Abstract
Sufficient attention should be attached to the large amount of fly ash containing high levels of toxic heavy metals generated after municipal solid waste incineration. Because heavy metals could be leached out of the fly ash under specific conditions, it is necessary to stabilize the heavy metals in fly ash before landfill disposal. Processing technologies of incineration fly ash include solidification/stabilization technology, thermal treatments, and separation processes. This study reviewed the current treatment technologies of municipal solid waste incineration (MSWI) fly ash, with the main focus on the treatment of heavy metals in fly ash with chemical stabilization. Chemical stabilization processes involve chemical precipitation of heavy metal and chelation of heavy metals. In multiple studies, chemical stabilization technology has shown practical feasibility in terms of technology, economy, and effect. In addition, the combination of two or more stabilization agents broadens the general applicability of the agents to heavy metals and reduces the cost. The application of joint processing technology realizes the remove of soluble salt from fly ash. To minimize pollutants while increase their usable value, effective use of waste and co-disposal of several kinds of wastes have gradually become the research hotspots. New developments in chemical stabilization are progressively moving towards the sustainable direction of harmlessness and resource utilization of MSWI fly ash.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the dramatic rise of municipal solid waste (MSW) and the reduction of land (Demirbas 2011), municipal solid waste incineration (MSWI) has been widely applied and spread in recent years (Cheng and Hu 2010; Lin and Ma 2012; Lu et al. 2017). Compared with the traditional methods of MSW treatment, such as compost and landfill (Eriksson et al. 2007), incineration is more efficient and more reductive in volume. Incineration can decrease the weight of waste by 80 to 85% and the volume by 90% and kill all pathogenic microorganisms and parasite eggs (Bie et al. 2016; Lam et al. 2010). Moreover, the heat energy produced by burning MSW is recyclable (Palanivel and Sulaiman 2014; Scarlat et al. 2015). Generally, incineration will make household waste reductive, harmless, and resourceful to the maximum and occupy less land resources (Kirby and Rimstidt 1993; Lu et al. 2017).
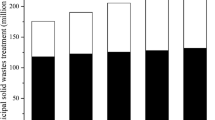
China is one of the countries with the heaviest municipal solid waste burden in the world (Zhou et al. 2014). The proportion of waste incineration for harmless treatment in China is increasing year by year. From 2008 to 2020, the number of sites for MSW incineration had risen from 74 to 463, and the disposal ratio had grown from 15.2 to 62.3% (Fig. 1) based on the data from the Statistical Yearbook of Chinese Ministry of Housing and Urban–Rural Development from 2008 to 2020 (MOHURD 2021). Since burning MSW can meet the needs of the reduction and harmless development in treating urban household MSW, it may become the mainstream way to dispose of MSW in the future. By 2015, there were 1179 waste incineration power plants generating electricity around the world, with a total capacity of more than 700,000 Mg/day (Mg is short for Megagram; 1 Mg equals to a metric ton) (Lu et al. 2017). The use of MSW in the power industry reduces the use of fossil fuels such as coal and oil and reduces the energy pressure for some countries and regions. Compared with the harmless treatment rate of waste in some representative developed countries such as Japan, the waste-incineration industry in China and some other regions still has broad room for growth.
However, waste incineration also has potential environmental pollution, which will produce a large number of residues, including bottom ash and fly ash (Assi et al. 2020). As a sort of secondary pollutants, fly ash are the small particulate matters trapped and settled in the process of purifying flue gases in an incineration system (Huber et al. 2018), accounting for about 3 ~ 5% of the whole volume of the waste put into incineration (Tian et al. 2015). After incineration, most heavy metals, such as Pb, Cr, Zn, and Cd, are concentrated in the fly ash, and their contents are 10 ~ 100 times higher than those in the soil. High levels of heavy metals and toxic organic pollutants such as dioxins and dibenzofuran make fly ash hazardous wastes (Jiao et al. 2016; Zhang et al. 2020a), which pose significant environmental risks and are regarded as hazardous wastes internationally (Huang et al. 2011b; Xiong et al. 2014). If not properly handled, heavy metals in fly ash will enter the soil and groundwater under certain conditions through leaching and other processes (Du et al. 2014; Long et al. 2022; Shim et al. 2003), causing pollution to water bodies and soils, and entering organisms through channels such as the atmosphere, water, and food, thereby posing potential threats to the environment and human health (Reijnders 2005; Wang et al. 2019). Therefore, MSWI fly ash must be harmlessly treated before buried or reused, and the safe disposal and resource utilization of fly ash have become a new challenge for national urban development.
The fly ash disposal technologies, with slight capacity increase, low cost, effective stability of heavy metals, and environmental friendliness, are critical approaches to waste disposal and management. In this paper, the current treatment technologies of MSWI fly ash are reviewed, focusing on the research and application of the chemical stabilization of heavy metals in fly ash, and the direction and prospect of the technology in the future are pointed out.
Treatment and disposal of heavy metals in fly ash
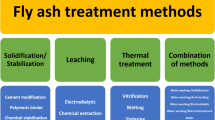
The color of waste incineration fly ash is usually grey or brown, related to its incineration technologies. Circulating fluidized bed and stoker grate are the two major MSWI technologies currently being used in China. The particle size of fly ash is primarily distributed in the range of several micrometers to hundreds of micrometers. Fly ash are mostly irregularly shaped particles, with small particles adsorbed on the surface, in addition to some microcrystalline and cenospheres dispersed in the particle–matrix (Marieta et al. 2021), which has complex structures and high hygroscopicity. Fly ash usually contain alkaline substances, mainly due to a large amount of lime sprayed into the exhaust gas during the desulfurization process. Chemical and physical characteristics of fly ash depend on the compositions of the raw waste, auxiliary fuel, incinerator body type, flue gas purification system, etc. (Dou et al. 2017; Luo et al. 2019a; Tian et al. 2015; Zacco et al. 2014). In most regions, the treatment goals of the flue gas purification system are still focused on acid gas and NOx controls. There is no significant difference in the mineral composition of fly ash before and after treatment by the flue gas purification system, but the mineral phase content is different. The compositions of the raw MSW vary over time and region. But under normal circumstances, fly ash usually contain the main components like SiO2, Al2O3, calcium salts, and chloride salts, and the main heavy metals with lower boiling points such as Cd, Pb, and Zn (Li et al. 2004; Pan et al. 2013), most of which are embraced in silicate or silicoaluminate in the form of insoluble substances (Jiang et al. 2007; Yakubu et al. 2018). To effectively prevent heavy metals in fly ash from leaching and reduce the impact on the environment, the heavy metals need to be treated harmlessly to reduce their toxicity before the final disposal of fly ash in waste incineration. The processing technologies of fly ash in incineration at home and abroad mainly include three categories: separation treatment, stabilization/solidification treatment, thermal treatment (Zacco et al. 2014). Table 1 shows a comparison of the typical fly ash treatment processes, and supplementary data shows the flow charts of different technologies of MSWI fly ash treatment.
Solidification/stabilization treatment technology
Solidification/stabilization technology can fix heavy metals through physical processes and chemical reactions. It is currently a widely used fly ash treatment technology, which can be specifically divided into stabilization technology and chemical stabilization technology. The curing process reduces the mobility of toxic substances by changing physical properties such as permeability and surface area and ultimately solidifies the toxic substance into the structure of the hydration product. This technology mainly relies on curing agents, such as cement (Bie et al. 2016; Yang et al. 2018), asphalt (Rani et al. 2008), and clay (Luna Galiano et al. 2011). They work through the means of solidification and inclusion to make the heavy metals wrapped in the hydration silicate, which is generated after hydration reaction, thus reducing the leaching toxicity of fly ash (Lampris et al. 2011). However, solidified products obtained from the curing treatment become larger in volume than initial fly ash, and thus, this technology has been restricted due to increased pressure of landfill (Luo et al. 2019a).
Chemical stabilization reduces the environmental risk of toxic substances by adding agents to transform poisonous substances into forms with lower solubility or toxicity through chemical reactions. With high treatment efficiency and simple operation, chemical stabilization can effectively realize innocuous waste and stabilization of heavy metals with small volume increase after treatment. Thus, it shows excellent advantages and development prospects in engineering applications. The technology has been well used in the USA, Japan, and other countries (Eighmy et al. 1997). However, there are some shortcomings in conventional stabilization technologies to be solved, such as the cost of the agent input and the long-term stability cannot be balanced. In practical application, a low-cost stabilizer is often unable to achieve long-term stability of heavy metals in a wide range of pH, and agents with long-term stability are often expensive.
Thermal treatment technology
Thermal treatments are the methods that are carried out at high temperatures, which can solidify most heavy metals in hard sinter with decomposing the dioxins, furans and other organic pollutants in fly ash under high temperature (Lindberg et al. 2015; Wey et al. 2006). According to the treatment temperature, thermal treatments can be generally divided into sintering treatment (700 ~ 1100℃) (Wey et al. 2006; Zhang et al. 2007) and vitrification/melting treatment (1000 ~ 1500℃) (Abe et al. 1996; Quina et al. 2008). Thermal treatment of fly ash in incineration achieves detoxification and volume reduction due to its high temperature and reducing volume by about 2/3 (Zhang et al. 2011). The molten slag obtained by melting is a glass matrix composed of a Si–O network structure and heavy metals are effectively wrapped in this disordered network structure so that the leachability of heavy metals in the slag is extremely low. The obtained materials can be used as basic glass and asphalt aggregate to produce roadbed materials and glass–ceramics to achieve resource reuse (Andreola et al. 2008; Ferreira et al. 2003). Many prior studies have shown that thermal plasma is a promising technology for the vitrification of hazardous fly ash (Carnogurska et al. 2015). Initiation and maintenance of low ionization plasma are simple and easily controlled (Chen et al. 2009; Cheng et al. 2002; Zhao et al. 2010). Although it seems to be technically feasible, the secondary pollution caused by the volatile heavy metal elements in fly ash at high temperature has to be considered (Kuo et al. 2004; Li et al. 2014; Polettini et al. 2004). In addition, the unstable quality of vitrified products caused by the large difference in the properties of incineration fly ash further limits the popularization of this technology. However, this technology, with high energy consumption and high cost (Fedje et al. 2010), is mainly used in countries with very limited landfill space (such as Japan) (Bernardo et al. 2010).
Separation technology
Separation technology refers to the separation and extraction of heavy metals and salts from fly ash under the action of microorganisms or specific chemical agents to improve the quality of residue and make waste recyclable (Funari et al. 2017; Zacco et al. 2014). Chemical leaching removes heavy metals and soluble salts by adding water, acids, or other solvents as washing agents (Huang et al. 2011a; Wang et al. 2018, 2001a), which is usually as the washing pre-treatment of fly ash. The existing researches have shown that Sulfobacillus and Acidiphilium have been used as microorganisms to extract heavy metals (Dominguez-Benetton et al. 2018; Ishigaki et al. 2005; Karwowska et al. 2015), although less research has been done on fly ash. Although many heavy metals with toxicity can be extracted, recycled, and sequestered by way of separation (De Boom and Degrez 2015; Huang et al. 2011a), the process is complex, and the waste water generated needs follow-up treatment before discharge. Otherwise, secondary pollution will be produced (Wang et al. 2015a).
Other MSWI fly ash treatment technologies
Hydrothermal treatment technology is to synthesize silicoaluminate minerals under alkaline conditions in which the heavy metals are stabilized by using Al and Si sources from fly ash or added extra Al and Si sources, taking water as a medium under hydrothermal conditions (Bayuseno et al. 2009). In essence, hydrothermal treatment technology is a unique chemical stabilization technology that occurs under specific conditions. This process is often accompanied by the formation of zeolites, such as tobermorite (Luo et al. 2019b), which has a strong ability to adsorb heavy metals (Querol et al. 2002; Shoumkova and Stoyanova 2013). Although hydrothermal treatment does not require high-temperature conditions as thermal treatments, it still needs enough heat to support the reactions. Therefore, hydrothermal treatment is still accompanied by high energy consumption. For this reason, microwave technology has been applied to hydrothermal treatment to reduce energy consumption ( Qiu et al. 2016, 2017). Qiu et al. (2017) have studied that microwave-assisted hydrothermal treatment was performed to stabilize the heavy metals in MSWI fly ash, which confirmed the high efficiency of the microwave-assisted hydrothermal treatment. However, the efficiency of the microwave-assisted hydrothermal treatment is greatly affected by factors such as liquid–solid ratio, alkaline substance addition (Bayuseno et al. 2009), the content of Al, Si, and other elements (Shan et al. 2011). For this reason, the engineering practicality of this technology still needs to be tested.
Electrodialysis technology has been widely used in the treatment process of sea water desalination (Mohammadi and Kaviani 2003) and soil pollution (Ottosen et al. 1997) to realize the separation, concentration, and removal of related substances. However, as a new type of extraction method, it has been used for fly ash treatment research only in the past 20 years (Pedersen 2002). The principle of electrodialysis technology is to apply an electric field to the polluted material, undergoing four steps of acidification-desorption-transfer-removal to enrich and remove heavy metals distributed on mineral and non-mineral components (Kirkelund and Jensen 2018). The experimental model study of electrodialysis technology by Kristine et al. showed that the effect of electrodialysis treatment to remove heavy metals from pollutants is related to many factors, such as current density, remediation time, stirring rate, dry/wet sediment, cell setup as well as sediment properties (Pedersen et al. 2015).
Research progress of chemical stabilization technology
Although the heavy metal leaching rate of the thermal-treated fly ash product is lower than that after chemical stabilization treatment, the chemical stabilization treatment has unique advantages under the condition of lower control cost and small compatibilization. If the performance and structure of the reagents are improved, the long-term stability of the stabilized products can also be enhanced to avoid the secondary leaching of toxic materials. Therefore, the research and development of new environmentally friendly reagents with high efficiency and low price and the rational use of conventional agents have gradually become a hot spot in developing the stabilization technology of MSWI fly ash.
The precipitation-dissolution technology
The precipitation-dissolution technology is based on the precipitation-dissolution equilibrium theory, using specific agents to form precipitates with positively low solubility of heavy metals (Table 2), thereby achieving the stability of heavy metals (Lewis 2010). Inorganic agents are selected in this technology, including sulfides, soluble phosphates, ferrites, and other relatively inexpensive inorganic salts (Atanes et al. 2019; Eighmy et al. 1997; Liu et al. 2015; Lundtorp et al. 2002). The solubility of most metal sulfides is minimal. Therefore, adding sulfides to fly ash can transform heavy metals from a soluble state to an insoluble state. Thus, heavy metals exist in nature as mineral precipitate for a long time (Lewis 2010; Zhao et al. 2002). Sodium sulfide and thiourea, the commonly used sulfide agents, are considered to be one kind of excellent chemical agents for effective treatment of fly ash and have shown a more significant stabilizing effect on heavy metals than NaOH and ethylene diamine tetraacetic acid (EDTA) (Kim et al. 2002; Zacco et al. 2014; Zhao et al. 2002). The leaching concentration of fly ash can be reduced to less than 0.1 mg/L with the addition of 2% thiourea, while the limits of Pb in landfill pollution control standard for domestic waste is 0.25 mg/L (Ma et al. 2019a).
Compared with sulfides, phosphates have a better long-term stabilizing effect on those heavy metals which leaching amount generally exceeds the standard limit such as Cd, Pb, and Zn (Jiang et al. 2005; McGowen et al. 2001; Quina et al. 2014; Zhu et al. 2020). Especially Pb, in the pH range of 4 ~ 13, the leaching amount of Pb is very low after phosphate treatment (Jiang et al. 2005). Phosphates are also recommended to stabilize heavy metals in industrial wastewater and Pb contaminated soil (McGowen et al. 2001; Oliva et al. 2011; Wang et al. 2001b), which is known as the most promising development and in-depth study of the chemical species (Vavva et al. 2017; Zacco et al. 2014). Eighmy et al. (1997) found that soluble phosphate can react with particles at a nanometer level after mixing with fly ash for a certain time, which effectively reduces the leaching concentration of Cd2+, Cu2+, Pb2+, and Zn2+. Besides, they pointed out that it is helpful to optimize the stabilization effect of heavy metals with the sequence of promoting metal dissolution first, and then the metal precipitates with the phosphate. It is worth noting that the reaction mechanism of phosphate stabilizing heavy metals is not a simple chemical precipitation. Therefore, the mechanism needs to be further explored (Grubb et al. 2000).
Ferrite agents are mainly ferrous salts, such as FeSO4, which can be oxidized into iron oxide or hydrated iron oxide crystals under alkaline heating conditions. The oxidized products obtained by mixing the ferrite agent with fly ash and water bind the heavy metals firmly in the crystal lattice to reduce the leaching rate (Hu et al. 2015; Mizutani et al. 2000). Hu (2005) used a mixed solution of ferrous sulfate/iron sulfate to treat MSWI fly ash at room temperature. The results showed this mixed solution reacts with the ash on the surface of the dust particles forming a Ca4Fe9O17 coating which tightly stabilizes the heavy metals, and the heavy metal concentration meets the regulation limits of toxicity characteristic leaching procedure (TCLP) test.
Due to the simple principle and easy availability of materials, the precipitation-dissolution technology to stabilize heavy metals has been applied relatively early. The products obtained from heavy metals in MSWI fly ash treated with inorganic chemicals are highly affected by the environment and are easy to be leached in an acidic environment; thus, the stabilization effect is difficult to meet the environmental protection requirements of entering the domestic waste landfill (Zhang et al. 2016a). Therefore, with the improvement of the long-term stable safety requirements of hazardous waste and the emergence of more efficient agents (Jiang et al. 2004; Mizutani et al. 2000; Wang et al. 2015a), these inorganic agents have gradually become auxiliary agents in the stabilization of heavy metals, and the research on the combination of these inorganic agents with cement or organic chelating agents is increasing progressively (Quina et al. 2014; Zhu et al. 2020).
The complex precipitation technology
The complex precipitation technology refers to the technology based on the complexation mechanism, where certain organic agents, represented by organic chelator, coordinate with metal ions to form stable coordinate bonds (Table 2). Organic chelating agents involve coordination bonds formed by heavy metals and their own coordination atoms, containing lone pair electrons to generate stable complexes to achieve stabilization. In recent years, organic chelating agents have received more and more attention in terms of reducing heavy metal pollution due to their relatively low cost and tolerance in different environments. Compared with inorganic chemicals, organic reagents, particularly the organic polymer ones, have a more potent binding force with heavy metals and can form more stable insoluble substances (Zhang et al. 2020b). Heavy metal chelating agents (dithiocarbamate), synthesized by Jiang et al. (2004) in the laboratory, are useful heavy metal chelators for fly ash. Compared with inorganic chemicals Na2S and lime, dithiocarbamate has a more significant advantage in terms of stabilization, and its efficiency in capturing heavy metals is as high as 97%. Meanwhile, it is immune to microbial activities. Organic chelating agents mainly include dithiocarbamates and their derivatives (DTCs), mercaptopolyamines, EDTA polymers, and chitosan and its derivatives, etc. (Hu 2005; Ma et al. 2019a; Mizutani et al. 2000; Zhang et al. 2020b). In terms of stabilization, synthetic organic chelating agents are superior to the inorganic ones and the natural ones, and they are more adaptable to the environment (Ma et al. 2019a; Wang et al. 2015a; Zhang et al. 2016a).
In recent years, DTCs have been extensively studied because of their strong ability to coordinate with metals (Jiang et al. 1999; Wang et al. 2015a; Zheng et al. 2019). DTCs are synthesized by the nucleophilic reaction of organic polyamines and carbon disulfide. According to their solubility (Jiang et al. 2004; Zheng et al. 2019), organic chelating agents are mainly divided into two forms of utilization: chelating agent and chelating resin. Among them, DTCs can be divided into single-DTC chelating agents and multi-DTC linear chelating agents according to the number of DTC groups in the molecule (Wang et al. 2015a; Zhang et al. 2020b). Having more DTC groups, high-molecular-weight DTC thus owns more sites to react with heavy metals and chelated products with more complex structures will be formed that firmly embeds heavy metals therein to reduce the amount of leaching (Xu et al. 2013). For this reason, high-molecular-weight DTC is often used for the stable treatment of heavy metal pollutants. The comparative test between single-DTC chelating agents (sodium dimethyldithiocarbamate) and multi-DTC linear chelating agents (sixthio guanidine acid and tetrathio bicarbamic acid) on disposing of fly ash has shown that multi-DTC linear chelating agents can bind with heavy metals more effectively and present more excellent overall curing performance due to their multiple hydrosulfide groups (Wang et al. 2015a). Many studies have shown that DTC can steadily work within a broad range of pH, and in the entire pH range (3 ~ 11) the chelation effect of DTCs to most of the heavy metals keeps reliable and stable (Jiang et al. 2004; Mizutani et al. 2000; Zheng et al. 2019). In addition, some macromolecular DTC chelating agents can achieve better overall stability performance at low doses (3%) and prevent heavy metals from leaching in a wide pH range (2 ~ 13), which cannot be achieved by small molecule chelating agents (Zhang et al. 2020b).
Polymer DTC chelating resin is a water-insoluble chelating resin synthesized by grafting DTC groups on different polymer matrixes, which usually contain an amine group or connect an amine group after modification. Polymer chelating resins are mostly applied to the separation and recycling of heavy metals. Although there are many pieces of research on it (Liu et al. 2016; Ma et al. 2019a), chelating resins, due to their high cost, are seldom used to dispose of MSWI fly ash in practical application.
Moreover, the natural polymer DTC also has got a lot of attention. Based on natural polymers, these chelating agents are one kind of heavy metal trapping agents that introduce DTC groups through cross-linking, etherification, and polyamine substitution. Natural polymers commonly used mainly include cellulose, lignin, chitosan, and starch (Babel and Kurniawan 2003; Xiang et al. 2016). Cheng et al. (2013), using starch as raw material, synthesized DTC modified glycidyl methacrylate starch DMGS that can quickly and effectively complex metal ions. Some researchers created new types of DTC composite collectors through modifying sesbania gum and montmorillonite (Say et al. 2008; Zhang et al. 2008). The natural organic polymer materials are abundant, cheap, and easy to degrade that their modified products can capture the heavy metal ions with high efficiency and low toxicity. Exactly because of the benefits in economy and environment, the natural organic polymer materials may develop into a new research focus in the future.
Mercaptopolyamines are mainly organic substances containing sulfhydryl functional groups. Among them, thiourea can convert Pb and Zn into more stable substances, far better than Na2S in terms of stability. The stability of the functionalized product of sulfhydryl is better than that of single thiourea (Liu et al. 2016; Zhang et al. 2016a). Trithiocyanuric acid trisodium salt (TMT) is also a favorable stabilizer. For example, TMT-15 can effectively reduce the leaching concentration of heavy metals when a low dose (4.2%) is added (Zhu et al. 2020). Studies on dendrimer macromolecules have begun to increase because researchers want to achieve the goal of forming a complex structure to strengthen the binding with heavy metals and improve the resistance to acid and alkali. Using functional groups during the synthesis of dendrimers to possess certain functions has achieved the goal (Zhang et al. 2016a, 2020b). A team from Tongji University in China has developed a series of functionalized dendrimer chelator, such as TEPA-SNa (Zhang et al. 2016a) and TEM-CSSNa (Li et al. 2019a). Studies have revealed that these chelating agents have a strong chelating ability of heavy metals such as Cd and Pb in fly ash. The three-dimensional dendritic polymer, formed by the chelator, leads the heavy metals to strong stability in the harsh environment with strong acid and strong alkali (Zhang et al. 2020b). Some researchers also grafted polyamide dendrimers with chitosan, carbon nanotubes, and other types of compounds, and the obtained materials have shown good effects in combining with heavy metals such as Pb2+ (Al Hamouz et al. 2017; Hayati et al. 2017; Zarghami et al. 2016). Although organic chelating agents have outstanding advantages, like high compatibilization ratio, good stabilization effect, and has wide sources, there is a problem that the organic chelating agent buried underground itself may have adverse effects on the soil, groundwater, and surroundings, which need to be verified by experiments and time. Table 3 summarizes some typical studies on chemical stabilization of heavy metals in MSWI fly ash.
The adsorption stabilization technology
The adsorbents used to dispose of heavy metal include activated carbon, clay, montmorillonite, zeolite, and other natural modified or artificial materials (Babel and Kurniawan 2003; Liu et al. 2020; Tillman et al. 2005; Zhang and Pu 2011). Mineral materials enable to effectively adsorb heavy metals and organic pollutants for their small particles and large specific surface area, reducing the leaching rate of soluble heavy metals and reducing pollution to soil and water (Usman et al. 2005). Belviso et al. (2010) used synthetic zeolite to treat soil containing incineration fly ash, and the results showed that the zeolite played a good role in stabilizing Ni. In recent years, the research on apatite used to dispose of the heavy metals in fly ash has gradually increased (He et al. 2013). Materials containing apatite, such as animal bones, also have similar effects (Dybowska et al. 2009; Mu et al. 2018; Sneddon et al. 2006). Based on the phosphate mineral, apatite materials have a complicated reaction mechanism with heavy metals. In the existed literature, the mechanisms including ion-exchange, surface complexation, dissolution–precipitation, and co-precipitation have been proposed to act separately or jointly in the adsorption reaction (Fig. 2) (Corami et al. 2008; del Rio et al. 2004; Elouear et al. 2008; He et al. 2013; Oliva et al. 2011; Saxena and D'Souza 2006). In addition, there is also a view that the mechanisms may vary from metal to metal (Dybowska et al. 2009; Ndiba et al. 2008; Sneddon et al. 2006).
Compared with other stabilization technologies, most adsorption materials such as ores and clay are relatively inexpensive, and the stabilization effect is relatively satisfactory (Babel and Kurniawan 2003). Meanwhile, some adsorbents from waste materials are also actively explored, and adsorption stabilization technologies are popular in the field of pollutant stabilization (Mu et al. 2018; Saxena and D'Souza 2006). However, adsorbents take a longer time to work, not as fast as the chelating agent (Quina et al. 2014). Furthermore, the adsorbed substances are easily detached under extreme conditions and their stabilization effects are affected by the fluctuation of pH. Therefore, the adsorption stabilization method usually needs to be used to achieve the expected goal in conjunction with the stabilization technologies of other agents (Quina et al. 2014). Of course, developing new adsorbents is also an option. For example, Bai et al. (2011), grafting dithioamino polyethyleneimine onto modified silica, devised a new type of silicon-based DTC adsorbent used for heavy metal treatment.
New developments in chemical stabilization
A combination of chemical agents
The organic chelating agent is difficult to be widely used because of its high cost. Moreover, the chelation of heavy metals is selective, and the stabilization effects of different heavy metals vary to a certain extent, and thus, it is hard to guarantee the stabilization effect of all heavy metals; inorganic agents are greatly affected by the environment and have relatively poor long-term stability but with low costs. Therefore, a treatment method in which organic chelating agents and inorganic agents are combined is adopted. Adopting the agent that mixed appropriate inorganic agents and organic agents to treat heavy metals in MSWI fly ash can prolong stabilization time while achieving a better effect and sound product expansion ratio and economic benefits. Zhu et al. (2020) designed single and composite stabilization agents to deal with the waste incineration fly ash. The comparison of the treatment results shows that although the single agent with a certain concentration can make the heavy metals leached into the environment below the prescribed limit, the method of mixed agents is more effective and even cheaper than single-agent treatment. Most outstanding organic chelating agents with high prices are synthesized or patented in the laboratory (Zhang et al. 2016a, 2020b). Accordingly, the complementarity of different chemical agents that are common in the market can be used to achieve adequate stability of different metals, thereby reducing costs. The combination of two or more agents is worth trying.
Application of joint processing technology
The soluble salts contained in fly ash hinder the stabilization of heavy metals, especially large amounts of soluble chloride salts, which increase the solubility of some heavy metals such as Cd, Pb, and Zn in the processed product, thereby increasing the difficulty of processing (Chen et al. 2012; Mu et al. 2018; Wang et al. 2001a). Moreover, after fly ash treatment, resource utilization is mainly used as construction materials, and high levels of metal chlorides are very harmful to that (Zhu et al. 2018). The removal of chlorine from fly ash is very important. However, chemical stabilization technology is often specifically for heavy metals and has little effect on removing soluble substances in fly ash (Zacco et al. 2014). Therefore, it is necessary to perform pre-treatment operations before chemically treating, which is usually proceeded with the support of water washing or pickling (Chimenos et al. 2005; De Boom and Degrez 2015; Loginova et al. 2019; Vavva et al. 2017). The fly ash undergoes a combined treatment of two technologies: water washing and phosphoric acid stabilization, which can effectively stabilize the Pb in the fly ash while removing the salts in the ash and getting harmless landfill materials that meet the disposal standards (Vavva et al. 2017). Loginova et al. (2019) adopted a three-step combined treatment of water washing, stabilization by ethylenediaminetetraacetate and gluconate, which greatly improved the elution capacity of metals, among which the metal Cd in this aspect increased by 1800 times in comparison with plain water washing. Washing treatment is a pre-treatment step that can be involved in other stabilization technologies (Fig. 3), and many related attempts have worked well (Benassi et al. 2016; Liu et al. 2009; Wang et al. 2010; Zhu et al. 2018). However, the waste water after washing contains a large amount of soluble salts and some heavy metals, causing serious harm to the environment if it is directly discharged (McGowen et al. 2001). Therefore, the wastewater generated in this way must undergo subsequent treatment to reduce the content of soluble salts and heavy metals before it can be discharged or recycled (McGowen et al. 2001; Quina et al. 2009; Wang et al. 2001a). Studies have shown that changing the order of treatment can produce cleaner wastewater (Vavva et al. 2017).
The product of stabilizing fly ash with chemicals alone has a large surface area and lose structure, facilitating the leaching of heavy metals and harmful substances again (Ma et al. 2019a). For stronger fixation of heavy metals, the experiments of combining the stabilization process of the agent and the solidification technology were carried out (Hu et al. 2015; Ma et al. 2019a). The high physical strength and low porosity of cured or heat-treated product reduced the fluidity of pollutants (Batchelor 2006; Quina et al. 2014), before which the process of the chemical treatment has converted heavy metals into less toxic forms. Adding cement to the fly ash will produce C-S–H gel which can wrap the heavy metals in the cement-based solidification product to prevent the migration of heavy metals (Ma et al. 2019a). The process reduced the processing cost and prolonged the stable life cycle of the product, providing technical and economic supports for the industrial processing of fly ash. In addition, hydrothermal treatment of fly ash has been regarded as a promising approach for its significant advantages in economic, technical, and environmental performance (Ferreira et al. 2003; Hu et al. 2015; Sun et al. 2011). The combined use of multiple fly ash treatment technologies is a new development trend. Like the combined use of physical, chemical, and biological methods to treat contaminated soil and wastewater, the technologies are complemented with each other to achieve optimal economic and environmental benefits.
Effective use of waste
With the continuous promotion of sustainable development concept worldwide, people’s views on waste have changed, and their consciousness of “turning waste into treasure” has been strengthened (Baek et al. 2017). Waste from production and daily life also plays a role in the management and disposal of fly ash. The waste generated by MSWI includes not only fly ash but also bottom ash. Assi et al. (2020) attempted to treat fly ash with bottom ash and mixed flue gas desulfurization residues and fly ash to produce a material with pozzolana characteristics, which would provide a new way to achieve zero waste in MSWI industries. However, this process has the problems of elevated metal contents and leaching toxicity in themselves (Poykio et al. 2016; Saqib and Backstrom 2015; Zhu et al. 2020), which seems to be common problems of processing waste materials. The University of Brescia developed a new sustainable method named COSMOS for heavy metal stabilization based on the use of colloidal silica, producing a colloidal silica medium eventually (Bontempi et al. 2010). Their recent research utilized waste and by-product materials (such as coal fly ash, flue gas desulfurization residues, and rice husk ash) to replace colloidal silica, which also traps heavy metals into the silica network successfully (Benassi et al. 2015; Bosio et al. 2013, 2014), and this technology had been verified by engineering (Benassi et al. 2016). However, problems also exist in this technology (Dou et al. 2017; Schnell et al. 2020). Other wastes have been applied in the field of fly ash stabilization as well, such as red mud (Li et al. 2019c), electrolytic manganese residues (Zhan et al. 2018), and fishbone waste (Mu et al. 2018) (Li et al. 2014; Xu et al. 2018), although their principles are different. There are also studies using waste liquids (Sun et al. 2019; Tian et al. 2020). For example, Tian et al. (2020) investigated the stabilization effect of MSWI fly ash by using spent caustic as alkaline activators and adding blast furnace slag. Moreover, co-disposal of several kinds of wastes, while trying to get usable products as much as possible, seems to be a trend nowadays (Geng et al. 2020; Luo et al. 2020; Yue et al. 2019; Zhan et al. 2021; Zhao et al. 2019, 2020). Zhan et al. (2021) successfully co-sintered MSWI fly ash, water-washed fly ash, coal fly ash, and electrolytic manganese residue to produce lightweight MFCE ceramisite, which provided a feasibility method for the development of urban sources while producing green materials. Significantly, the compositions of waste and fly ash vary from sources and treatment methods. Therefore, there are some uncertainties in the promotion and application of using (Zhu et al. 2020). However, it is undeniable that waste resource recovery from fly ash treatment is distinctly a very rewarding and sustainable idea.
As an available resource, fly ash itself is also used in roads, construction, industry, and other fields. The most common one is to use fly ash as one of the raw materials for cement production (Aubert et al. 2006; Guo et al. 2014). Some researchers have used the characteristics of heavy metals in fly ash to be volatile during high-temperature treatment to conduct high-temperature thermal separation of heavy metals such as high content of Cd, Cu, Pb, and Zn in fly ash (Jakob et al. 1996; Syc et al. 2020). The fly ash processed by high-temperature thermal separation technology can be used as ordinary waste landfill or construction raw materials. The secondary fly ash formed by the condensation of heavy metal volatiles with the flue gas has a high heavy metal content, which is equivalent to a special heavy metal–rich resource that can be used as raw material for metallurgy (Geng et al. 2020). For the recycling of fly ash, except for the existing standard toxicity leaching test, it is also necessary to evaluate its environmental compatibility through a long-term leaching test to confirm that the product will not cause secondary pollution to the environment in the future. The ultimate goal of MSW management is to build and perfect the integrated management systems on the basis of optimizing and utilizing available technologies (He et al. 2019), and it is a key link in sustainable development.
Influencing factors of chemical stabilization technology
Effects of ash properties
The key to stabilization is to reduce the leaching of hazardous components from the waste. Then, the leaching behavior of MSWI fly ash can be influenced by ash properties such as the soluble salt content, particle size distribution, and chemical speciation (Luo et al. 2019a; Saqib and Backstrom 2015). The fly ash particles are easily filled by heavy metals, increasing toxicity due to the small ash particle size (Mangialardi 2003). Besides, the fly ash after flue gas purification is often highly alkaline, but the landfill is generally neutral or acidic, which leads to heavy metals leaching out easily during the disposal process (Cornelis et al. 2008; Saqib and Backstrom 2015). The remaining alkaline substances (mainly calcium-bearing compounds) are the important factor of metal leaching behavior in fly ash. Anions play a major role in soluble substances (Tong et al. 2019). Anions promote or inhibit the leaching of heavy metals by forming corresponding salts with heavy metals. For example, SO42− stabilizes most of the heavy metals by forming sulfate phases and inhibits leaching and consequently contributes to the stabilization effect (Verhulst et al. 1996). On the contrary, the existence of Cl− is not conducive to the stabilization of heavy metals, which has been widely studied in the current fly ash disposal technologies (Zhang et al. 2016a). Some researchers have adopted water washing pre-treatment in the treatment program to reduce the negative effects of soluble chloride salts on the stability effect (Atanes et al. 2019; Chimenos et al. 2005; Loginova et al. 2019; Wey et al. 2006).
The treating result varied among metals in fly ash. For example, the leaching of some heavy metals such as Ba, Pb, Sb, V, and Zn is mainly affected by the reaction system. Under the high liquid–solid ratio (L/S ratio), the release of target elements will increase with the dissolution of the mineral phase, leading to a higher cumulative release (Allegrini et al. 2015). In contrast, for As, Cd, Cu, Ni, and Se, the leaching procedure seems to have a greater influence (Luo et al. 2019a). Therefore, the properties of the residue itself are critical to selecting proper management and disposal strategies for MSWI fly ash.
Effects of agents
The stability ability of different agents to metal elements varies greatly due to their mechanism of action and differences in molecular weight (Ma et al. 2019a; Wang et al. 2015a). Combination products of agents and heavy metals based on the precipitation-dissolution principle or the adsorption principle are greatly affected by environmental changes. If the metal and the agents are combined in a complexation mechanism, they will have stronger environmental resistance. There are differences in the molecular weight of organic chelating agents, and macromolecular organic chelation agents perform better due to a large number of coordinating groups. Some macromolecular organic chelating agents can form two-dimensional or even three-dimensional structures (Li et al. 2019a; Zhang et al. 2016a), which firmly bind heavy metals. Besides, functional groups will affect the selectivity of the agent to heavy metals. Research on chemical stabilization has focused on organic chelation agents with N, P, and S as coordinating atoms, and the corresponding groups include amine, sulfhydryl, and dithiocarboxylate acid groups (Ahmad et al. 2020). It has induced a research trend: the integration of multiple groups into one chelation agent.
Any kind of agents has an optimal dosage, which is the concentration effect. In general, the stability of heavy metals in fly ash after reaction increases with the increase in the amount of chemical added. Still, when the additive chemical exceeds a certain amount, the leaching concentration of heavy metals in fly ash is almost in a constant state after treatment (Assi et al. 2020; Jiang et al. 1999; Ma et al. 2019a; Xu et al. 2013; Zhang et al. 2020b). In this process, there is an optimal additive amount, that is, the minimum additive amount to achieve this state. Space for landfill construction and operation is so scarce that the volume increase ratio must be taken into account (Yakubu et al. 2018). Therefore, the chemical agents in a small additional amount as well as effective are more advantageous and more popular in the market.
The influence of the leaching system
Disposed fly ash is in a non-static environment. Thus, as landfill time grows, waste that is initially classified as non-hazardous may turn hazardous again due to long-term interactions with the surrounding environment. When fly ash is disposed of at the landfill, the heavy metals may leach out again depending on the change of L/S ratio, temperature, pH, and other factors in the environmental system (Luo et al. 2019a). Among the various factors that affect the leaching behavior of heavy metals, the pH of the leaching solution has the most direct and greatest influences (Li et al. 2019b; Quina et al. 2009). Changing field conditions caused by temperature, soil type, rain (especially acid rain), the activity of soil living organisms, and vegetation may result in pH fluctuations which might favor an increase in the levels of heavy metals in a particular waste (Yakubu et al. 2018). Relevant studies have shown that the leached concentrations of the majority of the elements from fly ash increased with the decrease of pH, which means that it is easier to leach large amounts of heavy metals under acidic conditions (Jiang et al. 1999; Zhang et al. 2016a, 2020b), while Pb, Zn, and Cr may also be leached under strong alkali conditions due to their amphoteric nature (Quina et al. 2009; Yakubu et al. 2018). The pH of the leaching solution and the adsorption process are the main factors affecting the leaching (Zhang et al. 2016b). Therefore, the width of pH at which metal substances can be effectively stabilized is an important indicator for evaluating whether a chemical agent is practical and feasible. Although the pH is the most critical variable in the leaching system, the liquid–solid ratio (L/S ratio) also is an innegligible factor in leaching processes (Quina et al. 2011) because leaching of inorganic constituents from MSWI ash such as heavy metals is controlled by solubility on the whole (Luo et al. 2019a). A higher L/S ratio promotes the dissolution of minerals and accelerates the release of heavy metals (Quina et al. 2011), although this is not the case for all metals.
Many studies have investigated the leaching behavior of heavy metals from residual materials (Allegrini et al. 2015; Jiang et al. 2007; Jiao et al. 2016; Quina et al. 2009; Saqib and Backstrom 2015; Zhang et al. 2016b). Luo et al. (2019a) reviewed the factors affecting the leaching behavior of pollutants in fly ash, including weathering and aging treatments and biological activity, in addition to those as mentioned earlier. There has been a lot of researches into different factors, but in reality, they always work together and are often complex and changeable. Waste managers should put the processed fly ash, which may become hazardous waste, in a stable environment as much as possible to increase their life stability and reduce the environmental risks they may bring.
Conclusion
Faced with the ever-increasing world population and limited available land area, adequate disposal of the generated municipal solid waste without pollution is an urgent problem facing the world. Incineration is widely adopted in urban waste management because it provides an efficient path to compress the volume of waste needed to be dumped in landfills and to realize resource recycling at the same time. However, this has resulted in a rapid increase in the amount of MSWI fly ash. Among the treatments of fly ash, solidification technology and thermal treatment technology have obvious defects and are not suitable for the future urban development direction. Hydrothermal treatment technology has a high potential for promotion due to its energy-saving, high efficiency, and vital applicability, but it will take some time to apply mature technology to practical projects. By contrast, the development of new chemical agents environmentally friendly with high efficiency and low price to stabilize heavy metals in fly ash is an easier control way. For better stable performance, the development of high-efficiency and multifunctional stabilizing agents is required. Besides, compound agents may have a broad market prospect due to their good prices. Reasonable addition of washing pre-treatment steps that can improve the quality of waste ashes is beneficial to removing harmful substances in fly ash. Designing an effective chemical stabilization process and quantifying the relationship between heavy metal pollutants and stabilizing agents will enhance the universal applicability of chemical stabilization technology in different regions. Besides, municipal solid waste classification should be advocated, and necessary pre-treatment should be implemented, to realize the harmless and resource-based disposal of municipal solid waste and promote sustainable development.
Data Availability
Some data that support the findings of this study are available from the Statistical Yearbook of the Chinese Ministry of Housing and Urban–Rural Development. Other data presented in this paper are from references.
References
Abe S, Kambayashi F, Okada M (1996) ASH melting treatment by rotating type surface melting furnace. Waste Manage 16:431–443. https://doi.org/10.1016/s0956-053x(96)00102-x
Ahmad SZN, Salleh WNW, Ismail AF, Yusof N, Yusop MZM, Aziz F (2020) Adsorptive removal of heavy metal ions using graphene-based nanomaterials: toxicity, roles of functional groups and mechanisms. Chemosphere 248. https://doi.org/10.1016/j.chemosphere.2020.126008
Al Hamouz OCS, Adelabu IO, Saleh TA (2017) Novel cross-linked melamine based polyamine/CNT composites for lead ions removal. J Environ Manage 192:163–170. https://doi.org/10.1016/j.jenvman.2017.01.056
Allegrini E, Butera S, Kosson DS, Van Zomeren A, Van der Sloot HA, Astrup TF (2015) Life cycle assessment and residue leaching: the importance of parameter, scenario and leaching data selection. Waste Manage 38:474–485. https://doi.org/10.1016/j.wasman.2014.12.018
Andreola F, Barbieri L, Hreglich S, Lancellotti I, Morselli L, Passarini F, Vassura I (2008) Reuse of incinerator bottom and fly ashes to obtain glassy materials. J Hazard Mater 153:1270–1274. https://doi.org/10.1016/j.jhazmat.2007.09.103
Assi A, Bilo F, Zanoletti A, Ponti J, Valsesia A, La Spina R, Zacco A, Bontempi E (2020) Zero-waste approach in municipal solid waste incineration: reuse of bottom ash to stabilize fly ash. J Clean Prod 245.https://doi.org/10.1016/j.jclepro.2019.118779
Atanes E, Cuesta-Garcia B, Nieto-Marquez A, Fernandez-Martinez F (2019) A mixed separation-immobilization method for soluble salts removal and stabilization of heavy metals in municipal solid waste incineration fly ash. J Environ Manage 240:359–367. https://doi.org/10.1016/j.jenvman.2019.03.122
Aubert JE, Husson B, Sarramone N (2006) Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement. Part 1: Processing and characterization of MSWI fly ash. J Hazard Mater 136:624–631. https://doi.org/10.1016/j.jhazmat.2005.12.041
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243. https://doi.org/10.1016/s0304-3894(02)00263-7
Baek JW, Choi AES, Park HS (2017) Solidification/stabilization of ASR fly ash using Thiomer material: optimization of compressive strength and heavy metals leaching. Waste Manage 70:139–148. https://doi.org/10.1016/j.wasman.2017.09.010
Bai L, Hu H, Fu W, Wan J, Cheng X, Zhuge L, Xiong L, Chen Q (2011) Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J Hazard Mater 195:261–275. https://doi.org/10.1016/j.jhazmat.2011.08.038
Batchelor B (2006) Overview of waste stabilization with cement. Waste Manage 26:689–698. https://doi.org/10.1016/j.wasman.2006.01.020
Bayuseno AP, Schmahl WW, Muellejans T (2009) Hydrothermal processing of MSWI Fly Ash-towards new stable minerals and fixation of heavy metals. J Hazard Mater 167:250–259. https://doi.org/10.1016/j.jhazmat.2008.12.119
Belviso C, Cavalcante F, Ragone P, Fiore S (2010) Immobilization of Ni by synthesising zeolite at low temperatures in a polluted soil. Chemosphere 78:1172–1176. https://doi.org/10.1016/j.chemosphere.2009.12.046
Benassi L, Bosio A, Dalipi R, Borgese L, Rodella N, Pasquali M, Depero LE, Bergese P, Bontempi E (2015) Comparison between rice husk ash grown in different regions for stabilizing fly ash from a solid waste incinerator. J Environ Manage 159:128–134. https://doi.org/10.1016/j.jenvman.2015.05.015
Benassi L, Pasquali M, Zanoletti A, Dalipi R, Borgese L, Depero LE, Vassura I, Quina MJ, Bontempi E (2016) Chemical stabilization of municipal solid waste incineration fly ash without any commercial chemicals: first pilot-plant scaling up. Acs Sustain Chem Eng 4:5561–5569. https://doi.org/10.1021/acssuschemeng.6b01294
Bernardo E, Bonomo E, Dattoli A (2010) Optimisation of sintered glass-ceramics from an industrial waste glass. Ceram Int 36:1675–1680. https://doi.org/10.1016/j.ceramint.2010.02.047
Bie R, Chen P, Song X, Ji X (2016) Characteristics of municipal solid waste incineration fly ash with cement solidification treatment. J Energy Inst 89:704–712. https://doi.org/10.1016/j.joei.2015.04.006
Bontempi E, Zacco A, Borgese L, Gianoncelli A, Ardesi R, Depero LE (2010) A new method for municipal solid waste incinerator (MSWI) fly ash inertization, based on colloidal silica. J Environ Monit 12:2093–2099. https://doi.org/10.1039/c0em00168f
Bosio A, Rodella N, Gianoncelli A, Zacco A, Borgese L, Depero LE, Bingham PA, Bontempi E (2013) A new method to inertize incinerator toxic fly ash with silica from rice husk ash. Environ Chem Lett 11:329–333. https://doi.org/10.1007/s10311-013-0411-9
Bosio A, Zacco A, Borgese L, Rodella N, Colombi P, Benassi L, Depero LE, Bontempi E (2014) A sustainable technology for Pb and Zn stabilization based on the use of only waste materials: a green chemistry approach to avoid chemicals and promote CO2 sequestration. Chem Eng J 253:377–384. https://doi.org/10.1016/j.cej.2014.04.080
Carnogurska M, Lazar M, Puskar M, Lengyelova M, Vaclav J, Sirillova L (2015) Measurement and evaluation of properties of MSW fly ash treated by plasma. Measurement 62:155–161. https://doi.org/10.1016/j.measurement.2014.11.014
Chen M, Meng Y, Shi J, Ja K, Ni G, Liu W, Jiang Y (2009) DC Arc plasma furnace melting of waste incinerator fly ash. Plasma Sci Technol 11:62–65
Chen W-S, Chang F-C, Shen Y-H, Tsai M-S, Ko C-H (2012) Removal of chloride from MSWI fly ash. J Hazard Mater 237:116–120. https://doi.org/10.1016/j.jhazmat.2012.08.010
Chen WM, Wang F, Li Z, Li QB (2020) A comprehensive evaluation of the treatment of lead in MSWI fly ash by the combined cement solidification and phosphate stabilization process. Waste Manage 114:107–114. https://doi.org/10.1016/j.wasman.2020.06.041
Cheng H, Hu Y (2010) Curbing dioxin emissions from municipal solid waste incineration in China: Re-thinking about management policies and practices. Environ Pollut 158:2809–2814. https://doi.org/10.1016/j.envpol.2010.06.014
Cheng TW, Chu JP, Tzeng CC, Chen YS (2002) Treatment and recycling of incinerated ash using thermal plasma technology. Waste Manage 22:485–490. https://doi.org/10.1016/s0956-053x(01)00043-5
Cheng X, Cheng R, Ou S, Li Y (2013) Synthesis and adsorption performance of dithiocarbamate-modified glycidyl methacrylate starch. Carbohyd Polym 96:320–325. https://doi.org/10.1016/j.carbpol.2013.04.001
Chimenos JM, Fernandez AI, Cervantes A, Miralles L, Fernandez MA, Espiell F (2005) Optimizing the APC residue washing process to minimize the release of chloride and heavy metals. Waste Manage 25:686–693. https://doi.org/10.1016/j.wasman.2004.12.014
Corami A, Mignardi S, Ferrini V (2008) Cadmium removal from single- and multi-metal (Cd plus Pb plus Zn plus Cu) solutions by sorption on hydroxyapatite. J Colloid Interface Sci 317:402–408. https://doi.org/10.1016/j.jcis.2007.09.075
Cornelis G, Johnson CA, Van Gerven T, Vandecasteele C (2008) Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: a review. Appl Geochem 23:955–976. https://doi.org/10.1016/j.apgeochem.2008.02.001
De Boom A, Degrez M (2015) Combining sieving and washing, a way to treat MSWI boiler fly ash. Waste Manage 39:179–188. https://doi.org/10.1016/j.wasman.2015.01.040
del Rio JAG, Morando PJ, Cicerone DS (2004) Natural materials for treatment of industrial effluents: comparative study of the retention of Cd, Zn and Co by calcite and hydroxyapatite. Part 1: batch experiments. J Environ Manage 71:169–177. https://doi.org/10.1016/j.jenvman.2004.02.004
Demirbas A (2011) Waste management, waste resource facilities and waste conversion processes. Energy Convers Manage 52:1280–1287. https://doi.org/10.1016/j.enconman.2010.09.025
Dominguez-Benetton X, Varia JC, Pozo G, Modin O, Ter Heijne A, Fransaer J, Rabaey K (2018) Metal recovery by microbial electro-metallurgy. Prog Mater Sci 94:435–461. https://doi.org/10.1016/j.pmatsci.2018.01.007
Dou X, Ren F, Minh Quan N, Ahamed A, Yin K, Chan WP, Chang VW-C (2017) Review of MSWI bottom ash utilization from perspectives of collective characterization, treatment and existing application. Renew Sustain Energy Rev 79:24–38. https://doi.org/10.1016/j.rser.2017.05.044
Du Y-J, Wei M-L, Reddy KR, Jin F, Wu H-L, Liu Z-B (2014) New phosphate-based binder for stabilization of soils contaminated with heavy metals: leaching, strength and microstructure characterization. J Environ Manage 146:179–188. https://doi.org/10.1016/j.jenvman.2014.07.035
Dybowska A, Manning DAC, Collins MJ, Wess T, Woodgate S, Valsami-Jones E (2009) An evaluation of the reactivity of synthetic and natural apatites in the presence of aqueous metals. Sci Total Environ 407:2953–2965. https://doi.org/10.1016/j.scitotenv.2008.12.053
Eighmy TT, Crannell BS, Butler LG, Cartledge FK, Emery EF, Oblas D, Krzanowski JE, Eusden JD, Shaw EL, Francis CA (1997) Heavy metal stabilization in municipal solid waste combustion dry scrubber residue using soluble phosphate. Environ Sci Technol 31:3330–3338. https://doi.org/10.1021/es970407c
Elouear Z, Bouzid J, Boujelben N, Feki M, Jamoussi F, Montiel A (2008) Heavy metal removal from aqueous solutions by activated phosphate rock. J Hazard Mater 156:412–420. https://doi.org/10.1016/j.jhazmat.2007.12.036
Eriksson O, Finnveden G, Ekvall T, Bjorklund A (2007) Life cycle assessment of fuels for district heating: a comparison of waste incineration, biomass- and natural gas combustion. Energy Policy 35:1346–1362. https://doi.org/10.1016/j.enpol.2006.04.005
Fedje KK, Ekberg C, Skarnemark G, Steenari B-M (2010) Removal of hazardous metals from MSW fly ash-an evaluation of ash leaching methods. J Hazard Mater 173:310–317. https://doi.org/10.1016/j.jhazmat.2009.08.094
Ferreira C, Ribeiro A, Ottosen L (2003) Possible applications for municipal solid waste fly ash. J Hazard Mater 96:201–216. https://doi.org/10.1016/s0304-3894(02)00201-7
Funari V, Makinen J, Salminen J, Braga R, Dinelli E, Revitzer H (2017) Metal removal from Municipal Solid Waste Incineration fly ash: a comparison between chemical leaching and bioleaching. Waste Manage 60:397–406. https://doi.org/10.1016/j.wasman.2016.07.025
Geng C, Liu JG, Wu SC, Jia YF, Du B, Yu SY (2020) Novel method for comprehensive utilization of MSWI fly ash through co-reduction with red mud to prepare crude alloy and cleaned slag. J Hazard Mater 384.https://doi.org/10.1016/j.jhazmat.2019.121315
Grubb DG, Guimaraes MS, Valencia R (2000) Phosphate immobilization using an acidic type F fly ash. J Hazard Mater 76:217–236. https://doi.org/10.1016/s0304-3894(00)00200-4
Guo X, Shi H, Hu W, Wu K (2014) Durability and microstructure of CSA cement-based materials from MSWI fly ash. Cement Concr Compos 46:26–31. https://doi.org/10.1016/j.cemconcomp.2013.10.015
Hayati B, Maleki A, Najafi F, Daraei H, Gharibi F, McKay G (2017) Super high removal capacities of heavy metals (Pb2+ and Cu2+) using CNT dendrimer. J Hazard Mater 336:146–157. https://doi.org/10.1016/j.jhazmat.2017.02.059
He L, Zhong H, Liu G, Dai Z, Brookes PC, Xu J (2019) Remediation of heavy metal contaminated soils by biochar: mechanisms, potential risks and applications in China. Environ Pollut 252:846–855. https://doi.org/10.1016/j.envpol.2019.05.151
He M, Shi H, Zhao X, Yu Y, Qu B (2013) Immobilization of Pb and Cd in contaminated soil using nano-crystallite hydroxyapatite. In: Quan X (ed) 2013 Int Symp Environ Sci Technol. pp 657–665
Hu SH (2005) Stabilization of heavy metals in municipal solid waste incineration ash using mixed ferrous/ferric sulfate solution. J Hazard Mater 123:158–164. https://doi.org/10.1016/j.jhazmat.2005.03.044
Hu Y, Zhang P, Li J, Chen D (2015) Stabilization and separation of heavy metals in incineration fly ash during the hydrothermal treatment process. J Hazard Mater 299:149–157. https://doi.org/10.1016/j.jhazmat.2015.06.002
Huang K, Inoue K, Harada H, Kawakita H, Ohto K (2011a) Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants. Trans Nonferrous Met Soc Chin 21:1422–1427. https://doi.org/10.1016/s1003-6326(11)60876-5
Huang K, Inoue K, Harada H, Kawakita H, Ohto K (2011b) Leaching of heavy metals by citric acid from fly ash generated in municipal waste incineration plants. J Mater Cycles Waste Manage 13:118–126. https://doi.org/10.1007/s10163-011-0001-5
Huber F, Laner D, Fellner J (2018) Comparative life cycle assessment of MSWI fly ash treatment and disposal. Waste Manage 73:392–403. https://doi.org/10.1016/j.wasman.2017.06.004
Ishigaki T, Nakanishi A, Tateda M, Ike M, Fujita M (2005) Bioleaching of metal from municipal waste incineration fly ash using a mixed culture of sulfur-oxidizing and iron-oxidizing bacteria. Chemosphere 60:1087–1094. https://doi.org/10.1016/j.chemosphere.2004.12.060
Jakob A, Stucki S, Struis R (1996) Complete heavy metal removal from fly ash by heat treatment: influence of chlorides an evaporation rates. Environ Sci Technol 30:3275–3283. https://doi.org/10.1021/es960059z
Jiang J-g, Xu X, Wang J, Yang S-j, Zhang Y (2007) Investigation of basic properties of fly ash from urban waste incinerators in China. J Environ Sci 19:458–463. https://doi.org/10.1016/s1001-0742(07)60076-x
Jiang J-g, Zhang Y, Xu X, Wang J, Deng Z, Zhao Z-z (2005) Heavy metal stabilization in municipal solid waste incineration fly ash using soluble phosphate. Huanjing Kexue 26:191–194
Jiang J, Wang W, Li G, Na C, Zeng X, Zhao X (1999) Experimental study on the chemical stabilization technology in treating with fly ash using heavy metal chelating agent. Huanjing Kexue 20:13–17
Jiang JG, Wang J, Xu X, Wang W, Deng Z, Zhang Y (2004) Heavy metal stabilization in municipal solid waste incineration flyash using heavy metal chelating agents. J Hazard Mater 113:141–146. https://doi.org/10.1016/j.jhazmat.2004.05.030
Jiao F, Zhang L, Dong Z, Namioka T, Yamada N, Ninomiya Y (2016) Study on the species of heavy metals in MSW incineration fly ash and their leaching behavior. Fuel Process Technol 152:108–115. https://doi.org/10.1016/j.fuproc.2016.06.013
Karwowska E, Wojtkowska M, Andrzejewska D (2015) The influence of metal speciation in combustion waste on the efficiency of Cu, Pb, Zn, Cd, Ni and Cr bioleaching in a mixed culture of sulfur-oxidizing and biosurfactant-producing bacteria. J Hazard Mater 299:35–41. https://doi.org/10.1016/j.jhazmat.2015.06.006
Kim BR, Gaines WA, Szafranski MJ, Bernath EF, Miles AM (2002) Removal of heavy metals from automotive wastewater by sulfide precipitation. J Environ Eng 128:612–623. https://doi.org/10.1061/(asce)0733-9372(2002)128:7(612)
Kirby CS, Rimstidt JD (1993) Mineralogy and surface-properties of municipal solid-waste ash. Environ Sci Technol 27:652–660. https://doi.org/10.1021/es00041a008
Kirkelund GM, Jensen PE (2018) Electrodialytic treatment of Greenlandic municipal solid waste incineration fly ash. Waste Manage 80:241–251. https://doi.org/10.1016/j.wasman.2018.09.019
Kuo YM, Lin TC, Tsai PJ (2004) Metal behavior during vitrification of incinerator ash in a coke bed furnace. J Hazard Mater 109:79–84. https://doi.org/10.1016/j.jhazmat.2004.02.053
Lam CHK, Ip AWM, Barford JP, McKay G (2010) Use of Incineration MSW Ash: a Review. Sustainability 2:1943–1968. https://doi.org/10.3390/su2071943
Lampris C, Stegemann JA, Pellizon-Birelli M, Fowler GD, Cheeseman CR (2011) Metal leaching from monolithic stabilised/solidified air pollution control residues. J Hazard Mater 185:1115–1123. https://doi.org/10.1016/j.jhazmat.2010.10.021
Lewis AE (2010) Review of metal sulphide precipitation. Hydrometallurgy 104:222–234. https://doi.org/10.1016/j.hydromet.2010.06.010
Li M, Xiang J, Hu S, Sun LS, Su S, Li PS, Sun XX (2004) Characterization of solid residues from municipal solid waste incinerator. Fuel 83:1397–1405. https://doi.org/10.1016/j.fuel.2004.01.005
Li R, Zhang B, Wang Y, Zhao Y, Li F (2019a) Leaching potential of stabilized fly ash from the incineration of municipal solid waste with a new polymer. J Environ Manage 232:286–294. https://doi.org/10.1016/j.jenvman.2018.11.036
Li W, Sun Y, Huang Y, Shimaoka T, Wang H, Wang Y-n, Ma L, Zhang D (2019b) Evaluation of chemical speciation and environmental risk levels of heavy metals during varied acid corrosion conditions for raw and solidified/stabilized MSWI fly ash. Waste Manage 87:407–416. https://doi.org/10.1016/j.wasman.2019.02.033
Li X, Chen Q, Zhou Y, Tyrer M, Yu Y (2014) Stabilization of heavy metals in MSWI fly ash using silica fume. Waste Manage 34:2494–2504. https://doi.org/10.1016/j.wasman.2014.08.027
Li Y, Min X, Ke Y, Liu D, Tang C (2019c) Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manage 83:202–208. https://doi.org/10.1016/j.wasman.2018.11.019
Lin H, Ma X (2012) Simulation of co-incineration of sewage sludge with municipal solid waste in a grate furnace incinerator. Waste Manage 32:561–567. https://doi.org/10.1016/j.wasman.2011.10.032
Lindberg D, Molin C, Hupa M (2015) Thermal treatment of solid residues from WtE units: a review. Waste Manage 37:82–94. https://doi.org/10.1016/j.wasman.2014.12.009
Liu S-J, Guo Y-P, Yang H-Y, Wang S, Ding H, Qi Y (2016) Synthesis of a water-soluble thiourea-formaldehyde (WTF) resin and its application to immobilize the heavy metal in MSWI fly ash. J Environ Manage 182:328–334. https://doi.org/10.1016/j.jenvman.2016.07.086
Liu W, Wei D, Mi J, Shen Y, Cui B, Han C (2015) Immobilization of Cu(II) and Zn(II) in simulated polluted soil using sulfurizing agent. Chem Eng J 277:312–317. https://doi.org/10.1016/j.cej.2015.04.106
Liu Y, Liu F, Ding N, Hu X, Shen C, Li F, Huang M, Wang Z, Sand W, Wang C-C (2020) Recent advances on electroactive CNT-based membranes for environmental applications: The perfect match of electrochemistry and membrane separation. Chin Chem Lett 31:2539–2548. https://doi.org/10.1016/j.cclet.2020.03.011
Liu Y, Zheng L, Li X, Xie S (2009) SEM/EDS and XRD characterization of raw and washed MSWI fly ash sintered at different temperatures. J Hazard Mater 162:161–173. https://doi.org/10.1016/j.jhazmat.2008.05.029
Loginova E, Proskurnin M, Brouwers HJH (2019) Municipal solid waste incineration (MSWI) fly ash composition analysis: a case study of combined chelatant-based washing treatment efficiency. J Environ Manage 235:480–488. https://doi.org/10.1016/j.jenvman.2019.01.096
Long L, Jiang XG, Lv GJ, Chen Q, Liu XB, Chi Y, Yan JH, Zhao XL, Kong LT, Qiu QL (2022) Comparison of MSWI fly ash from grate-type and circulating fluidized bed incinerators under landfill leachate corrosion scenarios: the long-term leaching behavior and speciation of heavy metals. Environ Sci Pollut Res 29:15057–15067. https://doi.org/10.1007/s11356-021-16618-z
Lu J-W, Zhang S, Hai J, Lei M (2017) Status and perspectives of municipal solid waste incineration in China: a comparison with developed regions. Waste Manage 69:170–186. https://doi.org/10.1016/j.wasman.2017.04.014
Luna Galiano Y, Fernandez Pereira C, Vale J (2011) Stabilization/solidification of a municipal solid waste incineration residue using fly ash-based geopolymers. J Hazard Mater 185:373–381. https://doi.org/10.1016/j.jhazmat.2010.08.127
Lundtorp K, Jensen DL, Sorensen MA, Christensen TH, Mogensen EPB (2002) Treatment of waste incinerator air-pollution-control residues with FeSO4: Concept and product characterisation. Waste Manage Res 20:69–79. https://doi.org/10.1177/0734242x0202000108
Luo H, Cheng Y, He D, Yang E-H (2019a) Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci Total Environ 668:90–103. https://doi.org/10.1016/j.scitotenv.2019.03.004
Luo H, He D, Zhu W, Wu Y, Chen Z, Yang E-H (2019b) Humic acid-induced formation of tobermorite upon hydrothermal treatment with municipal solid waste incineration bottom ash and its application for efficient removal of Cu(II) ions. Waste Manage 84:83–90. https://doi.org/10.1016/j.wasman.2018.11.037
Luo ZT, Chen LG, Zhang MX, Liu L, Zhao J, Mu YD (2020) Analysis of melting reconstruction treatment and cement solidification on ultra-risk municipal solid waste incinerator fly ash-blast furnace slag mixtures. Environ Sci Pollut Res 27:32139–32151. https://doi.org/10.1007/s11356-020-09395-8
Ma W, Chen D, Pan M, Gu T, Zhong L, Chen G, Yan B, Cheng Z (2019) Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: a comparative study. J Environ Manage 247:169–177. https://doi.org/10.1016/j.jenvman.2019.06.089
Ma WC, Chen DM, Pan MH, Gu TB, Zhong L, Chen GY, Yan BB, Cheng ZJ (2019) Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: a comparative study. J Environ Manage 247:169–177. https://doi.org/10.1016/j.jenvman.2019.06.089
Mangialardi T (2003) Disposal of MSWI fly ash through a combined washing-immobilisation process. J Hazard Mater 98:225–240. https://doi.org/10.1016/s0304-3894(02)00359-x
Marieta C, Guerrero A, Leon I (2021) Municipal solid waste incineration fly ash to produce eco-friendly binders for sustainable building construction. Waste Manage 120:114–124. https://doi.org/10.1016/j.wasman.2020.11.034
McGowen SL, Basta NT, Brown GO (2001) Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J Environ Qual 30:493–500. https://doi.org/10.2134/jeq2001.302493x
Mizutani S, van der Sloot HA, Sakai S (2000) Evaluation of treatment of gas cleaning residues from MSWI with chemical agents. Waste Manage 20:233–240. https://doi.org/10.1016/s0956-053x(99)00317-7
Mohammadi T, Kaviani A (2003) Water shortage and seawater desalination by electrodialysis. Desalination 158:267–270. https://doi.org/10.1016/s0011-9164(03)00462-4
MOHURD (2021) 2020 China Urban Construction Statistical Yearbook.
Mu Y, Saffarzadeh A, Shimaoka T (2018) Utilization of waste natural fishbone for heavy metal stabilization in municipal solid waste incineration fly ash. J Clean Prod 172:3111–3118. https://doi.org/10.1016/j.jclepro.2017.11.099
Ndiba P, Axe L, Boonfueng T (2008) Heavy metal immobilization through phosphate and thermal treatment of dredged sediments. Environ Sci Technol 42:920–926. https://doi.org/10.1021/es072082y
Oliva J, De Pablo J, Cortina J-L, Cama J, Ayora C (2011) Removal of cadmium, copper, nickel, cobalt and mercury from water by Apatite II (TM): Column experiments. J Hazard Mater 194:312–323. https://doi.org/10.1016/j.jhazmat.2011.07.104
Ottosen LM, Hansen HK, Laursen S, Villumsen A (1997) Electrodialytic remediation of soil polluted with copper from wood preservation industry. Environ Sci Technol 31:1711–1715. https://doi.org/10.1021/es9605883
Palanivel TM, Sulaiman H (2014) Generation and composition of municipal solid waste (MSW) in Muscat, Sultanate of Oman. In: Baby S, Yang D (eds) 5th Int Confer Environ Sci Develop - Icesd 2014. pp 96–102
Pan Y, Wu Z, Zhou J, Zhao J, Ruan X, Liu J, Qian G (2013) Chemical characteristics and risk assessment of typical municipal solid waste incineration (MSWI) fly ash in China. J Hazard Mater 261:269–276. https://doi.org/10.1016/j.jhazmat.2013.07.038
Pedersen KB, Kirkelund GM, Ottosen LM, Jensen PE, Lejon T (2015) Multivariate methods for evaluating the efficiency of electrodialytic removal of heavy metals from polluted harbour sediments. J Hazard Mater 283:712–720. https://doi.org/10.1016/j.jhazmat.2014.10.016
Pedersen PJ (2002) Evaluation of assisting agents for electrodialytic removal of Cd, Pb, Zn, Cu and Cr from MSWI fly ash. J Hazard Mater 95:185–198. https://doi.org/10.1016/s0304-3894(02)00138-3
Polettini A, Pomi R, Trinci L, Muntoni A, Lo Mastro S (2004) Engineering and environmental properties of thermally treated mixtures containing MSWI fly ash and low-cost additives. Chemosphere 56:901–910. https://doi.org/10.1016/j.chemosphere.2004.05.004
Poykio R, Makela M, Watkins G, Nurmesniemi H, Dahl O (2016) Heavy metals leaching in bottom ash and fly ash fractions from industrial-scale BFB-boiler for environmental risks assessment. Trans Nonferrous Met Soc Chin 26:256–264. https://doi.org/10.1016/s1003-6326(16)64112-2
Qiu Q, Jiang X, Chen Z, Lu S, Ni M (2017) Microwave-assisted hydrothermal treatment with soluble phosphate added for heavy metals solidification in MSWI fly ash. Energy Fuels 31:5222–5232. https://doi.org/10.1021/acs.energyfuels.6b02516
Qiu Q, Jiang X, Lu S, Ni M (2016) Effects of microwave-assisted hydrothermal treatment on the major heavy metals of municipal solid waste incineration fly ash in a circulating fluidized bed. Energy Fuels 30:5945–5952. https://doi.org/10.1021/acs.energyfuels.6b00547
Querol X, Moreno N, Umana JC, Alastuey A, Hernandez E, Lopez-Soler A, Plana F (2002) Synthesis of zeolites from coal fly ash: an overview. Int J Coal Geol 50:413–423. https://doi.org/10.1016/s0166-5162(02)00124-6
Quina MJ, Bordado JC, Quinta-Ferreira RM (2008) Treatment and use of air pollution control residues from MSW incineration: an overview. Waste Manage 28:2097–2121. https://doi.org/10.1016/j.wasman.2007.08.030
Quina MJ, Bordado JCM, Quinta-Ferreira RM (2009) The influence of pH on the leaching behaviour of inorganic components from municipal solid waste APC residues. Waste Manage 29:2483–2493. https://doi.org/10.1016/j.wasman.2009.05.012
Quina MJ, Bordado JCM, Quinta-Ferreira RM (2011) Percolation and batch leaching tests to assess release of inorganic pollutants from municipal solid waste incinerator residues. Waste Manage 31:236–245. https://doi.org/10.1016/j.wasman.2010.10.015
Quina MJ, Bordado JCM, Quinta-Ferreira RM (2014) Stabilisation/solidification of APC residues from MSW incineration with hydraulic binders and chemical additives. J Hazard Mater 264:107–116. https://doi.org/10.1016/j.jhazmat.2013.11.014
Rani DA, Boccaccini AR, Deegan D, Cheeseman CR (2008) Air pollution control residues from waste incineration: current UK situation and assessment of alternative technologies. Waste Manage 28:2279–2292. https://doi.org/10.1016/j.wasman.2007.10.007
Reijnders L (2005) Disposal, uses and treatments of combustion ashes: a review. Resour Conserv Recycl 43:313–336. https://doi.org/10.1016/j.resconrec.2004.06.007
Saqib N, Backstrom M (2015) Distribution and leaching characteristics of trace elements in ashes as a function of different waste fuels and incineration technologies. J Environ Sci 36:9–21. https://doi.org/10.1016/j.jes.2015.03.006
Saxena S, D’Souza SF (2006) Heavy metal pollution abatement using rock phosphate mineral. Environ Int 32:199–202. https://doi.org/10.1016/j.envint.2005.08.011
Say R, Birlik E, Erdemgil Z, Denizli A, Ersoz A (2008) Removal of mercury species with dithiocarbamate-anchored polymer/organosmectite composites. J Hazard Mater 150:560–564. https://doi.org/10.1016/j.jhazmat.2007.03.089
Scarlat N, Motola V, Dallemand JF, Monforti-Ferrario F, Mofor L (2015) Evaluation of energy potential of municipal solid waste from African urban areas. Renew Sustain Energy Rev 50:1269–1286. https://doi.org/10.1016/j.rser.2015.05.067
Schnell M, Horst T, Quicker P (2020) Thermal treatment of sewage sludge in Germany: a review. J Environ Manage 263. https://doi.org/10.1016/j.jenvman.2020.110367
Shan C, Jing Z, Pan L, Zhou L, Pan X, Lu L (2011) Hydrothermal solidification of municipal solid waste incineration fly ash. Res Chem Intermed 37:551–565. https://doi.org/10.1007/s11164-011-0287-x
Shim YS, Kim YK, Kong SH, Rhee SW, Lee WK (2003) The adsorption characteristics of heavy metals by various particle sizes of MSWI bottom ash. Waste Manage 23:851–857. https://doi.org/10.1016/s0956-053x(02)00163-0
Shoumkova A, Stoyanova V (2013) Zeolites formation by hydrothermal alkali activation of coal fly ash from thermal power station “Maritsa 3”, Bulgaria. Fuel 103:533–541. https://doi.org/10.1016/j.fuel.2012.07.076
Sneddon IR, Orueetxebarria M, Hodson ME, Schofield PF, Valsami-Jones E (2006) Use of bone meal amendments to immobilise Pb, Zn and Cd in soil: a leaching column study. Environ Pollut 144:816–825. https://doi.org/10.1016/j.envpol.2006.02.008
Sun XL, Guo Y, Yan YB, Li JS, Shen JY, Han WQ, Sun XY, Wang LJ (2019) Co-processing of MSWI fly ash and copper smelting wastewater and the leaching behavior of the co-processing products in landfill leachate. Waste Manage 95:628–635. https://doi.org/10.1016/j.wasman.2019.07.003
Sun Y, Zheng J, Zou L, Liu Q, Zhu P, Qian G (2011) Reducing volatilization of heavy metals in phosphate-pretreated municipal solid waste incineration fly ash by forming pyromorphite-like minerals. Waste Manage 31:325–330. https://doi.org/10.1016/j.wasman.2010.10.011
Syc M, Simon FG, Hyks J, Braga R, Biganzoli L, Costa G, Funari V, Grosso M (2020) Metal recovery from incineration bottom ash: state-of-the-art and recent developments. J Hazard Mater 393.https://doi.org/10.1016/j.jhazmat.2020.122433
Tian X, Rao F, Leon-Patino CA, Song SX (2020) Co-disposal of MSWI fly ash and spent caustic through alkaline-activation: immobilization of heavy metals and organics. Cement Concr Compos 114. https://doi.org/10.1016/j.cemconcomp.2020.103824
Tian Z, Zhang B, He C, Tang R, Zhao H, Li F (2015) The physiochemical properties and heavy metal pollution of fly ash from municipal solid waste incineration. Process Saf Environ Prot 98:333–341. https://doi.org/10.1016/j.psep.2015.09.007
Tillman FD, Bartelt-Hunt SL, Craver VA, Smith JA, Alther GR (2005) Relative metal ion sorption on natural and engineered sorbents: batch and column studies. Environ Eng Sci 22:400–410. https://doi.org/10.1089/ees.2005.22.400
Tong LZ, Tang Y, Wang F, Hu B, Shi PX, Hu Q (2019) Investigation of controlling factors on toxic metal leaching behavior in municipal solid wastes incineration fly ash. Environ Sci Pollut Res 26:29316–29326. https://doi.org/10.1007/s11356-019-06123-9
Usman A, Kuzyakov Y, Stahr K (2005) Effect of clay minerals on immobilization of heavy metals and microbial activity in a sewage sludge-contaminated soil. J Soils Sediments 5:245–252. https://doi.org/10.1065/jss2005.05.141
Vavva C, Voutsas E, Magoulas K (2017) Process development for chemical stabilization of fly ash from municipal solid waste incineration. Chem Eng Res Des 125:57–71. https://doi.org/10.1016/j.cherd.2017.06.021
Verhulst D, Buekens A, Spencer PJ, Eriksson G (1996) Thermodynamic behavior of metal chlorides and sulfates under the conditions of incineration furnaces. Environ Sci Technol 30:50–56. https://doi.org/10.1021/es940780+
Wang F-H, Zhang F, Chen Y-J, Gao J, Zhao B (2015a) A comparative study on the heavy metal solidification/stabilization performance of four chemical solidifying agents in municipal solid waste incineration fly ash. J Hazard Mater 300:451–458. https://doi.org/10.1016/j.jhazmat.2015.07.037
Wang FH, Zhang F, Chen YJ, Gao J, Zhao B (2015) A comparative study on the heavy metal solidification/stabilization performance of four chemical solidifying agents in municipal solid waste incineration fly ash. J Hazard Mater 300:451–458. https://doi.org/10.1016/j.jhazmat.2015.07.037
Wang H, Fan X, Wang Y-n, Li W, Sun Y, Zhan M, Wu G (2018) Comparative leaching of six toxic metals from raw and chemically stabilized MSWI fly ash using citric acid. J Environ Manage 208:15–23. https://doi.org/10.1016/j.jenvman.2017.11.071
Wang KS, Chiang KY, Lin KL, Sun CJ (2001a) Effects of a water-extraction process on heavy metal behavior in municipal solid waste incinerator fly ash. Hydrometallurgy 62:73–81. https://doi.org/10.1016/s0304-386x(01)00186-4
Wang L, Jin Y, Nie Y, Li R (2010) Recycling of municipal solid waste incineration fly ash for ordinary Portland cement production: a real-scale test. Resour Conserv Recycl 54:1428–1435. https://doi.org/10.1016/j.resconrec.2010.06.006
Wang P, Hu Y, Cheng H (2019) Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ Pollut 252:461–475. https://doi.org/10.1016/j.envpol.2019.04.082
Wang YM, Chen TC, Yeh KJ, Shue MF (2001b) Stabilization of an elevated heavy metal contaminated site. J Hazard Mater 88:63–74. https://doi.org/10.1016/s0304-3894(01)00289-8
Wey M-Y, Liu K-Y, Tsai T-H, Chou J-T (2006) Thermal treatment of the fly ash from municipal solid waste incinerator with rotary kiln. J Hazard Mater 137:981–989. https://doi.org/10.1016/j.jhazmat.2006.03.024
Xiang B, Fan W, Yi X, Wang Z, Gao F, Li Y, Gu H (2016) Dithiocarbamate-modified starch derivatives with high heavy metal adsorption performance. Carbohyd Polym 136:30–37. https://doi.org/10.1016/j.carbpol.2015.08.065
Xiong Y, Zhu F, Zhao L, Jiang H, Zhang Z (2014) Heavy metal speciation in various types of fly ash from municipal solid waste incinerator. J Mater Cycles Waste Manage 16:608–615. https://doi.org/10.1007/s10163-014-0274-6
Xu Y, Chen Y, Feng Y (2013) Stabilization treatment of the heavy metals in fly ash from municipal solid waste incineration using diisopropyl dithiophosphate potassium. Environ Technol 34:1411–1419. https://doi.org/10.1080/09593330.2012.752871
Xu Y, Fu Y, Xia W, Zhang D, An D, Qian G (2018) Municipal solid waste incineration (MSWI) fly ash washing pretreatment by biochemical effluent of landfill leachate: a potential substitute for water. Environ Technol 39:1949–1954. https://doi.org/10.1080/09593330.2017.1345985
Yakubu Y, Zhou J, Ping D, Shu Z, Chen Y (2018) Effects of pH dynamics on solidification/stabilization of municipal solid waste incineration fly ash. J Environ Manage 207:243–248. https://doi.org/10.1016/j.jenvman.2017.11.042
Yang ZZ, Tian SC, Liu LL, Wang XD, Zhang ZT (2018) Application of washed MSWI fly ash in cement composites: long-term environmental impacts. Environ Sci Pollut Res 25:12127–12138. https://doi.org/10.1007/s11356-017-1181-x
Yue Y, Zhang J, Sun FC, Wu SM, Pan Y, Zhou JZ, Qian GR (2019) Heavy metal leaching and distribution in glass products from the co-melting treatment of electroplating sludge and MSWI fly ash. J Environ Manage 232:226–235. https://doi.org/10.1016/j.jenvman.2018.11.053
Zacco A, Borgese L, Gianoncelli A, Struis RPWJ, Depero LE, Bontempi E (2014) Review of fly ash inertisation treatments and recycling. Environ Chem Lett 12:153–175. https://doi.org/10.1007/s10311-014-0454-6
Zarghami Z, Akbari A, Latifi AM, Amani MA (2016) Design of a new integrated chitosan-PAMAM dendrimer biosorbent for heavy metals removing and study of its adsorption kinetics and thermodynamics. Biores Technol 205:230–238. https://doi.org/10.1016/j.biortech.2016.01.052
Zhan X, La Wang HuC, Gong J, Xu T, Li J, Yang L, Bai J, Zhong S (2018) Co-disposal of MSWI fly ash and electrolytic manganese residue based on geopolymeric system. Waste Manage 82:62–70. https://doi.org/10.1016/j.wasman.2018.10.014
Zhan X, Wang La, Wang L, Gong J, Wang X, Song X, Xu T (2021) Co-sintering MSWI fly ash with electrolytic manganese residue and coal fly ash for lightweight ceramisite. Chemosphere 263.
Zhang B, Zhou W, Zhao H, Tian Z, Li F, Wu Y (2016a) Stabilization/solidification of lead in MSWI fly ash with mercapto functionalized dendrimer Chelator. Waste Manage 50:105–112. https://doi.org/10.1016/j.wasman.2016.02.001
Zhang H, Zhao Y, Qi J (2007) Study on use of MSWI fly ash in ceramic tile. J Hazard Mater 141:106–114. https://doi.org/10.1016/j.jhazmat.2006.06.100
Zhang H, Zhao Y, Qi J (2011) Utilization of municipal solid waste incineration (MSWI) fly ash in ceramic brick: product characterization and environmental toxicity. Waste Manage 31:331–341. https://doi.org/10.1016/j.wasman.2010.10.017
Zhang J, Zhang S, Liu B (2020a) Degradation technologies and mechanisms of dioxins in municipal solid waste incineration fly ash: a review. J Clean Prod 250. https://doi.org/10.1016/j.jclepro.2019.119507
Zhang M, Guo M, Zhang B, Li F, Wang H, Zhang H (2020b) Stabilization of heavy metals in MSWI fly ash with a novel dithiocarboxylate-functionalized polyaminoamide dendrimer. Waste Manage 105:289–298. https://doi.org/10.1016/j.wasman.2020.02.004
Zhang M, Pu J (2011) Mineral materials as feasible amendments to stabilize heavy metals in polluted urban soils. J Environ Sci 23:607–615. https://doi.org/10.1016/s1001-0742(10)60455-x
Zhang Q, Gao Y, Zhai YA, Liu FQ, Gao G (2008) Synthesis of sesbania gum supported dithiocarbamate chelating resin and studies on its adsorption performance for metal ions. Carbohyd Polym 73:359–363. https://doi.org/10.1016/j.carbpol.2007.11.028
Zhang Y, Cetin B, Likos WJ, Edil TB (2016b) Impacts of pH on leaching potential of elements from MSW incineration fly ash. Fuel 184:815–825. https://doi.org/10.1016/j.fuel.2016.07.089
Zhao P, Ni G, Jiang Y, Chen L, Chen M, Meng Y (2010) Destruction of inorganic municipal solid waste incinerator fly ash in a DC arc plasma furnace. J Hazard Mater 181:580–585. https://doi.org/10.1016/j.jhazmat.2010.05.052
Zhao SJ, Muhammad F, Yu L, Xia M, Huang X, Jiao BQ, Lu N, Li DW (2019) Solidification/stabilization of municipal solid waste incineration fly ash using uncalcined coal gangue-based alkali-activated cementitious materials. Environ Sci Pollut Res 26:25609–25620. https://doi.org/10.1007/s11356-019-05832-5
Zhao SZ, Liu B, Ding YJ, Zhang JJ, Wen Q, Ekberg C, Zhang SG (2020) Study on glass-ceramics made from MSWI fly ash, pickling sludge and waste glass by one-step process. J Clean Prod 271.https://doi.org/10.1016/j.jclepro.2020.122674
Zhao YC, Song LJ, Li GJ (2002) Chemical stabilization of MSW incinerator fly ashes. J Hazard Mater 95:47–63. https://doi.org/10.1016/s0304-3894(02)00002-x
Zheng L, Wang W, Li Z, Zhang L, Cheng S (2019) Immobilization of heavy metal using dithiocarbamate agent. J Mater Cycles Waste Manage 21:652–658. https://doi.org/10.1007/s10163-019-00829-1
Zhou H, Meng A, Long Y, Li Q, Zhang Y (2014) An overview of characteristics of municipal solid waste fuel in China: physical, chemical composition and heating value. Renew Sustain Energy Rev 36:107–122. https://doi.org/10.1016/j.rser.2014.04.024
Zhu F, Xiong Y, Wang Y, Wei X, Zhu X, Yan F (2018) Heavy metal behavior in “Washing-Calcination-Changing with Bottom Ash” system for recycling of four types of fly ashes. Waste Manage 75:215–225. https://doi.org/10.1016/j.wasman.2018.01.032
Zhu J, Hao Q, Chen J, Hu M, Tu T, Jiang C (2020) Distribution characteristics and comparison of chemical stabilization ways of heavy metals from MSW incineration fly ashes. Waste Manage 113:488–496. https://doi.org/10.1016/j.wasman.2020.06.032
Funding
This study was supported by the National Natural Science Foundation of China (42077346); Chengdu Science and Technology Project (2018-YF05-00760-SN); the Strategic Cooperation Project Between Sichuan University and Panzhihua Municipal Government (20826041D4287); and the Strategic Cooperation Project Between Sichuan University and Yibin Municipal Government (2019CDYB-19). These funds provided support in the design of the study and collection, interpretation of data, and manuscript writing.
Author information
Authors and Affiliations
Contributions
NN was in charge of literature data collection. JYY and ZSY provided guidance on the conception and writing of the article. XYZ was responsible for reviewing and summarizing literatures in related fields and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Ta Yeong Wu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Xy., Yang, Jy., Ning, N. et al. Chemical stabilization of heavy metals in municipal solid waste incineration fly ash: a review. Environ Sci Pollut Res 29, 40384–40402 (2022). https://doi.org/10.1007/s11356-022-19649-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19649-2