Abstract
Lespedeza formosa is an economically important shrub in the agroecosystems of southern China, where acid rain (AR) is an increasingly serious environmental issue. However, the roles of arbuscular mycorrhizal fungi (AMF) in adapting the plants to AR stress are poorly understood. In this study, L. formosa seedlings were cultivated in a greenhouse, where the inoculated (colonization with Rhizophagus irregularis and Diversispora versiformis, alone and in combination) and non-inoculated plants were treated with three AR regimes (pH 5.6, 4.0, and 2.5) to evaluate the roles of AMF under acidic conditions. The results showed that AR individually suppressed plant growth by inhibiting photosynthetic parameters and induced Al phytotoxicity in non-mycorrhizal plants. However, mycorrhizal inoculation, especially in combination, significantly increased the total dry weight, photosynthetic capabilities, shoot nitrogen (N) concentration (average 15.8 and 16.7 mg g−1 for non-mycorrhizal and mycorrhizal plants, respectively) and plant phosphorus (P) concentration (average 1.6 and 2.3 mg g−1 for non-mycorrhizal and mycorrhizal plants, respectively) at pH 4.0, reduced N/P ratio (average 9.5 and 6.9 for non-mycorrhizal and mycorrhizal plants, respectively) at pH 4.0, and protected roots against Al phytotoxicity (average 2.0 and 1.4 mg g−1 for non-mycorrhizal and mycorrhizal roots, respectively), indicating that AMF could mitigate some of the detrimental effects of AR. Moreover, our findings suggest that AMF mainly benefited the plant through the combined effects of N concentrations and N/P ratios in shoots and Al3+ concentrations in roots under acidic conditions.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid rain (AR) is a critical global environmental issue that is mainly caused by atmospheric deposition of acidifying precursors, such as H2SO4 and HNO3, with annual average pH values below 5.6 (Liang et al. 2016; Gilani et al. 2021). Following Central Europe and Northeast America, the southern region of China has become the third area worldwide to be seriously affected by AR over the past several decades (Cao et al. 2009; Huang et al. 2019). Over 30% of China’s land area experiences AR, with SO42− being the dominant anion in AR (Gilani et al. 2021). Moreover, although SO2 emissions have decreased with the implementation of cleaner policies in recent years, AR-induced environmental challenges remain in southeastern China (Wang et al. 2021; Xia et al. 2021), where the annual mean pH of precipitation in most areas is < 4.5, and certain cities have recorded rainfall with a significantly low pH of 2.95 (Huang et al. 2008; Wei et al. 2017). Previous studies have indicated that AR can damage plants and induce soil acidification (Larssen et al. 1999; Li et al. 2020). The aboveground organs of plants are initially affected by AR, which directly suppresses their light-harvesting functions by reducing chlorophyll contents, limiting photosynthesis and transpiration, and increasing water loss in foliage (Du et al. 2017; Ju et al. 2020). Meanwhile, AR-induced soil acidification accelerates the leaching of soil nutrients and basic cations, releasing bound Al into the soil solution, thereby threatening the root development and microbial processes of plants (Muthukumar et al. 2014; Maltz et al. 2019). These alterations would ultimately reduce biomass and stress resistance and cause annual economic losses of up to US$2.4 billion in China (Li et al. 2020). Despite the conventional strategies to mitigate the detrimental effects of AR on plants, novel technologies involving biological approaches, such as building symbiotic associations between host plants and arbuscular mycorrhizal fungi (AMF), are promising tools (Larssen et al. 1999; Aguilera et al. 2015).
Mycorrhizal symbiosis can reportedly assist plant performance in accessing mineral nutrients with low mobility, such as phosphorus (P), from the soil as AM fungal hyphae can achieve greater exploration of soil, enlarge root absorption area, and subsequently improve tolerance towards biotic and abiotic stresses (Smith and Read 2008; Garg and Singh 2018; Pandey et al. 2019). Several AM fungi have been isolated from acidic edaphic environments, most of which belong to the Glomus and Acaulospora genera (Aguilera et al. 2015). Although AR might negatively affect the structure and development of mycorrhizae (Clark 1997; Liu et al. 2020), several studies have demonstrated improved growth and performance of mycorrhizal plants under a range of acidic conditions (Medeiros et al. 1994; He et al. 2019). For example, colonization with G. etunicatum and G. intraradices enhanced the dry matter yield and nutrient acquisition of Sorghum bicolor grown at lower pH values (Medeiros et al. 1994). Similarly, mycorrhizae improved the growth and P acquisition of Thuja occidentalis subjected to AR stress (Anwar et al. 2020). Furthermore, inoculation with A. tuberculata positively affected the shoot and root biomass of Calamagrostis villosa rather than that of Deschampsia flexuosa grown under acidic conditions (Vosátka and Dodd 1998). Moreover, the total biomass and photosynthetic capabilities of Zelkova serrata seedlings inoculated with Diversispora versiformis were higher than those of plants inoculated with Rhizophagus irregularis under AR stress (Wang et al. 2021). These findings indicate that the growth response tends to vary with host plants and AM fungi species (Muthukumar et al. 2014). The possible underlying mechanisms by which mycorrhizal plants enhance acidic tolerance mainly involve P (Anwar et al. 2020) or other mineral nutrient acquisition (Medeiros et al. 1994; Clark 1997). However, Chen and Lei (2019) found that mycorrhizal inoculation did not improve the N and P contents in the leaves of Koelreuteria paniculata under AR stress. It has been suggested that enhanced stress tolerance in plants due to AM association results from a combination of nutritional and physiological effects (Pandey et al. 2019). In addition, plants consisting of woody tissue appear to be more sensitive to acidification than dwarf or herbaceous plants (Zvereva et al. 2010). Therefore, it is imperative to investigate the roles of AM fungi in regulating the growth of woody plants under AR stress.
Lespedeza formosa (Vog.) Koehne, a legume widely distributed in Asia and North America (Nemoto and Ohashi 1993), is an economically important shrub in agroecosystems, and is used for livestock forage, reclamation of degraded environments, and medicine (Zhao et al. 2006). Zhejiang Province, the main planting area of L. formosa in southeastern China, has exposed to AR most frequently, with pH values ranging from 5.6 to 3.8 (Gilani et al. 2021). Moreover, L. formosa reportedly cannot grow satisfactorily under acidic conditions, with the preferred pH value ranging from 5.5 to 6.0 (Zhao et al. 2006). Given that L. formosa is a mycorrhizal plant with root colonization of up to 40% (He et al. 2019), we hypothesized that AM fungal inoculation would mitigate the AR-induced detrimental effects on L. formosa with enhanced nutrient acquisition. To test this hypothesis, a greenhouse experiment was conducted to investigate the potential of AM fungi in improving the acid tolerance of L. formosa by using plant growth parameters, photosynthetic properties, and mineral nutrient acquisition as indicators of improved plant performance in relation to mycorrhizal efficiency.
Materials and methods
Plant material and AMF preparation
The L. formosa seeds and topsoil (0–20 cm) used in this experiment were collected from the experimental field near Zhejiang A & F University, Hangzhou City, Zhejiang Province, China (30˚140ʹ N, 119˚42ʹ E). On May 11, 2018, the seeds were surface-disinfected using 10% (v/v) hydrogen peroxide for 5 min and rinsed five times with sterilized deionized water. Six seeds were subsequently sown in each plastic pot (21 × 22 × 17 cm) filled with 4 kg of soil, which comprised of a mixture of field soil and peat at a ratio of 1:3 (v/v) and was sterilized by gamma irradiation at a dose of 25 kGy (McNamara et al. 2003). The local soil classified as Ultisols in the US Soil Taxonomy (Soil Survey Staff 2010) had the following distinct properties: organic matter, 22.1 mg·g−1; total N, 0.85 mg·g−1; Olson P, 0.42 mg·g−1; and pH 5.6 (water: soil = 5:1). Thirty days after sowing, the plants (6 cm in height and eight leaves per plant) were thinned to two seedlings per pot.
Two dominant mycorrhizal fungal species, Rhizophagus irregularis [(N.C. Schenck & G.S. Sm.) C. Walker & A. Schüßler] (previously named G. intraradices) (BGC BJ09), and Diversispora versiformis [(P. Karst.) Oehl, G. A. Silva & Sieverd.] (previously named G. versiforme) (BGC GD01C) were purchased from the Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry Science, China. Rhizophagus irregularis and D. versiformis were originally isolated from neutral and acidic soil, respectively (Wang et al. 2021). These two AMF were cultured individually using S. bicolor as a trap plant in a plastic pot with autoclaved fine sand as the substrate (Wang et al. 2018). After five months of culturing, the AMF inocula used in the experiment consisted of spores, hyphae, sand, and colonized root fragments of the host plant. The inocula contained approximately 210 spores per 10 g of soil, which was determined following the method provided by Brundett et al. (1996). The corresponding AMF inoculum was placed in the growth substrate at a depth of 5 cm immediately before sowing the L. formosa seeds.
Experimental design
The experiments were conducted in a greenhouse near Zhejiang A & F University using a full factorial design consisting of three acid rain regimes (pH 5.6, 4.0, or 2.5) and four AMF inoculation regimes (inoculation with sterilized AMF as the control, R. irregularis, D. versiformis, or the combined inoculation of the two AMF). Each treatment included 10 replicates for a total of 12 treatment combinations, resulting in a total of 120 pots (two seedlings per pot). The plants to be inoculated with AMF (AM plants) were provided with 40 g of R. irregularis, D. versiformis, or a combined inoculum comprising equal proportions of the two AMF taxa per plant. Non-mycorrhizal control plants (NM plants) received 40 g of autoclaved (121 °C, 0.11 MPa, 2 h) inoculum combining the two AMF and 40 mL of filtrate to minimize the differences in soil microflora (Evelin et al. 2012).
According to the reports of AR in most areas of Zhejiang Province (Zhang et al. 2007), H2SO4 and HNO3 (8:1 by mole) were selected to prepare the acidic stock solution. To mimic the precipitation pH in the most AR-threatened areas of southern China, the pH values of AR treatments were set at 5.6, 4.0, and 2.5 (Gilani et al. 2021; Wang et al. 2021). Acid solutions with pH levels of 5.6, 4.0, and 2.5 were obtained by separately diluting the stock solution with distilled water (average pH was approximately 6.8). The simulated AR of pH 5.6 was treated as a control. Approximately 400 mL of acid solution per pot was sprinkled on the top of the plants every two days, which was calculated from the mean annual precipitation in Hangzhou City (1420 mm) (Song et al. 2015) and the area of plastic pot (346 cm2). At the end of the experiment, the corresponding AR (pH 5.6, 4.0, and 2.5) treated plants achieved average soil pH values of 5.6, 4.5, and 3.8, respectively. To avoid acid shock during fungal establishment, acid rain treatment was initiated on June 14, 2018, which was one month after the start of the experiment. Throughout the experiment, the average temperature and relative humidity in the greenhouse were maintained at 28.8 °C and 66.5%, respectively. Seedlings were harvested on June 14, 2019, after more than 12 months of AR treatment.
During harvest, the plants were carefully washed and separated into shoots and roots to determine the initial fresh biomass. The roots were further divided into two subsamples and their fresh weights were recorded. A root subsample of a known weight was used to determine the mycorrhizal colonization. The remaining parts of the plants were oven-dried at 60 ºC to constant weight. The dry weight of the root subsample, used for measuring the AM colonization, was determined following the method described by Veiga et al. (2013). The root biomass was subsequently calculated by adding the dry weight of the subsample to that of the remaining roots.
Determination of chlorophyll contents and photosynthetic parameters
Immediately before harvesting, the relative chlorophyll contents in ten randomly selected leaves from each treatment combination were determined using a chlorophyll meter (SPAD-502Plus, Konica Minolta, Japan). Subsequently, the net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) were measured from 8:30 to 11:30 using an LI-6400 portable photosynthesis system (LI-COR Bioscience, Lincoln, NE, USA). Leaf photosynthetic measurements were conducted on leaves of four plants completely exposed to sunlight, which were randomly selected from each treatment combination, following the methods described by León-Sánchez et al. (2016). During the measurements, the leaf temperature, CO2 concentration, relative humidity, and photosynthetically active radiation were set at 27 °C, 400 μmol mol−1, 70%, and 1200 μmol m−2 s−1, respectively. The instantaneous water use efficiency (WUE) was calculated as Pn/Tr.
Determination of AMF colonization
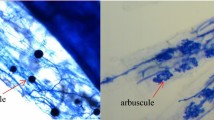
To measure the AM colonization, root subsamples of three plants per treatment combination were cleaned using 10% KOH at 90 °C for 1 h, acidified with 1 M HCl for 5 min, and stained with trypan blue (Phillips and Hayman 1970). Mycorrhizal colonization (hyphae or vehicle) was microscopically examined following the gridline intersect method (Giovannetti and Mosse 1980).
Measurement of plant elemental composition
Dried shoot and root samples of five randomly selected plants from each treatment combination were ground separately. The N and P concentrations were determined following the Kjeldahl acid-digestion and the molybdenum blue ascorbic acid methods, respectively (Allen 1989). The K+, Mg2+, Al3+, and S concentrations were determined following the methods described by Rončević et al. (2014) using a plasma atomic emission spectrometer (ICP-AES) (Optima 8000, Inc., PerkinElmer, USA).
Data analysis
To quantify the mycorrhizal efficiency, the mycorrhizal growth response (MGR) values were calculated as follows (Johnson et al. 2015):
where \({DW}_{AMF}\) and \(Avg\left({DW}_{non-AMF}\right)\) are the total dry weight of the mycorrhizal plants and mean dry weight of the NM plants under the corresponding pH conditions (n = 10), respectively.
The acid tolerance index (ATI) of each plant was determined as follows (He et al. 2019):
where \({B}_{i,max}\) is the largest shoot biomass of the ten plants at pH 5.6 with identical AMF inoculation, and Bi is the shoot biomass of each of the ten plants at pH 4.0 and 2.5 (i.e., the pH stress conditions applied in our study). In this experiment, target species with ATI values of 0.3 < ATI < 0.6 and ATI > 0.6 were considered moderately acid-sensitive and acid-tolerant, respectively.
Two-way analysis of variance (ANOVA) was performed to estimate the responses of plant parameters to AR and AMF treatments using the “aov” function of the R programming language. Furthermore, to compare which pairs of treatment combinations had significantly different trait values, least significance difference (LSD) analyses were conducted using the “LSD.test” function of the “agricolae” package (Mendiburu 2020) in R software. In addition, we checked the normality using the “shapiro.test” function and homoscedasticity of the residuals of the performed ANOVA using the “levene Test” function in the “car” package (Fox and Weisberg 2019) of R. Partial regression was fitted to evaluate the effects of plant growth parameters, photosynthetic properties, and nutritional acquisition on mycorrhizal efficiency, and Akaike’s Information Criterion values were used to identify the best-fit model (Burnham and Anderson 2002). All analyses were performed using R 4.0.3 (R Core Team 2020). Bar graphs were generated using Origin 2018 (Origin Lab Co., Northampton, MA, USA), and the graphs for partial regression were produced using the “ggforestplot” package (Scheinin et al. 2021) in R 4.0.3.
Results
Mycorrhizal colonization and plant growth
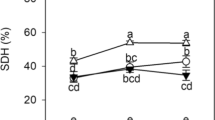
This study showed that nearly no root colonization (2.8–5%) was observed for L. formosa plants grown without AMF (Fig. 1). AMF inocula were highly infective at all pH levels, with root colonization ranging from 16.2% to 66.7%, whereas the extent of mycorrhizal colonization was significantly affected by AR (Fig. 1; Fig. S1). We observed significant differences in root colonization induced by various AMF inocula. The highest root colonization (54.7%) was recorded in plants inoculated with the combination of R. irregularis and D. versiformis under highly acidic conditions (pH 2.5).
Effects of arbuscular mycorrhizal fungi (AMF) on mycorrhizal colonization of Lespedeza formosa under acid rain with pH 2.5, 4.0, and 5.6. NM, Ri, Dv, and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, with Diversispora versiformis and with the mixtures of the two fungi inoculum, respectively. Values are presented as the mean ± SE (n = 3). Different letters indicate a significant difference (P < 0.05)
AR alone did not affect the total dry weight of NM plants (Fig. 2A). Mycorrhizal inoculation greatly improved biomass at all pH levels. At pH 4.0, the total dry weights of plants inoculated individually with R. irregularis and D. versiformis, and their combination were 28.7%, 19.2%, and 34.9% higher than those of NM plants, respectively, whereas the corresponding improvements with mycorrhizal assistance at pH 2.5 were 20.3%, 13.9%, and 41.6%, respectively. Significant differences were observed in the efficiencies of AMF on total dry weight, where the highest improvement was induced by the combined AMF inoculation at lower pH levels (pH 4.0 or 2.5). Additionally, AR decreased the root/shoot ratio of AM plants, which was not affected by mycorrhizal inoculation (Fig. 2B). Significant interactive effects between AR and AMF were observed for mycorrhizal colonization and total dry weight (Table S1).
Effects of arbuscular mycorrhizal fungi (AMF) on total dry weight (A) and root/shoot ratio (B) of Lespedeza formosa under acid rain with pH 2.5, 4.0, and 5.6. NM, Ri, Dv, and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, with Diversispora versiformis, and with the mixtures of the two fungi inoculum, respectively. Values are presented as the mean ± SE (n = 10). Different letters indicate a significant difference (P < 0.05)
Leaf photosynthesis
AR and AMF independently exerted variable effects on SPAD and gs (Figs. 3A and B). AR alone significantly decreased the Pn of NM plants (Fig. 3C). At pH 4.0 and 2.5, the Pn of AM plants was higher than that of NM plants, and the highest Pn was observed in plants inoculated with combined AMF at pH 4.0. Moreover, AR significantly decreased the Ci of plants irrespective of mycorrhizal inoculation (Fig. 3D). However, the Ci of AM plants was drastically higher than that of NM plants, where the Ci of plants inoculated with R. irregularis, D. versiformis, and their combination was 29.3%, 34.1%, and 33.6% (at pH 4.0) and 120.0%, 120.1%, and 138.1% (at pH 2.5) higher than that of their non-mycorrhizal counterparts, respectively. Furthermore, AR alone significantly increased the Tr (Fig. 3E), which was significantly decreased by AMF, especially at lower pH levels (4.0 and 2.5). The lowest Tr was found in plants inoculated with the combined AMF at all pH levels. AR considerably decreased the WUE of L. formosa plants (Fig. 3F). In addition, the WUE of AM plants was significantly higher than that of NM plants, especially with the inoculation of combined fungi at pH 4.0 and 2.5, which was 160.3% and 358.8% higher than that of their NM counterparts, respectively. Significant interactive effects were observed between AR and AMF on the aforementioned six photosynthetic traits (Table S1).
Effects of arbuscular mycorrhizal fungi (AMF) on relative chlorophyll contents (SPAD) (A), stomatal conductance (gs) (B), net photosynthetic rate (Pn) (C), intercellular CO2 concentration (Ci) (D), transpiration Rate (Tr) (E), and instantaneous water use efficiency (WUE) (F) of Lespedeza formosa under acid rain with pH 2.5, 4.0, and 5.6. NM, Ri, Dv, and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, with Diversispora versiformis, and with the mixtures of the two fungi inoculum, respectively. Values for SPAD are presented as the mean ± SE (n = 10), and those for the other traits are stated as mean ± SE (n = 4). Different letters indicate a significant difference (P < 0.05)
Mineral nutrient concentrations
The N concentrations in NM plants were significantly increased by AR alone, which exhibited variable effects on N concentrations in the shoots and roots (Fig. 4A). Mycorrhizal inoculation significantly decreased the N concentrations in roots, and their benefits were only observed in the N concentrations of shoots inoculated with R. irregularis at pH 4.0. Meanwhile, AR had variable effects on the P and K+ concentrations in shoots and roots, where positive mycorrhizal efficiencies were only observed in the P concentrations of shoots and roots, and K+ concentrations in roots inoculated with R. irregularis and D. versiformis at pH 4.0 (Fig. 4B and C). In addition, AR significantly increased the Mg2+ concentrations in shoots and roots, whereas the mycorrhizal benefits varied with AMF inoculation, soil pH, and organs (Fig. 4D). In the shoots of AM plants, the highest Mg2+ concentrations were detected in plants inoculated with combined AMF at all pH levels. However, in mycorrhizal plant roots, the highest values were observed in the D. versiformis-inoculated plants at pH 5.6 or 4.0 and in R. irregularis-inoculated plants at pH 2.5. In this study, AR increased the Al3+ concentrations in the shoots and roots of NM plants, whereas mycorrhizal inoculation substantially decreased the Al3+ concentrations both in the shoots and roots, and the lowest values were consistently detected in plants inoculated with combined AMF (Fig. 4E). Moreover, both AR and AMF exerted various effects on the S concentrations in shoots and roots (Fig. 4F).
Effects of arbuscular mycorrhizal fungi (AMF) on nutrient concentrations of nitrogen (N) (A), phosphorus (P) (B), potassium (K+) (C), magnesium (Mg2+) (D), aluminum (Al3+) (E), and sulfur (S) (F) in shoot and root of Lespedeza formosa under acid rain with pH 5.6, 4.0, and 2.5. NM, Ri, Dv, and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, with Diversispora versiformis, and with the combination of the two fungi inoculum, respectively. Values are presented as the mean ± SE (n = 5). Different letters indicate a significant difference (P < 0.05)
Furthermore, neither AR nor AMF constantly affected the N/P ratios of shoots and roots, which were maintained below 10 (Fig. 5). At pH 4.0, mycorrhizal inoculation significantly decreased the N/P ratio of roots, and this type of mycorrhizal efficiency was only observed in the N/P ratio of the shoots of D. versiformis-inoculated plants. At pH 2.5, mycorrhizal inoculation improved the N/P ratios of shoots and roots, especially in plants with D. versiformis and combined AMF, and no significant differences in mycorrhizal efficiencies were observed. Significant interactive effects were observed between AR and AMF on all the above nutrients.
Effects of arbuscular mycorrhizal fungi (AMF) on the ratios of nitrogen to phosphorus (N/P ratio) in shoot and root of Lespedeza formosa under acid rain with pH 5.6, 4.0, and 2.5. NM, Ri, Dv, and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, with Diversispora versiformis, and with the combination of the two fungi inoculum, respectively. Values are presented as the mean ± SE (n = 5). Different letters indicate a significant difference (P < 0.05)
Mycorrhizal benefits and their relationships with other plant traits
The acid tolerance index (ATI) was not affected by AR alone (Fig. 6A). Mycorrhizal inoculation significantly increased the ATI in plants, with no variations observed between the AM plants. The ATI values suggest that AM plants were considerably more tolerant to AR than NM plants (ATI > 0.6). Although AR alone significantly decreased the MGR of all plants, AMF inoculation effectively improved the MGR, which promoted the growth of L. formosa (MGR > 0) (Fig. 6B). In addition, the highest MGR was consistently observed in the combined AMF-inoculated plants at lower pH levels (pH 4.0, 2.5). To quantify the relationship between MGR and other plant traits, we built a model (R2 = 0.398, P < 0.05) represented as follows:
Effects of arbuscular mycorrhizal fungi (AMF) on acid tolerance index (ATI) (A) and mycorrhizal growth response (MGR) (B) of Lespedeza formosa under acid rain with pH 5.6, 4.0, and 2.5. NM, Ri, Dv, and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, with Diversispora versiformis, and with the combination of the two fungi inoculum, respectively. Values are presented as the mean ± SE (n = 10). Different letters indicate a significant difference (P < 0.05)
where X1, X2, X3, X4, X5, and X6 represent the shoot-N, shoot-K+, root-Al3+, root-S, shoot-Mg2+, and shoot N/P ratios, respectively. The N concentrations in shoots (shoot-N) and N/P ratios in shoots (shoot N/P ratio) were positively correlated with MGR, whereas the Al3+ concentration in roots (root-Al3+) was negatively correlated with MGR (Fig. 7A; Fig. S2). Furthermore, shoot-N, root-Al3+, and the shoot N/P ratios together accounted for 70.1% of the variance in MGR, which signified their vital contributions to MGR (Fig. 7B).
Mean effect sizes of nutritional variables on mycorrhizal growth response (MGR) (A) and the relative contributions of variances of nutritional variables to MGR in Lespedeza formosa (B). Solid lines with filled circles demonstrate significant effects (P < 0.05), and the dotted lines with hollowed circles represent nonsignificant effects (P > 0.05). Abbreviations are as follows: Shoot-N, nitrogen concentration in shoot; Root-Al3+, aluminum concentration in root; Shoot-K+, potassium concentration in shoot; Shoot N/P ratio, the ratio of nitrogen to phosphorus concentration in shoot; Root-S, sulfur concentration in root; Shoot-Mg2+, magnesium concentration in shoot
Discussion
In this study, AR individually suppressed plant growth by decreasing photosynthetic properties (such as Pn, Ci, and WUE) and increasing the Tr and Al3+ concentrations in NM plants. In contrast, mycorrhizal inoculation, especially inoculating with combined AMF, could mitigate some of the detrimental effects of AR by increasing the total dry weight, photosynthetic capabilities, and nutrient acquisition, whereas decreasing the Al3+ concentrations in roots, supporting our hypothesis that AMF inoculation can alleviate the detrimental effects of AR on L. formosa. Furthermore, our findings revealed that mycorrhizal benefits were mainly attributed to shoot-N, shoot N/P ratio, and root-Al3+ in L. formosa plants grown under acidic conditions.
Enhanced leaf photosynthetic properties and biomass with mycorrhizal inoculation
Evidently, AR can exert direct and indirect impacts on various plant physiological functions, with photosynthesis being the most affected (Sun et al. 2016; Chen et al. 2019). Notably, photosynthesis is the key metabolic process for plant growth and development (Ramlall et al. 2015). In our study, AR alone negatively affected the photosynthetic parameters (Pn, Ci, and WUE) (Fig. 3), which is consistent with previous studies (Wang et al. 2017; Chen et al. 2019). However, AMF inoculation significantly improved Pn with higher Ci and WUE at lower pH levels, indicating that AMF might enhance leaf gas exchange in L. formosa by increasing the intercellular CO2 concentration and WUE. These mycorrhizal benefits enhance carbohydrate accumulation and water storage in reserves when AR limits water uptake through roots (Chen et al. 2019). Correspondingly, mycorrhizal inoculation considerably improved the total dry weight of L. formosa under acidic conditions, which is consistent with previous studies conducted on C. villosa grown under acidic conditions (Vosátka and Dodd 1998), legumes grown at pH 4.36 (Saif 1987), cowpea grown at pH 4.7 and 4.9 (Rohyadi et al. 2004), and Torreya grandis grown at pH 4.0 and 2.5 (Xia et al. 2021). The effect of mycorrhizal support on dry matter production would improve plant fitness under stressful conditions, allowing AMF to access additional photosynthates from the host plant (Pandey et al. 2019). It is suggested that mycorrhizal efficiencies vary with host plant species and the environmental conditions exposed to the plants. Meanwhile, the selection of sterilization methods, such as autoclaving or γ-irradiation, which could exert varying secondary effects on soil properties, may also cause differences in the mycorrhizal efficacies (McNamara et al. 2003).
Improving N acquisition and decreasing Al3+ concentrations with mycorrhizal inoculation
Our study shows that the mycorrhizal efficiencies on nutrient acquisition were mainly correlated with the N concentration in shoots rather than the P concentration; however, a clear P benefit was observed (Fig. 4 and Fig. 7). This finding corresponded with that of mycorrhizal Chrysanthemum morifolium under salinity stress (Wang et al. 2018), but contradicted those of mycorrhizal T. occidentalis (Anwar et al. 2020) and T. grandis (Xia et al. 2021) grown under acidic conditions. There are several possible reasons for these contradictions. First, N is often limited other than P under acidic conditions (Muthukumar et al. 2014). Liebig’s law of the minimum states that plant growth is predominantly determined by the most limiting factor (Anwar et al. 2020). Johnson et al. (2015) suggested that whether or not mycorrhizal benefits outweigh their costs depends on the relative availability of soil N and P. The plant N/P ratio is a useful indicator of the gradual and dynamic character of nutrient limitation (N-limited vs P-limited), and N/P ratios < 10 and > 20 generally correspond to N- and P- limited biomass production (Güsewell 2004). In this experiment, the N/P ratios in shoots and roots were < 10, indicating that they likely underwent N-limited biomass production (Fig. 5). Second, there were functional differences between AM fungal species (Koide 2000). Although AMF can access N in either inorganic or organic forms (Smith and Smith 2011), most studies stated that AMF do not increase the N availability to the same extent as P (Vosátka and Dodd 1998; Rohyadi et al. 2004; Rohyadi 2008). This may be because AM fungi require more N than their hosts (Johnson et al. 2015), and it is assumed that AM fungi must use N to fulfill their own nutritional requirements before supplying it to the host (Hodge and Fitter 2013). Subsequently, the mycorrhizal benefits vary with the dependence of AM fungi on N. Third, the characteristics of host plants differ. Lespedeza formosa is a legume, which needs more N for the growth and building association with AMF than the aforementioned non-legume host species (Johnson et al. 2015).
Moreover, our study found that mycorrhizal benefits correlated with a decrease in the root Al3+ concentrations (Fig. 4E and Fig. 7). In contrast with the shoot, more Al3+ were bound to roots, and mycorrhizal L. formosa plants contained lower concentrations of root Al3+ than their NM counterparts at pH 4.0 and 2.5. A similar decrease in the concentration of root Al3+ has also been reported previously (Raju et al. 1988; Rohyadi et al. 2004). Acidic soils reportedly reduce plant growth mainly due to Al phytotoxicity, which inhibits water and nutrient acquisition from soils and severely limits root growth (Aguilera et al. 2015). The lower Al3+ concentration in the roots of AM plants seemingly improved AR resistance. Aguilera et al. (2015) speculated that the mechanisms underlying reduced Al3+ concentration may be related to the chelation of Al3+ in the rhizosphere or AMF-mediated sequestration, both of which reduce the availability of Al3+ ions and their phytotoxicity. However, the exact mechanism underlying the mycorrhizal benefits on Al3+ concentration in L. formosa remains unclear; therefore, this subject requires further study.
Mycorrhizal efficiency variations with AM fungal species and the underlying mechanisms
The current results showed that mycorrhizal inoculation improved acid tolerance in L. formosa plants, and the mycorrhizal efficiencies were almost positive at all pH levels (Fig. 6). Most interestingly, although there were no differences in the acid tolerance abilities of mycorrhizal L. formosa plants, the mycorrhizal benefits varied within AMF species. This functional difference may be attributed to AMF specificity and the compatibility between AMF and host plants (van der Heijden et al. 1998). In comparison with single AMF, the highest MGR was observed in combined-fungi inoculum under AR stress, which is inconsistent with the studies on T. grandis (Xia et al. 2021) and Z. serrata (Wang et al. 2021), where R. irregularis and D. versiformis were proven to be preferable choices under acidic conditions, respectively. Boyer et al. (2015) found that single AMF species behave identically to the AMF mixtures when associations were built with strawberries. This enhanced mycorrhizal efficiency with combined fungi is generally attributed to functional complementarity among AMF, which is explained by niche segregation and facilitation (Koide 2000). Jansa et al. (2008) suggested that multispecies mixtures of AMF could provide additional P and better enhance plant growth than the single AMF species. However, the exact mechanism of the ability of combined-AMF to bolster stress tolerance in L. formosa plants remains unclear.
Several mechanisms have been proposed to explain the mechanism by which AM symbiosis can alleviate the detrimental effects of AR as follows (Medeiros et al. 1994; Muthukumar et al. 2014; Aguilera et al. 2015; Wang et al. 2021; Xia et al. 2021): (1) enhancement of nutrient acquisition, such as P, which is often deficient in acidic soil and can be achieved by two ways, i.e., by changing the root structure and function and the secretion of phosphatase or phytase enzymes by mycorrhizal roots or AM fungal hyphae to dissociate the bound P; (2) changes in plant physiology, metabolic, and biochemical activities, which are known to activate plant growth; (3) reducing the acquisition of Al by roots and its translocation within plants; (4) the production of exudates by extraradical fungal hyphae, which results in the chelation of toxic ions (especially Al or Mn); and (5) binding of toxic ions to AM fungal structures. In our study, the combination of the improved N concentrations, shoot N/P ratios, and reduction in the Al3+ concentration in roots likely work collectively to protect the mycorrhizal L. formosa plants from AR stress (Fig. 7). However, Xia et al. (2021) suggested that the benefits imparted by AM fungi on T. grandis under acidic conditions could be due to the joint effects of root colonization, acid tolerance, shoot P, shoot Zn2+, and root Fe2+. Otherwise, Wang et al. (2021) speculated that the higher acid tolerance of mycorrhizal Z. serrata seedlings is attributed to their enhanced photosynthetic capabilities over those of non-mycorrhizal plants. These contradictories are induced by the variations in AM fungi, host, and the parameters determined under acidic conditions.
Conclusions
Our results showed that AMF could benefit the growth of L. formosa and consequently increase the AR resistance in plants by enhancing photosynthetic properties, increasing nutrient acquisition (especially shoot N, root K+, P, and Mg2+), improving dry matter production and releasing the host plant from Al phytotoxicity. Furthermore, the mycorrhizal efficiencies differed between AMF species; the combined inoculation of R. irregularis and D. versiformis enhanced the performance better than the two fungi individually. These results suggest that AM fungi play a vital role in the survival and growth of L. formosa, which might increase their strength in AR-dominant regions. Moreover, this information highlights the need for further research on the contribution of AMF in field studies. Further studies are needed to demonstrate whether this conclusion is true for other host/AMF combinations.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguilera P, Cumming J, Oehl F, Cornejo P, Borie F (2015) Diversity of Arbuscular Mycorrhizal Fungi in Acidic Soils and Their Contribution to Aluminum Phytotoxicity Alleviation. In: Panda S, Baluška F (eds) Aluminum Stress Adaptation in Plants, Signaling and Communication in Plants. Springer, Switzerland, pp 203–228
Allen SE (1989) Chemical Analysis of Ecological Materials, 2nd edn.Blackwell Scientific Publications, London.
Anwar G, Lilleskov EA, Chimner RA (2020) Arbuscular mycorrhizal inoculation has similar benefits to fertilization for Thuja occidentalis L. seedling nutrition and growth on peat soil over a range of pH: implications for restoration. New for 51:297–311. https://doi.org/10.1007/s11056-019-09732-x
Boyer LR, Brain P, Xu XM, Jeffries P (2015) Inoculation of drought-stressed strawberry with a mixed inoculum of two arbuscular mycorrhizal fungi: Effects on population dynamics of fungal species in roots and consequential plant tolerance to water deficiency. Mycorrhiza 25:215–227. https://doi.org/10.1007/s00572-014-0603-6
Brundett M, Bougher N, Dell B, Grove T, Malajcuk N (1996) Working with Mycorrhiza in Forestry and Agriculture. ACIAR & CSIRO Resereach Forest Product, Embley, Western Australia, pp 155–157.
Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd edn. Springer, New York
Cao YZ, Wang S, Zhang G, Luo J, Lu S (2009) Chemical characteristics of wet precipitation at an urban site of Guangzhou, South China. Atmos Res 94:462–469. https://doi.org/10.1016/j.atmosres.2009.07.004
Chen S, Sun L, Zhang X, Shen X, Liu Y, Ren J (2019) Contrasting effects of long-term acid rain simulation on temperature sensitivity of soil respiration and enzymatic activities in a subtropical forest. J Soil Sediment 20:412–424. https://doi.org/10.1007/s11368-019-02385-5
Chen L, Lei N (2019) Effect of soil microbe inoculation on koelreuteria paniculata seedlings growth under simulated acid rain stress. Ecol Environ 28:438–445 (in Chinese). https://doi.org/10.16258/j.cnki.1674-5906.2019.03.002
Clark RB (1997) Arbuscular mycorrhizal adaptation, spore germination, root colonization, and host plant growth and mineral acquisiiton at low pH. Plant Soil 192:15–22. https://doi.org/10.1023/A:1004218915413
Du E, Dong D, Zeng X, Sun Z, Jiang X, De Vries W (2017) Direct effect of acid rain on leaf chlorophyll content of terrestrial plants in China. Sci Total Environ 605–606:764–769. https://doi.org/10.1016/j.scitotenv.2017.06.044
Evelin H, Giri B, Kapoor R (2012) Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 22:203–217. https://doi.org/10.1007/s00572-011-0392-0
Fox J, Weisberg S (2019) An {R} Companion to Applied Regression, 3rd edn. Thousand Oaks CA, Sage. https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Garg N, Singh S (2018) Mycorrhizal inoculations and silicon fortifications improve rhizobial symbiosis, antioxidant defense, trehalose turnover in pigeon pea genotypes under cadmium and zinc stress. Plant Growth Regul 86:105–119. https://doi.org/10.1007/s10725-018-0414-4
Gilani MM, Tigabu M, Liu B, Farooq TH, Rashid MHU, Ramzan M, Ma XQ (2021) Seed germination and seedling emergence of four tree species of southern China in response to acid rain. J for Res 32:471–481. https://doi.org/10.1007/s11676-020-01102-0
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrbizal infection of roots. New Phytol 84:489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266. https://doi.org/10.1111/j.1469-8137.2004.01192.x
He L, Xu J, Hu L, Ren M, Tang J, Chen X (2019) Nurse effects mediated by acid-tolerance of target species and arbuscular mycorrhizal colonization in an acid soil. Plant Soil 441:161–172. https://doi.org/10.1007/s11104-019-04103-z
Hodge A, Fitter AH (2013) Microbial mediation of plant competition and community structure. Funct Ecol 27:865–875. https://doi.org/10.1111/1365-2435.12002
Huang K, Zhuang G, Xu C, Wang Y, Tang A (2008) The chemistry of the severe acidic precipitation in Shanghai, China. Atmos Res 89:149–160. https://doi.org/10.1016/j.atmosres.2008.01.006
Huang J, Wang H, Zhong Y, Huang J, Fu X, Wang L, Teng W (2019) Growth and physiological response of an endangered tree, Horsfieldia hainanensis merr., to simulated sulfuric and nitric acid rain in southern China. Plant Physiol Bioch 144:118–126. https://doi.org/10.1016/j.plaphy.2019.09.029
Jansa J, Smith FA, Smith SE (2008) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789. https://doi.org/10.1111/j.1469-8137.2007.02294.x
Johnson NC, Wilson GWT, Wilson JA, Miller RM, Bowker MA (2015) Mycorrhizal phenotypes and the Law of the Minimum. New Phytol 205:1473–1484. https://doi.org/10.1111/nph.13172
Ju S, Wang L, Chen J (2020) Effects of silicon on the growth, photosynthesis and chloroplast ultrastructure of Oryza sativa L. seedlings under acid rain stress. SILICON 12:655–664. https://doi.org/10.1007/s12633-019-00176-8
Koide RT (2000) Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytol 147:233–235. https://doi.org/10.1046/j.1469-8137.2000.00710.x
Larssen T, Seip HM, Semb A, Mulder J, Muniz IP, Vogt RD, Lydersen E, Angell V, Dagang T, Eilertsen O (1999) Acid deposition and its effects in China: an overview. Environ Sci Policy 2:9–24. https://doi.org/10.1016/S1462-9011(98)00043-4
León-Sánchez L, Nicolás E, Nortes PA, Maestre FT, Querejeta JI (2016) Photosynthesis and growth reduction with warming are driven by nonstomatal limitations in a Mediterranean semi-arid shrub. Ecol Evol 6:2725–2738. https://doi.org/10.1002/ece3.2074
Li HR, Xiang HM, Zhong JW, Ren XQ, Wei H, Zhang JE, Xu QY, Zhao BL (2020) Acid rain increases impact of rice blast on crop health via inhibition of resistance. Enzymes Plants 9:881. https://doi.org/10.3390/plants9070881
Liang G, Hui D, Wu X, Wu J, Liu J, Zhou G, Zhang D (2016) Effects of simulated acid rain on soil respiration and its components in a subtropical mixed conifer and broadleaf forest in southern China. Environ Sci-Proc Imp 18:246–255. https://doi.org/10.1039/C5EM00434A
Liu X, Feng Z, Zhao Z, Zhu H, Yao Q (2020) Acidic soil inhibits the functionality of arbuscular mycorrhizal fungi by reducing arbuscule formation in tomato roots. Soil Sci Plant Nutr 66:1–10. https://doi.org/10.1080/00380768.2020.1721320
Maltz MR, Chen Z, Cao J, Arogyaswamy K, Shulman H, Aronson EL (2019) Inoculation with Pisolithus tinctorius may ameliorate acid rain impacts on soil microbial communities associated with Pinus massoniana seedlings. Fungal Ecol 40:50–61. https://doi.org/10.1016/j.funeco.2018.11.011
McNamara NP, Black HIJ, Beresford NA, Parekh NR (2003) Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl Soil Ecol 24:117–132. https://doi.org/10.1016/S0929-1393(03)00073-8
Medeiros CAB, Clark RB, Ellis JR (1994) Growth and nutrient uptake of sorghum cultivated with vesicular-arbuscular mycorrhiza isolates at varying pH. Mycorrhiza 4:185–191. https://doi.org/10.1007/BF00206778
Mendiburu FD (2020) Agricolae: Statistical Procedures for Agricultural Research. R package version 1.3–3. https://CRAN.R-project.org/package=agricolae
Muthukumar T, Priyadharsini P, Uma E, Jaison S, Pandey RR (2014) Role of Arbuscular Mycorrhizal Fungi in Alleviation of Acidity Stress on Plant Growth. In: Miransari M (ed) Use of Microbes for the Alleviation of Soil Stresses. Springer, New York, pp 43–71
Nemoto T, Ohashi H (1993) Seedling morphology of Lespedeza (Leguminosae). J Plant Res 106:121–128. https://doi.org/10.1007/BF02344415
Pandey D, Kehri HK, Zoomi I, Akhtar O, Singh AK (2019) Mycorrhizal Fungi: Biodiversity, Ecological Significance, and Industrial Applications In: Yadav A, Mishra S, Singh S, Gupta A (eds) Recent Advancement in White Biotechnology Through Fungi. Springer, Switzerland, pp 181–199. https://doi.org/10.1007/978-3-030-10480-1_5
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161. https://doi.org/10.1016/S0007-1536(70)80110-3
R Core Team (2020) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
Raju PS, Clark RB, Ellis JR, Maranville JW (1988) Effects of Va mycorrhizae on growth and mineral uptake of sorghum grown at varied levels of soil acidity. Commun Soil Sci Plant Anal 19:919–931. https://doi.org/10.1080/00103628809367985
Ramlall C, Varghese B, Ramdhani S, Pammenter NW, Bhatt A, Berjak P, Sershen, (2015) Effects of simulated acid rain on germination, seedling growth and oxidative metabolism of recalcitrant-seeded Trichilia dregeana grown in its natural seed bank. Physiol Plantarum 153:149–160. https://doi.org/10.1111/ppl.12230
Rohyadi A (2008) Growth responses of external hyphae of arbuscular mycorrhizal fungi to acidic soil conditions and their effects on cowpea growth. Microbiology Indonesia 2:22–26. https://doi.org/10.5454/mi.2.1.5
Rohyadi A, Smith FA, Murray RS, Smith SE (2004) Effects of pH on mycorrhizal colonization and nutrient uptake in cowpea under conditions that minimise confounding effects of elevated available aluminium. Plant Soil 260:283–290. https://doi.org/10.1023/B:PLSO.0000030183.87228.0b
Rončević S, Svedružić LP, Nemet I (2014) Elemental composition and chemometric characterization of pyrethrum plant materials and insecticidal flower extracts. Anal Lett 47:627–640. https://doi.org/10.1080/00032719.2013.845898
Saif SR (1987) Growth responses of tropical forage plant species to vesicular-arbuscular mycorrhizae: I. Growth, mineral uptake and mycorrhizal dependency. Plant Soil 97:25–35. https://doi.org/10.1007/bf02149820
Scheinin I, Kalimeri M, Jagerroos V, Parkkinen J, Tikkanen E, Würtz P, Kangas A (2021) Ggforestplot: Forestplots of Measures of Effects and Their Confidence Intervals. https://github.com/nightingalehealth/ggforestplot
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, 2nd edn. Academic Press, London
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250. https://doi.org/10.1146/annurev-arplant-042110-103846
Soil Survey Staff (2010) Keys to Soil Taxonomy, 11th edn. USDA-Natural Resources Conservation Service, Washington, DC
Song X, Zhou G, Gu H, Qi L (2015) Management practices amplify the effects of N deposition on leaf litter decomposition of the Moso bamboo forest. Plant Soil 395:391–400. https://doi.org/10.1007/s11104-015-2578-2
Sun J, Hu H, Li Y, Wang L, Zhou Q, Huang X (2016) Effects and mechanism of acid rain on plant chloroplast ATP synthase. Environ Sci Pollut Res 23:18296–18306. https://doi.org/10.1007/s11356-016-7016-3
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72. https://doi.org/10.1038/23932
Veiga RSL, Faccio A, Genre A, Pieterse CMJ, Bonfante P, van der Heijden MGA (2013) Arbuscular mycorrhizal fungi reduce growth and infect roots of the non-host plant Arabidopsis thaliana. Plant Cell Environ 36:1926–1937. https://doi.org/10.1111/pce.12102
Vosátka M, Dodd JC (1998) The role of different arbuscular mycorrhizal fungi in the growth of Calamagrostis villosa and Deschampsia flexuosa, in experiments with simulated acid rain. Plant Soil 200:251–263. https://doi.org/10.1023/A:1004366822682
Wang YH, Wang MQ, Li Y, Wu AP, Huang JY (2018) Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 13:e0196408. https://doi.org/10.1371/journal.pone.0196408
Wang YH, Liu SY, Shao CL, Wu AP, He XB, Xia LN, Wang XD, Qiu YJ, Yu SQ, Pei J, Zhang NL (2021) Enhancement of photosynthetic parameters and growth of Zelkova serrata by arbuscular mycorrhizal fungi under simulated sulfuric acid rain. Plant Ecol 222:1361–1374. https://doi.org/10.1007/s11258-021-01184-8
Wang LH, Sun JW, Wang W, Zhou, Q (2017) Research advances in effects of acid rain on plant photosynthesis. J Saf Environ 17:775–780 (in Chinese). https://doi.org/10.13637/j.issn.1009-6094.2017.02.069
Wei H, Liu W, Zhang J, Qin Z (2017) Effects of simulated acid rain on soil fauna community composition and their ecological niches. Environ Pollut 220:460–468. https://doi.org/10.1016/j.envpol.2016.09.088
Xia LN, Shao CL, Zhang NL, Wu AP, Xie JB, Qiu YJ, He XB, Pei J, Wang XD, Wang YH (2021) Improved tolerance of mycorrhizal Torreya grandis seedlings to sulfuric acid rain related to phosphorus and zinc contents in shoots. J Fungi 7:296. https://doi.org/10.3390/jof7040296
Zhang M, Wang S, Wu F, Yuan X, Zhang Y (2007) Chemical compositions of wet precipitation and anthropogenic influences at a developing urban site in southeastern China. Atmos Res 84:311–322. https://doi.org/10.1016/j.atmosres.2006.09.003
Zhao Y, Chen XY, Pian RQ, Wang XR (2006) Research Advances of Lespedeza Michx. J Northwest Forestry Univ 21:71–75 (in Chinese). https://doi.org/10.3969/j.issn.1001-7461.2006.02.020
Zvereva EL, Roitto M, Kozlov MV (2010) Growth and reproduction of vascular plants in polluted environments: A synthesis of existing knowledge. Environ Rev 18:355–367. https://doi.org/10.1139/A10-017
Acknowledgements
We are grateful to four anonymous reviewers for their constructive comments. Additionally, we would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the National Natural Science Foundation of China (32071644 and 31400366), the Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (LTY22C030003), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB 31030000), and the Special Foundation for National Science and Technology Basic Research Program of China (2019FY102000).
Author information
Authors and Affiliations
Contributions
Yanhong Wang proposed and organized the overall experiment, wrote and revised the main manuscript text. Naili Zhang and Changliang Shao gave assistance with interpretation of the results and reviewed the final manuscript. Aiping Wu reviewed the final manuscript. Xiaobin He contributed to data analysis and figures preparation. Lina Xia, Tiantian Li, and Jia Pei contributed to laboratory work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue.
Consent for publication
All authors approved the final manuscript as submitted for publication.
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, X., Shao, C., Wu, A. et al. Arbuscular mycorrhizal fungi enhance nutrient acquisition and reduce aluminum toxicity in Lespedeza formosa under acid rain. Environ Sci Pollut Res 29, 29904–29916 (2022). https://doi.org/10.1007/s11356-021-18248-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18248-x