voltage operating, despite the effect on bacterial metabolic activity, did not cause any remarkable apoptosis. In this regard, there was the only little impact on the destruction of the bacterial membrane and the cell damage. Biochemical events leading to morphological changes were also relatively affected by electrical stimulation. The findings could improve the knowledge on the interaction effects of the characteristics of AC on the bioelectrical stimulation. Accordingly, we can practically use low-frequency low-voltage AC current to promote denitrification processes based on electrical stimulation.
References
Adibzadeh A, Rezaee A, Salehi Z (2016) Enhancement of lipase activity for the oily wastewater treatment by an electrostimulation process. RSC Adv 6:115290–115297
Ailijiang N, Chang J, Liang P, Li P, Wu Q, Zhang X, Hua X (2016) Electrical stimulation on biodegradation of phenol and responses of microbial communities in conductive carriers supported biofilms of the bioelectrochemical reactor. Bioresour Technol 201:1–7
Alshawabkeh AN, Shen Y, Maillacheruvu KY (2004) Effect of DC electric fields on COD in aerobic mixed sludge processes. Environ Eng Sci 21:321–329
Asadi MR, Torkaman G (2014) Bacterial inhibition by electrical stimulation. Adv Wound Care 3:91–97
Dehghani S, Rezaee A, Hosseinkhani S (2018a) Biostimulation of heterotrophic-autotrophic denitrification in a microbial electrochemical system using alternating electrical current. J Cleaner Prod 200:1100–1110
Dehghani S, Rezaee A, Moghiseh Z (2018b) Phenol biodegradation in an aerobic fixed-film process using conductive bioelectrodes: biokinetic and kinetic studies. Desalin Water Treat 105:126–131
Feng H, Zhang X, Guo K, Vaiopoulou E, Shen D, Long Y, Yin J, Wang M (2015) Electrical stimulation improves microbial salinity resistance and organofluorine removal in bioelectrochemical systems. Appl Environ Microbiol 81:3737–3744
Hammes F, Goldschmidt F, Vital M, Wang Y, Egli T (2010) Measurement and interpretation of microbial adenosine tri-phosphate (ATP) in aquatic environments. Water Res 44:3915–3923
Hones I, Pospischil A, Berg H (1998) Electrostimulation of proliferation of the denitrifying bacterium Pseudomonas stutzeri. Bioelectrochem Bioenerg 44:275–277
Hoseinzadeh E, Rezaee A, Farzadkia M (2017a) Enhanced biological nitrate removal by alternating electric current bioelectrical reactor: selectivity and mechanism. J Mol Liq 246:93–102
Hoseinzadeh E, Rezaee A, Farzadkia M (2017b) Low frequency-low voltage alternating electric current-inducedanoxic granulation in biofilm-electrode reactor: a study of granuleproperties. Process Biochem 56:154–162
Hoseinzadeh E, Rezaee A, Farzadkia M (2018) Nitrate removal from pharmaceutical wastewater using microbial electrochemical system supplied through low frequency-low voltage alternating electric current. Bioelectrochemistry 120:49–56
Hossini H, Rezaee A, Ayati B, Mahvi AH (2015) Simultaneous nitrification and denitrification using a polypyrrole/microbial cellulose electrode in a membraneless bio-electrochemical system. RSC Adv 5:72699–72708
Lee SY, Ryu S, Kang DH (2013) Effect of frequency and waveform on inactivation of Escherichia coli O157: H7 and Salmonella enterica serovar Typhimurium in salsa by ohmic heating. Appl Environ Microbiol 79:10–17
Li H, Zuo W, Tian Y, Zhang J, Di S, Li L, Su X (2017) Simultaneous nitrification and denitrification in a novel membrane bioelectrochemical reactor with low membrane fouling tendency. Environ Sci Pollut Res 24:5106–5117
Liu H, Chen N, Feng C, Tong S, Li R (2017) Impact of electro-stimulation on denitrifying bacterial growth and analysis of bacterial growth kinetics using a modified Gompertz model in a bio-electrochemical denitrification reactor. Bioresour Technol 232:344–353
Loloi M, Rezaee A, Sabour Roohaghdam A, Aliofkhazraei M (2017) Conductive microbial cellulose as a novel biocathode for Cr (VI)bioreduction. Carbohydr Polym 162:56–61
Maadi H, Haghi M, Delshad R, Kangarloo H, Nezhady MA, Hemmatyar GR (2010) Effect of alternating and direct currents on Pseudomonas aeruginosa growth in vitro. Afracian J Biotechnol 9:6373–6379
Mesquita N, Portugal A, Pinar G, Loureiro J, Coutinho AP, Trovão J, Nunes I, Botelho ML, .Freitas H (2013) Flow cytometry as a tool to assess the effects of gamma radiation on the viability, growth and metabolic activity of fungal spores. Int Biodeterior Biodegrad, 84, 250–257
Neumeyer A, Hübschmann T, Müller S, Frunzke J (2013) Monitoring of population dynamics of Corynebacterium glutamicum by multiparameter flow cytometry. Microbiol Biotechnol 6:157–167
Nguyen VK, Park Y, Yu J, Lee T (2016) Bioelectrochemical denitrification on biocathode buried in simulated aquifer saturated with nitrate-contaminated groundwater. Environ Sci Pollut Res 23:15443–15451
Rezaee A, Godini H, Bakhtou H (2008) Microbial cellulose as support material for the immobilization of denitrifying bacteria. Environ Eng Manag J 7:589–594
Rezaee A, Safari M, Hossini H (2015) Bioelectrochemical denitrification using carbon felt/multiwall carbon nanotube. Environ Technol 36:1057–1062
Safari M, Rezaee A, Ayati B, Jonidi-Jafari A (2014) Bio-electrochemical reduction of nitrate utilization MWCNT supported on carbon base electrode: a comparision study. J Taiwan Inst Chem Eng 45:2212–2216
Tzur A, Moore JK, Jorgensen P, Shapiro HM, Kirschner MW (2011) Optimizing optical flow cytometry for cell volume-based sorting and analysis. PLoS One 6:e16053
Velasco-Alvarez N, González I, Damian-Matsumura P, Gutiérrez-Rojas M (2011) Enhanced hexadecane degradation and low biomass production by Aspergillus niger exposed to an electric current in a model system. Bioresour Technol 102:1509–1515
Wang T, Huang X (2006) Biodegradation of high concentration phenol containing heavy metal ions by functional biofilm in bioelectro-reactor. J Environ Sc 18:639–643
Wang H, Ren ZJ (2013) A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol Adv 31:1796–1807
Wang H, Hang Q, Crittenden J, Zhou Y, Yuan Q, Liu H (2016a) Combined autotrophic nitritation and bioelectrochemical-sulfur denitrification for treatment of ammonium rich wastewater with low C/N ratio. Environ Sci Pollut Res 23:2329–2340
Wang X, Zhou L, Lobo FL, Li N, Wang H, Park J, Ren ZJ (2016b) Alternating current influences anaerobic electroactive biofilm activity. Environ Sci Technol 50:9169–9176
Wei V, Elektorowicz M, Oleszkiewicz J (2011) Influence of electric current on bacterial viability in wastewater treatment. Water Res 45:5058–5062
Zeyoudi M, Altenaiji E, Ozer LY, Ahmed I, Yousef AF, Hasan SW (2015) Impact of continuous and intermittent supply of electric field on the function and microbial community of wastewater treatment electro-bioreactors. Electrochim Acta 181:271–279
Zhang B, Liu Y, Tong S, Zheng M, Zhao Y, Tian C, Liu H, Feng C (2014) Enhancement of bacterial denitrification for nitrate removal in groundwater with electrical stimulation from microbial fuel cells. J Power Sources 268:423–429
Acknowledgments
The authors gratefully acknowledge Mrs. Maryam Moradi, Mr. Yousefi, Mrs. Mohseni, and Mrs. Masoudi from Tarbiat Modares University for technical assistance.
Funding
This study was supported by Tarbiat Modares University, Ph.D. thesis support. This study was financially supported by grant no: 950603 of the Biotechnology Development Council of the Islamic Republic of Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Dehghani, S., Rezaee, A. & Hosseinkhani, S. Effect of alternating electrical current on denitrifying bacteria in a microbial electrochemical system: biofilm viability and ATP assessment. Environ Sci Pollut Res 25, 33591–33598 (2018). https://doi.org/10.1007/s11356-018-3170-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3170-0