Abstract
Sediment dredging can permanently remove pollutants from an aquatic ecosystem, which is considered an effective approach to aquatic ecosystem restoration. In this work, a 2-year field simulation test was carried out to investigate the effect of dredging on nitrogen cycling across the sediment-water interface (SWI) in Lake Taihu, China. The results showed that simulated dredging applied to an area rich in total organic carbon (TOC) and total nitrogen (TN) slightly reduced the NH4 +-N release from sediments while temporarily enhanced the NH4 +-N release in an area with lower TOC and/or TN (in the first 180 days), although the application had a limited effect on the fluxes of NO2 −-N and NO3 −-N in both areas. Further analysis indicated that dredging induced decreases in nitrification, denitrification, and anaerobic ammonium oxidation (anammox) in sediments, notably by 76.9, 49.0, and 89.9 %, respectively, in the TOC and/or TN-rich area. Therefore, dredging slowed down nitrogen cycling rates in sediments but did not increase N loading to overlying water. The main reason for the above phenomenon could be attributed to the removal of the surface sediments enriched with more TOC and/or TN (compared with the bottom sediments). Overall, to minimize internal N pollution, dredging may be more applicable to nutrient-rich sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication has caused a number of threats to freshwater ecosystems and has become the most prominent problem of lakes all over the world (Qin and Zhu 2006; Fan et al. 2004; Jin 2003; Smith et al. 1999). Many approaches have been applied during the past decades to improve lake water quality (Pan et al. 2012; Gustavson et al. 2008; Yin et al. 2013; Wang et al. 2014; 2015). Sediment dredging is used as a significant eco-environmental engineering method to permanently remove surface-contaminated sediments from aquatic ecosystems. However, the effectiveness of sediment dredging is still debatable because it may not be as ideal as people have expected for unclear reasons (Fan et al. 2004; Pu et al. 2000). At the same time, there are also questions concerning the efficacy of sediment dredging and the degree to which dredging results in reduced risk to biological health and the aquatic environment. In such cases, numerous studies have examined the biological effects of dredging in lakes, which mainly focused on razor fish community characterization (Tuck et al. 2000), scallops (Maguire et al. 2002), benthos and algal-periphyton community composition (Lewis et al. 2001), and the restoration of the benthic habitat (Szymelfenig et al. 2006). To better understand the environmental effectiveness and to elaborate on mechanisms of pollutant removal by dredging, much attention has been paid to the influence of dredging on changes in sediment resuspension and transport processes (Yu et al. 2012), the release of persistent organic pollutants (POPs) and heavy metals following the removal of contaminated sediments (Bremle and Larsson 1998; Bowman et al. 2003; Van den berg et al. 2001), and the long term effectiveness of dredging on internal contaminant control (Kleeberg and Kohl 1999; Reddy et al. 2007).
Generally, dredging is primarily applied to remove the POPs, heavy metals, and phosphorus in eutrophic lakes (Kleeberg and Kohl 1999; Reddy et al. 2007; Zhong et al. 2008; Yenilmez and Aksoy 2013; Recknagel et al. 1996). Nitrogen in eutrophic lakes is typically one of the limiting nutrients; however, the effectiveness of dredging on nitrogen removal is unknown. In terms of the methodology, most field studies (Fan et al. 2004; Wang and Feng 2007) and laboratory simulations (Spencer et al. 2006; Zhong et al. 2009) have been deployed by monitoring nitrogen content in pore water and overlying water to evaluate the short-term and long-term effects of nitrogen removal after dredging based on flux calculation or instantaneous measurements alone (Fan et al. 2004; Wang and Feng 2007). The results are usually contradictory among various studies (Fan et al. 2004;Yu et al. 2012; Lohrer and Wetz 2003), and these discrepancies may be attributed, to a great extent, to the implications of pollution status of the sediments in which dredging is implemented. Thus, it is not possible to definitively conclude whether variations in water quality of lakes are natural in origin or are a result of dredging practices under different pollution statuses of sediments (Gustavson et al. 2008).
The cycling of nitrogen in sediments is driven by the rate and lability of organic matter deposition, sediment accumulation rates and bioturbation/bioadvection, and rates of microbial nitrogen transformation (Cornwell and Owens 2011). Dredging is one type of large-scale, anthropogenic disturbance associated with high costs, and it often destroys the balance of the sediment-water interface, including the physical environment (soil texture, particulate deposition, suspension, and advection), chemical processes (mineralization, adsorption, and desorption), and biological processes (bioturbation, bioirrigation, and excretion) (Pu et al. 2000; Qin et al. 2004). Taken together, these processes must inevitably influence nutrient cycling processes, reaction rates, diagenetic properties, transport in surface sediments, and nutrient exchange across the sediment-water interface (SWI) (Falcào et al. 2003; Yu et al. 2012; Qin et al. 2004). To date, there is little information available concerning the effect of dredging on micro-interface nitrogen cycling. The study published by Jing et al. (Jing et al. 2013) measured the variations of nitrogen fractions in overlying water from undredged, fresh-dredged, and post-dredged sediments to evaluate the effects of dredging on nitrogen cycling across the SWI based on nitrogen diffusive flux from sediment to overlying water. Cornwell and Owens (2011) used sediment pore water NH4 +-N chemistry, adsorbed NH4 +-N concentrations, sediment-water NH4 +-N exchange, and N2-N flux to quantify the mass of labile N that can be released during dredging in the Chesapeake Bay. However, published studies have not addressed the elaborate mechanisms of nitrogen removal processes and furthermore have not distinguished the nitrogen release induced by dredging from the environmental effects of natural processes and have not quantified the nitrogen releases under the condition of overall N cycling in aquatic ecosystems. Only fragmentary studies conducted by Zhong et al. (2010a, b) and Graca et al. (2004) noted that dredging can reduce the denitrification rates by changing the pattern of inherent cycling of nitrogen. However, the basic microbial processes to which nitrogen is subjected in the sediment are denitrification, nitrification, ammonification, anammox, and dissimilatory NO3 −-N reduction to ammonium (DNRA), of which the denitrification, anammox, and DNRA processes can permanently remove nitrogen from aquatic ecosystems. In addition, denitrification seems to be the most significant process for removing nitrogen from freshwater bodies (Liu et al. 2015; Hanaki et al. 1990; Zhong et al. 2010a, b; Trimmer et al. 2003). Therefore, it is important to identify the specific microbial processes of nitrogen release induced by dredging under different pollution states of sediments in aquatic ecosystem restoration.
After dredging, we hypothesized that N cycling would be slowed down, particularly the dissimilatory microbial processes of nitrogen removal, owing to lower values of TOC and/or TN and of microbe abundance. We further hypothesized that the NO3 −-N and NO2 −-N removal from aquatic ecosystem would be weakened because the de nitrogen processes were inhibited substantially. Therefore, the main objectives of this study were first to determine the effect of dredging on inorganic nitrogen releases from sediments across the SWI; second, to construct rough budgets for the nitrogen removal induced by dredging on the basis of nitrification, denitrification, and anammox; and third, to further understand the implications of dredging projects to nitrogen mass balance under different pollution states of sediments based on the results of the first two objectives. To better interpret the effects of dredging on nitrogen cycling, this in situ microcosm study was deployed in the lake for 2 years.
Methods and materials
Study site
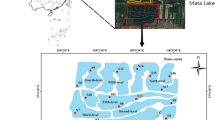
Lake Taihu is the third largest freshwater lake located in the southern part of the Delta of the Yangtze River in China (Fig. 1). Since the mid-1980s, Microcystis algal blooms have occurred every summer in the northern part of the lake, with increasing severity in recent years. Subsequently, drinking water supply has often been limited, which has caused massive economic loss (Qin et al. 2006). Some dredging projects have been conducted starting in 2009, and many more dredging plans are being developed or will be put into practice for pollution control of Lake Taihu. Meiliang Bay is located at the north of Lake Taihu with an area of 130 km2. The water at the study site has vital biological functions, not only as landscape water used for navigational purposes and fishing but also as a source of drinking water for the city of Wuxi. Two stations, in the inner bay and the outer bay, were selected considering the pollution state characteristics of Meiliang Bay. The inner bay (31° 31′ 33.9″ N, 120° 12′ 35.2″ E) is enriched with TOC, TN, and TP in sediments by receiving a greater proportion of municipal and industrial waste water from the Liangxihe River (yearly average values of overlying water NH4 +-N, NO3 −-N, and NO2 −-N were 3.92, 0.66, and 0.12 mg L−1 from January 2006 to January 2007, respectively), and the TOC and TN content were much greater than those of paddy soil in the Taihu region (ranging from 0.18 to 2.8 % and 0.047 to 0.09 3% of TOC and TN) (Xu et al. 1980). The outer bay (31° 25′ 49.3″ N, 120° 12′ 27.7″ E) is situated at the mouth of the Meiliang Bay with a distance of 1 km away from the Taihu Laboratory for Lake Ecosystem Research (TLLER) (31° 25′ 10.78″ N, 120° 12′ 50.7″E), with less TOC, TN, and TP than the inner bay, and with TOC and TN contents less than those of the paddy soil of the Taihu region (Xu et al. 1980; Zhong et al. 2010a, b).
Experimental set-up of in situ dredging simulation study
A total of 30 intact sediment cores were collected with a large caliber core sampler (Φ110 mm, length 500 mm, Rigo Co., Japan) at either site. To decrease the heterogeneity of sediments, the cores were systematically collected in a grid within the 2 × 2 m square. The cores collected were generally more than 400 mm deep, and they were filled up with near-bottom water from the same location and closed with rubber stoppers to avoid sediment oxidation during sampling and transportation. With this sampling procedure, the surfaces of sediment core remained undisturbed. Simultaneously, some subsamples of surface sediments (0–2 cm) were rapidly sliced onboard within a few minutes to minimize changes resulting from exposure of sediment surfaces to the atmosphere. Overlying water samples were also collected at each location using 25 L polyethylene bottles.
In the laboratory, sediment cores from either site were treated as follows. The overlying water of all sediment cores was siphoned. The simulating dredging depth was 25 cm sediment based on practical dredging projects in Lake Taihu, and the sediment height used in the study was approximately 12 cm. Subsequently, the surface sediments of three cores for each site were removed by suction pump, and the underlying 12 cm layer of sediment was filled into the tubes undisturbed as the dredged treatments. As for the control treatment, the top 12 cm sediment layers of three cores for each site were cut and carefully transplanted into the Plexiglas cylinders (11 cm inner diameter, 17 cm height). A total of 48 cores were selected to determine the rates of nitrification, denitrification, and anammox over the course of 1 year, and sampled in October 2011, and January, April, and July 2012. The overlying water was carefully dropped onto the sediment cores immediately. To better mimic the environmental conditions in the lake, undredged cores and dredged cores were incubated on the lake bottom, situated at the observation platform of the TLLER. All prepared cores were put into the stainless shelf with six units for each shelf. In addition, each core was covered completely with fine mesh (ca. 0.0374 mm) nylon screening to restrict access of large aquatic fauna. Then, the shelf was fastened to the bridge of the observation platform using nylon line, which was put down to the sediment surface of the lake (ca. 1.5 m). In the incubation period from August 2011 to July 2013, sampling was carried out six times (at 3, 30, 90, 180, 360, and 720 days after dredging). At the end of the experiments, the sediment cores were sliced at 1 cm intervals, and the sediment subsamples were freeze-dried and sieved through 100 mesh sieves for further analysis.
Collection of pore water
The pore water sample was collected by using a nondestructive method to portray the nutrient profiles in interstitial water at different dredging times. Rhizon soil moisture samplers (Rhizon SMS: Rhizosphere Research Products, the Netherlands) were used to extract successive pore water samples from the microcosm sediments at depths of 0.5, 1.5, 2.5,3.5, 5, 7, and 9 cm. Holes for Rhizon SMS Samplers in the Plexiglass tube walls were sealed in advance with hydrophobic tape. Owing to the pore water samples separated using negative pressure extraction, the overlying water in the sediment cores were extracted using a syringe before sampling to avoid the diffusion of overlying water into the interstitial water under negative pressure. At each site, approximately 5 mL of pore water was obtained through suction and was filtered in the field by 0.45 μm aperture GF/F filter membranes. Then, the pretreated samples were immediately transported to the laboratory in ice boxes for determination of NH4 +-N, NO3 −-N, and NO2 −-N using a Skalar flow-injection analyzer (Skalar Sanplus, the Netherlands).
Nitrification potential rate measurement
The potential nitrification rate of sediments was measured by using the modified method (Dollhopf et al. 2005; Hall, 1984) with the specific inhibitor of nitrification, allylthiourea (ATU), which blocks the NH4 +-N to the NH2OH step of ammonium oxidation to ensure that nitrification does not occur. Each subsample of surface sediment was sampled and homogenized, and one aliquot of sediment (ca. 5 g of wet sediment) was placed into 250 mL Erlenmeyer flasks. Each treatment had three replicates, and the known amount of NH4 +-N was added as (NH4)2SO4 to obtain the initial NH4 +-N concentration of 14 mg L−1 (1000 μmol L−1). The ATU was added to the flasks of the inhibited group to attain a final ATU concentration of 10 mg L−1, and the uninhibited group only contained the (NH4)2SO4 solution. Subsequently, all of the flasks were capped with gauze to exclude external bacteria, then covered with aluminum foil and incubated in a shaker incubator at 25 °C. There were five sampling times carried out at 0, 6, 12, 18, and 24 h during the whole incubation. After incubation, the overlying water of each flask was filtered through previously ignited and washed Whatman GF/F filters, and the NH4 +-N and NO3 −-N concentrations were further determined. Potential nitrification rates were calculated according to the NH4 +-N concentration differences between the inhibited and uninhibited flasks as follows:
where NP is the potential nitrification rate (mmol m−2 h−1), ρ is the bulk density of sediment (kg m−3), h is the sampling depth of sediment (m), and V 1 and V 2 are solution volume and pore water of sediment (L), respectively, k1 and k0 represent the variations of NH4 +-N between the inhibited and uninhibited group with time (μmol L−1 h−1), and w is the dry weight of sediment used in incubation.
Simultaneous determination of denitrification and anammox
The system described by Nishio et al. (1982) and Usui et al. (2001) was used to simultaneously measure denitrification and anammox with some modifications. Specifically, the intact sediment cores were capped with a Plexiglas lid with an O-ring to avoid leaving headspace in the cores, were wrapped with aluminum foil to keep light out, and were placed in a temperature-controlled incubation water bath under in situ temperature. The continuous flow-through chamber setup consisted of an intake water vessel, Teflon flow tubes, a peristaltic pump, and a sample collection vessel. The influent water was sampled from in situ near-bottom lake water, of which the concentration was adjusted to 100 μmol L−1 using 15NO3 −-N (final % 15N ca.90–99 %, depending on the background nitrate concentration). Then, the cores were incubated under in situ conditions for approximately 24 h to achieve an equilibrium state. During the course of incubation, the intake water was pumped into the chamber using a peristaltic pump. The inlet vessel was lower than that of the output vessel, which was located at the position approximately 1 cm above the SWI. Resulting from horizontal and vertical flow in cores, the intake water could be mixed completely. As for field conditions, it was crucial to select the appropriate water flow used in the experiment mainly based on the oxygen consumption of the overlying water and the characteristics of SWI. In this study, according to the monthly field observation in Lake Taihu (Peter et al. 2005), to avoid air introduction to the mixing chamber, total flow rates of waters into the mixing chamber were set at 1 mL L−1. Oxygen saturation was retained ranging from 7 to 29 %, with a mean value of 16 % (7.62 mg L−1 inlet water, 5.40 mg L−1 output water), and oxygen concentration in the influent and effluent were determined simultaneously. Incubation temperature was set at 15 °C in spring and autumn, 30 °C in summer, and 5 °C in winter. The experiments were continued for 24 h, and the influent water and overflowed effluent samples were collected at the 12-h interval using gas-tight polyethylene bottles with 250 μL of 50 % ZnCl2 solution and analyzed within 6 h. At that moment, several samples were collected and filtered by baked Whatman GF/F filters to determine the NO3 −-N concentration in the water samples. Dissolved N2 compound were determined by membrane inlet mass spectrometry (MIMS) with a detection limit of 0.06 μmol L−1 (Yin et al. 2015).
The rates of the denitrification and anammox processes across the SWI were calculated from the differences in concentrations of dissolved nitrogen gases between influent and effluent samples, cross-sectional area of the core, and flow rates, which were expressed as micromoles of N per square meter per hour. Both the calculations of denitrification rates and anammox rates adopted the method used by Yin et al. (2015) and Nielsen (1992) and Thamdrup and Dalsgaard (2002).
Quantification of physicochemical characteristics
To determine the sediment core oxygen microprofiles, oxygen concentration was measured by an optical fiber O2 microelectrode with a tip diameter of less than 0.1 mm (Presens, Germany). Sediment water content was determined by drying fresh sediment to a constant weight at 105 °C. Porosity and bulk density were measured with the use of a cutting ring (Graca et al. 2004). TN and TP in sediments were measured by the potassium persulfate oxidation method, and the TOC in sediments was measured by the method of potassium dichromate oxidation-ferrous sulfate titrimetry (Jin and Tu 1990). The NH4 +-N, NO3 −-N, and NO2 −-N concentrations in surface water and pore water were filtered (Whatman GF/F) and measured by a flow-injection autoanalyzer (Skalar Sanplus, The Netherlands).
Calculation of nutrient fluxes
Under steady state conditions, the nutrient fluxes across the SWI occurred mainly through chemical diffusion induced by the concentration gradient. The amounts of nutrient fluxes across the SWI can be calculated according to Fick’s first law of diffusion (Ullman and Aller 1982):
where ∅ is the porosity of surficial sediment, and \( \frac{\partial C}{\partial x}\;\left|x=0\right. \) is the concentration gradient between the surficial sediment and bottom water, determined as the concentration difference between the interstitial water sample and the bottom lake water. The elaborated operation for the concentration gradient was adopted from the study performed by Zhong et al. (2008). D s is the sediment diffusion coefficient for various nutrients.
There is an empirical equation provided by Ullman and Aller (1982) for the relationship between the real diffusion coefficient and the porosity:
where D o is the ideal diffusion coefficient of the infinite dilution solution. The diffusion coefficients of NH4 +, NO3 −, and NO2 − at 25 °C are 17.6 × 10−6 cm2 s−1, 19.0 × 10−6 cm2 s−1, and 19.1 × 10−6 cm2 s−1, respectively. The D s value was corrected for the field temperature and was calculated using the means of three replicates of porosity in the upmost 2 cm depth sediments.
Mass balance in individual sediment cores
The rough budgets constructed for N loss from sediments induced by dredging was mainly including the diffusion fluxes of inorganic N from pore water and the microbial denitrogen processes associated with denitrification and anammox processes. Simultaneously, we also took the N fixation process into consideration, because the N fixation rate in Lake Taihu was an important source compared with microbial denitrogen mass (Zhang 2012). The loss of N from sediments caused by dredging can be calculated as follows:
where M represents the total amount of N loss from sediments induced by dredging, R 1, R 2, R 3, and R 4 are the denitrification rates, anammox rates, diffusion fluxes of inorganic N, and N fixation rates in control sediments, respectively; r 1, r 2, r 3, and r 4 represents the denitrification rates, anammox rates, diffusion fluxes of inorganic N, and N fixation rates in dredged sediments. S is the area, and the constant 365 represents 1 year.
Statistical analysis
The data shown in this study were represented as the means and standard deviations of three replicates. The differences of NH4 +-N, NO3 −-N, and NO2 −-N concentrations of pore water in different experimental treatments, sampling times, and sediment depths were tested by two-way repeated measures analysis of variance (RM-ANOVA) with the time as the repeated factor. If a significant difference was determined, Tukey’s post hoc test was used to detect the differences between treatments and depths. Likewise, the differences in NH4 +-N, NO3 −-N, and NO2 −-N fluxes across the SWI were also tested by one-way RM-ANOVA with time as the repeated factor. The differences in the nitrification rates, denitrification rates, denitrification rates of nitrate from coupled nitrification (Dn), and from the overlying water (Dw) and anammox rates across SWI were detected by one-way RM-ANOVA, with time as the repeated factor. Pearson correlation analysis was used to test the correlation between the denitrification rates and anammox rates. The paired sample t-test was used to test for significant differences in the variables of the mass of nitrogen loss between undredged and dredged samples. All statistical analyses in this study were performed by SPSS 13.0 (SPSS, USA).
Results
Sediment characterization
The data for undredged and dredged sediments showed large spatial variances between the inner bay and the outer bay; the TOC, TN, and TP contents of the inner bay were generally much greater than those of the outer bay (Fig. 2). The tendencies of water content, porosity, TOC, TN, and TP of sediments generally decreased with depth, whereas the bulk density increased with depth in both the inner bay and the outer bay. Elevated water content and porosity in the outer bay were recorded in the undredged treatment all over the depths. The bulk density, TOC, TN, and TP in the outer bay exhibited higher values in undredged samples at depths of 0–4 cm than those of the dredged samples and changed with a reciprocating oscillation trend at depths of 4–9 cm. In the inner bay, porosity and bulk density in dredged sediments were higher at depths of 2 and 9 cm, respectively, compared with undredged sediments, and others were similar to the changes in the outer bay, which indicated an opposite effect of dredging in these two sites for porosity and bulk density. The penetration depths of oxygen in dredged sediments were generally shallower than those in undredged samples (Fig. 3).
Fluxes of inorganic nitrogen release across the SWI
The inorganic nitrogen fluxes were calculated on the basis of Fick’s first law. During the experiment, sediment acted as a nitrogen source to the water column, and the nitrogen release was dominated by NH4 +-N (Table 1). In the inner bay, the release rates of NH4 +-N in undredged sediments were much higher than those in dredged sediments (Tukey’s HSD test, P < 0.05), and the peak fluxes were observed after dredging at day 720. In the outer bay, the dredging significantly increased the release of NH4 +-N from day 3 to day 180 (Tukey’s HSD test, P < 0.05). However, sediment was a sink of NO3 −-N in overlying water, except for the NO3 −-N fluxes on day 720. The NO2 −-N fluxes were similar between different treatments (Tukey’s HSD test, P > 0.05), and the sediment served as a sink initially and then served as a source in both sites (Table 1).
Nitrification
Nitrification rates of undredged sediments were much greater than those of dredged sediments (Fig. 4), and the differences between control and dredged sediments were significant in the inner bay (P < 0.05) but not for the outer bay (Table 2). As for the chronological changes in nitrification rates, the nitrification rates of dredged sediments attained the peak value after dredging for 180 days at both sites, whereas the values of the control group had no similar pattern.
Nitrification potential rates in the undredged and dredged sediments of the inner bay (a) and the outer bay (b) after different dredging times. All results are expressed as the mean ± SD of three replicates. The P value shown in figures indicate the significant difference between control and dredged sediments during the entire experiment using one-way repeated analysis of variance (RM-ANVOA) with time as the repeated factor
Denitrification
Figure 5 shows the denitrification variations of the control and dredged treatments with time ongoing. The denitrification rates of undredged and dredged sediments increased gradually from day 90 to day 270 in both the inner bay and the outer bay (Table 2) but drastically decreased on day 360 (Fig. 5). In principle, the denitrification rates of the undredged treatment group were significantly greater than those of the dredged treatment over the whole experiment in the inner bay (Table 2; Fig. 5a) but not for the outer bay (Fig. 5b).
Denitrification rates and denitrification rates of nitrate from coupled nitrification (Dn) and from the overlying water (Dw) across the sediment-water interface in the undredged and dredged sediments of the inner bay (a, c, e) and the outer bay (b, d, f) after different dredging times. All results are expressed as the mean ± SD of three replicates. The P value showed in figures indicate the significant difference between control and dredged sediments during the entire experiment using one-way repeated analysis of variance (RM-ANVOA) with time as the repeated factor
In the inner bay and the outer bay, Dn and Dw rates were significantly correlated with both the denitrification and anammox rates (P < 0.01; Figs. 5 and 6). Both the rates of Dn and Dw increased from day 90 to day 270 but sharply decreased on day 360, and the maximum values and much lower values were achieved on day 270 and days 90 and 360 in the inner and outer bays (Fig. 5). There was a significant difference in Dn between the undredged and dredged sediments but no significant difference in Dn in the outer bay and Dw in both the inner and outer bays (Fig. 5).
Anammox
Anammox rates changed in a similar pattern to denitrification rates (Figs. 5 and 6). Significant differences in anammox rates were determined between the control and dredged treatments, and the anammox rates of the undredged group were generally greater than those of the dredged treatment (Fig. 6). The maximum values of both control and dredged treatments appeared on day 270, but much lower values occurred on day 90 and day 360 at both sites (Fig. 6).
Anammox rates across the sediment-water interface in the undredged and dredged sediments of the inner bay (a) and the outer bay (b) after different dredging times. All results are expressed as the mean ± SD of three replicates. The P value showed in figures indicate the significant difference between control and dredged sediments during the entire experiment using one-way repeated analysis of variance (RM-ANVOA) with time as the repeated factor
Discussion
The effects of dredging on nitrogen removal across the SWI
The fluxes of ammonium demonstrated that the sediment was the main source of the overlying water, but not for NO3 −-N and NO2 −-N (Table 1). The ammonium fluxes induced by dredging were effectively reduced in the inner bay, which were identical to previous study by Zhong et al. (2009). In the inner bay, this result may be attributed to the following four reasons: (1) Ammonium release was due to the basic processes of ammonification and DNRA; similarly, dredging resulted in substantially lower redox potentials and oxygen depletion rates in sediment, which enhanced the DNRA and ammonification processes (Yin et al. 2002; Fig. 3). (2) The porosities of dredged sediment were lower than those of the control treatment (Fig. 2). Consequently, the diffusion of ammonium into the overlying water is hampered by the compact sediment of dredged cores (Zhong et al. 2009). (3) The presence of major factors blocking ammonium migration in sediment that was adsorbed by soil colloids such as Fe/Mn oxides and organic matter, and precipitated with fine-grained sediment particles. Fan et al. (2004) showed that the average pore water NH4 +-N concentration showed a downward increasing trend from SWI to bottom sediment in Meiliang Bay, resulting from the lower redox potential and dissolved oxygen in subsurface sediment (Fan et al. 2000; Fig. 3). Therefore, the NH4 +-N−rich sediment layer was overturned to the surface. (4) The result may be driven by ammonium concentrations in preexisting pore water and adsorbed NH4 +-N concentrations plus any ongoing N regeneration (Morin and Morse 1999; Reddy et al. 2007).
Dredging had minor effect on the fluxes of NO3 −-N and NO2 −-N, which demonstrated that the concentrations of NO3 −-N and NO2 −-N were similar between pore water and overlying water (Table 1; Fig. S1) despite the potential nitrification rates of undredged sediments that were much greater than those of dredged sediments, and it was true for the denitrification rates (Fig. 5). Therefore, the reasons for these results were mostly due to nitrification undergoing anammox or denitrification in the sediment; furthermore, it could also be assimilated by the phytoplankton.
Basic microbial processes to which nitrogen was subjected in the sediment included denitrification, nitrification, ammonification, anammox, and DNRA processes, all of which may result in nitrogen removal from the sediment (Hanaki et al. 1990; Zhong et al. 2010a, b; Trimmer et al. 2003; Wang and Li, 2015). In the present study, the rates of denitrification and anammox induced by dredging were significantly reduced in the inner bay, but not for the denitrification rates of the outer bay (Figs. 5 and 6). These differences can be attributed to complicated environmental factors such as nitrate concentration (Liu et al. 2015; Venterrink et al. 2003; Zhong et al. 2010a, b; Seitzinger 1994), quality and quantity of the organic carbon (Bastviken et al. 2005), soil texture (D’Haene et al. 2003), oxygen concentration (Cavari and Phelps 1977), temperature (Mogge et al. 1999), light conditions, salinity (Toet et al. 2003), and bioturbation of benthic animals (Svensson and Leonardson 1996). There were five reasons for this given as follows. (1) The new surface sediments after dredging were buried in the deeper depth below the bottom for several decades, even for hundreds of years, and the labile organic matter content and bioavailability were limited in sediments after dredging (Pinay et al. 2003; Bastviken et al. 2005). (2) The microbial activity and functional diversity of the microbial community in dredged sediments were significantly lower than those of in undredged sediments (Zhong et al. 2010a, b; Wang and Li, 2015). Furthermore, previous study showed that adding glucose can increase the denitrification rate in the outer bay (Zhong et al. 2010a, b; Chen et al. 2015), which can also be confirmed by the lower TOC content in dredged sediments compared with undredged sediments (Fig. 2). (3) There were lower water contents, porosities, oxygen depletion rates, and oxygen penetration depths in dredged sediments than those of in the undredged treatment (Figs. 2 and 3). (4) Bioturbation stimulated the denitrification in the undredged sediments, whereas bioturbation in dredged sediments was weakened as benthic animals located in the surface sediment were removed by dredging (Svensson and Leonardson 1996). (5) Above all, nitrate from the water and sediment was considered to be the key factor in regulating the denitrification. In the present study, the denitrification rates of nitrate from overlying water in dredged sediments was much higher than that in un-dredged sediments during the experiment (Table 2). This result was accordant with results from Christensen et al. (1990) who also demonstrated that diffusion of nitrate from the overlying water into the sediment was the major nitrate source for denitrification in a Danish nitrate-rich stream.
Note that the rates of anammox decrease was partly due to the denitrification process. It was unexpected that the temporal changing in denitrification rates was significantly correlated with the anammox rates during the entire simulation course (P < 0.01; Figs. 5 and 6). Previous work showed that anaerobic ammonium oxidation coupled the oxidation of NH4 + to the reduction of NO2 − as in the following equation:
As in the above general reaction, the concentration of NO2 −, which serves as an electron acceptor, rapidly increases by denitrification. This process can effectively enhance the anammox process in dredged sediment (Trimmer et al. 2003). The results also indicated that the denitrification rates significantly correlated with anammox rates in sediments of both the inner bay and the outer bay (R2 = 0.959 for undredged sediments in the inner bay, R2 = 0.990 for dredged sediments in the inner bay, R2 = 0.993 for undredged sediments in the outer bay, and R2 = 0.933 for dredged sediments in the outer bay), which was also verified by previous study (Rysgaard et al. 2004).
The implications of dredging on nitrogen cycling under different pollution statuses of sediments
Nitrogen cycling in sediments was commonly regulated by organic matter decomposition, sediment deposition rates, bioturbation/bioadvection, and rates of microbial nitrogen transformation (Joye and Anderson 2008). There were different water quality statuses and diverse ecological types across Lake Taihu (Duan et al. 2009; Ma et al. 2008). In such cases, some dredging plans were implemented or will be put into practice mainly in the northern bays to reduce internal nutrient loading of sediments. To date, more attention has been paid to evaluate the environmental effectiveness of dredging projects, but little knowledge is available concerning the implications of dredging to nitrogen mass balance under different pollution statuses of sediments. On the other hand, the TOC, TP, and TN contents of the inner bay sediments were generally greater than those of the outer bay, and the TOC and TP in the inner bay were approximately twice as high as those of the outer bay (Fig. 2). Therefore, nitrate removal after dredging was inevitably influenced by pollution status (Fan et al. 2004). Taken together, it was vital to assess the implications of dredging to nitrogen cycling in lake ecosystem restoration.
First, note that the patterns of change in NH4 +-N fluxes of the inner bay and the outer bay were opposite(Table 1). The opposite changing pattern for the inner bay and the outer bay may be attributed to several reasons as follows: (1) The NH4 +-N flux was regulated by the flux of preexisting pore water and adsorbed NH4 +-N concentrations plus any ongoing N regeneration, and this process was mainly driven by the decomposition of organic nitrogen (Reddy et al. 2007; Zhong et al. 2009). This result could be further validated by the TOC content of sediments in inner bay (Fig. 2). (2) The TOC and TN contents generally decreased with depth at both sites, and the NH4 +-N release of the outer bay may be driven by the preexisting pore water because the NH4 +-N-rich sediment layer was overturned to the surface (Fan et al. 2000; Fig. 2). However, the TOC and TN-rich sediment was removed by dredging, and the fresh surface sediment had a lower NH4 +-N gradient potential between the pore water and the overlying water, which resulted in a lower NH4 +-N flux (Yu et al. 2012). (3) The main basic microbial processes for NH4 +-N release usually include denitrification, nitrification, ammonification, anammox and DNRA processes, all of which may result in changes in NH4 +-N fluxes (Hanaki et al. 1990; Zhong et al. 2010a, b; Trimmer et al. 2003). The yearly average potential nitrification rates of dredged sediments for the inner bay were greater than those of the outer bay. The same was true for the yearly average denitrification and anammox rates (Figs. 4, 5, and 6), which demonstrated the lower capacity of the outer bay to transform NH4 +-N to NO3 −-N compared with that of the inner bay, resulting from the lower abundance of nitrifying bacteria, the lower energy yield of nitrification, and the lower nitrifier biomass of freshly generated surface sediments in the outer bay (Cornwell and Owens 2011).
A second important issue was how to budget the associated denitrogen mass caused by dredging in the inner bay and the outer bay. Using the information from the fluxes of inorganic nitrogen and dissimilatory microbial processing of N, we can construct a rough budget for N induced by dredging in both the inner bay and the outer bay (Fig. 7). The total mass of nitrogen removal associated with denitrification and anammox processes was estimated to be 486.80 and 240.36 μmol m−2 day−1 of undredged and dredged sediments, respectively; in the inner bay, 440.62 and 397.41 μmol m−2 day−1 of undredged and dredged sediments in the outer bay (Fig. 7). More specifically, the rates of denitrification were 457.25 and 233.07 μmol m−2 day−1 for undredged and dredged sediments in the inner bay and 417.13 and 375.89μmol m−2 day−1 for undredged and dredged sediments in the outer bay, respectively. The rates of anammox were 23.39 and 2.35 μmol m−2 day−1 for undredged and dredged sediments in the inner bay and 17.9 and 14.58 μmol m−2 day−1 for undredged and dredged sediments in the outer bay, respectively. In terms of nitrogen releases from sediment to overlying water, the sediment was a main source of ammonium based on Fick’s first law, but not for NO3 −-N and NO2 −-N (Table 1; Fig. S1). Additionally, taking into consideration the nitrogen fixation rates in the overlying water and sediment at the above two sites in Meiliang Bay, the rates of microbial nitrogen fixation in Meiliang Bay were 10.03 and 6.10 μmol m−2 day−1 for the dredged and undredged treatments in the inner bay and 10.04 and 6.10 μmol m−2 day−1 for the dredged and undredged treatments in the outer bay, respectively (Zhang 2012). Moreover, the final denitrogen mass was 476.77and 234.26 μmol m−2 day−1 for the control and dredged treatments in the inner bay and 430.58 and 391.31 μmol m−2 day−1 for the control and dredged treatments in the outer bay, respectively (Fig. 7). Based on the above analyses, the mass of denitrogen and diffusive fluxes induced by dredging in Meiliang Bay were decreased by 161.10 and 26.09 t N a−1 for the inner bay and the outer bay, respectively (with an area of 130 km2 for Meiliang Bay), suggesting that dredging had a negative effect on decreasing nitrogen removal from sediments in Meiliang Bay, Lake Taihu. In addition, the nitrogen removal induced by dredging was mainly by denitrification process (Liu et al. 2014), accounting for 90.97 and 95.44 % for the inner bay and the outer bay, respectively. Therefore, the results demonstrated that dredging may be more applicable to heavily polluted sediments, and the reasons may be attributed to the fact that higher nutrient concentrations and organic matter contents in sediments will enhance water-sediment interface processes and nutrient regeneration (Fan et al. 2004). Dredging with certain depth can effectively reduce the nutrient loading in sediments but also can reduce the release of ammonium across the SWI.
Rough budgets constructed for the nitrogen balance of undredged and dredged sediments at two sites in Meiliang Bay, Lake Taihu: a inner bay + undredged; b inner bay + dredged; c outer bay + undredged; d outer bay + dredged. Units are μmol m−2 day−1 for all processes, which are denoted with superscript. Data for N fixation rates in the overlying water and sediments at the two sites in Meiliang Bay originate from Zhang’s Master’s degree thesis. The red line represents the N loss out of sediments, and the blue line represents the N transfer into sediment
Conclusions
The results showed that, with regard to the pollution status of sediments, simulated dredging implemented in TOC and/or TN-rich sediments can slightly reduce the NH4 +-N release rates from sediments, whereas the temporary NH4 +-N release (from day 3 to day 180 after dredging) induced by dredging in sediments with less TOC and/or TN would be enhanced. Moreover, dredging had minimal effects on the fluxes of NO3 −-N and NO2 −-N from sediments of both sites. Further analysis demonstrated that dredging would decrease the rates of nitrification, denitrification, and anaerobic ammonium oxidation in sediments, especially in TOC and/or TN-rich areas. After the sediment dredging, the nitrogen removal was reduced by 161.10 and 26.09 t N a−1 for the inner bay and the outer bay, respectively. Taken together, this field study demonstrated that dredging may be more applicable to nutrients and TOC-rich sediments in minimizing the internal N pollution once the external N pollution was controlled.
References
Bastviken SK, Eriksson PG, Premrow A, Tonderski K (2005) Potential denitrification in wetland sediments with different plant species detritus. Ecol Eng 25:183–190
Bowman JC, Readman JW, Zhou JL (2003) Seasonal variability in the concentrations of Irgarol 1051 in Brighton Marina, UK; including the impact of dredging. Mar Pollut Bull 46:444–451
Bremle G, Larsson P (1998) PCB concentration in fish in a river system after remediation of contaminated sediment. Environ Sci Technol 32(22):3491–3495
Cavari BZ, Phelps G (1977) Denitrification in lake Kinneret in the presence of oxygen. Freshwater Biol 7:385–391
Chen HB, Wang DB, Li XM, Yang Q, Zeng GM (2015) Enhancement of post-anoxic denitrification for biological nutrient removal: effect of different carbon sources. Environ Sci Pollut Res 22:5887–5894
Christensen PB, Nielsen LP, SØrensen J, Revsbech NP (1990) Denitrification in nitrate-rich streams: diurnal and seasonal variation related to benthic oxygen metabolism. Limnol Oceanogr 35:640–651
Cornwell JC, Owens MS (2011) Quantifying sediment nitrogen releases associated with estuarine dredging. Aquat Geochem 17:499–517
D’Haene K, Moreels E, De Neve S, Daguilar BC, Boeckx P, Hofman G, Cleemput OV (2003) Soil properties influencing the denitrification potential of Flemish agricultural soils. Biol Fertile Soils 38:358–366
Dollhopf SL, Hyun JH, Smith AC, Adams HJ, O’Brien S, Kostka JE (2005) Quantification of ammonia-oxidizing bacteria and factors controlling nitrification in salt marsh sediments. Appl Environ Microb 71(1):240–246
Duan HT, Ma RH, Xu XF, Kong FX, Zhang SX, Kong WJ, Hao JY, Sheng LL (2009) Two-decade reconstruction of algal blooms in China’s Lake Taihu. Environ Sci Technol 43(10):3522–3528
Falcào M, Gaspar MB, Caetano M, Santos MN, Vale C (2003) Short-term environmental impact of clam dredging in coastal waters (south of Portugal): chemical disturbance and subsequent recovery of seabed. Mar Environ Res 56:649–664
Fan CX, Yang LY, Zhang L (2000) The vertical distributions of nitrogen and phosphorus in the sediment and interstitial water in Taihu Lake and their interrelations. J Lake Sci 12(4):359–366 (in China)
Fan CX, Zhang L, Wang JJ, Zheng CH, Gao G, Wang SM (2004) Processes and mechanism of effects of sludge dredging on internal source release in lakes. Chinese Sci Bull 49(17):1853–1859 (in China)
Graca B, Burska D, Matuszewska K (2004) The impact of dredging deep pits on organic matter decomposition in sediments. Water Air Soil Poll 158:237–259
Gustavson K, Allen Burton G, Francingues NJR, Reible DD, Vorhees DJ, Wolfe JR (2008) Evaluating the effectiveness of contaminated-sediment dredging: as the science of environmental dredging and sediment management changes, adaptive management strategies can help long-term remediation projects keep pace. Environmen Sci Technol 15:5042–5047
Hall GH (1984) Measurement of nitrification rates in lake sediments: comparison of the nitrification inhibitors nitrapyrin and allylthiourea. Microbial Ecol 10:25–36
Hanaki K, Wantawin C, Ohgaki S (1990) Nitrification at low levels of dissolve oxygen with and without organic loading in a suspended-growth reactor. Water Res 24(3):297–302
Jin XC, Tu QY (1990) The standard methods in lake eutrophication investigation, 2nd edn. China Environmental Science Press, Beijing
Jin XC (2003) Analysis of eutrophication state and trend for lakes in China. J Limnol 62(2):60–66
Jing LD, Wu CX, Liu JT, Wang HG, Ao HY (2013) The effects of dredging on nitrogen balance in sediment-water microcosms and implications to dredging projects. Ecol Eng 52:167–174
Joye SB, Anderson IC (2008) Nitrogen cycling in coastal sediments. In: Capone DG, Bronk DA, Mulholland MR, Carpenter EJ (eds) Nitrogen in the marine environment, 2nd edn. Academic, Amsterdam, pp 868–915
Kleeberg A, Kohl JG (1999) Assessment of the long-term effectiveness of sediment dredging to reduce benthic phosphorus release in shallow lake Müggelsee (Germany). Hydrobiologia 394:153–161
Lewis MA, Weber DE, Stanley RS, Moore JC (2001) Dredging impact on an urbanized Florida bayou: effects on benthos and algal-periphyton. Environ Pollut 115:161–171
Liu DD, Li ZK, Zhang WG (2014) Nitrate removal under different ecological remediation measures in Taihu Lake: a 15N mass-balance approach. Environ Sci Pollut Res 21:14138–14145
Liu TZ, Yuan JJ, Dong WY, Wu HC, Wang HJ (2015) Effects on inorganic nitrogen compounds release of contaminated sediment treatment with in situ calcium nitrate injection. Environ Sci Pollut Res 22:1250–1260
Lohrer AM, Wetz JJ (2003) Dredging-induced nutrient release from sediments to the water column in a southeastern salt marsh tidal creek. Mar Pollut Bull 46:1156–1163
Ma RH, Duan HT, Gu XH, Zhang SX (2008) Detecting aquatic vegetation changes in Taihu Lake, China using multi-temporal satellite imagery. Sensors 8(6):3988–4005
Maguire JA, Coleman A, Jenkins S, Burnell GM (2002) Effects of dredging on undersized scallops. Fish Res 56:155–165
Mogge B, Kaiser EA, Munch JC (1999) Nitrous oxide emissions and denitrification N-losses from agricultural soils in the Bornhoved lake region: influence of organic fertilizers and land-use. Soil Biol Biochem 31:1245–1252
Morin J, Morse JW (1999) Ammonium release from resuspended sediments in the Laguna Madre estuary. Mar Chem 65:97–110
Nielsen LP (1992) Denitrification in sediment determined from nitrogen isotope pairing. Fems Microbiol Ecol 86:357–362
Nishio T, Koike I, Hattori A (1982) Denitrification, nitrate reduction, and oxygen consumption in coastal and estuarine sediments. Appl Environ Microb 43(3):648–653
Pan G, Dai LC, Li L, He LC, Li H, Bi L, Gulati RD (2012) Reducing the recruitment of sedimented algae and nutrient release into the overlying water using modified soil/sand flocculation-capping in Eutrophic lakes. Environ Sci Technol 46:5077–5084
Peter K, Zhu W, Mario M (2005) Water and mass budgets for estimating phosphorus sediment-water exchange in Lake Taihu (China P. R). Hydrobiologia 544:167–175
Pinay G, O’Keefe T, Edwards R, Naiman RJ (2003) Potential denitrification activity in the landscape of a western Alaska drainage basin. Ecosystems 6:336–343
Pu PM, Wang GX, Hu CH, Fan CX (2000) Can we control lake eutrophication by dredging? J Lake Sci 12(3):269–279 (in China)
Qin BQ, Yang LY, Chen FZ, Zhu GW, Zhang L, Chen YY (2006) Mechanism and control of lake eutrophication. Chinese Sci Bull 51(19):2401–2412 (in China)
Qin BQ, Zhu GW (2006) The nutrient forms, cycling and exchange flux in the sediment and overlying water system in lakes from the middle and lower reaches of Yangtze River. Sci China (SerD) 49(1):1–13
Qin BQ, Hu WP, Gao G, Luo LC, Zhang JS (2004) Dynamics of sediment resuspension and the conceptual schema of nutrient release in the large shallow Lake Taihu, China. Chinese Sci Bull 49:54–64 (in China)
Recknagel F, Hosomi M, Fukushima T, Kong DS (1996) Short- and long-term control of external and internal phosphorus loads in lakes—a scenario analysis. Water Res 29:1767–1779
Reddy KR, Fisher MM, Wang Y, White JR, Thomas James R (2007) Potential effects of sediment dredging on internal phosphorus loading in a shallow, subtropical lake. Lake Reserv Manage 23:27–38
Rysgaard S, Glud RN, Risgaard-Petersen N, Dalsgaard T (2004) Denitrification and anammox activity in Arctic marine sediment. Limnol Oceanogr 49(5):1493–1502
Seitzinger SP (1994) Linkages between organic matter mineralization and denitrification in eight riparian wetlands. Biogeochemistry 25:19–39
Smith VH, Tilman GD, Nekola JC (1999) Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut 100:179–196
Spencer KL, Dewhurst RE, Penna P (2006) Potential impacts of water injection dredging on water quality and ecotoxicity in Limehouse Basin, River Thames, SE England, UK. Chemosphere 63:509–521
Svensson JM, Leonardson L (1996) Effects of bioturbation by tube-dwelling chironomid larvae on oxygen uptake and denitrification in eutrophic lake sediments. Freshwater Biol 35:289–300
Szymelfenig M, Kotwicki L, Graca B (2006) Benthic re-colonization in post-dredging pits in the Puck bay (southern Baltic sea). Estuar Coast Shelf S 68:489–498
Thamdrup B, Dalsgaard T (2002) Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microb 68(3):1312–1318
Toet S, Huibers LHFA, Van Logtesijn RSP (2003) Denitrification in the periphyton associated with plant shoots and in the sediment of a wetland system supplied with sewage treatment plant effluent. Hydrobiologia 501:29–44
Trimmer M, Nicholls JC, Deflandre D (2003) Anaerobic ammonium oxidation measured in sediments along the Thames estuary, United Kingdom. Appl Environ Microb 69(11):6447–6454
Tuck ID, Bailey N, Harding M, Sangster G, Howell T, Graham N, Breen M (2000) The impact of water jet dredging for razor clams, Ensis spp., in a shallow sandy subtidal environment. Journal of Sea Research 43:65–81
Ullman WJ, Aller RC (1982) Diffusion coefficients in nearshore marine sediments. Limnol Oceanogr 27:552–556
Usui T, Koike I, Ogura N (2001) N2O production, nitrification and denitrification in an estuarine sediment. Estuar Coast Shelf S 52:769–781
Van den berg GA, Meijers GA, Van der heijdt LM, Zwolsman JJG (2001) Dredging-related mobilization of trace metals: a case study in the Netherlands. Water Res 35(8):1979–1986
Venterrink HO, Hummerlink E, Van den hoorn MW (2003) Denitrification potential of a river floodplain during flooding with nitrate-rich water: grasslands versus reedbeds. Biogeochemistry 65:233–244
Wang L, Li T (2015) Effects of seasonal temperature variation on nitrification, anammox process, and bacteria involved in a pilot-scale constructed wetland. Environ Sci Pollut Res 22:3774–3783
Wang XY, Feng J (2007) Assessment of the effectiveness of environmental dredging in south lake, China. Environ Manage 40:314–322
Wang CH, Liu JF, Wang ZX, Pei YS (2014) Nitrification in lake sediment with addition of drinking water treatment residuals. Water Res 56:234–245
Wang CH, Yuan NN, Pei YS, Jiang HL (2015) Aging of aluminum/iron-based drinking water treatment residuals in lake water and their association with phosphorus immobilization capability. J Environ Manage 159:178–185
Xu Q, Lu YC, Liu YC, Zhu HG (1980) The paddy soil of Taihu region in China. Shanghai Scientific and Technical Press, Shanghai
Yenilmez F, Aksoy A (2013) Comparison of phosphorus reduction alternatives in control of nutrient concentrations in Lake Uluabat (Bursa, Turkey): Partial versus full sediment dredging. Limnologica 43:1–9
Yin GY, Hou LJ, Zong HB, Ding PX, Liu M, Zhang SF, Cheng XL, Zhou JL (2015) Denitrification and anaerobic ammonium oxidization across the sediment-water interface in the hypereutrophic ecosystem, Jinpu Bay, in the northeastern coast of China. Estuar and Coast 38:211–219
Yin HB, Kong M, Fan CX (2013) Batch investigations on P immobilization from wastewaters and sediment using natural calcium rich sepiolite as a reactive material. Water Res 47:4247–4285
Yin SX, Chen D, Chen LM, Edis R (2002) Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils. Soil Biol Biochem 34:1131–1137
Yu JH, Zhong JC, Zhang YL, Fan CX, He W, Zhang L, Tang ZW (2012) Influence of dredging on sediment resuspension and phosphorus transfer in lake: a simulation study. Environ Sci 22(7):961–967 (in China)
Zhang B (2012) The study of nitrogen fixation of water and sediment in Taihu Lake and the factors. Dissertation, University of Chinese Academy of Sciences (in China)
Zhong JC, Fan CX, Liu GF, Zhang L, Shang JG, Gu XZ (2010a) Seasonal variation of potential denitrification rates of surface sediment from Meiliang Bay, Taihu Lake, China. J Environ Sci 22(1):68–75 (in China)
Zhong JC, Liu GF, Fan CX, Bai XL, Li B, Zhang L, Ding SM (2010b) Environmental effect of sediment dredging in lake: IV. Influence of dredging on microbial activity and functional diversity of microbial community in sediments and its significance. J Lake Sci 22(1):21–28 (in China)
Zhong JC, Liu GF, Fan CX, Li B, Zhang L, Ding SM (2009) Environmental effect of sediment dredging in lake: II. The role of sediment dredging in reducing internal nitrogen release. J Lake Sci 21(3):335–344 (in China)
Zhong JC, You BS, Fan CX, Li B, Zhang L, Ding SM (2008) Influence of sediment dredging on chemical forms and release of phosphorus. Pedosphere 18(1):34–44 (in China)
Acknowledgments
This study was jointly sponsored by the research projects of the Major State Water Pollution Control and Treatment Technique Programs of China (Nos. 2012ZX07101010 and 2013ZX07113001) and the National Natural Science Foundation of China (Nos. 41171367 and 41371457). We do appreciate Dr. Zhonghua Zhao for their assistance in improving our manuscript. Furthermore, we appreciate the anonymous reviewers for their valuable comments and suggestions to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Pore water profiles of NH4 +-N, NO3 --N and NO2 --N in undredged and dredged sediments after different dredging times in the inner bay (square) and the outer bay (circle). The hollow and filled lines represent the undredged and dredged cores, respectively, and values are expressed as the mean±SD of three replicates.. Pore water chemistry. The concentrations of NH4 +-N, NO3 --N and NO2 --N in pore water of the undredged and dredged sediments are shown in Fig. S1. With respect to the concentration of nutrients in pore water between undredged and dredged treatments during the entire experiment, there were no significant differences (Tukey’s HSD test, P>0.05). Concentration gradient depths of NH4 +-N in both the inner and outer bays were generally similar, and much higher values were observed from day 3 to day 180 after dredging in dredged than in control treatments. On day 360, the NH4 +-N concentrations in dredged samples were positively controlled (Tukey’s HSD test, P<0.01) but reversed on day 720 (Fig. 2). In terms of the pore water NO3 --N concentration changes after different dredging times, the concentration gradient depths of NO3 --N of dredged sediments could be better controlled over the course of the experiment, with the exception of day 180 and day 360 in the inner bay and the outer bay, respectively. Because the concentration of NO2 --N in pore water was fairly low, ranging from 0 to 0.0339 mg·L-1 and 0 to 0.0115 mg·L-1 in the inner bay and the outer bay, respectively, the contribution of dredging to controlling the concentration of NO2 --N was also insignificant in this study (Tukey’s HSD test, P>0.05) (DOCX 289 kb)
Rights and permissions
About this article
Cite this article
Yu, J., Fan, C., Zhong, J. et al. Effects of sediment dredging on nitrogen cycling in Lake Taihu, China: Insight from mass balance based on a 2-year field study. Environ Sci Pollut Res 23, 3871–3883 (2016). https://doi.org/10.1007/s11356-015-5517-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5517-0