Abstract
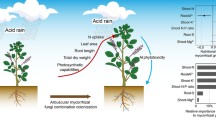
Acid rain (AR) is a frequent environmental issue in southern China that causes damage to the growth and photosystems of subtropical tree species. Arbuscular mycorrhizal fungi (AMF) can improve plant tolerance to acidic conditions; however, how AMF mediate the detrimental effects of AR on the growth and photosynthetic parameters of tree species is yet to be understood. In this study, we inoculated Zelkova serrata, an important economic tree species in China, with Rhizophagus irregularis, and Diversispora versiformis, alone and in combination, under three simulated AR regimes (pH 2.5, 4.0, and 5.6). Mycorrhizal colonization, the concentrations of succinate dehydrogenase (SDH) and alkaline phosphatase (ALP) in hyphae, leaf chlorophyll fluorescence and photosynthetic parameters, and growth were all subsequently measured. Our results revealed that AR sharply reduced photosynthetic ability and total biomass of non-mycorrhizal plants, whereas AMF inoculation significantly improved ALP, SDH, total biomass, net photosynthetic rate, and acid tolerance under acidic conditions compared to the non-mycorrhizal controls. Moreover, the acid tolerance of Z. serrata was positively correlated with net photosynthetic rate. Furthermore, our results indicated that mycorrhizal efficiencies varied with the intensity of AR and AMF identities, with D. versiformis proving much more efficient than the other fungi under acidic conditions. Overall, our findings highlight the significance of AMF associations for tree species suffering from AR stress and provide insight into strategies for improving the acid tolerance of plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Given that plants are confined to their growing locations, they are typically unable to avoid unpredictable and unfavourable changes in their environment (Bussotti and Pollastrini 2021). Acid rain (AR) has become a growing environmental issue worldwide, and ongoing global changes are likely to enhance the severity and exposure of plants to this problems in the coming decades (Andrade et al. 2020; Liu et al. 2019). In China, over the past three decades, more than 30% of the land area has suffered from AR, particularly in southern China (Liang et al. 2016; Wei et al. 2017; Zhang et al. 2007). Zhejiang Province is an economically developed region in southeastern China, and in recent years, with the rapid growth of the economy and the increasing intensity of fuel consumption, 91.3% of the county-level cities received AR pollution in 2016, with sulfuric AR being the main type of AR (Zhejiang Ecology and Environment Bureau 2016). Furthermore, economic losses associated with AR in China are estimated to reach 110 billion RMB each year (Bao et al. 2020; Singh and Agrawal 2008; Wei et al. 2017). Therefore, AR has aroused widespread public concern, with tree damage and loss of particular concern because of their vulnerability to this form of pollution (Larssen et al. 1999; Li et al. 2021; Liu et al. 2019).
AR usually has adverse effects on a range of soil properties, microorganisms and microbial processes, and plant morphological traits, which can subsequently affect plant physiological functioning (Bao et al. 2020; Gilani et al. 2020; Rodriguez-Sanchez et al. 2020; Singh and Agrawal 2008). Compared to other plant organs, leaves are much more sensitive to AR (Macaulay and Enahoro 2015). For example, as one important group of leaf functional traits, photosynthetic parameters are considered the best indicators for understanding and evaluating the effects of AR on plant performance (Wang et al. 2017). Nevertheless, the photosynthetic responses of plant species to acidic conditions differ with the intensity of AR. For example, the photosynthetic activity of Platanus occidentalis is decreased at pH 2.0 (Singh and Agrawal 2008), and the chlorophyll content and maximum quantum efficiency of PSII photochemistry (Fv/Fm) are reduced in Camellia sinensis at pH 3.5 (Zhang et al. 2020). For soybean (Glycine max), chlorophyll content and net photosynthesis rate are impeded at pH 4.5 (Wen et al. 2011). All of these effects decrease plant production. Effort is currently being made to reduce such deleterious effects using appropriate measures, such as liming, and adopting policy measures to control the emission of acid precursors (i.e., SO2 and NOX). However, these approaches are often either expensive or unpractical (Rengel 2003; Singh and Agrawal 2008). Among the available biotechnological methods, generating mutualistic symbioses between plants and arbuscular mycorrhizal fungi (AMF) has proved effective under acidic conditions (Aguilera et al. 2015; Vosátka and Dodd 1998).
It is generally accepted that AMF can form mycorrhizal associations with approximately 80–90% vascular plant species, which facilitate mineral nutrient acquisition (such as N or P) from the soil. In return, the host plants provide carbon compounds for fungal development (Albornoz et al. 2020; Grilli et al. 2014; Smith and Read 2008). There is strong evidence that AMF can benefit plants by stimulating mineral nutrient absorption, enhancing photosynthesis, improving soil properties, and increasing tolerance to abiotic stresses (Al-Karaki 2006; Anwar et al. 2019; Li et al. 2021; Ruiz-Lozano et al. 2012). It has been reported that AMF can survive in acidic soils with pH values ranging from 2.7 to 9.2, while pH tolerance appears to vary between different fungal isolates (Clark 1997; Date et al. 1995; Liu et al. 2020; Rohyadi 2008). Some studies have shown that AMF can stimulate the growth of grasses and crop species under acidic conditions (Medeiros et al. 1994; Rohyadi 2008; Vosátka and Dodd 1998); however, few studies have been conducted on trees. Furthermore, while promoting nutrient acquisition is the prominent effect of AMF on plants under unfavorable conditions, Albornoz et al. (2020) recommended that non-nutritional mycorrhizal efficiency deserves more attention. Indeed, the photo-physiological mechanisms underlying the beneficial mycorrhizal effects on tree species subject to AR remain poorly understood.
Zelkova serrata (Thunb.) Makino, a Class II protected tree species in China, is a culturally and economically valuable indigenous species in Zhejiang Province, widely used in the construction, horticultural, shipbuilding, and pharmaceutical industries (Wang et al. 2019). It has been reported that Z. serrata can form mycorrhizal associations with enhanced photosynthetic ability under stressful conditions, and frequent AR events have become a severe limiting factor for the survival and growth of Z. serrata (Jiang et al. 2012; Wang et al. 2019; Zhu et al. 2018). Therefore, this study was conducted based on the hypothesis that AMF can mediate the adverse effects of AR on the performance of Z. serrata. Furthermore, previous studies suggest that the efficacy of mycorrhizal fungi in alleviating plant stress is usually stress-dependent (van der Heijden and Sanders 2003) and varies with AMF species (Amanifar and Toghranegar 2020). Therefore, we also hypothesized that the protective effects of AMF under acidic conditions may vary with the intensity of AR and fungal species. Here, we describe a factorial experiment investigating the effects of various AMF types on the growth and photosynthesis of Z. serrata seedlings under simulated AR conditions. In doing so, we sought to better understand the potential application of AMF in vegetation restoration and afforestation programs.
Materials and methods
Plant materials, AMF inoculum, and soil
Z. serrata seeds were obtained from Richu Seeds Co., Ltd., China. After being surface sterilized with 5% sodium hypochlorite for 10 min and rinsed with distilled water, the seeds were sown in autoclaved river sand in a growth chamber set to a 19/15 °C day/night regime with a 12/12 h photoperiod. After emergence, 100 seedlings of similar size were selected for the following treatments.
Two broad-spectrum mycorrhizal fungal species, Diversispora versiformis (P. Karst.) Oehl, G. A. Silva and Sieverd (BGC GD01C) and Rhizophagus irregularis (N.C. Schenck & G.S. Sm.) C. Walker & A. Schüßler (BGC BJ09) were provided by the Glomales Germplasm Bank of the Beijing Municipal Academy of Agriculture and Forestry Science in China. D. versiformis and R. irregularis were originally isolated from Eremochloa ciliaris rhizosphere in Shaoguan, Guangdong Province, southern China, and from tomato plants in Langfang, Hebei Province, northern China, respectively. Moreover, D. versiformis and R. irregularis are acidic- and non-acidic-tolerant fungi, respectively. These two AMF were propagated individually using Sorghum bicolor as trap plants in plastic pots with sterilized fine sand as the substrate (Qiu et al. 2020). After five months of culturing, the fungal inoculum collected from the host rhizosphere consisted of spores, hyphae, sand, and colonized root fragments. Both types of AMF contained approximately 210 spores per 10 g of soil and were subjected to the highest possible number test.
The soil used in the experiment was composed of a mixture of field soil and peat (3:1, w/w), which was sterilized with γ-irradiation at a dose of 25 kGy (McNamara et al. 2003). The sterilized growth medium had the following properties: organic matter = 22.1 mg g−1, total N = 0.85 mg g−1, Olson P = 0.42 mg g−1, and pH = 5.6 (water:soil = 5:1).
Experimental design
The experiment was conducted using a full factorial design consisting of 12 factorial combinations of AR regimes and AMF regimes. Three AR regimes (pH 5.6, 4.0, or 2.5) and four AMF inoculation regimes (inoculated with sterilized inoculum or R. irregularis and D. versiformis, either alone or the combination) were tested, with five replicates for each treatment combination. On May 11, 2018, the seedlings were moved into a greenhouse located at the Pingshan Research Station of Zhejiang A & F University (30°15 N, 119°43 E) for acclimation. Nine days later, 60 seedlings of similar size were selected and transplanted into plastic pots (20.5 × 21 × 16.5 cm; one seedling per pot). These pots contained 4 kg of sterilized soil medium. The seedlings were replaced with new, healthy ones if they died within one month. Immediately before transplanting, the corresponding inoculum was placed below the roots of the seedlings. The AMF-inoculated plants (AM plants) received 40 g of R. irregularis, D. versiformis, or the combined inoculum comprising an equal proportion of the two AMF taxa per plant. Non-mycorrhizal plants (NM plants) were provided with the same amount of autoclaved mixed inoculum with 40 mL of soil extract to compensate for the differences in soil microflora (Evelin et al. 2012). To avoid acid shock during fungal establishment, we began the AR treatment on June 20, 2018. To simulate the AR status in most regions of Zhejiang Province, a stock acid solution was prepared with H2SO4 and HNO3 at a ratio of 8:1 (Zhang et al. 2007). Furthermore, in southeastern China, the pH of AR varies between 4.5 and 3.5, reaching as low as 2.95 in some cities (Cao et al. 2009; Huang et al. 2008; Niu et al. 2017; Zhang et al. 2007). Therefore, the stock acid solution was subsequently diluted to pH 5.6, 4.0, and 2.5 with distilled water (the average pH was approximately 6.8). According to the mean annual precipitation in Hangzhou, Zhejiang Province (Song et al. 2015) and the surface area of the plant pots, the daily spraying amount from June to December was calculated. Thus, 398 mL of the corresponding acid solution was applied to each pot every two days. The pot positions were changed every two weeks to reduce edge effects. The experiment ended on December 26, 2018, lasting for more than six months. During the experiment, the average temperature and relative humidity in the greenhouse were 28.2 °C and 68.3%, respectively, and the mid-day photosynthetic photon flux density was approximately 950 μmol m−2 s−1.
Determination of leaf chlorophyll fluorescence and photosynthetic parameters
Before harvesting, chlorophyll fluorescence parameters (Fv/Fm, the maximum quantum yield of PSII; Yield, the actual photosynthetic quantum yield; qN, non-photochemical quenching; and qP, photochemical quenching) were measured using a portable chlorophyll fluorometer (PAM-2500 WALZ, Effeltrich, Germany) following the saturation pulse method as described by Wang et al. (2019). Four plants from each treatment combination and three fully expanded leaves on the upper part of the plants were randomly selected for chlorophyll fluorescence measurements. Before measurement, the leaves were dark-adapted for 30 min, and then the determination was made at 1-h intervals between 9:00 am and 14:00 pm.
Subsequently, photosynthetic parameters (A, leaf net photosynthetic rate; gs, stomatal conductance; and E, transpiration rate) were determined for the same leaves as used for the chlorophyll fluorescence measurements using a LI-6400 portable photosynthesis system (LI-COR, Inc., Lincoln, USA). During these measurements, the leaf temperature was set at 27 °C in line with the mean ambient temperature and the relative humidity of the leaf chamber was set at 70%. Measurements were taken with a photosynthesis active radiation of 1200 μmol m−2 s−1, an airflow of 500 μmol s−1, and an ambient CO2 concentration of 400 μmol mol−1 (León-Sánchez et al. 2016). Instantaneous water use efficiency (WUE) was also calculated as A/E.
Measurements of leaf area and plant biomass
At the end of the experiment, one fully expanded fresh leaf was selected randomly from each replicate pot. These leaves were then scanned (Epson V330, Japan) and their leaf areas (LA) determined using Image J software (1.44p; National Institutes of Health, Bethesda, MD, USA) (Wang et al. 2018). Specific leaf area (SLA) was calculated as the leaf area divided by the dry biomass of each leaf. Subsequently, the plants were rinsed with distilled water three times and separated into roots and shoots. The dry weights of the roots and shoots were then weighed after oven-drying at 70 °C for 48 h.
Determination of mycorrhizal colonization and fungal activity
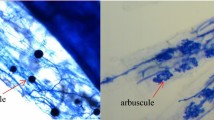
After harvesting, 3 g of root samples were taken randomly from three plants in each treatment combination. The root samples from each replicate pot were then evenly divided into three subsamples; the first subsample was stained with adjusted trypan blue (Wang et al. 2018) and microscopically examined for mycorrhizal colonization (hyphae or vehicle) using the gridline intersect method (Giovannetti and Mosse 1980); the second and third subsamples were used to measure succinate dehydrogenase (SDH) activity (Zhao et al. 1997) and alkaline phosphatase (ALP) (Tisserant et al. 1993) in the hyphae.
Statistical analysis
To quantify the mycorrhizal efficiency, mycorrhizal growth response (MGR) values were calculated as follows (Johnson et al. 2015):
where \({\text{DW}}_{{{\text{AMF}}}}\) and \(\overline{{{\text{DW}}_{{\text{non - AMF}}} }}\) represent the total dry weight of the mycorrhizal plants and mean dry weight of the non-mycorrhizal plants under the same pH conditions (n = 5), respectively.
The acid tolerance index (ATI) of each plant was determined as follows (He et al. 2019):
where Bi,max is the largest shoot biomass of the five plants at pH 5.6 with identical AMF inoculation, and Bi is the shoot biomass of each of the five plants at pH 4.0 and 2.5, respectively (i.e., the stressful pH conditions applied in our study). In this experiment, target species with ATI values of 0.3 < ATI < 0.6 and ATI > 0.6 were considered moderately acid-sensitive and acid-tolerant, respectively.
A two-way ANOVA (SPSS 23.0; SPSS Inc., Chicago, IL, USA) was performed to estimate the responses of different plant parameters to the AR and AMF treatments. Before analysis, all data were subjected to Levene’s tests to check for equality of variance, and the Shapiro–Wilk test was used to test for normality. When the interactive effects of AR and AMF were significant (P < 0.05), the Fisher’s least significant difference test was applied to compare differences between the treatments. Otherwise, pairwise trait relationships between all the measured Z. serrata parameters were assessed via Pearson’s correlation analysis across the 12 treatment combinations in the R 4.0.3 statistical platform (http://www.R-project.org/).
Results
Mycorrhizal colonization and AMF activity
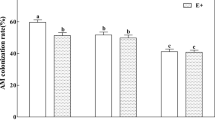
The percentage of root colonization was significantly higher in the mycorrhizal (AM) plants compared to the non-mycorrhizal (NM) plants (Fig. 1a, Fig. S1). The maximum percentage colonization (60%) was observed in the roots of the combined inoculated Z. serrata seedlings at pH 5.6. The root colonization of the combined inoculated plants tended to slowly decrease, while colonization of the plants inoculated with R. irregularis initially increased and then typically decreased under the harsher acidic conditions. In contrast, the mycorrhizal colonization of the D. versiformis-inoculated plants drastically increased with increasing AR intensity. Therefore, D. versiformis was notably more tolerant of the acidic experimental conditions. Non-inoculated seedlings also exhibited a small degree of root colonization (ranging from 1.33 to 2.46%), but there were no differences between the pH levels. Mycorrhizal colonization was significantly dependent on the fungal species as well as the interaction of the AR treatment and AMF, but not by AR alone (Table 1).
Effects of arbuscular mycorrhizal fungi on mycorrhizal coloniztion (a), succinate dehydrogenase (SDH) (b) and alkaline phosphatase (ALP) (c) of Zelkova serrata under sulfuric acid rain (SAR) with pH 2.5, 4.0 and 5.6. NM, Ri, Dv and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, Diversispora versiformis, either alone or the combination, respectively. Values are presented as the means ± SE (n = 3). Two-way ANOVA is performed to compare the effects of acid rain and mycorrhizal inoculation and their interactions on plants. Different letters indicate a significant difference according to LSD at P < 0.05
Both AR and AMF had significant effects on SDH (Table 1). Specifically, at lower pH values (pH 4.0 and 2.5), the SDH activity of the hyphae in the AM plants significantly increased, with the exception of the D. versiformis-inoculated plants (Fig. 1b). The highest proportion of SDH-active hyphae was recorded in the plants inoculated with the combined fungi at all pH values. The ALP activity of the hyphae in the AM plants significantly increased as pH decreased, specifically in the combined inoculated plants, in which approximately 57% of the hyphae showed ALP activity under the most acidic treatment (Fig. 1c). Moreover, the proportions of metabolically active (SDH and ALP) hyphae in the D. versiformis-inoculated and NM plants were consistent across all pH values.
Plant growth
AR alone significantly decreased the total dry weight of the NM plants. In comparison, the total dry weights of the AM plants were significantly higher, with the highest values recorded for the R. irregularis-inoculated plants at pH 5.6 and the D. versiformis-inoculated plants at lower pH values (pH 4.0 and 2.5) (Fig. 2a). AR alone had no effect on the root:shoot ratio, although the effects of AMF on the ratio depending on the pH and AMF species (Fig. 2b). Significant interactive effects of AR and AMF were detected on total dry weight and the root:shoot ratio (Table 1).
Effects of arbuscular mycorrhizal fungi on total dry weight (a) and root: shoot ratio (b) of Zelkova serrata under sulfuric acid rain (SAR) with pH 2.5, 4.0 and 5.6. NM, Ri, Dv and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, Diversispora versiformis, either alone or the combination, respectively. Values are presented as the means ± SE (n = 5). Two-way ANOVA is performed to compare the effects of acid rain and mycorrhizal inoculation and their interactions on plants. Different letters indicate a significant difference according to LSD at P < 0.05
Leaf photosynthetic parameters and chlorophyll fluorescence
AR, AMF, and their interaction had significant effects on A and gs; additionally, AR alone and the interaction between AR and AMF had significant effects on E and WUE (Table 1). AR alone had negative effects on A, gs, and WUE (Fig. 3). There were no differences in A between the AM and NM plants at pH 5.6, whereas under the lower pH conditions (pH 4.0 and 2.5), the A of the AM plants was significantly higher than that of the NM plants, with the highest values observed for the D. versiformis-inoculated plants (Fig. 3a). Except for the E of the plants with mixed inoculation at pH 4.0, AMF inoculation had no positive effects on gs, E, and WUE at any pH level (Fig. 3b–d).
Effects of arbuscular mycorrhizal fungus on net photosynthetic rate (A) (a), stomatal conductance (gs) (b), transpiration rate (E) (c) and instantaneous water-use efficiency (WUE) (d) of Zelkova serrata under sulfuric acid rain (SAR) with pH 2.5, 4.0 and 5.6. NM, Ri, Dv and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, Diversispora versiformis, either alone or the combination, respectively. Values are presented as the means ± SE (n = 4). Two-way ANOVA is performed to compare the effects of acid rain and mycorrhizal inoculation and their interactions on plants. Different letters indicate a significant difference according to LSD at P < 0.05
AR alone exhibited no effect on Fv/Fm but had a slight effect on qN; AMF alone had no effect on these two variables (Table 1; Fig. 4a, b). With an increase in AR intensity, the Yield and qP of the plants initially decreased and then frequently increased. At pH 5.6, the Yield and qP of the AM plants were lower than for the NM plants; at lower pH values (pH 4.0 and 2.5), although there were no differences in Yield and qP between the AM and NM plants, the highest values were detected in the D. versiformis-inoculated plants. Significant interactive effects of AR and AMF were detected in the case of Fv/Fm, Yield, and qP (Table 1).
Effects of arbuscular mycorrhizal fungus on the maximum quantum yield of PSII (Fv/Fm) (a), non-photochemical quenching (qN) (b), the actural photosynthetic quantum yield (Yield) (c), and photochemical quenching (qP) (d) of Zelkova serrata under sulfuric acid rain (SAR) with pH 2.5, 4.0 and 5.6. NM, Ri, Dv and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, Diversispora versiformis, either alone or the combination, respectively. Values are presented as the means ± SE (n = 4). Two-way ANOVA is performed to compare the effects of acid rain and mycorrhizal inoculation and their interactions on plants. Different letters indicate a significant difference according to LSD at P < 0.05
Mycorrhizal efficiency and acid tolerance
AR alone did not affect ATI, whereas AMF treatment had a significant effect in this regard (Table 1). The ATI values of the plants inoculated with D. versiformis, R. irregularis, and the mixture of the two were 175.6, 115.1, and 84.7% higher at pH 4.0, and 121, 63.4, and 127.6% higher at pH 2.5, respectively, compared to those of the NM plants (Fig. 5a). Mycorrhizal efficiencies (MGR) also varied with pH and AMF species (Fig. 5b). Specifically, at pH 5.6 and 4.0, the MGR values of D. versiformis and R. irregularis alone were higher than those of the mixture of the two, while under the most acidic condition (pH 2.5), the MGR value of D. versiformis was higher than those with the other two mycorrhizal inoculations. Furthermore, MGR was negatively correlated with A, gs and WUE, whereas ATI was positively correlated with A (Fig. S2).
Effects of arbuscular mycorrhizal fungi on acid-tolerance index (ATI) (a) and mycorrhizal growth response (MGR) (b) of Zelkova serrata under sulfuric acid rain (SAR) with pH 2.5, 4.0 and 5.6. NM, Ri, Dv and Ri + Dv represent the four AMF treatments: inoculation with sterilized mycorrhizal fungi, with Rhizophagus irregularis, Diversispora versiformis, either alone or the combination, respectively. Values represent means ± SE (n = 5). Two-way ANOVA is performed to compare the effects of acid rain and mycorrhizal inoculation and their interactions on plants. Different letters indicate a significant difference according to LSD at P < 0.05
Discussion
Our results show that AR had negative effects on A, gs, E, and total dry weight, whereas AMF inoculation, especially with D. versiformis, positively influenced ALP, SDH, total dry weight, A, and acid tolerance under acidic conditions compared to the NM controls. Nevertheless, mycorrhizal efficiencies varied with the intensity of AR. These observations support our hypotheses that AMF can mediate the detrimental effects of AR on Z. serrata seedlings, and mycorrhizal efficiencies varied with different AR levels and with different AMF isolates.
Biomass can be an appropriate indicator of plant growth and development under AR stress, which reflects differences in resource capture and biomass production (Dovrat et al. 2019; Liu et al. 2019). In our study, AR alone substantially decreased the total dry weight of NM plants (Fig. 2a), and we found that pH 4.0 was critical for the growth of Z. serrata seedlings compared to pH 3.0 in the case of rice (Oryza sativa) and pH 4.5 for soybean (Glycine max) (Liang et al. 2020). Inoculation with all three of the AMF treatments (R. irregularis, D. versiformis, and in combination) significantly increased the total biomass of Z. serrata seedlings under acidic conditions (i.e., pH 4.0 and 2.5). Such mycorrhizal efficiency conforms with observations of 24 tropical forage legumes and grasses inoculated with a combination of Glomus manihotis, Acaulospora longula, and Entrophospora colombiana at pH 4.36 (Saif 1987), Calamagrostis villosa inoculated with A. tuberculata at pH 3.2 (Vosátka and Dodd 1998), S. bicolor inoculated with G. deserticola at pH 4.5 (Raju et al. 1988), and Torreya grandis inoculated with mycorrhizal fungi at pH 4.0 (Xia et al. 2021). In contrast, mycorrhizal inoculation was found to have no effect on the biomass accumulation of Deschampsia flexuosa grown under acidic conditions (Vosátka and Dodd 1998). Thus, mycorrhizal benefits depend on the fungal taxa, the host plant species, and the intensity of AR. Additionally, the AMF inoculation in our study negatively affected biomass allocation to roots (Fig. 2b), which conforms to the responses observed in 14 other host plant species (Saif 1987) but is contrary to previous observations of cowpea plants (Rohyadi et al. 2004). Dovrat et al. (2019) postulated that variations in biomass partitioning could reflect species adaption and adjustment to environmental perturbations. Such a response indicates that mycorrhizal fungi prefer the development of shoots rather than roots, thereby potentially protecting shoots from acid deposition.
Photosynthesis is a primary process affected by AR (Du et al. 2017). We observed significant reductions in the A, gs, and E of the non-mycorrhizal Z. serrata plants as the intensity of AR was increased (Fig. 3), which is in accordance with previous studies on Pinus massoniana (Tong and Zhang 2014) and rice (da Fonseca et al. 2020). Jiao et al. (2017) suggested that photosynthetic activity could be regulated by stomatal factors (i.e., conductance and stomatal behaviors) as well as non-stomatal factors. This is also supported by our observation that stomatal factors, such as lower gs, could be related to reductions in A. However, the AM plants had higher A, gs, and E values than the NM plants in the lower pH treatments, which is consistent with the observations under salt stress made by Ruiz-Lozano et al. (2012) and Wu et al. (2010). The mycorrhizal-induced enhancement in photosynthetic ability could modulate the damage to photosynthetic organelles incurred by over-reduction of the reaction centers in the Photosystem II of plants subjected to AR stress (Ruiz-Lozano et al. 2012). Furthermore, this photosynthetic improvement would increase carbon assimilation rates, enhance its availability for plant growth and fungal development, and, thereby, improve plant acid tolerance as seen from the positive relationship between ATI and A (Fig. S2). However, in our experiment, mycorrhizal efficacy was negatively correlated with photosynthetic capability (Fig. S2), suggesting that some other mechanisms was responsible for the observed responses. Clark (1997) suggested that the enhanced uptake of some commonly deficient minerals (P, Ca, Mg, and K) could be responsible for the beneficial effects of mycorrhizal fungi in plants grown under acidic conditions, which has been verified for T. grandis under acidic conditions (Xia et al. 2021). Thus, the importance of AMF in buffering the detrimental effects of AR on Z. serrata plants warrants further examination.
Based on our observations, mycorrhizal efficacy is expected to vary with the intensity of AR, which is in disagreement with the previous theoretical models that predict that the magnitude of positive effects of neighbors on a target species will be higher under harsh abiotic stresses (Bertness and Callaway 1994). Raju et al. (1988) and Medeiros et al. (1994) reported similar mycorrhizal effectiveness under AR stress in sorghum. Nevertheless, other studies report that mycorrhizal benefits are enhanced under more acidic conditions (Chen and Lei 2019; Rohyadi 2008; Xia et al. 2021). Furthermore, in our experiment, plant parameters responded differently even when inoculated with the same fungal species under AR stress. For example, the total dry weight of the D. versiformis-inoculated plants was constant across all pH levels, whereas the photosynthetic capabilities (e.g., A and gs) of these plants decreased at lower pH values (Figs. 2, 3). As Maestre et al. (2005) suggest such departures from theoretical predictions can be induced by local environmental conditions, meaning that the variability in the estimators of plant performance can occur within target plant species.
Moreover, there were differences in mycorrhizal efficiencies between the AMF isolates, with D. versiformis proving more effective than both R. irregularis alone and their use in combination. It has been suggested that AR can have negative effects on the germination of spores, germ tube growth, the development of extra-radical mycelia, and mycorrhizal viability (expressed by SDH and ALP activities in hyphae) following colonization of the host plant (Liu et al. 2020; Vosátka and Dodd 1998; Vosátka et al. 1999). These effects are dependent on host preferences, pH tolerance, and functional diversity (Aguilera et al. 2015). For example, the optimal pH for R. irregularis is approximatly 5.0 (Medeiros et al. 1994), compared to that ranging from pH 3.8 to 8.0 for D. versiformis (Sieverding 1991). In our experiment, D. versiformis was isolated from acid soils and R. irregularis was isolated from agricultural soils with neutral pH, indicating that the former was more tolerant of acidic conditions. Smith et al. (2004) argued that such functional differences between fungal isolates may reflect stress-specific adaption mechanisms, such as variations in P uptake. Our results indicated that individual mycorrhizal species, i.e., D. versiformis in this case, provide a greater degree of acid tolerance in Z. serrata plants than when occurring in combination (i.e., with R. irregularis). Similarly, Xia et al. (2021) found that individually, R. irregularis had a more beneficial effect on T. grandis than in combination with Funneliformis mosseae. These observations provide empirical evidence for functional redundancy, as suggested by Gosling et al. (2016), although there is some evidence of enhanced nutrient uptake and plant growth as a result of functional complementarity of dual inoculation (Jansa et al. 2008; Koide 2000). Such contradictory resluts may be attributed to variations in the physiology of the different fungal isolates and host plant species or the experimental procedures and conditions employed (Jakobsen et al. 1992).
Conclusions
This study demonstrates that AR negatively affects the growth and photosynthetic characteristics of Z. serrata seedlings, while these detrimental effects were mitigated by AMF inoculation, especially in the case of D. versiformis. Furthermore, the magnitude of these effects varied with AR intensity and the type of AMF. Therefore, our observations indicate that AMF inoculation could offer a viable management strategy in areas subject to AR pollution, although two factors require further consideration. First, our experiment was conducted under greenhouse conditions, thus eliminating plant competitive effects. Second, mycorrhizal efficacy is affected by several environmental factors, and nitric AR is becoming more common than sulfuric acid deposition in some areas. Therefore, field trials are now required to examine the efficacy of mycorrhizal inoculation of Z. serrata under different AR conditions (both sulfuric and nitric types, and with different intensities) to further inform the development of AR mitigation strategies.
References
Aguilera P, Cumming J, Oehl F, Cornejo P, Borie F (2015) Diversity of arbuscular mycorrhizal fungi in acidic soils and their contribution to aluminum phytotoxicity alleviation. In: Panda SK, Baluška F (eds) Aluminum stress adaptation in plants, signaling and communication in plants. Springer, Switzerland, pp 203–228
Albornoz FE, Dixon KW, Lambers H (2020) Revisiting mycorrhizal dogmas: are mycorrhizas really functioning as they are widely believed to do? Soil Ecol Lett. https://doi.org/10.1007/s42832-020-0070-2
Al-Karaki GN (2006) Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci Hortic 109:1–7. https://doi.org/10.1016/j.scienta.2006.02.019
Amanifar S, Toghranegar Z (2020) The efficiency of arbuscular mycorrhiza for improving tolerance of Valeriana officinalis L. and enhancing valerenic acid accumulation under salinity stress. Ind Crops Prod 147:112234. https://doi.org/10.1016/j.indcrop.2020.112234
Andrade GC, Castro LN, da Silva LC (2020) Micromorphological alterations induced by simulated acid rain on the leaf surface of Joannesia princeps Vell. (Euphorbiaceae). Ecol Indic 116:106526. https://doi.org/10.1016/j.ecolind.2020.106526
Anwar G, Lilleskov EA, Chimner RA (2019) Arbuscular mycorrhizal inoculation has similar benefits to fertilization for Thuja occidentalis L. seedling nutrition and growth on peat soil over a range of pH: implications for restoration. New Forest 51:297–311. https://doi.org/10.1007/s11056-019-09732-x
Bao GZ, Tang WY, An QR, Liu YX, Tian JQ, Zhao N, Zhu SN (2020) Physiological effects of the combined stresses of freezing-thawing, acid precipitation and deicing salt on alfalfa seedlings. BMC Plant Biol 20:204. https://doi.org/10.1186/s12870-020-02413-4
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193. https://doi.org/10.1016/0169-5347(94)90088-4
Bussotti F, Pollastrini M (2021) Revisiting the concept of stress in forest trees at the time of global change and issues for stress monitoring. Plant Stress 2:100013. https://doi.org/10.1016/j.stress.2021.100013
Cao Y, Wang S, Zhang G, Luo J, Lu S (2009) Chemical characteristics of wet precipitation at an urban site of Guangzhou, South China. Atmos Res 94:462–469. https://doi.org/10.1016/j.atmosres.2009.07.004
Chen L, Lei N (2019) Effect of soil microbe inoculation on Koelreuteria paniculata seedlings growth under simulated acid rain stress. Ecol Environ Sci 28:438–445. https://doi.org/CNKI:SUN:TRYJ.0.2019-03-002
Clark RB (1997) Arbuscular mycorrhizal adaptation, spore germination, root colonization, and host plant growth and mineral acquisiiton at low pH. Plant Soil 192:15–22. https://doi.org/10.1023/A:1004218915413
da Fonseca SS, da Silva BRS, Lobato AKD (2020) 24-Epibrassinolide positively modulate leaf structures, antioxidant system and photosynthetic machinery in rice under simulated acid rain. J Plant Growth Regul 39:1559–1576. https://doi.org/10.1007/s00344-020-10167-4
Date RA, Grundon NJ, Rayment GE, Probert ME (1995) Plant-soil interactions at low pH: principles and management. Kluwer Academic Publishers, Dordrecht
Dovrat G, Meron E, Shachak M, Golodets C, Osem Y (2019) Plant size is related to biomass partitioning and stress resistance in water-limited annual plant communities. J Arid Environ 165:1–9. https://doi.org/10.1016/j.jaridenv.2019.04.006
Du EZ, Dong D, Zeng XT, Sun ZZ, Jiang XF, de Vries W (2017) Direct effect of acid rain on leaf chlorophyll content of terrestrial plants in China. Sci Total Environ 605:764–769. https://doi.org/10.1016/j.scitotenv.2017.06.044
Evelin H, Giri B, Kapoor R (2012) Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 22:203–217. https://doi.org/10.1007/s00572-011-0392-0
Gilani MM, Tigabu M, Liu B, Farooq TH, Rashid MHU, Ramzan M, Ma XQ (2020) Seed germination and seedling emergence of four tree species of southern China in response to acid rain. J for Res 32:471–481. https://doi.org/10.1007/s11676-020-01102-0
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Gosling P, Jones J, Bending GD (2016) Evidence for functional redundancy in arbuscular mycorrhizal fungi and implications for agroecosystem management. Mycorrhiza 26:77–83. https://doi.org/10.1007/s00572-015-0651-6
Grilli G, Urcelay C, Longo MS, Galetto L (2014) Mycorrhizal fungi affect plant growth: experimental evidence comparing native and invasive hosts in the context of forest fragmentation. Plant Ecol 215:1513–1525. https://doi.org/10.1007/s11258-014-0410-3
He L, Xu J, Hu LL, Ren ML, Tang JJ, Chen X (2019) Nurse effects mediated by acid-tolerance of target species and arbuscular mycorrhizal colonization in an acid soil. Plant Soil 441:161–172. https://doi.org/10.1007/s11104-019-04103-z
Huang K, Zhuang G, Xu C, Wang Y, Tang A (2008) The chemistry of the severe acidic precipitation in Shanghai, China. Atmos Res 89:149–160. https://doi.org/10.1016/j.atmosres.2008.01.006
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L.: 1. Spread of hyphae and phosphorus inflow into roots. New Phytol 120:371–379. https://doi.org/10.1111/j.1469-8137.1992.tb01077.x
Jansa J, Smith FA, Smith SE (2008) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789. https://doi.org/10.1111/j.1469-8137.2007.02294.x
Jiang HW, Jiang HW, Li JH, Li X (2012) Research progress of Zelkova Spach. J Jiangsu for Sci Technol 39:51–54. https://doi.org/10.3969/j.issn.1001-7380.2012.05.012
Jiao L, Wang L, Zhou Q, Huang X (2017) Stomatal and non-somatal factors regulated the photosynthesis of soybean seedligns in the present of exogenous bisphenol A. Ecotox Environ Safe 145:150–160. https://doi.org/10.1016/j.ecoenv.2017.07.028
Johnson NC, Wilson GWT, Wilson JA, Miller RM, Bowker MA (2015) Mycorrhizal phenotypes and the law of the minimum. New Phytol 205:1473–1484. https://doi.org/10.1111/nph.13172
Koide RT (2000) Functional complementary in the arbuscular mycorrhizal symbiosis. New Phytol 147:233–235. https://doi.org/10.1046/j.1469-8137.2000.00710.x
Larssen T, Seip HM, Semb A, Mulder J, Muniz IP, Vogt RD, Lydersen E, Angell V, Dagang T, Eilertsen O (1999) Acid deposition and its effects in China: an overview. Environ Sci Policy 2:9–24. https://doi.org/10.1016/S1462-9011(98)00043-4
León-Sánchez L, Nicolás E, Nortes PA, Maestre FT, Querejeta JI (2016) Photosynthesis and growth reduction with warming are driven by nonstomatal limitations in a Mediterranean semi-arid shrub. Ecol Evol 6:2725–2738. https://doi.org/10.1002/ece3.2074
Li X, Wang Y, Zhang Y, Wang Y, Pei C (2021) Response of soil chemical properties and enzyme activity of four species in the three gorges reservoir area to simulated acid rain. Ecotox Environ Safe 208:111457. https://doi.org/10.1016/j.ecoenv.2020.111457
Liang G, Hui D, Wu X, Wu J, Liu J, Zhou G, Zhang D (2016) Effects of simulated acid rain on soil respiration and its components in a subtropical mixed conifer and broadleaf forest in southern China. Environ Sci-Proc Imp 18:246–255. https://doi.org/10.1039/C5EM00434A
Liang C, Ma Y, Li L (2020) Comparison of plasma membrane H+-ATPase response to acid rain stress between rice and soybean. Environ Sci Pollut Res 27:6389–6400. https://doi.org/10.1007/s11356-019-07285-2
Liu Z, Yang J, Zhang J, Xiang H, Wei H (2019) A bibliometric analysis of research on acid rain. Sustainability 11:3077. https://doi.org/10.3390/su11113077
Liu X, Feng Z, Zhao Z, Zhu H, Yao Q (2020) Acidic soil inhibits the functionality of arbuscular mycorrhizal fungi by reducing arbuscule formation in tomato roots. Soil Sci Plant Nutr 66:1–10. https://doi.org/10.1080/00380768.2020.1721320
Macaulay BM, Enahoro GE (2015) Effects of simulated acid rain on the morphology, phenology and dry biomass of a local variety of maize (Suwan-1) in Southwestern Nigeria. Environ Monit Assess 187:622. https://doi.org/10.1007/s10661-015-4844-4
Maestre FT, Valladares F, Reynolds JF (2005) Is the change of plant-plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. J Ecol 93:748–757. https://doi.org/10.1111/j.1365-2745.2005.01017.x
McNamara NP, Black HIJ, Beresford NA, Parekh NR (2003) Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl Soil Ecol 24:117–132. https://doi.org/10.1016/S0929-1393(03)00073-8
Medeiros CAB, Clark RB, Ellis JR (1994) Growth and nutrient uptake of sorghum cultivated with vesicular-arbuscular mycorrhiza isolates at varying pH. Mycorrhiza 4:185–191. https://doi.org/10.1007/BF00206778
Niu YW, Pu JJ, Deng FP, Qi B (2017) Analysis on spatial and temporal evolution of acid rain and its causes from 1992 to 2012 in Zhejiang. Environ Monitor China 33:55–62. https://doi.org/10.19316/j.issn.1002-6002.2017.06.08
Qiu YJ, Zhang NL, Zhang LL, Zhang XL, Wu AP, Huang JY, Yu SQ, Wang YH (2020) Mediation of arbuscular mycorrhizal fungi on growth and biochemical parameters of Ligustrum vicaryi in response to salinity. Physiol Mol Plant P 112:101522. https://doi.org/10.1016/j.pmpp.2020.101522
Raju PS, Clark RB, Ellis JR, Maranville JW (1988) Effects of Va mycorrhizae on growth and mineral uptake of sorghum grown at varied levels of soil acidity. Commun Soil Sci Plant Anal 19:919–931. https://doi.org/10.1080/00103628809367985
Rengel Z (2003) Handbook of soil acidity. CRC Press, New York
Rodriguez-Sanchez VM, Rosas U, Calva-Vasquez G, Sandoval-Zapotitla E (2020) Does acid rain alter the leaf anatomy and photosynthetic pigments in urban trees? Plants 9:862. https://doi.org/10.3390/plants9070862
Rohyadi A (2008) Growth responses of external hyphae of arbuscular mycorrhizal fungi to acidic soil conditions and their effects on cowpea growth. Microbiol Indones 2:22–26. https://doi.org/10.5454/mi.2.1.5
Rohyadi A, Smith FA, Murray RS, Smith SE (2004) Effects of pH on mycorrhizal colonization and nutrient uptake in cowpea under conditions that minimise confounding effects of elevated available aluminium. Plant Soil 260:283–290
Ruiz-Lozano JM, Porcel R, Azcon C, Aroca R (2012) Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J Exp Bot 63:4033–4044. https://doi.org/10.1093/jxb/ers126
Saif SR (1987) Growth responses of tropical forage plant species to vesicular-arbuscular mycorrhizae: I. Growth, mineral uptake and mycorrhizal dependency. Plant Soil 97:25–35. https://doi.org/10.1007/BF02149820
Sieverding E (1991) Vesicular-arbuscular mycorrhiza management in tropical agrosystems. Deutsche Gesellschaft Technische Zusammenarbeit (GTZ) GmbH, Eschborn
Singh A, Agrawal M (2008) Acid ran and its ecological consequences. J Environ Biol 29:15–24. https://doi.org/10.2112/07A-0018.1
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic press, London
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524. https://doi.org/10.1111/j.1469-8137.2004.01039.x
Song XZ, Zhou GM, Gu HH, Qi LH (2015) Management practices amplify the effect of N deposition on leaf litter cecomposition of the Moso bamboo forest. Plant Soil 395:391–400. https://doi.org/10.1007/s11104-015-2578-2
Tisserant B, Gianiazzi-Pearson V, Gianinazzi S, Gollotte A (1993) In planta histochemical staining of fungal alkaline phosphatase activity for analysis of efficienty arbuscular mycorrhizal infections. Mycol Res 97:245–250. https://doi.org/10.1016/S0953-7562(09)80248-7
Tong S, Zhang L (2014) Differential sensitivity of growth and net photosynthetic rates in five tree species seedlings under simulated acid rain stress. Pol J Environl Stud 23:2259–2264. https://doi.org/10.15244/pjoes/24930
van der Heijden MGA, Sanders IR (2003) Mycorrhizal ecology. Springer, Heiderlberg, pp 184–185
Vosátka M, Dodd JC (1998) The role of different arbuscular mycorrhizal fungi in the growth of Calamagrostis villosa and Deschampsia flexuosa, in experiments with simulated acid rain. Plant Soil 200:251–263. https://doi.org/10.1023/a:1004366822682
Vosátka M, Batkhuugyin E, Albrechtová J (1999) Response of three arbuscular mycorrhizal fungi to simulated acid rain and aluminium stress. Biol Plant 42:289–296. https://doi.org/10.1023/A:1002125005497
Wang JP, Fu ZY, Ren Q, Zhu LJ, Lin J, Zhang JC, Cheng XF, Ma JY, Yue JM (2019) Effects of arbuscular mycorrhizal fungi on growth, photosynthesis, and nutrient uptake of Zelkova serrata (Thunb.) Makino seedlings under salt stress. Forests 10:186-201. https://doi.org/10.3390/f10020186
Wang LH, Sun JW, Wang W, Zhou Q (2017) Research advances in effects of acid arin on plant photosynthesis. J Saf Environ 17:775–780. https://doi.org/10.13637/j.issn.1009-6094.2017.02.069
Wang YH, Wang MQ, Li Y, Wu AP, Huang JY (2018) Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 13:e0196408. https://doi.org/10.1371/journal.pone.0196408
Wei H, Liu W, Zhang J, Qin Z (2017) Effects of simulated acid rain on soil fauna community composition and their ecological niches. Environ Pollut 220:460–468. https://doi.org/10.1016/j.envpol.2016.09.088
Wen K, Liang C, Wang L, Hu G, Zhou Q (2011) Combined effects of lanthanum ion and acid rain on growth, photosynthesis and chloroplast ultrastructure in soybean seedlings. Chemosphere 84:601–608. https://doi.org/10.1016/j.chemosphere.2011.03.054
Wu QS, Zou YN, He XH (2010) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ion balance of citrus seedlings under salt stress. Acta Physiol Plant 32:297–304. https://doi.org/10.1007/s11738-009-0407-z
Xia LN, Shao CL, Zhang NL, Wu AP, Xie JB, Qiu YJ, He XB, Pei J, Wang XD, Wang YH (2021) Improved tolerance of mycorrhizal Torreya grandis seedlings to sulfuric acid rain related to phosphorus and zinc contents in shoots. J Fungi 7:296. https://doi.org/10.3390/jof7040296
Zhang M, Wang S, Wu F, Yuan X, Zhang Y (2007) Chemical compositions of wet precipitation and anthropogenic influences at a developing urban site in southeastern China. Atmos Res 84:311–322. https://doi.org/10.1016/j.atmosres.2006.09.003
Zhang CY, Yi XQ, Gao XZ, Wang MH, Shao CY, Lv ZD, Chen JJ, Liu ZH, Shen CW (2020) Physiological and biochemical responses of tea seedlings (Camellia sinensis) to simulated acid rain conditions. Ecotoxicol Environ Saf 192:110315. https://doi.org/10.1016/j.ecoenv.2020.110315
Zhao B, Trouvelot A, Gianiazzi S, Gianiazzi-Pearson V (1997) Influence of two legume species on hyphal production and activity of two arbuscular mycorrhizal fungi. Mycorrhiza 7:179–185. https://doi.org/10.1007/s005720050179
Zhejiang Ecology and Environment Bureau (2016) Ecological and environmental status bulletin of Zhejiang Province in 2016. http://sthjt.zj.gov.cn/art/2017/6/2/art_1201912_13471748.html
Zhu LJ, Fu ZY, Zhang JC, Wang JP, Lin J, Yuan ZM, Cheng XF, Chu DS (2018) Effects of mycorrhizal fungi on photosynthetic characteristics of Zelkova serrata Thunb. J Nanjing for Univ 42:121–127. https://doi.org/10.3969/j.issn.1000-2006.201801031
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32071644; 31400366), Special Foundation for National Science and Technology Basic Research Program of China (2019FY102000), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB 31030000) and the Key Research and Development Plan of Zhejiang Province (No. 2017C02028). We are grateful to two anonymous reviewers for their constructive comments for the improvement of the manuscript in the revision process. We would like to thank Editage (www.editage.cn) for English language editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Additional information
Communicated by George Yan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, S., Shao, C. et al. Enhancement of photosynthetic parameters and growth of Zelkova serrata by arbuscular mycorrhizal fungi under simulated sulfuric acid rain. Plant Ecol 222, 1361–1374 (2021). https://doi.org/10.1007/s11258-021-01184-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-021-01184-8