Abstract
During rainfall, phosphorus in drainage pipe sediments is easily washed and released. This study investigates the migration of phosphorus between sediments and water in storm and sewage sewers, the microbial community structure in sediments, and phosphorus transformation under biological action. Results showed that when the initial concentration of phosphorus in stormwater (water column) in storm sewer was high (1–2 mg/L), the total phosphorus (TP) level decreased in the water column but increased in the sediments, showing a trend of phosphorus migration from the water column to the sediments. Moreover, under high concentration (2 mg/L), the TP level decreased by 83.19% in the water column within 210 min, which was greater than 64.9% of the medium-concentration stormwater (1 mg/L). In sewage sewer, when the initial concentration of phosphorus in sewage was about 2 mg/L, phosphorus would migrate from the sediments and interstitial water to the water column because of the high concentration of phosphorus in the sediments. In addition, the variation in phosphorus was caused not only by concentration gradient but also by microbial communities. Phosphate accumulating organisms, such as Alphaproteobacteria, Gammaproteobacteria, and Actinobacteria, existed in the storm and sewage sewers, which could ingest dissolved reactive phosphorus in the water column and interstitial water and convert it into phosphorus in organisms. In storm sewers, Acidimicrobiia transferred phosphorus from the water column and interstitial water to the sediments through biochemical reactions and physical adsorption. In sewage sewers, organic acids secreted by Clostridia, Bacteroidia, and Bacilli could dissolve some insoluble phosphorus in sediments and then transfer them to interstitial water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediments in drainage pipes mainly come from two ways, namely, solid particles flushed by stormwater runoff into the drainage pipe at different catchment surfaces in the city and settlement of suspended solids in sewage sewer (Shahsavari et al. 2017). Various pollutants, including nitrogen, phosphorus, and sulfur, exist in sewer sediments (Kaeseberg et al. 2018; Shi et al. 2018). When sediments are flushed, some pollutants could be released again. If they enter the receiving water, then they might cause water eutrophication (Meng et al. 2019). Among them, phosphorus, as the main limiting factor of water eutrophication, has various occurrence forms and is greatly affected by environmental factors (House and Denison 2000; Chen et al. 2018).
Phosphorus in the sediments of drainage pipes comes from stormwater runoff flushing, atmospheric sedimentation, human excreta, and domestic garbage (detergents with phosphorus content) (Cotham and Bidleman 1995; Shi et al. 2018). In the drainage pipe, sediments, interstitial water, and water column constitute the sediment–water system. Phosphorus exists in different forms in this system. In the water column, phosphorus mainly exists in dissolved state and granular state. The higher active part of dissolved phosphorus is called dissolve reactive phosphorus (DRP), which is mainly composed of inorganic orthophosphate. Phosphorus in sediments can be divided into inorganic and organic phosphorus. Exchangeable phosphorus (Ex-P) is an inorganic phosphorus that is adsorbed on the surface of particles; it has weak binding ability and is easy to release (Liu et al. 2016). Ex-P in surface sediments is easily released to water column by desorption.
The sediment–water system is an environmental boundary composed of water and sediments. Under certain conditions, substance accumulated in sediments could affect the water column through morphological changes, release, and migration (Zhou et al. 2001). This process often occurs with the participation of microorganisms (Luca and Jack 2001). Three pollutant exchange pathways are proposed in sewage sewers: physical pollutant deposition, biological transformation adsorption, and biological transformation release, among which physical deposition is the main pathway (Shi et al. 2018). The concentration of DRP in the interstitial water of sediments is affected by organic matter in sediments, as well as Fe (II) concentration, bacterial activity, and water depth (Eckerrot and Petterson 1993). Under anoxic conditions, sediments would release water-soluble phosphorus, and it is directly involved in phosphorus migration and transformation at the sediment–water interface (Rydin 2000). After 2 years of sediment collection and analysis, Valdemarsen et al. (2015) found that phosphorus in sediments is mainly derived from microbial mineralization. Microorganisms play three main roles in the migration and transformation of phosphorus at the sediment–water interface: (1) secreting phosphohydrolase to mineralize organic phosphorus; (2) secreting organic acids to dissolve insoluble phosphorus, mainly calcium phosphate (Ca-P), and (3) fixing phosphorus by assimilation (Holdren and Armstrong 1980; Katherine and Emily 2013).
Phosphorus migration and transformation in the sediment–water system in drainage pipes is poorly understood. In the long-term operation of the pipe, various forms of phosphorus exist in sediments. The mechanism through which it migrates and transforms with phosphorus in the water column remains unclear. By studying the sediment–water system of lakes, sediment resuspension is beneficial to long-term P retention from the water column (Geng et al. 2021). The larval bioturbation increases the DRP and labile P adsorption capability of sediments, and Fe (II) oxidation and its enhanced adsorption are the major mechanisms (Yan et al. 2020). The release capacity and intensity of sediments could be directly reflected by the pollutant concentration in interstitial water, which have a direct impact on the material exchange process in the sediment–water system (Yu et al. 2016). In the study of phosphorus migration and transformation in the sediment–water system in drainage pipes, the role of interstitial water is also worth investigating. In addition, the changes in water quality in storm and sewage sewers and the influence of differences in microbial communities on the migration and transformation of phosphorus are all worthy to be studied. The dominant species of microorganism changes under the alternation of aerobic and anoxic states along the rainwater pipeline, resulting in different pathways of nitrogen transformation (Liu et al. 2021).
This work aims to explore differences in microbial communities in storm and sewage sewers and their role in the migration and transformation of phosphorus in sediments, interstitial water, and water column and determine the effect of water column concentration on the migration direction of phosphorus. This study analyzed the different storm and sewage concentrations in storm and sewage sewers and explored the migration and transformation mechanisms of phosphorus in the sediment–water system under biological action. Results could further predict the phosphorus exchange process between sediments and water in different sewers, thereby providing a theoretical basis for subsequent quantitative calculation of phosphorus release and its pollution load from sediments to the downstream water.
Materials and methods
Reactor
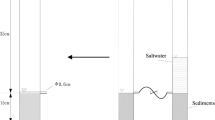
In laboratory, a static experimental device was built to simulate the sediment–water system in storm and sewage sewers (Fig. 1). The device is composed of plexiglass with the height of 600 mm and the diameter of 200 mm. Sediments and stormwater (sewage) are placed in the lower and upper parts of the device, respectively. In the experiment, water samples are taken from the sampling ports, which are set on the side wall of the upper part of the device at 75 mm intervals, and the sediment samples are absorbed by a needle tube.
The sediments used in the experiment were obtained from outdoor storm sewer and sewage sewer and were evenly filled in the bottom of the devices. Based on pipeline deposition survey results and documentary records (Charles et al. 2014), the ratio of the deposition thickness to the pipe diameter ranges from 0.10 to 38.19%. In the experiment, the sediment thickness was determined to be the same as the height of the water column, about 250 mm. The background concentrations of phosphorus in the two sediments are listed in Table 1. According to the actual quality of stormwater in storm sewer (Zhang et al. 2017; Qin et al. 2016) and sewage in sewage sewer (Jin et al. 2016; Sang et al. 2017), stormwater and sewage of high, medium, and low concentrations were used as the water column in the experiments (Table 2). Stormwater or sewage was poured in the two devices carefully, and the pH and dissolved oxygen (DO) in the water column measured after standing are listed in Table 2.
Sample collection and analysis methods
Static simulation experiments of storm and sewage sewers were carried out simultaneously. Water samples were obtained 5 mm above the sediment–water interface (50 ml for every sample), and sediment samples were collected at 5 mm below the interface with a needle tube. All the samples were taken at 0, 15, 30, 45, 60, 90, 120, 150, 180, and 210 min in the experiments. Samples were collected three times at different positions in the same cross-section and then mixed.
The concentrations of TP, dissolved TP (DTP), and DRP in the water column and interstitial water samples and the concentrations of TP and Ex-P in the pretreated sediment samples were determined by molybdate colorimetry (Wang 2014).
High-throughput sequencing
Three sediment samples were collected from the sediments obtained from outdoor storm canals and sewage sewers, respectively. The polymerase chain reaction (PCR) amplification of the 16S rRNA gene of sediment samples was performed by using PCR primers specific for the 515–806 (V3-V4) regions. The PCR assays were carried out in triplicate. All PCR products were visualized on an agarose gel (2% Tris acetate-EDTA buffer) that contained ethidium bromide and purified using a Deoxyribonucleic acid (DNA) gel extraction kit (Axygen, China). Before sequencing, the DNA concentration of each PCR product was determined with a QuantiFluor™-ST fluorescent quantitative system (Promega, USA) and mixed with the appropriate proportion based on the sequencing requirements. PE amplicon libraries were constructed, and sequencing was performed using the Illumina Miseq platform at Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai China).
Results and discussion
Phosphorus migration in sediment–water system in storm sewer
In the case of different initial TP concentrations (e.g., high, medium, and low concentrations) in stormwater (Table 1), the TP, DRP, and Ex-P variations with time in different positions (e.g., water column, interstitial water, and sediments) are shown in Fig. 2. Under the conditions of high and medium stormwater concentrations (Fig. 2a and b), the TP level in the water column and interstitial water decreased and that in the sediments increased and then tended to be stable. The high phosphorus concentrations in the stormwater showed a trend of migrating from the water to the sediments. Moreover, the TP variations at high and medium concentrations were different. At high concentrations, the decline percentages of TP in the water column and interstitial water within 0–210 min (83.19%, 60.30%) were greater than those in medium-concentration stormwater (64.90%, 41.54%), and the increase percentage of TP in sediments (63%) was greater than that in medium-concentration stormwater (7.55%). This finding showed that the greater the concentration gradient was, the more phosphorus migrated from the water column to the sediments. In addition, some phosphates in the water column combined with Fe3+ produced by biotransformation and then migrated to the sediments through sedimentation and deposition (Song et al. 2019). In low-concentration stormwater (Fig. 2c), the TP variations in the three positions obviously slowed down, the TP in the water column and interstitial water increased slightly, and that in the sediments decreased slightly. When the TP concentration in the water column was low, the TP in the sediments migrated upward through the interstitial water under the influence of environmental factors and organisms (Tammeorg et al. 2015).
Variations of TP, DRP, and Ex-P at different positions in the sediment–water system of storm sewer under different initial TP concentrations: a TP at high concentration, b TP at medium concentration, c TP at low concentration, d DRP/Ex-P at high concentration, e DRP/Ex-P at medium concentration, f DRP/Ex-P at low concentration
Under high and medium concentrations of stormwater, the DRP level in the water column and interstitial water decreased, and the Ex-P concentration in the sediments increased and stabilized at approximately 120 min (Fig. 2d and e). The situation in the low-concentration stormwater was exactly the opposite under the test conditions (Fig. 2f). In studies of phosphorus release from lake sediments (Lin et al. 2021), the release of phosphorus from the sediments into the water column could last for several days if the phosphorus concentration in the water column is very low (close to zero), leading to an increase in the concentration of phosphorus in the water column. Comparison of variations in TP and DRP during 210 min showed that the increments were very close. For example, at medium concentration, the TP in the water column decreased from 1.61 to 0.565 mg/L with the increment of − 1.045 mg/L (Fig. 2b), whereas the DRP decreased from 1.375 to 0.228 mg/L with the increment of − 1.147 mg/L (Fig. 2e). The migration of phosphorus in the sediment–water system could be mainly caused by DRP migration. Microorganisms preferred to use DRP in water. Microorganisms could convert other forms of phosphorus, such as organic phosphorus, into bioavailable phosphorus through enzymatic hydrolysis and hydrolysis only when the DRP was low (Müller et al. 2016). However, when the phosphorus concentration in the water column was low (Fig. 2f), the phosphorus in sediments could be transported to the water column by static or dynamic release, in which the static release would diffuse the dissolved phosphorus in the interstitial water to the water column by concentration gradient (Tammeorg et al. 2015).
Phosphorus migration in sediment–water system in sewage sewer
Under different sewage concentrations, the TP, DRP, and Ex-P variations with time in different positions are shown in Fig. 3. The TP variation trends in high and medium sewage concentrations were close (Fig. 3a and b): the water column was basically unchanged, the interstitial water decreased, and the TP level in the sediments increased slightly. In the low-concentration sewage (Fig. 3c), the TP in the water column increased obviously, whereas those in the interstitial water and sediments decreased. The background concentration of phosphorus in the sewage sewer sediments was high. When the TP concentration in the water column was also high, the migration between the water column, interstitial water, and sediments was not very evident, so the TP decrease in the interstitial water could be related to biological transformation. When the TP concentration in the water column was low, a concentration gradient was formed between the sediments (interstitial water) and water column, and TP could move from the sediments and interstitial water to the water column. In addition to the influence of concentration gradient, phosphate-solubilizing microorganisms played an important role. Phosphate-solubilizing bacteria could dissolve part of Ca–P in sediments, resulting in a decrease in the TP level (Katherine and Emily 2013).
Variations of TP, DRP, and Ex-P at different positions in the sediment–water system of sewage sewer under different initial TP concentrations: a TP at high concentration, b TP at medium concentration, c TP at low concentration, d DRP/Ex-P at high concentration, e DRP/Ex-P at medium concentration, f DRP/Ex-P at low concentration
The variations of DRP in the water column and interstitial water and Ex-P in sediments (Fig. 3d, e, and f) were close to the trend of TP. For example, at high concentrations, the TP content in the interstitial water decreased from 12.614 to 6.574 mg/L (Fig. 3a), whereas the DRP decreased from 12.127 to 6.556 mg/L (Fig. 3d), and the increments were similar. At low concentrations, the TP content in the water column increased from 2.568 to 5.274 mg/L (Fig. 3c), whereas the DRP increased from 1.701 to 5.055 mg/L (Fig. 3f), and the increments were close. This finding showed that DRP was easily migrated and transformed at the sediment–water interface, similar to that in the storm sewer.
Comparison of phosphorus migration in sediment–water system in storm and sewage sewers
Microorganisms exist in sediments and play an important role in phosphorus migration and transformation at the sediment–water interface in sewers (Valdemarsen et al. 2015). The differences in the microbial community structure in the storm and sewage sewers were compared and analyzed through the molecular biological technology of high-throughput sequencing to explore the role of microorganisms in phosphorus migration and transformation.
Fifteen dominant species with relative abundance greater than 1% at the phylum level in the sediments of storm and sewage sewer are displayed in Fig. 4a. In the two sewers, the dominant species were basically the same, but their relative abundance varied. The main dominant species included Proteobacteria, Actinobacteria, Firmicutes, and Acidobateriota.
The dominant microbial community structures in the storm and sewer sediments at the class level are compared in Fig. 4b. Significant differences were observed in the relative abundance of various microorganisms between the two sediments (*P < 0.05). The relative abundance of Acidimicrobiia and Chloroflexia in the storm sewer was high. Acidimicrobiia could oxidize Fe2+ into Fe3+, and colloidal Fe3+ becomes the protective layer of iron phosphate (Fe–P), thereby inhibiting PO43− detachment from the sediments; some Fe3+ combined with phosphate and formed insoluble FePO4. Moreover, the generated Fe(OH)3 easily adsorbed DRP in the water column and interstitial water (Holdren and Armstrong 1980). Chloroflexia tended to grow in high eutrophication environment (Klatt et al. 2013). In the sewage sewer, the relative abundance levels of Clostridia, Bacterioidia, and Bacilli were relatively high. These microorganisms could transform insoluble phosphorus (Ca-P, Fe–P, etc.) into phosphates that can be absorbed and utilized through their own metabolites (e.g., organic acids and phosphatases) or exhibit synergistic effect with other organisms (Zhu et al. 2018). In addition, Alphaproteobacteria, Gammaproteobacteria, and Actinobacteria (Nielsen et al. 2012; Katherine and Emily 2013) belong to phosphate accumulating organisms (PAOs). PAOs could uptake phosphorus in the water column and interstitial water and store it in their own cells in the form of polyphosphate (poly-P) (Deinema et al. 1980).
When the initial concentrations of TP in the two kinds of water column were close to 2.1 mg/L in stormwater and 2.5 mg/L in sewage, the TP variations at different positions in the storm and sewage sewers are shown in Fig. 5. In the storm sewer, the TP levels in the water column and interstitial water decreased, and that in the sediments increased, reaching the equilibrium at approximately 150 min (Fig. 5a). In the storm sewer, Acidimicrobiia was the dominant species, and it would promote the formation of the colloidal Fe3+ protective layer around Fe–P and inhibit PO43− detachment from the sediments into the interstitial water and water column. Moreover, the formed Fe(OH)3 could easily adsorb DRP in the water column and interstitial water (Holdren and Armstrong 1980), thereby promoting the migration of phosphorus from the water column and interstitial water to the sediments. In addition, the gradual increase in the TP content in the sediments of the storm sewer provided conditions for the growth of Chloroflexia.
In the sewage sewer, the TP level in the water column increased, while those in the interstitial water and sediments decreased (Fig. 5b). Clostridia, Bacteroidia, and Bacilli were the dominant species in the sewage sewer. They could dissolve some insoluble phosphorus (Ca-P, etc.) in the water column by secreting organic acids (Wong et al. 2013; Zheng et al. 2013). In addition to the microbial action, the TP in the interstitial water of the sewage sewer was higher than that in water column, and the existence of concentration difference would cause some phosphorus to move from the interstitial water to the water column. In the sediment–water system, the water column was more than the interstitial water. Thus, the increment in the phosphorus concentration in the water column was less than its reduction in interstitial water.
Alphaproteobacteria, Gammaproteobacteria, and Actinobacteria were observed in the storm and sewage sewers (Fig. 4b). These PAOs could uptake phosphorus in the water column and interstitial water and store it in their own cells; they could also transfer some phosphorus to sediments.
In the case of the similar initial concentration of DRP in the water column, the variations in DRP and Ex-P over time in the storm and sewage sewers are shown in Fig. 6. The DRP level in the water column and interstitial water in the storm sewer decreased, and the Ex-P content in the sediments increased (Fig. 6a). Based on comparison between Figs. 5a and 6a, the variations in the DRP level in the water column and interstitial water were close to those in TP. For example, the TP content in the water column decreased from 2.058 to 0.346 mg/L, whereas the DRP level decreased from 1.146 mg/L to 0.188 mg/L. Hence, microorganisms in the storm sewer would give priority to utilize DRP, and the generated products were partially transferred to the sediments. The Ex-P concentration in the sediments of the two sewers changed in the opposite direction. The increased Ex-P level in the storm sewer was partly derived from the biotransformation products in the water column and interstitial water.
The DRP concentration in the interstitial water of the sewage sewer decreased obviously, and the Ex-P concentration in the sediments declined slightly; meanwhile, the DRP concentration in the water column increased gradually (Fig. 6b). On the one hand, Ex-P with weak surface binding capacity of sediments in the sewage sewer was released to the water column through desorption. On the other hand, the background concentration of DRP in the interstitial water of the sewage sewer was higher than that in the storm sewer, thereby forming a large concentration gradient between the interstitial water and water column. Thus, DRP moved to the water column along the concentration gradient.
Transfer and transformation paths of phosphorus in sediment–water system of storm and sewage sewers
The background concentration of phosphorus in the sediments of the sewage sewer was higher than that in the storm sewer, and the two sewers had different dominant microbial species. The migration and transformation paths of phosphorus in the sediment–water system of the two varied obviously (Fig. 7).
In the storm sewer, the acid microorganism Acidimicrobiia (ACI) oxidized Fe2+ into Fe3+ formed a colloidal protective layer of Fe3+ around Fe–P, combined with phosphate to form insoluble FePO4, and formed Fe(OH)3 to adsorb DRP in the water column and interstitial water. These phenomena could promote the transfer of phosphorus from the water column and interstitial water to the sediments.
In the sewage sewer, the position migration and biotransformation of phosphorus in the sediment–water system existed simultaneously. In general, the initial TP and DRP concentrations in the interstitial water of the sewage sewer were higher than those in the water column, and these concentration gradient between interstitial water and water column could cause some phosphorus to move from the interstitial water to water column. Part of insoluble phosphorus (Ca-P, etc.) dissolved by organic acids secreted by Clostridia (CL), Bacterioidia (BAA), and Bacilli (BAI) was transferred to the interstitial water.
PAOs, such as Alphaproteobacteria (AL), Gammaproteobacteria (GA), and Actinobacteria (ACT), existed in both sewers. They could uptake DRP in the water column and interstitial water and store it in their own cells in the form of poly-P. Thus, PAOs transferred part of phosphorus from the water column and interstitial water to the sediments through biotransformation.
Conclusion
In storm and sewage sewers, changes in the concentrations of various forms of phosphorus were commonly due to position migration and biological transformation in the sediment–water system. The migration and transformation showed the following rules. When the stormwater and sewage (water column) concentrations were in different ranges, the migration direction of phosphorus in the sediment–water system in the sewers was different. In the storm sewer, when the phosphorus concentration in the stormwater was high (1–2 mg/L), the TP level decreased in the water column but increased in the sediments, showing a trend of phosphorus migration from the water column to the sediments. Moreover, at a high concentration (2 mg/L), the TP level decreased by 83.19% in the water column within 210 min, which was greater than 64.9% of the level in the medium-concentration stormwater (1 mg/L). In the sewage sewer, when the initial concentration of phosphorus in the sewage was about 2 mg/L, phosphorus would migrate from the sediments and interstitial water to the water column because of the high concentration of phosphorus in the sediments. When the phosphorus concentration in the sewage was too high (6–10 mg/L), the phosphorus migration among the water column, interstitial water, and sediments was not very obvious. Microbial communities also had important effects on the biological transformation of phosphorus. PAOs, such as Alphaproteobacteria, Gammaproteobacteria, and Actinobacteria, were found in both sewers. Under the action of PAOs, some DRP in the water column and interstitial water was absorbed and transformed into phosphorus in the organisms, and part of phosphorus was transferred to the sediments from the water column and interstitial water. In the storm sewer, Acidimicrobiia transferred phosphorus to the sediments from the water column and interstitial water through biochemical reactions and physical adsorption. In the sewage sewer, Clostridia, Bacterioidia, and Bacilli secreted organic acids and dissolved some insoluble phosphorus in the sediments, and the dissolved phosphorus was transferred to the interstitial water.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Charles HJB, Tze LL, Aminuddin AG (2014) Sediment size and deposition characteristics in Malaysian urban concrete drains-a case study of Kuching City. Urban Water J 11(1):74–89. https://doi.org/10.1080/1573062X.2012.750371
Chen MS, Ding SM, Chen X, Sun Q, Fan XF, Lin J, Ren MY, Yang LY, Zhang CS (2018) Mechanisms driving phosphorus release during algal blooms based on hourly changes in iron and phosphorus concentrations in sediments. Water Res 133:153–164. https://doi.org/10.1016/j.watres.2018.01.040
Cotham WE, Bidleman TF (1995) Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in air at an urban and a rural site near lake michigan. Environ Sci Technol 29(11):2782–2789. https://doi.org/10.1021/es00011a013
Deinema MH, Habets LHA, Scholten J, Turkstra E, Webers HAAM (1980) The accumulation of polyphosphate in Acinetobacter spp. FEMS Microbiol Lett 9(4):275–279. https://doi.org/10.1111/j.1574-6968.1980.tb05652.x
Eckerrot A, Petterson K (1993) Pore water phosphorus and iron concentrations in a shallow, eutrophic lake-indications of bacterial regulation. Hydrobiologia 253:165–177. https://doi.org/10.1007/bf00050736
Geng X, Li DP, Xu CT, Sun PR (2021) Using sediment resuspension to immobilize sedimentary phosphorus. Environ Sci Pollut R 28(2):1837–1849. https://doi.org/10.1007/s11356-020-10602-9
Holdren GC, Armstrong DE (1980) Factors affecting phosphorus release from intact lake sediment cores. Environ Sci Technol 14:79–87. https://doi.org/10.1021/es60161a014
House WA, Denison FH (2000) Factors influencing the measurement of equilibrium phosphate concentrations in river sediments. Water Res 34:1187–1200. https://doi.org/10.1016/S0043-1354(99)00249-3
Jin PK, Bian XZ, Jiao D, Wang B, Shi S (2016) On the pollutant deposition and erosion release in the urban sewer networks. J Saf Environ 16(5):253–257. https://doi.org/10.13637/j.issn.1009-6094.2016.05.049 (in Chinese)
Kaeseberg T, Schubert S, Oertel R, Zhang J, Berendonk TU, Krebs P (2018) Hot spots of antibiotic tolerant and resistant bacterial subpopulations in natural freshwater biofilm communities due to inevitable urban drainage system overflows. Environ Pollut 242:164–170. https://doi.org/10.1016/j.envpol.2018.06.081
Katherine DM, Emily KR (2013) Microbial contributions to phosphorus cycling in eutrophic lakes and wastewater. Annu Rev Microbiol 67:199–219. https://doi.org/10.1146/annurev-micro-092412-155713
Klatt CG, Liu Z, Ludwig M, Kühl M, Jensen SI, Bryant DA, Ward DM (2013) Temporal metatranscriptomic patterning in phototrophic Chloroflexi inhabiting a microbial mat in a geothermal spring. ISME J 7(9):1775–1789. https://doi.org/10.1038/ismej.2013.52
Lin CY, Li SW, Zhu BL, Liu SP, Li F, Zhou ZM, Li T (2021) Al-PHOSLOCK thin-layer capping to control phosphorus release from sediment: effect of hydraulic retention time and phosphorus migration/transformation mechanism. J Soil Sediment 21:2474–2482. https://doi.org/10.1007/s11368-021-02958-3
Liu JZ, Luo XX, Zhang NM, Wu YH (2016) Phosphorus released from sediment of Dianchi Lake and its effect on growth of Microcystis aeruginosa. Environ Sci Pollut R 23(16):16321–16328. https://doi.org/10.1007/s11356-016-6816-9
Liu CY, Yang YT, Zhou JQ, Chen YZ, Zhou J, Wang YY, Fu DF (2021) Migration and transformation of nitrogen in sediment–water system within storm sewers. J Environ Manage 287:112355. https://doi.org/10.1016/j.jenvman.2021.112355
Luca AVD, Jack JM (2001) The benthic boundary layer: transport processes and biogeochemistry. Oxford University Press, New York. https://doi.org/10.1029/01eo00381
Meng DZ, Wu J, Chen KL, Li HZ, Jin W, Shu SZ, Zhang J (2019) Effects of extracellular polymeric substances and microbial community on the anti-scouribility of sewer sediment. Sci Total Environ 687:494–504. https://doi.org/10.1016/j.scitotenv.2019.05.387
Müller S, Mitrovic SM, Baldwin DS (2016) Oxygen and dissolved organic carbon control release of N, P and Fe from the sediments of a shallow, polymictic lake. J Soil Sediment 16:1109–1120. https://doi.org/10.1007/s11368-015-1298-9
Nielsen PH, Saunders AM, Hansen AA, Larsen P, NielsenJ L (2012) Microbial communities involved in enhanced biological phosphorus removal from wastewater-a model system in environmental biotechnology. Curr Opin Biotech 23(3):452–459. https://doi.org/10.1016/j.copbio.2011.11.027
Qin HP, He KM, Fu GT (2016) Modeling middle and final flush effects of urban runoff pollution in an urbanizing catchment. J Hydrol 534:638–647. https://doi.org/10.1016/j.jhydrol.2016.01.038
Rydin E (2000) Potentially mobile phosphorus in Lake Erken sediment. Water Res 34(7):2037–2042. https://doi.org/10.1016/s0043-1354(99)00375-9
Sang LT, Shi X, Zhang T, Fu BW, Jin PK (2017) Law of pollutant erosion and deposition in urban sewage network. Environ Sci 38(5):1965–1971. https://doi.org/10.13227/j.hjkx.201610033 (in Chinese)
Shahsavari G, Arnaud-Fassetta G, Campisano A (2017) A field experiment to evaluate the cleaning performance of sewer flushing on non-uniform sediment deposits. Water Res 118:59–69. https://doi.org/10.1016/j.watres.2017.04.026
Shi X, Sang LT, Wang XC, Jin PK (2018) Pollutant exchange between sewage and sediment in urban sewer systems. Chem Eng J 351:240–247. https://doi.org/10.1016/j.cej.2018.06.096
Song CL, Cao XY, Zhou YY, Azzaro M, Monticelli LS, Maimone G, Azzaro F, Ferla RL, Caruso G (2019) Nutrient regeneration mediated by extracellular enzymes in water column and interstitial water through a microcosm experiment. Sci Total Environ 670:982–992. https://doi.org/10.1016/j.scitotenv.2019.03.297
Tammeorg O, Horppila J, Laugaste R, Haldna M, Niemistö J (2015) Importance of diffusion and resuspension for phosphorus cycling during the growing season in large, shallow Lake Peipsi. Hydrobiologia 760:133–144. https://doi.org/10.1007/s10750-015-2319-9
Valdemarsen T, Quintana CO, Flindt MR, Kristensen E (2015) Organic N and P in eutrophic fjord sediments-rates of mineralization and consequences for internal nutrient loading. Biogeosciences 12(6):1765–1779. https://doi.org/10.5194/bg-12-1765-2015
Wang SR (2014) Sediment–water interface process of lakes. Science Press, Beijing
Wong MT, Zhang D, LiJ HRKH, Tun HM, Brar MS, Park TJ, Chen YG, Leung FC (2013) Towards a metagenomic understanding on enhanced biomethane production from waste activated sludge after pH 10 pretreatment. Biotechnol Biofuels 6:38. https://doi.org/10.1186/1754-6834-6-38
Yan WM, Chen MS, Liu L, Wu TF, Zhang Y, Wang H, Xing XG, Fan KM (2020) Mechanism of phosphorus mobility in sediments with larval (Propsilocerus akamusi) bioturbation. Environ Sci Pollut R 27(7):7538–7548. https://doi.org/10.1007/s11356-019-07404-z
Yu JH, Fan CX, Zhong JC, Zhang L, Zhang L, Wang CH, Yao XL (2016) Effects of sediment dredging on nitrogen cycling in Lake Taihu, China: insight from mass balance based on a 2-year field study. Environ Sci Pollut R 23:3871–3883. https://doi.org/10.1007/s11356-015-5517-0
Zhang YT, Chen YH, Tang CF, Wei W, Li JB, Fu XL, Liu J (2017) Analysis on pollution characteristics of initial rainwater runoff from different functional areas of high risk tributary in Dongjiang upriver. Ecol Environ Sci 26(11):1942–1949. https://doi.org/10.16258/j.cnki.1674-5906.2017.11.015 (in Chinese)
Zheng X, Su YL, Li X, Xiao ND, Wang DB, Chen YG (2013) Pyrosequencing reveals the key microorganisms involved in sludge alkaline fermentation for efficient short-chain fatty acids production. Environ Sci Technol 47(9):4262–4268. https://doi.org/10.1021/es400210v
Zhou QX, Gibson CE, Zhu YM (2001) Evaluation of phosphorus bioavailability in sediments of three contrasting lakes in China and the UK. Chemosphere 42(2):221–225. https://doi.org/10.1016/S0045-6535(00)00129-6
Zhu J, Li M, Whelan M (2018) Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: a review. Sci Total Environ 612:522–537. https://doi.org/10.1016/j.scitotenv.2017.08.095
Funding
This work was supported by the National Natural Science Foundation of China (No. 51808285), Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. SJCX21-0450).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this study. The first draft of the manuscript was written by Qi Liu and Yanzhi Chen. The oversight and leadership responsibility for the research activity planning and execution, as well as the critical review and revision of the first draft, were performed by Cuiyun Liu. The material preparation, data collection, and analysis were performed by Qi Liu, Yanzhi Chen, Haodong Wei, Yiyang Wang, Jie Zhou, and Wenke Lv. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Q., Chen, Y., Liu, C. et al. Migration and transformation of phosphorus in sediment–water system in storm and sewage sewers. Environ Sci Pollut Res 29, 50085–50095 (2022). https://doi.org/10.1007/s11356-022-19491-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19491-6