Abstract
Microbial sulfate reduction, a vital mechanism for microorganisms living in anaerobic, sulfate-rich environments, is an essential aspect of the sulfur biogeochemical cycle. However, there has been no detailed investigation of the diversity and biogenesis contribution of sulfate-reducing bacteria in arsenic-contaminated soils from realgar deposits. To elucidate this issue, soil samples from representative abandoned realgar deposits were collected. Microcosm assays illustrated that all three samples (2–1, 2–2, and 2–3) displayed efficient sulfate and As(V)-respiring activities. Furthermore, a total of 28 novel sequence variants of dissimilatory sulfite reductase genes and 2 new families of dsrAB genes were successfully identified. A novel dissimilatory sulfate-reducing bacterium, Desulfotomaculum sp. JL1, was also isolated from soils, and can efficiently respiratory reduce As(V) and sulfate in 4 and 5 days, respectively. JL1 can promote the generation of yellow precipitates in the presence of multiple electron acceptors (both contain sulfate and As(V) in the cultures), which indicated the biogenesis contribution of sulfate-reducing bacteria to the realgar mine. Moreover, this area had unique microbial communities; the most abundant populations belonged to the phyla Proteobacteria, Chloroflexi, and Acidobacteriota, which were attributed to the unique geochemistry characteristics, such as total organic carbon, total As, NO3−, and SO42−. The results of this study provide new insight into the diversity and biogenesis contributions of sulfate-reducing bacteria in arsenic-contaminated soils from realgar deposits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial-catalyzed sulfate reduction can be performed via assimilatory and dissimilatory metabolisms (Marietou et al. 2018; Rodrigues et al. 2019). In the assimilatory process, sulfate reduction is incorporated to synthesize sulfur-containing cell components. Second, sulfate-reducing organisms can utilize sulfate as a terminal electron acceptor through anaerobic respiration, along with organic compounds (formate, propionate, lactate, pyruvate, ethanol) or H2, methane, and even phosphite as electron donors (Kushkevych et al. 2018). From this electron transport chain, a conspicuous final product, hydrogen sulfide (H2S), is produced, which has been widely recognized as a poisonous chemical with a characteristic odor. Sulfate-reducing microorganisms are widely distributed in different environments, including paddy soils, petroleum, oil reservoirs, soda lakes, marine sediments, acid mine drainage, and thermal springs (Bao et al. 2012; Yan et al. 2018). Several enzymes are involved in this sulfate reduction process, such as ATP-sulfurylase, adenylylsulfate reductase and dissimilatory sulfite reductase. Dissimilatory sulfite reductase (DsrAB protein) promotes the conversion of sulfite to sulfide (Li et al. 2019a; Rodrigues et al., 2019). The dsrAB gene, which encodes the DsrAB protein, has widely detected a range of sulfate-reducing bacteria (SRB) community structures, containing more than 20 different genera, such as Desulforibrio, Desulfomonas, Desulfobulbus, Desulfobacter, Desulfococcus, Desulfosarcina, Desulfonema, Desulfotomaculum, and the genus Archaeoglobus within archaea (Guan et al. 2013; Lu et al. 2017). Sulfate reduction mediated by microbes is often coupled to other interactions, such as fundamental biogeochemical processes, oil biodeterioration and biocorrosion, food spoilage, bioremediation of heavy metals and biodegradation of organic matter (George et al., 2008; Lai et al. 2020; Sheoran et al. 2010). Hence, the biotechnologies of SRB could also be utilized in a variety of environmental treatments, such as microbial remediation of heavy metals in wastewater or contaminated soils, dichlorination of organochlorines and repair of acid mine drainage (Hu et al. 2020; Shan et al. 2019; Zhang et al. 2016). However, previously published studies on the biogenesis contributions of SRB to the arsenic sulfate coexisting pollution system are limited (Fan et al. 2018; Kirk et al., 2004).
As the largest source of natural realgar ore reserves in Asia with over 1500 years of intensive exploiting histories, the Shimen Realgar Mine was completely out of production in 2011 after a number of cases of As-poisoning (Tang et al., 2016). Large amounts of As-contaminated wastes, such as soils, water, air, plants, and animals have caused serious health risks to local residents. While previous studies have mainly focused on arsenic biogeochemistry cycles, such as As oxidation, reduction, and methylation, far too little attention has been given to the role of sulfur (Fan et al. 2018; Gao et al., 2017; Zhang et al. 2020). However, the fate of arsenic is influenced by abiotic or biological redox of sulfur, which can either mobilize or precipitate arsenic (Burton et al. 2013; Edwardson and Hollibaugh, 2017; Zhu et al. 2017). Moreover, the soil of this mine also contains a large amount of sulfur, which occupies 0.7–0.8% (Chen et al. 2017). Therefore, it is necessary to investigate the diversity and contribution of SRB in arsenic-contaminated soils.
In this context, the main issues addressed in this work are to (1) investigate the diversity and distribution of SRB in seriously arsenic-contaminated soils and (2) determine the ecological effect of SRB on the arsenic sulfate coexisting pollution system.
Materisls and methods
Sampling and geochemical analysis
Three soil samples were derived from the surface layer (0–20 cm) of an abandoned tailing realgar (As4S4) mine area located in Baiyun village, Changde City, Hunan Province, China, in July 2020. The sites of the three samples (named 2–1, 2–2, and 2–3) are presented in Table 1. After sampling, the soil samples (weighing 1.0 kg each) were carefully mixed, sealed, and stored in sterile polyethylene boxes (4 °C) and transported to the laboratory immediately. Certain soil samples were naturally air-dried, crushed, homogenized, and passed through a 2 mm nylon sieve for geochemical property analysis. Other soils were stored for subsequent batch microcosm assays (4 °C) and DNA extraction (− 20 °C) (Sun et al. 2016). Total As, soluble As, total organic carbon, and other characterizations were analyzed as described previously (Yang et al. 2018). Total soluble As(III) was detected using atomic fluorescence spectroscopy (AFS) (AFS-8510, Beijing). Soluble As(III) was separated using SupelClean™ (LC-SAX SPE-57017, Supelco, USA) from As(III) and As(V) to AFS measurements. Sulfate (SO42−) was measured using sulfate-barium chromate spectrophotometry (HJ/T 342–2007).
Determination of the sulfate and As(V)-respiring activities of the microbial communities
To detect the sulfate respiring abilities, 1.5 g of each evenly mixed soil sample was added into serum bottles containing 20 mL of deoxygenated and sterilized medium amended with 5.5 mM sulfate. To detect the As(V)-respiring activities of the microbial communities from the realgar mine soils, 1.5 g of each sample was mixed with 10 mL of deoxygenated medium supplemented with 1.0 mM As(V) (Yan et al. 2018). The medium consisted of (per liter of dd water, pH 6.8) 1.0 g of NH4Cl, 0.65 g of KH2PO4, 0.05 g of CaCl2, 3.5 g of sodium lactate, and 1.0 g of yeast extract. Duplicate controls were prepared by sterilized soils with the same reagents. All bottles were incubated at 30 °C with moderate shaking. Approximately 1.0 mL of suspension was removed at an interval of one day for the measurement of soluble As(V), As(III), and sulfate (Blum et al. 2012; Burton et al. 2013).
DNA extraction and sequence analysis of dissimilatory sulfite reductase (dsrAB) genes from the soils
Total DNA was extracted from a 0.5 g soil sample using the FastDNA® SPIN kit (MP bio, USA) for soil following the manufacturer’s protocol (Bao et al. 2021). The concentration and quality of extracted DNA were detected using a NanoDrop 2000 spectrophotometer. The DSRp2060F (5′-CAACATCGTYCAYACCCAGGG-3′) and DSR4R (5′-GTGTARCAGTTDCCRCA-3′) primers were chosen to amplify the dsrAB genes with PCRs (Besaury et al. 2012; Geets et al. 2006). PCR conditions consisted of an initial denaturation step of 94 °C for 4 min, followed by 35 cycles of 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min with a final extension at 72 °C for 10 min. Amplified PCR products were separated by 1.5% (w/v) agarose gel electrophoresis. DNA bands were cut out, and DNA was extracted and purified using a QIAquick Gel Extraction Kit, and then cloned into the pMD19-T vector for sequencing as described previously (Li et al. 2019a). The obtained DNA sequences were analyzed with the BLAST server and MEGA 6.0 software.
Isolation of a novel dissimilatory sulfate-reducing strain from arsenic-contaminated soils
A modified culture medium was applied in these microbial enrichment experiments. The medium used was the same as above, and 0.1 g of vitamin C (as an Eh regulator) and 1.2 g of Fe(NH4)2(SO4)26H2O (as an indicator) were added (Shan et al. 2019; Bao et al. 2012). Approximately 1.5 g of soil sample was added to 10.0 mL of anoxic sterile medium as described previously. Standard anaerobic incubations were performed at 30 °C for enrichment. When the color of the medium turned dark (the best proof of the positive growth of SRB), approximately 0.5 mL of culture was transferred into 10.0 mL of the same fresh medium for second-round enrichment. This step was repeated for several rounds. The enrichment culture was diluted to different gradient concentrations, and the agar shake tube technique was used to isolate single bacterial strains under strict anaerobic conditions (Dias et al. 2008).
Functional characterization of the novel cultivable isolate
Bacterial anaerobic functional characterization experiments were performed via the addition of different electron acceptors, such as As(V), SO42− and combined electron acceptors of As(V) and SO42−. JL1 cells (1107/mL) were inoculated into 10.0 mL of modified MMS medium containing 10.0 mM lactate acting as the sole electron donor and 2.0 mM SO42−, 1.5 mM As(V) or the same concentration of As(V) and SO42− as described above. Strict anaerobic incubations were performed at 30 °C and briefly shaken once a day. At an interval of half day, approximately 0.5 mL of the culture mixture was taken for measuring the concentration of total As(V), As(III) and sulfate as described above.
Cloning, sequencing, and data analysis of bacterial 16S rRNA and dsrAB genes
Genomic DNA was extracted using a simple boiling method described by Miyamoto. Briefly, several bacterial cells were collected into 1.0 mL centrifuge tubes, boiled at 100 °C for 15 min, and centrifuged at 5000 min−1 for 2 min. The 16S rRNA gene was amplified and sequenced using the universal bacterial primer set 27F/1492R (Kim et al. 2020). The dsrAB genes of dissimilatory sulfite reductase proteins were cloned, sequenced and analyzed using the primer set DSRp2060/DSR4R described above. Dendrogram analyses of the obtained. dsrAB genes and bacterial 16S rRNA gene sequence with 1,000 replicated bootstraps were constructed using the neighbor-joining method.
Determination of the microbial community structures
Total DNA of the soil sample was PCR amplified with the primer pair 341F/806R for the V3-V4 hypervariable regions of the 16S rRNA gene as described elsewhere (Li et al. 2019b). Then, high-throughput sequencing was performed on the MiSeq platform. The raw sequencing data was deposited to the Short Read Archive database at NCBI under accession number PRJNA784765.
Statistical analysis
Excel 2016 (Microsoft, USA), OriginPro 2020 (OriginLab, USA) IBM SPSS 22 (SPSS, USA) and R packages were used for statistical analyses. All data are expressed as the means and standard deviations. Significant differences were considered at P < 0.05 and assessed by ANOVA. The rarefaction curves were performed with Mothur. Principal coordinate analysis (PcoA) was analyzed by UPGMA according to the Bray–Curtis dissimilarity matrix. Heatmaps were employed to illustrate the correlation between community compositions and environmental factors using R packages (vegan). A Venn diagram shows the corresponding relationships between samples and phyla using R packages.
Results
Geochemical characterizations
The detailed physical properties of the three samples are presented in Table 1. The tailings soil samples were reddish-brown in appearance. As the largest deposit of realgar ore (As4S4) in China, the soils of the Shimen Realgar Mine contain relatively high concentrations of sulfate (73.54–117.05 mg/kg), total arsenic (832.38–4206.14 mg/kg), and soluble arsenic (1.06–379.88 mg/kg). According to the national soil quality standard of China (GB15618-2018), the total As concentration of the sampling site exceeded 27.75–140.20 fold the concentration grade II (30 mg/kg, 6 < pH ≤ 7.5) in agricultural land, revealing serious risks to the environment and human health (Wan et al. 2017; Wu et al. 2016). Moreover, the sample had a relatively high abundance of total organic carbon (TOC) (24.60–59.40 g/kg), which provided a suitable environment for the growth and reproduction of microorganisms.
Analysis of the sulfate and As(V)-respiring activities
The sulfate and As(V)-respiring activities of the microbial communities from the three tailing samples were detected using the microcosm technique. As shown in Fig. 1a–c, after 17.0 days of incubation, approximately 99.54%, 89.30%, and 93.74% sulfate was reduced by microcosms of 2–1, 2–2, and 2–3, respectively. Moreover, the concentration of As was increased by 190.19%, 91.05%, and 81.18%, at 2.0, 2.0, and 5.0 days, respectively, which may be attributed to two reasons: aqueous sulfide generated by sulfate respiration can directly reduce Fe-oxide compounds, thereby catalyzing As dissolution; in the presence of sulfide, it not only accelerated the mass production of thio-As species but also affected As release for these different affinities to Fe-bearing minerals (Wang et al. 2019). However, after 29.0 days of incubation, approximately 0.44, 0.04, and 0.28 mM As was detected, as a result of precipitation of As-sulfide minerals (such as realgar and orpiment) or adsorption and coprecipitation by Fe(II)-sulfide minerals, respectively (Sun et al. 2016). In comparison, the microbial community from sterilized controls possessed no significant sulfate or As(V)-respiring activities. As shown in Fig. 1d–e, after 2.0 days of incubation, approximately 82.67%, 89.24%, and 89.72% As(V) were reduced in microcosms 2–1, 2–2, and 2–3, respectively. Moreover, the sulfate concentration steadily decreased. However, no detectable As(V) or sulfate-reducing abilities were observed in these cultures.
Unique molecular diversity of dsrAB genes
There has been little quantitative analysis of the diversity of sulfate-reducing genes in the microbial communities of arsenic-contaminated soils. The phylogenetic affiliation of dsrAB gene sequences is presented in Fig. 2. Dissimilatory sulfite reductase is a key enzyme for sulfate reducers, which catalyzes the energy-generation step during anaerobic respiration of sulfite (Zhou et al. 2021). The alpha and beta subunits of dissimilatory sulfite reductase (dsrA and dsrB) have been used as makers to detect sulfate reducers in different ecosystems. We obtained 28 novel sequence variants of dissimilatory sulfite reductase genes by amplifying, cloning, and sequencing them from the total genomic DNA of the soil sample. Phylogenetic relationships of the dsrAB gene sequences revealed rich and unique diversity compared to the other known dsrAB genes from other bacteria. We found that these novel sequence variants of dsrAB genes from the soils share 66.18–98.33% sequence identities to those deposited in the GenBank database.
It is well established from a variety of studies that if a group of dsrAB genes from an independent cluster in a phylogenetic tree, shares less than 60% maximal homology with other known dsrAB genes, they can be classified as a new family. According to this rule, we successfully identified 2 new families of dsrAB genes from the soils: family 1 (SM1, SM2, SM3, SM4, SM6, SM7, SM10, SM12, SM13, SM14, SM16, SM18, SM19, SM22, SM23, SM25, SM26, SM27, SM29, SM30, SM34, SM44, SM46, SM48, SM51) and family2 (SM55, SM56, SM57). Specifically, family 1 is closely associated with the dissimilatory sulfite reductase alpha subunit (dsrA) and dissimilatory reductase beta subunit (dsrB) genes (DQ386233.1 and DQ211852.1) from Desulfotomaculum sp. Lac2 and Bacterium LS1701. Family 2 is closely related to the dsrB genes of.
Bacterium AMD. C1 (EU086051.1) and Desulfovibrio sp. strain S10 (MN792773.1). These findings demonstrate the rich and unique diversity of sulfate-reducing microbes in arsenic-contaminated soils from realgar deposits.
Anaerobic reduction activities of JL1
Figure 3 illustrates the sulfate- and arsenate-respiring activities of Desulfotomaculum sp. JL1. As shown in Fig. 3a, in the presence of 1.5 mM As(V), the As(V) reduction efficiency was slow at the beginning when lactate was used as the sole electron donor under anaerobic conditions. However, after 4 days of incubation, 1.5 mM As(V) was completely transformed into As(III). JL1 also has apparent activity to reduce sulfate. As shown in Fig. 3b, JL1 is highly effective in sulfate reduction under anaerobic conditions. It takes only 5 days for sulfate to be completely converted to sulfide catalyzed by JL1 in MMM medium (without sulfate) containing 2.0 mM sulfate and 10.0 mM sodium lactate.
Functional charaterization of Desulfotomaculum sp. JL1. The sulfate-respiring activity of JL1 (a); the arsenate-respiring activity of JL1 (b); the reduction abilities of JL1 in the presence of multiple electron acceptors (both contain sulfate ans As(V) in the cultures) (c); the appearance difference of serum bottle before and after 10 days culture (d)
To further explore the reduction abilities of bacterial cells in the presence of multiple electron acceptors, we performed a bacterial reduction assay containing 2.0 mM sulfate and 1.5 mM As(V) as electron acceptors and lactate as the sole electron donor under anaerobic conditions. As shown in Fig. 3c, when sulfate and As(V) were added to the cultures, compared to Fig. 3a, the sulfate reduction ability was more efficient than that achieved in the presence of only sulfate. After 4 days of incubation, 2.0 mM sulfate was fully reduced. Moreover, compared to Fig. 3b, the As(V) reduction ability is much slower than that when only As(V) is added; after 4 days, only half of As(V) is converted to As(III). After 10 days of incubation, significant amounts of yellow precipitates were generated in the culture (Fig. 3d). This phenomenon suggests that microorganisms can drive the reduction of As(V) and SO42− and can effectively promote the formation of precipitation, which also hints at the biogenesis of the realgar mine in Shimen.
Phylogenetic features of the bacterial strain JL1
To further reveal the functional characterization and environmental effects of sulfate-reducing bacteria in arsenic-contaminated soils, we isolated a representative dissimilatory sulfate-reducing bacterium using the microbial enrichment technique, which was referred to as JL1. 16S rRNA gene phylogenetic analysis revealed that the novel isolate belonged to Firmicutes and shared the closest relatives with Desulfotomaculum sp. GY-2 (HQ827821.1) with 98.72% sequence similarity (Fig. 4a). The phylogenetic dendrogram also illustrates that JL1 fell into the same cluster with high bootstrap value within Desulfotomaculum and was thus referred to as Desulfotomaculum sp.JL1 (MZ342818).
The dsrAB gene sequences of the present isolate were deposited in GenBank under the accession numbers MZ383093 and MZ383094, and were identified as JL1-dsrAB-1 and JL1-dsrAB-2, which share 99.61% sequence identity. According to the phylogenetic dendrogram showing dsrAB gene sequence comparison in Fig. 4b, 11 reference strains were chosen to display sequence similarity (78.28–96.10%). JL1-dsrAB-1 shares 96.10%, 95.80%, 95.03%, 94.97%, and 94.44% sequence identities with Desulfovibrio carbinoliphilus strain D41, Desulfovibrio sp. sul5, Desulfovibrio burkinensis strain DSM 6830, Desulfovibrio burkinensis, and Bacterium LS2003, respectively. These results showed that JL1 possesses new variant of dissimilatory sulfite reductase gene, and it can also be inferred that this isolate is a novel sulfate-reducing bacterial strain.
Unique microbial community compositions of arsenic-contaminated soils
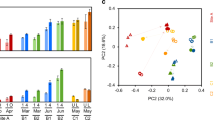
As shown in Fig. 5, the Illumina high-throughput sequencing technique was used to determine the microbial community compositions from the arsenic-contaminated soils. A total of 104,599 high-quality 16S rRNA gene sequences were obtained from samples 2–1, 2–2, and 2–3. As shown in Fig. 5a, the dominant phyla on average contained Proteobacteria (26.08%), Chloroflexi (19.64%), Acidobacteriota (19.14%), Actinobacteriota (8.06%), Gemmatimonadote (6.27%), Bacteroidota (4.34%), Myxococcota (2.82%), Methlomirabilota (2.03), unclassified_k_norank (1.78%), Deinococcota (1.45%), Patescibacteria (1.32%), and other bacteria (7.08%). These microbial community compositions were relatively consistent with previous reports at the site (Li et al. 2020; Yu et al. 2020). According to the Bray–Curtis dissimilarity analysis using UPGMA (Fig. 5a), the soil samples can be divided into two groups: I (2–1 and 2–2) and II (2–3). Rarefaction curves showed that these sequences covered 99.78%, 99.84%, and 99.60% of the microbial diversities of the corresponding soils, respectively (Fig. 5b). The corresponding relationships between samples and phyla are described in Fig. 5c. The number of common species of these three multi-samples was 26, and the number of unique species of these three multi-samples was 1, 0, and 2. Heatmap (Fig. 5d) analysis showed the relationships between the abundances of dominant phyla and the environmental parameters. Total As and sulfate showed significant positive correlations with Bacteroidota, Acidobacteriota, unclassified_k_norank, Patescibacteria, Cyanobacteria, Verrucomicrobiota, Firmicutes, Armatimonadota, Elusimicrobiota, WS4. Total As and sulfate showed significant negative correlations with Desulfobacterota, Entotheonellaeota, SAR324_cladeMarine_group_B, WPS-2, Nitrospirota, NB1-j, Myxococcota, Latescibacterota. In comparison, Mn showed the opposite correlation to the total As and sulfate.
The microbial community compositions and its correlations with environmental paerameters from the arsenic-contaminated soils. Relative abundances of the dominant phyla of the soil samples and UPGMA analysis (a); rarefaction curves (b); Venn analysis showed the corresponding relationships between samples and phyla (c); heatmap analysis showed the relationships between the abudances of dominant phyla and environmental factors (d)
Discussion
Unique geochemistry characteristics and microbial community structures
The Shimen realgar mine in China has been exploited for approximately 1500 years and is the largest source of realgar (As4S4) ore in Asia (Peng et al. 2019). Hence, the soils contain extremely high concentrations of arsenic and sulfate. However, previous studies have only focused on microbial-catalyzed arsenic metabolism, and have found that microorganisms here have a strong ability to drive As(III) oxidation, As(V) reduction, and As methylation. In this study, we focused on investigating the diversity and activities of sulfate-reducing microorganisms in tailings. We found that the microbial communities had efficient abilities to respire As(V) and sulfate (Fig. 1). The diversity of sulfate-reducing microbes in the soils of the Shimen Realgar Mine was closely related to Desulfotomaculum, Desulfovibrio, and Bacterium (Fig. 2), which is totally different from other sulfur-rich ecosystems, such as mangrove sediments, deep-sea anoxic brines of the Red Sea, petroleum reservoirs, and flooded rice paddy soils (Li et al. 2021; Guan et al. 2015; Santos et al. 2020; Liu et al. 2009). Moreover, compared to other arsenic-contaminated or sulfate-contaminated sites, this area had unique microbial communities, the most abundant populations belonged to the phyla Proteobacteria, Chloroflexi, and Acidobacteriota (Fig. 5), which were attributed to the unique geochemistry parameters, such as TOC, total As, NO3−, and SO42− (Li et al. 2015; Zheng et al. 2019).
Conceptual model for the biogenesis contribution of sulfate-reducing bacteria to the arsenic-contaminated soils from realgar mine
Realgar contains over 90% tetra-arsenic tetra-sulfide (As4S4), and has been commonly used as a drug to treat diseases in ancient China, India and other countries for thousands of years (Wu et al., 2017). In recent years, realgar has also been proven to be an effective clinical treatment for various forms of cancer in vivo and in vitro. Compared with the disadvantages of traditional methods, such as low solubility in water, poor gastrointestinal absorption, high toxicity and low bioavailability of realgar, microbial leaching technology can significantly improve the solubility and bioavailability of realgar, and has high efficiency, ecological safety, low cost and other advantages. In a previous study, Chen found that A.ferrooxidans can effectively bioleach realgar in different environments (Chen et al. 2011). Addition to this, our research found that the novel strain JL1 was a typical sulfate-reducing bacteria (Fig. 4) and can effectively promote the formation of yellow precipitates (Fig. 3). Therefore, we proposed a conceptual model for the ecological effect of sulfate-reducing bacteria on arsenic-contaminated soils (Fig. 6).
Can this sulfate-reducing bacterium be used for the bioremediation of As and sulfate-contaminated water?
Using SRB as a biological treatment for acid mine drainage (AMD), remediation has gained extensive attention (Kefeni et al., 2017; Sánchez-Andrea et al., 2014; Jung et al., 2015). Several studies have approved SRB as an effective AMD treatment option for the ability to produce alkalinity and remove heavy metals (Jung et al., 2012). However, our research provides new insight into the SRB used for the bioremediation of As and sulfate-contaminated water, which can efficiently bioleach realgar from the soil and has promising industrial potential.
Conclusion
The fate of arsenic is affected by abiotic or biological redox of sulfur. However, little is known about the diversity and biogenesis contribution of SRB in arsenic-contaminated soils from realgar deposits. Microcosm assays illustrated that soil samples displayed efficient sulfate and As(V)-respiring activities. The diversity of sulfate-reducing microbes in the soils of the Shimen Realgar Mine was closely related to Desulfotomaculum, Desulfovibrio, and Bacterium. A novel dissimilatory sulfate-reducing bacterium, Desulfotomaculum sp. JL1, was further isolated from soils, and can efficiently respiratory reduce As(V) and sulfate in 4 and 5 days, respectively. JL1 can promote the generation of yellow precipitates in the presence of multiple electron acceptors (both contain sulfate and As(V) in the cultures), which indicated the biogenesis contribution of SRB to the realgar mine. Moreover, this area had unique microbial communities, the most abundant populations belonged to the phyla Proteobacteria, Chloroflexi, Acidobacteriota, Actinobacteriota, and Gemmatimonadote, which were attributed to the unique geochemistry characteristics, such as TOC, total As, NO3−, and SO42−.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bao P, Hu Z, Wang X, Chen J, Ba Y, Hua J, Zhu C, Zhong M, Wu C (2012) Dechlorination of p, p′-DDTs coupled with sulfate reduction by novel sulfate-reducing bacterium Clostridium sp. BXM Environ Pollut 162:303–310. https://doi.org/10.1016/j.envpol.2011.11.037
Bao Y, Jin X, Guo C, Lu G, Dang Z (2021) Sulfate-reducing bacterial community shifts in response to acid mine drainage in the sediment of the Hengshi watershed. South China Environ Sci Pollut Res 28(3):2822–2834. https://doi.org/10.1007/s11356-020-10248-7
Besaury L, Ouddane B, Pavissich J, Dubrulle C, González B, Quillet L (2012) Impact of copper on the abundance and diversity of sulfate-reducing prokaryotes in two chilean marine sediments. Mar Pollut Bull 64(10):2135–2145. https://doi.org/10.1016/j.marpolbul.2012.07.042
Blum J, Kulp T, Han S, Lanoil B, Saltikov C, Stolz J, Miller L, Oremland R (2012) Desulfohalophilus alkaliarsenatis gen. nov., sp. nov., an extremely halophilic sulfate- and arsenate-respiring bacterium from Searles Lake. Calif Extremophiles 16(5):727–742. https://doi.org/10.1007/s00792-012-0468-6
Burton E, Johnston S, Kraal P, Bush R, Claff S (2013) Sulfate availability drives divergent evolution of arsenic speciation during microbially mediated reductive transformation of schwertmannite. Environ Sci Technol 47(5):2221–2229. https://doi.org/10.1021/es303867t
Chen P, Yan L, Leng F, Nan W, Yue X, Zheng Y, Feng N, Li H (2011) Bioleaching of realgar by Acidithiobacillus ferrooxidans using ferrous iron and elemental sulfur as the sole and mixed energy sources. Bioresour Technol 102(3):3260–3267. https://doi.org/10.1016/j.biortech.2010.11.059
Chen Z, Wang Y, Jiang X, Fu D, Xia D, Wang H, Dong G, Li Q (2017) Dual roles of AQDS as electron shuttles for microbes and dissolved organic matter involved in arsenic and iron mobilization in the arsenic-rich sediment. Sci Total Environ 574:1684–1694. https://doi.org/10.1016/j.scitotenv.2016.09.006
Dias M, Salvado J, Monperrus M, Caumette P, Amouroux D, Duran R, Guyoneaud R (2008) Characterization of Desulfomicrobium salsuginis sp. nov. and Desulfomicrobium aestuarii sp. nov., two new sulfate-reducing bacteria isolated from the Adour estuary (French Atlantic coast) with specific mercury methylation potentials. Syst and Appl Microbiol 31(1):30–37. https://doi.org/10.1016/j.syapm.2007.09.002
Edwardson C, Hollibaugh J (2017) Metatranscriptomic analysis of prokaryotic communities active in sulfur and arsenic cycling in Mono Lake, California, USA. ISME J 11(10):2195–2208. https://doi.org/10.1038/ismej.2017.80
Fan L, Zhao F, Liu J, Frost R (2018) The As behavior of natural arsenical-containing colloidal ferric oxyhydroxide reacted with sulfate reducing bacteria. Chem Eng J 332:183–191. https://doi.org/10.1016/j.cej.2017.09.078
Gao P, Zeng X, Bai L, Wang Y, Wu C, Duan R, Su S (2017) As(V) resistance and reduction by bacteria and their performances in As removal from As-contaminated soils. Curr Microbiol 74(9):1108–1113. https://doi.org/10.1007/s00284-017-1293-z
Geets J, Borremans B, Diels L, Springael D, Vangronsveld J, Lelie D, Vanbroekhoven K (2006) DsrB gene-based DGGE for community and diversity surveys of sulfate-reducing bacteria. J Microbiol Methods 66(2):194–205. https://doi.org/10.1016/j.mimet.2005.11.002
George J, Purushothaman C, Shouche Y (2008) Isolation and characterization of sulphate-reducing bacteria Desulfovibrio vulgaris from Vajreshwari thermal springs in Maharashtra. India World J Microbiol Biotechnol 24(5):681–685. https://doi.org/10.1007/s11274-007-9524-2
Guan J, Xia L, Wang L, Liu J, Gu J, Mu B (2013) Diversity and distribution of sulfate-reducing bacteria in four petroleum reservoirs detected by using 16S rRNA and dsrAB genes. Int Biodeterior Biodegrad 76:58–66. https://doi.org/10.1016/j.ibiod.2012.06.021
Guan Y, Hikmawan T, Antunes A, Ngugi D, Stingl U (2015) Diversity of methanogens and sulfate-reducing bacteria in the interfaces of five deep-sea anoxic brines of the Red Sea. Res Microbiol 166(9):688–699. https://doi.org/10.1016/j.resmic.2015.07.002
Hu K, Xu D, Chen Y (2020) An assessment of sulfate reducing bacteria on treating sulfate-rich metal-laden wastewater from electroplating plant. J Hazard Mater 393:122376. https://doi.org/10.1016/j.jhazmat.2020.122376
Jung S, Cheong Y, Yim G, Ji S, Kang H (2015) Erratum to: Performance and bacterial communities of successive alkalinity-producing systems (SAPSs) in passive treatment processes treating mine drainages differing in acidity and metal levels. Environ Sci Pollut Res 22(6):4788–4788. https://doi.org/10.1007/s11356-014-3949-6
Jung S, Ji S, Kang H, Yim G, Cheong Y (2012) Biotechnology in passive treatment of acid mine drainage: a review. J of the Korean Soc of Mineral and Energy Resour Eng 49:844. https://doi.org/10.12972/ksmer.2012.49.6.844
Kefeni K, Msagati T, Mamba B (2017) Acid mine drainage: prevention, treatment options, and resource recovery: a review. J Cleaner pro 151:475–493. https://doi.org/10.1016/j.jclepro.2017.03.082
Kim Y, Yang J, Lim J, Park M, Yang S, Lee H, Kang S, Lee J, Kwon K (2020) Paradesulfovibrio onnuriensis gen. nov., sp. nov., a chemolithoautotrophic sulfate-reducing bacterium isolated from the Onnuri vent field of the Indian Ocean and reclassification of Desulfovibrio senegalensis as Paradesulfovibrio senegalensis comb. nov. J of Microbiol 58(4):252–259. https://doi.org/10.1007/s12275-020-9376-0
Kirk M, Holm T, Park J, Jin Q, Sanford R, Fouke B, Bethke C (2004) Bacterial sulfate reduction limits natural arsenic contamination in groundwater. Geology 32(11):953. https://doi.org/10.1130/G20842.1
Kushkevych I, Kováč J, Vítězová M, Vítěz T, Bartoš M (2018) The diversity of sulfate-reducing bacteria in the seven bioreactors. Arch Microbiol 200(6):945–950. https://doi.org/10.1007/s00203-018-1510-6
Lai R, Li Q, Cheng C, Shen H, Liu S, Luo Y, Zhang Z, Sun S (2020) Bio-competitive exclusion of sulfate-reducing bacteria and its anticorrosion property. J Petrol Sci Eng 194:107480. https://doi.org/10.1016/j.petrol.2020.107480
Li P, Wang Y, Dai X, Zhang R, Jiang Z, Jiang D, Wang S, Jiang H, Dong H (2015) Microbial community in high arsenic shallow groundwater aquifers in Hetao Basin of Inner Mongolia. China Plos One 10(5):e0125844. https://doi.org/10.1371/journal.pone.0125844
Li J, Cai M, Miao Y, Luo G, Li W, Li Y, Li A (2019a) Bacterial community structure and predicted function in an acidogenic sulfate-reducing reactor: Effect of organic carbon to sulfate ratios. Bioresour Technol 293:122020. https://doi.org/10.1016/j.biortech.2019.122020
Li X, Xiao H, Zhang W, Li Y, Tang X, Duan J, Yang Z, Wang J, Guan F, Ding G (2019b) Analysis of cultivable aerobic bacterial community composition and screening for facultative sulfate-reducing bacteria in marine corrosive steel. Journal of Oceanology and Limnology 37(2):600–614. https://doi.org/10.1007/s00343-019-7400-1
Li W, Liu J, Hudson-Edwards K (2020) Seasonal variations in arsenic mobility and bacterial diversity: the case study of Huangshui Creek, Shimen Realgar Mine, Hunan Province. China Sci Total Environ 749:142353. https://doi.org/10.1016/j.scitotenv.2020.142353
Li M, Fang A, Yu X, Zhang K, He Z, Wang C, Peng Y, Xiao F, Yang T, Zhang W, Zheng X, Zhong Q, Liu X, Yan Q (2021) Microbially-driven sulfur cycling microbial communities in different mangrove sediments. Chemosphere 273:128597. https://doi.org/10.1016/j.chemosphere.2020.128597
Liu X, Zhang L, Prosser J, He J (2009) Abundance and community structure of sulfate reducing prokaryotes in a paddy soil of southern China under different fertilization regimes. Soil Biol Biochem 41(4):687–694. https://doi.org/10.1016/j.soilbio.2009.01.001
Lu S, Han S, Du Y, Liu H, Nie H, Luo X, Huang Q, Chen W (2017) The shift of sulfate-reducing bacterial communities from the upland to the paddy stage in a rapeseed-rice rotation system, and the effect from the long-term straw returning. Appl Soil Ecol 124(3):124–130. https://doi.org/10.1016/j.apsoil.2017.10.032
Marietou A, Roy H, Jorgensen B, Kjeldsen K (2018) Sulfate transporters in dissimilatory sulfate reducing microorganisms: a comparative genomics analysis. Front Microbiol 9:309. https://doi.org/10.3389/fmicb.2018.00309
Peng H, Ji X, Jian Z, Wei W, Jiapei C, Bocharnikova E, Matichenkov V (2019) Effect of Si on As speciation and distribution in rice near the Shimen Realgar Mine. Mine Water Environ 38(4):808–816. https://doi.org/10.1007/s10230-019-00625-1
Rodrigues C, Núñez-Gómez D, Silveira D, Lapolli F, Lobo-Recio M (2019) Chitin as a substrate for the biostimulation of sulfate-reducing bacteria in the treatment of mine-impacted water (MIW). J Hazard Mater 375:330–338. https://doi.org/10.1016/j.jhazmat.2019.02.086
Sánchez-Andrea I, Sanz J, Bijmans M, Stams A (2014) Sulfate reduction at low pH to remediate acid mine drainage. J Hazard Mater 269:98–109. https://doi.org/10.1016/j.jhazmat.2013.12.032
Shan S, Guo Z, Lei P, Wang Y, Li Y, Cheng W, Zhang M, Wu S, Yi H (2019) Simultaneous mitigation of tissue cadmium and lead accumulation in rice via sulfate-reducing bacterium. Ecotoxicol Environ Saf 169:292–300. https://doi.org/10.1016/j.ecoenv.2018.11.030
Santos J, Lopes D, Da Silva J, De Oliveira M, Dias R, Lima H, De Sousa M, De Paula S, Silva C (2020) Diversity of sulfate-reducing prokaryotes in petroleum production water and oil samples. Int Biodeterior Biodegrad 151:104966. https://doi.org/10.1016/j.ibiod.2020.104966
Sheoran A, Sheoran V, Choudhary R (2010) Bioremediation of acid-rock drainage by sulphate-reducing prokaryotes: a review. Miner Eng 23(14):1073–1100. https://doi.org/10.1016/j.mineng.2010.07.001
Sun J, Quicksall A, Chillrud S, Mailloux B, Bostick B (2016) Arsenic mobilization from sediments in microcosms under sulfate reduction. Chemosphere 153:254–261. https://doi.org/10.1016/j.chemosphere.2016.02.117
Tang J, Liao Y, Yang Z, Chai L, Yang W (2016) Characterization of arsenic serious-contaminated soils from Shimen realgar mine area, the Asian largest realgar deposit in China. J Soils Sediments 16(5):1519–1528. https://doi.org/10.1007/s11368-015-1345-6
Wan X, Dong H, Feng L, Lin Z, Luo Q (2017) Comparison of three sequential extraction procedures for arsenic fractionation in highly polluted sites. Chemosphere 178:402–410. https://doi.org/10.1016/j.chemosphere.2017.03.078
Wang Y, Pi K, Fendorf S, Deng Y, Xie X (2019) Sedimentogenesis and hydrobiogeochemistry of high arsenic Late Pleistocene-Holocene aquifer systems. Earth-Sci Rev 189:79–98. https://doi.org/10.1016/j.earscirev.2017.10.007
Wu F, Wang J, Yang J, Li J, Zheng Y (2016) Does arsenic play an important role in the soil microbial community around a typical arsenic mining area? Environ Pollut 213:949–956. https://doi.org/10.1016/j.envpol.2016.03.057
Wu Y, Zhou X, Lei M, Yang J, Ma J, Qiao P, Chen T (2017) Migration and transformation of arsenic: contamination control and remediation in realgar mining areas. Appl Geochem 77:44–51. https://doi.org/10.1016/j.apgeochem.2016.05.012
Yan J, Zhong K, Wang S, Chen Z, Hu H, Jian Z, Wen H, Zhang H (2018) Carbon metabolism and sulfate respiration by a non-conventional Citrobacter freundii strain SR10 with potential application in removal of metals and metalloids. Int Biodeterior Biodegrad 133:238–246. https://doi.org/10.1016/j.ibiod.2018.05.010
Yang F, Xie S, Wei C, Liu J, Zhang H, Chen T, Zhang J (2018) Arsenic characteristics in the terrestrial environment in the vicinity of the Shimen realgar mine, China. Sci Total Environ 626:77–86. https://doi.org/10.1016/j.scitotenv.2018.01.079
Yu Z, Liu X, Zeng X, Yin H, Yu R, Zeng W (2020) Effect of arsenic pollution extent on microbial community in Shimen long-term arsenic-contaminated soil. Water Air Soil Pollut 231(7):340. https://doi.org/10.1007/s11270-020-04716-6
Zhang G, Ouyang X, Li H, Fu Z, Chen J (2016) Bio-removal of antimony from contaminated waters by a mixed batch culture of sulfate-reducing bacteria. Int Biodeterior Biodegrad 115:148–155. https://doi.org/10.1016/j.ibiod.2016.08.007
Zhang M, Li Z, Häggblom M, Young L, He Z, Li F, Xu R, Sun X, Sun W (2020) Characterization of nitrate-dependent As(III)-oxidizing communities in arsenic-contaminated soil and investigation of their metabolic potentials by the combination of DNA-stable isotope probing and metagenomics. Environ Sci Technol 54(12):7366–7377. https://doi.org/10.1021/acs.est.0c01601
Zhou L, Ou P, Zhao B, Zhang W, Yu K, Xie K, Zhuang W (2021) Assimilatory and dissimilatory sulfate reduction in the bacterial diversity of biofoulant from a full-scale biofilm-membrane bioreactor for textile wastewater treatment. Sci Total Environ 772:145464. https://doi.org/10.1016/j.scitotenv.2021.145464
Zheng T, Deng Y, Wang Y, Jiang H, O’Loughlin E, Flynn T, Gan Y, Ma T (2019) Seasonal microbial variation accounts for arsenic dynamics in shallow alluvial aquifer systems. J Hazard Mater 367:109–119. https://doi.org/10.1016/j.jhazmat.2018.12.087
Zhu Y, Xue X, Kappler A, Rosen B, Meharg A (2017) Linking genes to microbial biogeochemical cycling: lessons from arsenic. Environ Sci Technol 51(13):7326–7339. https://doi.org/10.1021/acs.est.7b00689
Funding
This work is supported by the Educational Commission of Hubei Province of China (No. Q20211310), Engineering Research Center of Ecology and Agricultural Use of Wetland, Ministry of Education (No. KFT201903), and Hubei Key Laboratory of Intelligent Yangtze and Hydroelectric Science (No. ZH2002000113).
Author information
Authors and Affiliations
Contributions
Hongzhong Pan and Xianbin Zhu designed research; Xianbin Zhu performed research; Xianbin Zhu and Liyuan Chen analyzed data; Xianbin Zhu wrote the manuscript. Lei Wang, Xun Zhang, and Dan Wang revised and reviewed the drafts.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All participants gave informed consent at enrollment.
Consent to publish
All participants gave final approval for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Robert Duran
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, X., Chen, L., Pan, H. et al. Diversity and biogenesis contribution of sulfate-reducing bacteria in arsenic-contaminated soils from realgar deposits. Environ Sci Pollut Res 29, 31110–31120 (2022). https://doi.org/10.1007/s11356-022-18595-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18595-3