Abstract
Food waste is a cheap and abundant organic resource that can be used as a substrate for the production of the broad-spectrum antifungal compound iturin A. To increase the efficiency of food waste biotransformation, different artificial consortia incorporating the iturin A producer Bacillus amyloliquefaciens HM618 together with engineered Bacillus subtilis WB800N producing lipase or amylase were constructed. The results showed that recombinant B. subtilis WB-A13 had the highest amylase activity of 23406.4 U/mL, and that the lipase activity of recombinant B. subtilis WB-L01 was 57.5 U/mL. When strain HM618 was co-cultured with strain WB-A14, the higher yield of iturin A reached to 7.66 mg/L, representing a 32.9% increase compared to the pure culture of strain HM618. In the three-strain consortium comprising strains HM618, WB-L02, and WB-A14 with initial OD600 values of 0.2, 0.15, and 0.15, respectively, the yield of iturin A reached 8.12 mg/L, which was 38.6% higher than the control. Taken together, artificial consortia of B. amyloliquefaciens and recombinant B. subtilis can produce an increased yield of iturin A, which provides a new strategy for the valorization of food waste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food waste (FW) is generally discharged from restaurants, family kitchens, and public canteens (Zhang et al. 2014). In China, about 60 million tons of FW were generated annually in 2011, and this value was expected to reach 140 million in 2020 (Zhang et al. 2016; Ye et al. 2018). As such, a large amount of FW is a significant sanitary and ecological problem; there is an urgent need for rational and effective methods to valorize FW. Biochemically, FW is a mixture of organic substances, including starch, proteins, lipids, and cellulose (Dinesh et al. 2018; Kim et al. 2011). Accordingly, FW is rich in nutrients that can be used by microorganisms and converted into value-added products (Banu et al. 2020). In recent studies, FW has been biotransformed into pullulan (Rishi et al. 2020), Bt biopesticide (Zhang et al. 2015), bioflocculants (Liu et al. 2019), lipids and proteins (Zeng et al. 2017), biosurfactants (Chen et al. 2018), xanthan (Li et al. 2017), surfactin (Pan et al. 2021), ethanol (Wang et al. 2008), and L-lactic acid (Tashiro et al. 2016) through microorganism aerobic fermentation, as well as methane (Park et al. 2019; Yu et al. 2018), hydrogen (Kuang et al. 2020), and volatile fatty acid (Cheah et al. 2019) through anaerobic digestion. Compared with traditional disposal methods such as incineration or landfilling (Tang et al. 2017), biotransformation of FW is much more environmentally friendly and sustainable.
Iturin A, an antifungal cyclic lipopeptide mainly produced by Bacillus species, is composed of a cyclic heptapeptide and a β-amino fatty acid chain with 13–17 carbon atoms (Zhao et al. 2019b; Kawagoe et al. 2015). Iturin A can be embedded in the fungal cell membrane to increase its permeability to K+ cations, which results broad-spectrum antifungal activity (Cochrane and Vederas 2016). Therefore, iturin A possess bright application prospects in the biological control of plant fungal pathogens (Wang et al. 2020; Calvo et al. 2019; Ambrico and Trupo 2017). Despite its high potential value, the high production cost coupled with low yield limits the further industrial application of iturin A (Dang et al. 2019; Shi et al. 2018). It is therefore imperative to reduce the production costs, and the use of cheaper raw materials is a promising strategy to achieve better process economics. In recent studies, various agro-industrial wastes or by-products such as rapeseed meal (Chen et al. 2021), rapeseed cake (Chen et al. 2019b), soybean curd residue (Mizumoto et al. 2006), soybean meal (Xu et al. 2020), and sunflower oil cake (Kumar et al. 2017) have been investigated as substrates for iturin A production. These studies have greatly stimulated the interest in exploring the utilization of FW to produce iturin A.
The degradation of complex substrates, such as FW, is generally considered the rate-limiting step for microbial fermentation (Choi et al. 2018). Usually, it is preferred to add commercial enzymes to degrade FW into energy-rich small molecules that can be directly used by microorganisms (Rishi et al. 2020; Al-Dhabi et al. 2020; Prasoulas et al. 2020). Although the effect of this approach is obvious, the cost is relatively high. Presently, the co-cultivation of degradation and production strains has been favored for the transformation of complex substrates into value-added products, by virtue of its advantages in the minimal operational cost and synergistic metabolism of two or more organisms (Mohapatra et al. 2020; Izmirlioglu and Demirci 2017; Hashem et al. 2021). Recently, a two-strain consortium composed of an enzyme-producing fungus and B. amyloliquefaciens HM618 is constructed to produce surfactin using FW as the substrate (Pan et al. 2021). In an earlier study, distillers’ grains are directly used to produce surfactin by the co-culture of B. amyloliquefaciens MT45 and B. amyloliquefaciens X82, whereby the maximum surfactin level reached 3.4 g/L (Zhi et al. 2017). In addition, the successful co-cultures of Bacillus coagulans and Bacillus thermoamylovorans to produce L-lactic acid, Saccharomyces cerevisiae and Pichia stipitis to produce ethanol, or Bacillus and Enterococcus to produce poly-3-hydroxybutyrate have been also reported (Tashiro et al. 2016; Ntaikou et al. 2018; Sindhu et al. 2020). In these studies, a target-product synthesizing strain was co-cultivated with an enzyme-producing strain, providing ideas for the valorization of FW to produce iturin A.
Bacillus subtilis WB800N, which has the advantages of a clear genetic background and strong protein secretion ability, is an excellent host for the production of heterologous proteins (Liu et al. 2018; Zhang et al. 2005). Because it cannot synthesize lipopeptides (Wu et al. 2018), strain WB800N is also suitable for co-cultivation with B. amyloliquefaciens HM618 to produce iturin A without affecting product analysis. In this study, recombinant B. subtilis was engineered to heterologously express amylase and lipase for the degradation of FW. Furthermore, recombinant B. subtilis was co-cultivated with HM618 to transform FW into iturin A. Finally, the inoculation sizes and time of strain HM618 and the engineered strains were optimized. To our knowledge, this is the first study to demonstrate the suitability of an artificial bacterial consortium to valorize FW for iturin A production.
Materials and methods
Strains, plasmids, and reagents
The strains used in this study are listed in Table S1. B. amyloliquefaciens HM618 (CGMCC 7097), which was used to produce iturin A, was isolated from the soil. B. subtilis WB800N and the plasmid pHT43, used for expressing the heterologous lipase and amylase, were purchased from Baosai Biological Co., Ltd. (Hangzhou, China). Escherichia coli DH5α was used to construct the plasmids listed in Table S2.
Phanta DNA Polymerase was purchased from Novazan Biotechnology Co., Ltd. (Nanjing, China). All restriction endonucleases and T4 DNA ligase were purchased from New England Biolabs (Beijing, China). The BM Seamless Cloning and Lethal Based Fast Cloning kits were purchased from Biomed (Beijing, China) and Tiangen (Beijing, China), respectively. Iturin A standard was purchased from Sigma (USA).
Cultivation conditions
When constructing the plasmids, E. coli DH5α and the recombinant B. subtilis were cultivated in Luria–Bertani (LB) medium (10.0 g/L NaCl, 10.0 g/L peptone, 5.0 g/L yeast extract). If necessary, 100.0 mg/L ampicillin or 6.0 mg/L chloramphenicol was added. Before fermentation for the production of iturin A, all bacterial glycerol stocks were activated by culturing twice in 250-mL flasks containing 50 mL seed medium (60.0 g/L glucose, 10.0 g/L peptone, 10.0 g/L NaCl, 5.0 g/L yeast extract, 10.0 g/L beef extract) at 37 ℃ and 220 rpm for 24 h. Subsequently, the activated B. amyloliquefaciens HM618 and recombinant B. subtilis were seeded to an initial OD600 of 0.20 into 500-mL flasks containing 200 mL of FW medium (60.0 g/L FW, 14.0 g/L sodium L-glutamate, 5.0 g/L yeast extract, 0.5 g/L KCl, 1.0 g/L KH2PO4, 1.0 g/L MgSO4•7H2O, 0.15 mg/L FeSO4, 5.0 mg/L MnSO4, 0.16 mg/L CuSO4), which was based on replacing glucose in Landy medium with FW and cultivated at 37 ℃ and 180 rpm for 168 h.
Plasmid construction
The lipase and amylase genes are listed in Table S3. The coding sequences of lip1a, lip2, lipA, and amyMH were synthetized and directly cloned into pHT43 by Tsingke Biotechnology Co., Ltd. (Beijing, China). For replacing the promoter Pgarc or signal peptide SPamyQ of plasmid pHT43 in situ, the fragments P43 and SPnprB were PCR amplified from the genome of B. subtilis 168 using the primer pairs P1/P2 and P3/P4 (Table 1), respectively. Then, the fragment P43 was assembled into the linearized pHT43 vector digested with SacI and NotI using the BM Seamless Cloning Kit, generating the promoter P43 replacement vector pHT-P43. To obtain the signal peptide SPnprB replacement vector pHT-SPnprB, the fragment SPnprB was ligated into the linearized vector pHT43, which was digested with NotI and BamHI, using T4 DNA Ligase. The promoter and signal peptide replacement vector pHT-P43SPnprB was generated analogously. Additionally, amyEBa was cloned from the genome of B. amyloliquefaciens HM618 using the primer pair P6/P7, and four pHT43 derivatives carrying amyEBa were generated through restriction coupled with ligation. The fragment encoding amyEBa was ligated into the linearized vector pHT-P43lip1a, which was digested with BamHI and XbaI, generating pHT-P43amyEBa.
Composition analysis of FW
The FW was collected from the Xueyi canteen of Tianjin University (Tianjin, China), and mainly included rice, vegetables, and small amounts of soup. After picking out the bones, paper towels, and other impurities, FW was ground using a mechanical homogenizer and stored at − 40℃. The starch content was determined according to the method described by Ben Taher et al. (2017). The lipid content was measured via Soxhlet extraction (Pan et al. 2021). The crude protein content was estimated using the Kjeldahl method as described by Chen et al. (2017). The composition of the FW used in this study is listed in Table 2.
Fermentation of the recombinant strains
All constructed plasmids were introduced into B. subtilis WB800N via natural competence (Anagnostopoulos and Spizizen 1961). The verified recombinant B. subtilis strains were cultured in LB medium at 37 ℃ and 180 rpm for 72 h. If necessary, 100.0 mg/L ampicillin, 6.0 mg/L chloramphenicol, or 0.5 mM IPTG was added. The biomass was assessed by measuring the OD600 using a spectrophotometer (TU-1810, Beijing, China). The enzyme activity in the fermentation supernatant was measured after removing the cells by centrifugation at 12000 rpm for 5 min.
Amylase activity assay
The amylase activity was determined by measuring the release of reducing sugars using the dinitrosalicylic acid (DNS) method. The 1.5 mL reaction system consisted of 500 μL phosphate buffer (50 mM, pH 6.0), 900 μL of soluble starch (1.0%, w/v) as substrate, and 100 μL bacterial culture supernatant. The mixture was reacted at 37 °C for 10 min, followed by adding 1.5 mL of DNS mixture to end the reaction. After boiling for 10 min, the absorbance at 540 nm (A540) was measured using a TU-1810 spectrophotometer. The control contained the same volume of fresh medium without inoculation. One unit of amylase activity was defined as the amount of enzyme required to produce 1.0 μmol of reducing sugars per minute.
Lipase activity assay
Lipase activity was determined using the method described by Liu et al. (2017b). The 1.0 mL reaction system consisted of 750 μL of Tris–HCl buffer (50 mM, pH 8.0), 50 μL of para-nitrophenyl laurate (p-NPL, 10 mM) as the substrate, and 200 μL of the bacterial culture supernatant. The mixture was reacted at 37 °C for 10 min, followed by boiling for 3 min to end the reaction. Finally, the A410 was measured using the TU-1810 spectrophotometer. The control contained the same volume of fresh medium without inoculation. One unit of lipase activity is defined as the amount of enzyme required to produce 1.0 μmol of para-nitrophenol per minute.
Iturin A isolation and high-performance liquid chromatography (HPLC) analysis
A sample comprising 100 mL of the fermentation supernatant was collected by centrifugation at 11000 rpm and 4 °C for 10 min, after which the pH was adjusted to 2.0 with 6 M HCl and the acidified supernatant was stored overnight at 4 °C. The lipopeptide precipitate was collected by centrifugation at 8000 rpm and 4 °C for 10 min, and then resuspended with 100 mL methanol (AR grade) for iturin A extraction overnight. After centrifuging at 8000 rpm for 10 min, and evaporating until almost completely dry, the iturin A precipitate finally dissolved in 5 mL methanol (HPLC grade). The crude iturin A filtered through a 0.22-μm pore-size nylon membrane for quantitative analysis (Zhao et al. 2019a; Shi et al. 2018).
The resulting iturin A sample (10.0 µL) was subjected to reversed-phase HPLC on an LC-20A system (Shimadzu, Japan) equipped with a C18 column (Thermo Scientific, 250 × 4.6 mm, 5 μm) and a UV detector set to 220 nm. The mobile phase was a mixture of water containing 0.1% formic acid and acetonitrile (55:45, v/v) at a flow rate of 1.0 mL/min (Dang et al. 2019). The iturin A concentration was quantified by comparing the peak area with the calibration curve made using the authentic iturin A standard.
Liquid chromatography-mass spectrometry (LC–MS) analysis
The iturin A produced by B. amyloliquefaciens HM618 was determined by LC–MS using a Waters HPLC interfaced with an electrospray ionization mass spectrometry instrument (Thermo Electron, USA) and micrOTOF-Q software (Bruker Daltonics, USA). The ESI source was operated in positive ion mode, and the m/z scanning range was 800–2000. The mobile phase and column were the same as described above.
Glucose analysis
The content of glucose in the fermentation broth is an important indicator of the growth status of the bacteria. The fermentation broth was centrifuged at 12000 rpm for 10 min and filtered through a 0.22-μm pore-size aqueous membrane. Then, a sample comprising 10.0 µL was subjected to reversed-phase HPLC using an Aminex HPX-87H Ion Exclusion particles column (7.8 mm × 300 mm, BIORAD) and a differential refractive index detector. The mobile phase was 5.0 mM sulfuric acid in water at a flow rate of 0.6 mL/min and the column temperature was maintained at 65℃ (Liu et al. 2017a). The retention time of glucose was 9.3 min.
Statistical analysis
All experiments were performed in triplicate. Statistical analysis was performed using Microsoft Excel 2019 (Microsoft, USA). Differences with p values < 0.05 (*), < 0.01 (**), and < 0.001 (***) were labeled with one, two, and three asterisks, respectively.
Results and discussion
Identification of iturin A produced by B. amyloliquefaciens HM618
Iturin A is a mixture of several homologues (Chen et al. 2019a). As reported, the hydrogen adduct ion m/z values of the four isoforms of iturin A with a C14 to C17 chain length were 1043.55, 1057.55, 1071.56, and 1085.58, respectively (Chen et al. 2019a; Dang et al. 2019). Consistent with the reported results, the LC–MS identification results showed that iturin A produced by B. amyloliquefaciens HM618 was composed of the four homologues of C14, C15, C16, and C17 (Fig. S1b). However, the tested C15 iturin A showed two liquid phase peaks, which may indicate two different sub-types. In addition, the C14 iturin A accounted for the largest proportion, while the amount of C17 iturin A was very small.
Construction of engineered B. subtilis expressing amylase and lipase
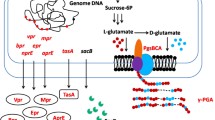
Since food waste is nearly half starch and about a quarter lipid, artificial consortia that use food waste as a substrate must produce amylases and lipases. As presented in Fig. 1, two amylase genes and three lipase genes were introduced into B. subtilis WB800N. During plasmid construction, the promoter and the signal peptide in plasmid pHT43 were also replaced. After screening, the recombinant WB800N-AmyE (engineered B. subtilis producing amylase) with higher amylase activity and the recombinant WB800N-Lip (engineered B. subtilis producing lipase) with higher lipase activity were designed to co-culture with B. amyloliquefaciens HM618. In the co-culture fermentation, the engineered B. subtilis are defined as the functional strains, secreting hydrolases to convert the FW into glucose and fatty acids as precursors for the synthesis of iturin A.
A total of twenty pHT43 expression plasmids were constructed (Figs. S2–S8), confirmed by sequencing, and introduced into B. subtilis WB800N for lipase and amylase production. Three controls, B. subtilis WB800N, WB + IPTG with added inducer during fermentation, and strain HM618, were also included. As shown in Figs. S9 and S10, the extracellular activities of the five enzymes consistently increased from 16 h, and reached a maximum at 48 h. According to the biomass curve (OD600), the increase of enzyme activity was largely caused by the accumulation of biomass. Further analysis found that the three lipase genes were better expressed under the control of the IPTG-inducible promoter Pgarc. With the assistance of the signal peptide SPamyQ, the extracellular lipase activity of the recombinant strains was further increased. In addition, the results showed that the signal peptide SPamyQ greatly promoted the secretion of amylase AmyEBa, and the amylase activity of the WB-A13 and WB-A14 strains with the SPamyQ gene was significantly higher than that of other strains. Although an earlier study reported that methyl parathion hydrolase activity was increased more than 100-fold in recombinant strain WB800 under the control of the P43 promoter and SPnprB signal peptide (Zhang et al. 2005), such a large increase could not be reproduced in this study.
After comparing the extracellular enzyme activity of each strain at 48 h, the recombinant strains with higher amylase and lipase activity were selected. As shown in Fig. 2, the recombinant strain WB-A13 had the highest amylase activity, reaching 23406.4 U/mL, which is 8.25 times higher than that of the control group WB + IPTG, as well as 5.26 times that of the strain HM618. In addition, the amylase activity of strain HM618 was 65% higher than that of WB800N. However, because of the difficult genetic manipulation of strain HM618, the amyEBa gene was transferred into WB800N as an alternative host. The recombinant strain WB-L01 had the highest lipase activity, reaching 57.5 U/mL, which was 96% higher than that of the control strain WB + IPTG. Accordingly, the recombinant strains WB-A13 and WB-L01 were selected for co-culture with strain HM618. Similarly, the recombinant strains WB-A14 with outstanding amylase activity and WB-L09 with higher lipase activity were also selected to construct artificial consortia with strain HM618. In addition, recombinant strain WB-L02 which had the highest lipase activity of all strains without adding IPTG was also selected for co-culture experiments.
Production of iturin A by artificial consortia consisting of strains HM618 and recombinant B. subtilis
Co-culture of strain HM618 with producing amylase recombinant B. subtilis
Considering the fact that starch accounts for nearly half of the organic components of FW, improving the degradation efficiency of starch is conducive to obtaining large amounts of reducing sugars from the substrate. Hence, artificial consortia of strain HM618 and recombinant B. subtilis expressing high levels of amylase were constructed for the production of iturin A. A pure culture of strain HM618 was included as a control. The iturin A production of the consortium comprising strains HM618 and WB800N-AmyE is shown in Fig. 3a. The iturin A level reached 7.66 mg/L in the co-culture of strains HM618 and WB-A14, representing a 32.9% increase over the pure culture of strain HM618 with the yield of 5.76 mg/L (p < 0.05). Compared with the co-culture of strains HM618 and WB-A14, the iturin A production was slightly decreased in the co-culture of strains HM618 and WB-A13. Thus, more iturin A was produced by the consortia than the pure culture, as anticipated. Accordingly, the choice of the co-cultured strain should be based on the composition of the complex substrate. In a previous study, the food waste contained more crude lipids than starch, and B. amyloliquefaciens produced more surfactin when co-cultured with lipase-producing fungi than with amylase-producing fungi (Pan et al. 2021). Furthermore, some studies investigated the biotransformation of cellulose-rich biomass, such as rice straw (Mohapatra et al. 2020) and corn stover (Minty et al. 2013), into biofuels.
The amylase activity in the co-culture was detected every 24 h. As shown in Fig. 3b, the changes of amylase activity in the co-culture fermentation were almost the same as those of strain HM618 in the pure culture, but they were obviously different from those of recombinant B. subtilis under the pure culture conditions. Specifically, the amylase activity in the pure culture fermentation of the recombinant strains reached about 6000 U/mL at 24 h, and remained high until the late fermentation stage. However, the amylase activity in the co-culture fermentation was lower, less than 3000 U/mL, which was almost equivalent to the amylase activity of strain HM618 under the pure culture conditions. According to the glucose consumption curve shown in Fig. S11, strain HM618 could utilize glucose at a faster rate than strain WB800N, and also reached the stationary phase earlier during fermentation. Hence, strain HM618 theoretically prioritized the use of energy to gain an advantage in terms of biomass, reducing the growth of recombinant B. subtilis in the co-culture fermentation. Thus, the amylase activity of the co-culture was significantly lower than that of the pure culture of engineered B. subtilis.
Iturin A belonged is a secondary metabolite, and its synthesis depends on the accumulation of biomass (Xu et al. 2020). The amylase activity is related to the amount of energy spent and biomass accumulated in the fermentation, which in turn might influence iturin A production. In the first 48 h of fermentation, the amylase activity of the consortium with strains HM618 and WB-A14 increased to 2216.35 U/mg, which was 62% higher than in the pure culture of strain HM618 (1369.68 U/mg) at the same stage of fermentation (Fig. 3b). High amylase activity provided more carbon sources for the growth and metabolism of strain HM618, which can explain why the consortium with strains HM618 and WB-A14 yielded more iturin A.
Co-culture of strain HM618 with lipase-producing recombinant B. subtilis
Lipids are the second most abundant nutrient in FW, and the fatty acids released by the hydrolysis of neutral lipids are also an important precursor for the synthesis of iturin A, making it promising to construct artificial consortia with lipase-producing recombinant B. subtilis. However, the iturin A production of the co-culture of strains HM618 and WB800N-Lip was even less satisfactory (Fig. 3c). Although the yield of iturin A in the co-culture of strains HM618 and WB-L02 reached 9.99 mg/L, which was the highest yield of iturin A in this co-culture fermentation, it was still less than the yield of the control group (10.51 mg/L). Interestingly, the yield of iturin A in the pure culture of strain HM618 was 10.51 mg/L (Fig. 3c), compared to only 5.76 mg/L in the first fermentation (Fig. 3a). The difference of iturin A yields might be caused by the instability of FW components that were pulverized into solid–liquid mixture. In general, relatively more iturin A was produced by the co-culture of strains HM618 and WB800N-AmyE, which may be related to the high starch content in FW. The change trend of lipase activity in the consortia was similar to that of amylase (Fig. 3d). Notably, the lipase activity of the consortia was almost indistinguishable from that of strain HM618 in the pure culture. In particular, the lipase activity at 24 h was higher in the pure culture of strain HM618 than in any of the three consortia, suggesting that the growth of the two stains might be affected in the consortia. Furthermore, less active lipase appeared to be insufficient to hydrolyze enough lipids for iturin A synthesis. These might explain why the pure culture of strain HM618 produced more iturin A.
In order to make full use of FW, the recombinant strains WB-A14 and WB-L02 were selected for co-culture with strain HM618. However, complex interactions between microorganisms inevitably occur in co-culture fermentation, including competition for resources and sharing of metabolites (Mee and Wang 2012). Therefore, a reasonable distribution of substrates and metabolic intermediates in the mixed culture is of great significance for increasing the yield of iturin A. It was reported that optimizing the inoculation sizes of Paenibacillus polymyxa, which can synthesize acetoin, as well as optimizing the inoculation time of an E. coli strain that produces vitamin B2, can result in an artificial consortium that can produce more acetoin (Liu et al. 2017a). Similarly, optimizing the inoculum sizes of two B. amyloliquefaciens strains in a co-culture fermentation with distiller’s grains resulted in an increase in the surfactant yield to 2.54 g/L (Zhi et al. 2017). Therefore, the inoculation method should be optimized to balance the energy and biomass distribution between B. amyloliquefaciens HM618 and the substrate-degrading recombinant B. subtilis strains as much as possible.
Effect of the inoculation method on iturin A production in the co-culture fermentation of strains HM618, WB-A14, and WB-L02
The recombinant strains that degrade the food waste were added into the FW medium earlier, to obtain more sufficient small molecules when the strain HM618 was inoculated. Taking into account the higher biomass of recombinant B. subtilis strains after 24 h, different inoculum sizes of strain HM618 were tested. As shown in Fig. 4a, when the strain HM618 was directly inoculated with strains WB-L02 and WB-A14, the yield of iturin A reached 8.12 mg/L, which was an increase of 38.6% compared with the control group (p < 0.01). When strain HM618 inoculation was delayed for 24 h, the yield of iturin A only reached between 2.05 and 2.52 mg/L, which was significantly less than the control group with a yield of 5.86 mg/L (p < 0.01), even if the initial OD600 of strain HM618 was increased to 0.6. This was not in line with our expectation that the yield of iturin A would be dramatically increased when strain HM618 inoculation was postponed for 24 h. This inconsistency might be explained by analyzing other results of the mixed fermentation. As shown in Fig. 4b, the amylase activity in consortia b–d at 24 h was higher than that of consortium a, and this advantage lasted until the later stage of fermentation. Accordingly, the glucose concentration in consortia b–d at 24 h was almost 4 times higher than in consortium a, and this gap gradually increased with the increase of fermentation time (Fig. 4d). These results indicated that the amount of B. subtilis was fully accumulated when strain HM618 was inoculated at 24 h. Consequently, the growth of strain HM618 was affected significantly, resulting in the decreased yield of iturin A in the mixed fermentation. In addition, the changes of lipase activity shown in Fig. 4c also indicated that strain HM618 no longer had a growth advantage in the three-strain consortium when its inoculation was postponed for 24 h. Although the three-strain consortium containing strains HM618, WB-L02, and WB-A14 showed certain advantages in the production of iturin A compared with other fermentation methods, the energy distribution between the three strains required additional optimization.
Effects of inoculation time in mixed culture fermentation of three-strain consortia. Iturin A production (a), amylase activity (b), lipase activity (c), and glucose content (d) under mixed culture of three-strain consortia. HM618 was solely cultured with initial OD600 of 0.2 as the control. Consortium a, L020.15 + A140.15 + HM0 h+0.2; b, L020.15 + A140.15 + HM24 h+0.2 (L02 and A14 were inoculated with OD600 0.15 and 0.15 respectively; after 24 h, HM618 was inoculated with OD600 of 0.2); c, L020.15 + A140.15 + HM24 h+0.4, d: L020.15 + A140.15 + HM24 h+0.6
The broader application of iturin A is still limited by its high production cost (Dang et al. 2019; Shi et al. 2018). Although the production of iturin A from FW in the pure culture of strain HM618 was unstable (Fig. 5), the co-culture of enzyme-producing B. subtilis and strain HM618 could generally improve the yield. Moreover, the feasibility of reducing the synthesis cost of iturin A by using FW was analyzed. As presented in Fig. 5, the yield of iturin A obtained by cultivating strain HM618 with Landy medium was 9.69 mg/L, compared with 5.76 mg/L, 10.51 mg/L, and 5.86 mg/L obtained using different consortia in FW medium. After replacing glucose with food waste, the production of iturin A did not change significantly. Therefore, this research verified the application value of FW in reducing the production cost of iturin A.
Comparison of iturin A production. Iturin A was synthetized from strain HM618 pure culture using Landy medium and FW medium, while from consortium comprising strains HM618 and WB-A14, consortium comprising strains HM618 and WB-L02, and the three-strain consortium comprising strains HM618, WB-L02, and WB-A14 respectively
The ability of the artificial consortia to synthetize iturin A was increased to some extent by adjusting the inoculation time. In agreement with previous studies (Chen et al. 2020; Ntaikou et al. 2018; Minty et al. 2013), our results confirm that the consortia contained different functional strains could convert the waste substrates into target product. Based on this research, it may be possible to develop consortia that encompass more species (Zhi et al. 2017) or are more purposefully modified via synthetic genetic circuits (Minty et al. 2013; Dang et al. 2019) to achieve the cost-effective biotransformation of food waste into iturin A.
Conclusion
Food waste is a suitable raw material for iturin A production. Consortia that incorporate the engineered B. subtilis strains with excellent amylase and lipase activity constructed via genetic methods resulted in the increase of iturin A production by B. amyloliquefaciens HM618 compared with the pure culture. Co-culture of strains HM618 and WB-A14 produced the higher iturin A yield among the tested two-strain consortia. Furthermore, the iturin A yield of the three-strain consortium comprising strains HM618, WB-A14, and WB-L02, respectively, inoculated at initial OD600 values of 0.2, 0.15, and 0.15, was 38.6% higher than that of strain HM618 pure culture. These results confirm that food waste can be converted into value-added chemicals using artificial consortia.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Al-Dhabi NA, Esmail GA, Arasu MV (2020) Co-fermentation of food waste and municipal sludge from the Saudi Arabian environment to improve lactic acid production by Lactobacillus rhamnosus AW3 isolated from date processing waste. Sustainability 12:6899. https://doi.org/10.3390/su12176899
Ambrico A, Trupo M (2017) Efficacy of cell free supernatant from Bacillus subtilis ET-1, an iturin A producer strain, on biocontrol of green and gray mold. Postharvest Biol Technol 134:5–10. https://doi.org/10.1016/j.postharvbio.2017.08.001
Anagnostopoulos C, Spizizen J (1961) Requirements for transformation in Bacillus Subtilis. J Bacteriol 81:741–746. https://doi.org/10.1128/jb.81.5.741-746.1961
Banu JR, Merrylin J, Usman TMM, Kannah RY, Gunasekaran M, Kim SH, Kumar G (2020) Impact of pretreatment on food waste for biohydrogen production: a review. Int J Hydrogen Energy 45:18211–18225. https://doi.org/10.1016/j.ijhydene.2019.09.176
Ben Taher I, Bennour H, Fickers P, Hassouna M (2017) Valorization of potato peels residues on cellulase production using a mixed culture of Aspergillus niger ATCC 16404 and Trichoderma reesei DSMZ 970. Waste Biomass Valor 8:183–192. https://doi.org/10.1007/s12649-016-9558-5
Calvo H, Mendiara I, Arias E, Blanco D, Venturini ME (2019) The role of iturin A from B. amyloliquefaciens BUZ-14 in the inhibition of the most common postharvest fruit rots. Food Microbiol 82:62–69. https://doi.org/10.1016/j.fm.2019.01.010
Cheah YK, Vidal-Antich C, Dosta J, Mata-Alvarez J (2019) Volatile fatty acid production from mesophilic acidogenic fermentation of organic fraction of municipal solid waste and food waste under acidic and alkaline pH. Environ Sci Pollut Res 26:35509–35522. https://doi.org/10.1007/s11356-019-05394-6
Chen H, Shen H, Su HF, Chen HZ, Tan F, Lin JF (2017) High-efficiency bioconversion of kitchen garbage to biobutanol using an enzymatic cocktail procedure. Bioresour Technol 245:1110–1121. https://doi.org/10.1016/j.biortech.2017.09.056
Chen CY, Sun N, Li DS, Long SH, Tang XY, Xiao GQ, Wang LY (2018) Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ Sci Pollut Res 25:14934–14943. https://doi.org/10.1007/s11356-018-1691-1
Chen MC, Wang JP, Zhu YJ, Liu B, Yang WJ, Ruan CQ (2019a) Antibacterial activity against Ralstonia solanacearum of the lipopeptides secreted from the Bacillus amyloliquefaciens strain FJAT-2349. J Appl Microbiol 126:1519–1529. https://doi.org/10.1111/jam.14213
Chen WH, Li X, Ma XL, Chen SW, Kang YP, Yang MM, Huang FH, Wan X (2019b) Simultaneous hydrolysis with lipase and fermentation of rapeseed cake for iturin A production by Bacillus amyloliquefaciens CX-20. BMC Biotechnol 19:98. https://doi.org/10.1186/s12896-019-0591-x
Chen H, Chen B, Su ZH, Wang K, Wang BX, Wang Y, Si ZH, Wu YL, Cai D, Qin PY (2020) Efficient lactic acid production from cassava bagasse by mixed culture of Bacillus coagulans and Lactobacillus rhamnosus using stepwise pH controlled simultaneous saccharification and co-fermentation. Ind Crops Prod 146:112175. https://doi.org/10.1016/j.indcrop.2020.112175
Chen WC, Wang M, Gong YM, Deng QC, Zheng MM, Chen SW, Wan X, Yang C, Huang FH (2021) The unconventional adverse effects of fungal pretreatment on iturin A fermentation by Bacillus amyloliquefaciens CX-20. Microb Biotechnol 14:587–599. https://doi.org/10.1111/1751-7915.13658
Choi JM, Han SK, Lee CY (2018) Enhancement of methane production in anaerobic digestion of sewage sludge by thermal hydrolysis pretreatment. Bioresour Technol 259:207–213. https://doi.org/10.1016/j.biortech.2018.02.123
Cochrane SA, Vederas JC (2016) Lipopeptides from Bacillus and Paenibacillus spp.: a gold mine of antibiotic candidates. Med Res Rev 36:4–31. https://doi.org/10.1002/med.21321
Dang YL, Zhao FJ, Liu XS, Fan X, Huang R, Gao WX, Wang SF, Yang C (2019) Enhanced production of antifungal lipopeptide iturin A by Bacillus amyloliquefaciens LL3 through metabolic engineering and culture conditions optimization. Microb Cell Fact 18:68. https://doi.org/10.1186/s12934-019-1121-1
Dinesh GK, Chauhan R, Chakma S (2018) Influence and strategies for enhanced biohydrogen production from food waste. Renew Sust Energ Rev 92:807–822. https://doi.org/10.1016/j.rser.2018.05.009
Hashem M, Alamri SA, Asseri TAY, Mostafa YS, Lyberatos G, Ntaikou I (2021) On the optimization of fermentation conditions for enhanced bioethanol yields from starchy biowaste via yeast co-cultures. Sustainability 13:1890. https://doi.org/10.3390/su13041890
Izmirlioglu G, Demirci A (2017) Simultaneous saccharification and fermentation of ethanol from potato waste by co-cultures of Aspergillus niger and Saccharomyces cerevisiae in biofilm reactors. Fuel 202:260–270. https://doi.org/10.1016/j.fuel.2017.04.047
Kawagoe Y, Shiraishi S, Kondo H, Yamamoto S, Aoki Y, Suzuki S (2015) Cyclic lipopeptide iturin A structure-dependently induces defense response in Arabidopsis plants by activating SA and JA signaling pathways. Biochem Biophys Res Commun 460:1015–1020. https://doi.org/10.1016/j.bbrc.2015.03.143
Kim DH, Kim SH, Kim HW, Kim MS, Shin HS (2011) Sewage sludge addition to food waste synergistically enhances hydrogen fermentation performance. Bioresour Technol 102:8501–8506. https://doi.org/10.1016/j.biortech.2011.04.089
Kuang Y, Zhao JW, Gao Y, Lu CG, Luo SY, Sun YJ, Zhang DL (2020) Enhanced hydrogen production from food waste dark fermentation by potassium ferrate pretreatment. Environ Sci Pollut Res 27:18145–18156. https://doi.org/10.1007/s11356-020-08207-3
Kumar PN, Swapna TH, Khan MY, Reddy G, Hameeda B (2017) Statistical optimization of antifungal iturin A production from Bacillus amyloliquefaciens RHNK22 using agro-industrial wastes. Saudi J Biol Sci 24:1722–1740. https://doi.org/10.1016/j.sjbs.2015.09.014
Li PY, Zeng Y, Xie Y, Li X, Kang Y, Wang YB, Xie TH, Zhang YK (2017) Effect of pretreatment on the enzymatic hydrolysis of kitchen waste for production. Bioresour Technol 223:84–90. https://doi.org/10.1016/j.biortech.2016.10.035
Liu L, Xu QM, Chen T, Cheng JS, Yuan YJ (2017a) Artificial consortium that produces riboflavin regulates distribution of acetoin and 2,3-butanediol by Paenibacillus polymyxa CJX518. Eng Life Sci 17:1039–1049. https://doi.org/10.1002/elsc.201600239
Liu W, Li MG, Yan YJ (2017b) Heterologous expression and characterization of a new lipase from Pseudomonas fluorescens Pf0-1 and used for biodiesel production. Sci Rep 7:15711. https://doi.org/10.1038/s41598-017-16036-7
Liu X, Wang H, Wang B, Pan L (2018) Efficient production of extracellular pullulanase in Bacillus subtilis ATCC6051 using the host strain construction and promoter optimization expression system. Microb Cell Fact 17:163. https://doi.org/10.1186/s12934-018-1011-y
Liu WJ, Dong Z, Sun D, Chen Y, Wang SW, Zhu JR, Liu C (2019) Bioconversion of kitchen wastes into bioflocculant and its pilot-scale application in treating iron mineral processing wastewater. Bioresour Technol 288:121505. https://doi.org/10.1016/j.biortech.2019.121505
Mee MT, Wang HH (2012) Engineering ecosystems and synthetic ecologies. Mol BioSyst 8:2470–2483. https://doi.org/10.1039/C2MB25133G
Minty JJ, Singer ME, Scholz SA, Bae CH, Ahn JH, Foster CE, Liao JC, Lin XN (2013) Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc Natl Acad Sci U S A 110:14592–14597. https://doi.org/10.1073/pnas.1218447110
Mizumoto S, Hirai M, Shoda M (2006) Production of lipopeptide antibiotic iturin A using soybean curd residue cultivated with Bacillus subtilis in solid-state fermentation. Appl Microbiol Biotechnol 72:869–875. https://doi.org/10.1007/s00253-006-0389-3
Mohapatra S, Mishra RR, Nayak B, Behera BC, Mohapatra PKD (2020) Development of co-culture yeast fermentation for efficient production of biobutanol from rice straw: a useful insight in valorization of agro industrial residues. Bioresour Technol 318:124070. https://doi.org/10.1016/j.biortech.2020.124070
Ntaikou I, Menis N, Alexandropoulou M, Antonopoulou G, Lyberatos G (2018) Valorization of kitchen biowaste for ethanol production via simultaneous saccharification and fermentation using co-cultures of the yeasts Saccharomyces cerevisiae and Pichia stipitis. Bioresour Technol 63:75–83. https://doi.org/10.1016/j.biortech.2018.04.109
Pan FD, Liu S, Chen XY, Cheng JS (2021) Bioconversion of kitchen waste to surfactin via simultaneous enzymolysis and fermentation using mixed-culture of enzyme-producing fungi and Bacillus amyloliquefaciens HM618. Biochem Eng J 172:108036. https://doi.org/10.1016/j.bej.2021.108036
Park JG, Lee B, Kwon HJ, Park HR, Jun HB (2019) Effects of a novel auxiliary bio-electrochemical reactor on methane production from highly concentrated food waste in an anaerobic digestion reactor. Chemosphere 220:403–411. https://doi.org/10.1016/j.chemosphere.2018.12.169
Prasoulas G, Gentikis A, Konti A, Kalantzi S, Kekos D, Mamma D (2020) Bioethanol production from food waste applying the multienzyme system produced on-site by Fusarium oxysporum F3 and mixed microbial cultures. Fermentation-Basel 6:39. https://doi.org/10.3390/fermentation6020039
Rishi V, Sandhu AK, Kaur A, Kaur J, Sharma S, Soni SK (2020) Utilization of kitchen waste for production of pullulan to develop biodegradable plastic. Appl Microbiol Biotechnol 104:1307–1317. https://doi.org/10.1007/s00253-019-10167-9
Shi JR, Zhu XY, Lu YJ, Zhao HZ, Lu FX, Lu ZX (2018) Improving iturin A production of Bacillus amyloliquefaciens by genome shuffling and its inhibition against Saccharomyces cerevisiae in orange juice. Front Microbiol 9:2683. https://doi.org/10.3389/fmicb.2018.02683
Sindhu R, Manju A, Mohan P, Rajesh RO, Madhavan A, Arun KB, Hazeena SH, Mohandas A, Rajamani SP, Puthiyamadam A, Binod P, Reshmy R (2020) Valorization of food and kitchen waste: an integrated strategy adopted for the production of poly-3-hydroxybutyrate, bioethanol, pectinase and 2, 3-butanediol. Bioresour Technol 310:123515. https://doi.org/10.1016/j.biortech.2020.123515
Tang YJ, Dong J, Chi Y, Zhou ZZ, Ni MJ (2017) Energy and exergy optimization of food waste pretreatment and incineration. Environ Sci Pollut Res 24:18434–18443. https://doi.org/10.1007/s11356-017-9396-4
Tashiro Y, Inokuchi S, Poudel P, Okugawa Y, Miyamoto H, Miayamoto H, Sakai K (2016) Novel pH control strategy for efficient production of optically active L-lactic acid from kitchen refuse using a mixed culture system. Bioresour Technol 216:52–59. https://doi.org/10.1016/j.biortech.2016.05.031
Wang QH, Ma HZ, Xu WL, Gong LJ, Zhang WY, Zou DX (2008) Ethanol production from kitchen garbage using response surface methodology. Biochem Eng J 39:604–610. https://doi.org/10.1016/j.bej.2007.12.018
Wang YY, Zhang CY, Liang J, Wu LF, Gao WB, Jiang JZ (2020) Iturin A extracted from Bacillus subtilis WL-2 affects phytophthora infestansvia cell structure disruption, oxidative stress, and energy supply dysfunction. Front Microbiol 11:536083. https://doi.org/10.3389/fmicb.2020.536083
Wu Q, Zhi Y, Xu Y (2018) Systematically engineering the biosynthesis of a green biosurfactant surfactin by Bacillus subtilis 168. Metab Eng 52:87–97. https://doi.org/10.1016/j.ymben.2018.11.004
Xu YX, Cai DB, Zhang H, Gao L, Yang Y, Gao JM, Li YY, Yang CL, Ji ZX, Yu J, Chen SW (2020) Enhanced production of iturin A in Bacillus amyloliquefaciens by genetic engineering and medium optimization. Process Biochem 90:50–57. https://doi.org/10.1016/j.procbio.2019.11.017
Ye M, Liu JY, Ma CN, Li YY, Zou LP, Qian GR, Xu ZP (2018) Improving the stability and efficiency of anaerobic digestion of food waste using additives: a critical review. J Cleaner Prod 192:316–326. https://doi.org/10.1016/j.jclepro.2018.04.244
Yu M, Gao M, Wang LH, Ren YY, Wu CF, Ma HZ, Wang QH (2018) Kinetic modelling and synergistic impact evaluation for the anaerobic co-digestion of distillers’ grains and food waste by ethanol pre-fermentation. Environ Sci Pollut Res 25:30281–30291. https://doi.org/10.1007/s11356-018-3027-6
Zeng Y, Bian DL, Xie Y, Jiang XL, Li X, Li PY, Zhang YK, Xie TH (2017) Utilization of food waste hydrolysate for microbial lipid and protein production by Rhodosporidium toruloides Y2. J Chem Technol Biotechnol 92:666–673. https://doi.org/10.1002/jctb.5049
Zhang XZ, Cui ZL, Hong Q, Li SP (2005) High-level expression and secretion of methyl parathion hydrolase in Bacillus subtilis WB800. Appl Environ Microbiol 71:4101–4103. https://doi.org/10.1128/AEM.71.7.4101-4103.2005
Zhang CS, Su HJ, Baeyens J, Tan TW (2014) Reviewing the anaerobic digestion of food waste for biogas production. Renew Sust Energy Rev 38:383–392. https://doi.org/10.1016/j.rser.2014.05.038
Zhang WY, Zou H, Jiang L, Yao JJ, Liang J, Wang QH (2015) Semi-solid state fermentation of food waste for production of Bacillus thuringiensis biopesticide. Biotechnol Bioprocess Eng 20:1123–1132. https://doi.org/10.1007/s12257-015-0347-y
Zhang JY, Lv C, Tong J, Liu JW, Liu JB, Yu DW, Wang YW, Chen MX, Wei YS (2016) Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour Technol 200:253–261. https://doi.org/10.1016/j.biortech.2015.10.037
Zhao HB, Xu XG, Lei SZ, Shao DY, Jiang CM, Shi JL, Zhang YW, Liu L, Lei SZ, Sun H, Huang QS (2019a) Iturin A-like lipopeptides from Bacillus subtilis trigger apoptosis, paraptosis, and autophagy in Caco-2 cells. J Cell Physiol 234:6414–6427. https://doi.org/10.1002/jcp.27377
Zhao HB, Zhao XX, Lei SZ, Zhang YW, Shao DY, Jiang CM, Sun H, Shi JL (2019b) Effect of cell culture models on the evaluation of anticancer activity and mechanism analysis of the potential bioactive compound, iturin A, produced by Bacillus subtilis. Food Funct 10:1478–1489. https://doi.org/10.1039/C8FO02433B
Zhi Y, Wu Q, Xu Y (2017) Production of surfactin from waste distillers’ grains by co-culture fermentation of two Bacillus amyloliquefaciens strains. Bioresour Technol 235:96–103. https://doi.org/10.1016/j.biortech.2017.03.090
Funding
This work was supported by the National Key R&D Program of China (grant number 2018YFA0902200) and the National Natural Science Foundation of China (program: 21878224).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Funding acquisition, resources, and project administration were performed by Qiu-Man Xu and Jing-Sheng Cheng. Material preparation, data collection, and analysis were performed by Chang-Hao Miao, Xiao-Feng Wang, Bin Qiao, and Chun-Yang Cao. The first draft of the manuscript was written by Chang-Hao Miao and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miao, CH., Wang, XF., Qiao, B. et al. Artificial consortia of Bacillus amyloliquefaciens HM618 and Bacillus subtilis for utilizing food waste to synthetize iturin A. Environ Sci Pollut Res 29, 72628–72638 (2022). https://doi.org/10.1007/s11356-022-21029-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21029-9