Abstract
We have previously reported that Ricinus communis is a good candidate for the phytoremediation of Cd- and Zn-contaminated soil and for fuel production. In this study, changes in the activity of antioxidant enzymes (superoxide dismutase, SOD; catalase, CAT; and guaiacol peroxidase, POD) and the contents of chlorophyll and malondialdehyde (MDA) in R. communis leaves under Cu, Zn, and Cd stress were examined. Compounds from the exudate of R. communis roots were collected and analyzed using GC-MS chromatograms. The results of enzyme activity showed that Cd treatment significantly increased the SOD content of R. communis leaves and slightly elevated the CAT content, whereas the POD content increased markedly at low Cd treatment concentrations and decreased as Cd concentrations increased. Zn treatment distinctly elevated SOD and POD content in R. communis leaves but had no great influence on CAT content. Cu treatment slightly increased CAT activity, while Cu did not evidently change SOD and POD activity. We found 17, 29, 18, 18, and 33 different compounds in the R. communis root exudates from the control group and Cd, Cu, Zn, and Cd+Cu+Zn treatment groups, respectively. The root exudates mainly included ester, alcohol, ether, amide, acid, phenol, alkanes, ketone, aromatic hydrocarbon, and nitrile compounds. However, the root exudates of R. communis grown in uncontaminated soils were dominated by esters, alcohols, and ethers. Single Cu or Zn treatment slightly changed the root exudates, which were dominated by esters, alcohols, and amides. In the Cd and Cd+Cu+Zn treatment groups, the compositions of root exudates apparently increased, with alkanes as the major species (> 88%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal (HM) contaminants in soil are a widespread global problem, posing risks to both human health and the environment. The removal of heavy metals from contaminated soils has emerged as an urgent issue over time. Phytoremediation, a process that utilizes plants for pollutant removal from contaminated soils, has attracted extensive attention because it is an economical, environmentally friendly, and aesthetically acceptable approach (Bauddh and Singh 2012; Cheng 2003). Ricinus communis, a plant used in fuel production, appears to possess great tolerance to high levels of heavy metals and has a strong ability to absorb Cd and Zn in soil (Wang et al. 2016). However, in excessive heavy metal contamination, R. communis may initiate a variety of subcellular responses such as oxidative stress. Oxidative stress can cause damage at the cellular level or lead to wider phytotoxic responses (Zhang et al. 2007). However, the biochemical mechanisms for heavy metal toxicity and the adaptation mechanisms of R. communis under heavy metal stress are not well understood.
The oxidative stress induced by heavy metals in plants may occur by generating reactive oxygen species (ROS) such as superoxide radicals (O2−·), singlet oxygen (1O2), hydrogen peroxide (H2O2), and hydroxyl radicals (OH·) (Dat et al. 2000; Sun et al. 2007). Under normal conditions, the formation and removal of ROS are balanced; however, under stress, ROS are highly reactive and damage membrane lipids, proteins, pigments, and nucleic acids, resulting in dramatic reductions of growth and productivity, and even the death of plants (Ekmekçi et al. 2008). Malondialdehyde (MDA) is a cytotoxic product of lipid peroxidation, which can serve as an indicator of free radical production and consequent tissue damage (Zhang et al. 2007). The MDA content of plants has been used widely to study the effects of stress on plants. To minimize the damaging effects of ROS, aerobic organisms have evolved non-enzymatic and enzymatic antioxidant defenses. The latter include catalases, peroxidases, superoxide dismutases, and glutathione S-transferases (Scandalios 2005). Within a cell, SOD constitutes the first line of defense against ROS and its enzymatic effect can disintegrate super oxygen free radicals into H2O2 and O2 (Alscher et al. 2002). H2O2 is still harmful to a plant, but both catalase (CAT) and guaiacol peroxidase (POD) can catalyze the dismutation of H2O2 into oxygen and water (Apel and Hirt 2004). CAT is located mainly in peroxisomes and mitochondria, while POD is located in the cytoplasm, membrane, and cell wall (Wang et al. 2009).
Allelopathy is defined as the direct/indirect harmful or beneficial effects of one plant on another through the production and release of chemical compounds (Rice 1984). Many of the compounds are water-soluble substances, which can be released into the environment through leaching, root exudation, volatilization, and decomposition of plant residues (Bertin et al. 2003). These substances are termed as allelochemicals (Whittaker and Feeny 1971). Root exudates are important pathways for the release of allelochemicals, which will produce a direct/indirect influence on the physical, chemical, and biological characters of soil, and thus produce beneficial or harmful effects on the growth of the plant itself or that of other plants. To adapt to the external environment, plants can change the status of the rhizosphere by regulating the composition of root exudates. Specifically, the species and quantity of plant root exudates will develop remarkable changes under heavy metal stress (Xu et al. 2006). The identification of root exudates includes three processes, namely, collection, isolation, and determination, which involve a variety of methods. The methods for collection mainly include the collection of solution, earth, or substrate cultures. The isolation methods are extraction, resin, chromatography, molecular film, and ultracentrifugation. The instruments commonly used for identifying allelochemicals are ultraviolet-visible spectrometers, infrared spectrometers, gas chromatographs, liquid chromatographs, ion chromatographs, mass spectrometers, and nuclear magnetic resonance spectrometers (Bertin et al. 2003).
This study aimed to investigate the changes in physiological indices, such as lipid peroxidation and antioxidase activities, and root exudate species of R. communis under single or combined pollution of Cd, Cu, and Zn. The results will be helpful for better understanding the physiological detoxification mechanism by which R. communis tolerates heavy metals.
Materials and methods
Experimental design
Soil for the pot experiments was collected from the surface layer (0–20 cm) of farmland soils in the suburbs of Beijing, China. The soil was air-dried and passed through a 2-mm nylon sieve. The physicochemical properties of the soil included 28.46 g/kg of organic matter, 0.076% of total N, 1.071% of total C, 1063 mg/kg of total P, 2.28 g/kg of CaO, and 2.56 g/kg of total K2O, pH 6.40. The concentrations of Cd, Cu, and Zn were 0.13, 21.4, and 74.6 mg/kg, respectively. The soil was mixed with an appropriate amount of CdCl2·2.5 H2O, CuSO4·5 H2O, and ZnCl2 to achieve different treatments (Table 1). The three types of treatments were the control, single-factor, and three-factor tests. The treated soil (8 kg) was transferred into each plastic pot (33.5 cm × 26 cm) and cultured for 3 months.
R. communis seeds (obtained from the Chinese Academy of Agricultural Sciences, Beijing, China) were sown in May. Each treatment was replicated in three pots. Two uniform plants were allowed to grow in each pot. To simulate field conditions, the plants were cultivated without fertilizers in an open field of the agricultural experimental station in the School of Environment, Beijing Normal University, China. Adequate moisture was maintained during the experiment. During the active growing season, the average temperature was 19.6 °C (0.2 °C higher than the average temperature over a period of 10 years). Between May and September, the rainfall was 667.4 mm (149.6 mm more than the average over a period of 10 years) and the sunshine was 1142 h. The enzyme activity and lipid peroxidation of R. communis in all treatment and the root exudate compounds in M-5 treatment were measured in September before harvesting.
Chlorophyll assay
The chlorophyll content was determined from an alcohol–acetone mixture extract (at the ratio of 1:2) using 0.1 g of leaves (Sartory and Grobbelaar 1984), which was expressed as milligram/gram fresh weight.
Malondialdehyde assay
Leaves (1.0-g fresh weight) were homogenized in 5% trichloroacetic acid solution and were then centrifuged at 7000g for 15 min. The supernatant was used for further analyses. MDA was measured in terms of 2-thiobarbituric acid reactive metabolite (Kosugi and Kikugawa 1985), which was expressed as nanomole per gram fresh weight.
Enzyme assay
Leaves (0.5-g fresh weight) were homogenized in 50 mmol/l cold Na-phosphate buffer (pH 7.8) with 1.0 mmol/l ethylenediaminetetraacetic acid and 2% (w/v) polyvinylpyrrolidone using a prechilled mortar and pestle in an ice bath. After centrifuging the sample at 7000g for 15 min at 4 °C, the supernatant was used for further analyses. The activity of SOD, POD, and CAT was measured as described by Beauchamp and Fridovich (1971), Passonneau and Lowry (1993), and Bergmeyer et al. (1974), respectively.
Root exudate assay
Root exudate compounds were collected by using the circulation method, extracted with anhydrous ether (Nardi et al. 2005; He et al. 2005) and determined by gas chromatography (Bruker 320GC-MS with DB-5 MS quartz capillary column).
The gas chromatography details were as follows: capillary column, 0.25 mm × 0.25 μm × 30 m; injector temperature, 250 °C; splitless injection volume, 1 μl; transfer line temperature, 250 °C; EI source temperature, 210 °C; electron energy, 70 eV. The column temperature started at 50 °C for 4 min and then was raised to 290 °C by 10 °C/min and remained at 290 °C for 12 min.
Statistical analysis
The data were subjected to statistical analysis of variance (ANOVA). The calculations and graphical analysis were performed using Excel 2007 and Origin 8.0 software. Differences at the level of p < 0.05 were considered significant.
Results
Effects of Cd, Cu, and Zn stress on chlorophyll content in leaves of R. communis
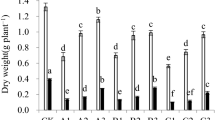
As observed in Fig. 1, the chlorophyll a, chlorophyll b, and total chlorophyll contents in R. communis leaves under Cd, Cu, or Zn single stress with low heavy metal concentration showed no remarkable difference when compared with those of the control group (p > 0.05). As Cd concentration in soils increased, the chlorophyll content in R. communis leaves decreased continuously (p < 0.05). The chlorophyll a, chlorophyll b, and the total chlorophyll content in R. communis leaves of the highest Cd (40 mg/kg) treatment group was reduced by 27.6%, 31.8%, and 29.3%, respectively, when compared with that of the control group. The chlorophyll content of R. communis leaves of the moderate Cu (200 mg/kg) and Zn (400 mg/kg) treatment groups displayed no distinct differences when compared with the control group (p > 0.05). Under the highest Cu (600 mg/kg) stress, the chlorophyll a and the total chlorophyll content slightly decreased by 17.9% and 15.7%, respectively. Under the highest Zn (800 mg/kg) stress, the chlorophyll a and the total chlorophyll content decreased by 25.8% and 22.7%, respectively. Cu and Zn stress had no effect on the chlorophyll b content. No significant differences were observed in the chlorophyll content of R. communis leaves under a low concentration of the combined pollutants when compared with the control group (p > 0.05). Chlorophyll content decreased continuously with an increase in the heavy metal concentrations. The chlorophyll a, the chlorophyll b, and the total chlorophyll content for the highest pollution group (M4-5) was only 37.8%, 38.9%, and 40.6%, respectively, compared to that of the control group.
Effects of Cd, Cu, and Zn stress on MDA content in leaves of R. communis
The effects of heavy metals on MDA content in R. communis leaves are shown in Fig. 2. The MDA content in R. communis leaves under a low concentration of single metal pollutant showed no obvious difference when compared with that of the control group (p > 0.05). The MDA concentration increased continuously with the increase in heavy metal concentration, the rising amplitude of which followed the order of Cd > Zn > Cu. In the highest heavy metal concentration group (M-5), Cd, Zn, and Cu increased the MDA content of R. communis leaves by 49.4%, 33.1%, and 14.0%, respectively, as compared with those of the control group. The MDA content of R. communis leaves under co-contaminated treatment was higher than that under a single heavy metal treatment. The rising amplitude for the highest concentration treatment group (M4-5) reached 73.0% when compared with that of the control group.
Effects of Cd, Cu, and Zn stress on SOD content in leaves of R. communis
Under single Cd or Zn treatment, the SOD content of R. communis leaves markedly increased when compared with that of the control group (p < 0.05), which also showed an increasing trend as the heavy metal concentration increased (Fig. 3). For the highest Cd or Zn concentration groups (40 mg/kg and 800 mg/kg, respectively), the SOD content of R. communis leaves increased by 72.6% and 83.4%, respectively, when compared with the SOD content of the control group. Moreover, Zn exerted a more obvious effect on SOD content than did Cd. Treatment with Cu alone appeared to have little effect on SOD content (p > 0.05). Under combined pollution by Cd+Cu+Zn, the SOD content first markedly increased, and then sharply decreased as the heavy metal content increased; that in the M4-3 group increased by 102% while that in the M4-5 treatment group declined by 25.2%.
Effects of Cd, Cu, and Zn stress on POD content in leaves of R. communis
The effects of heavy metals on POD content in the leaves of R. communis displayed various trends (Fig. 4). Under single pollution conditions, a low concentration of Cd did not greatly affect the POD content (p > 0.05). As the Cd concentration increased, the POD content first increased and then decreased. It achieved its maximum with the addition of 10 mg/kg of Cd, which was 109% higher than that of the control group, and then decreased in the highest Cd treatment group (40 mg/kg), but was still 35.8% higher than that of the control group. The POD content of R. communis leaves in the low concentration Cu group (50 mg/kg) increased by 53.3% when compared with that of the control group. It gradually decreased as the Cu concentration increased, and the difference in POD content of R. communis leaves between the highest Cu treatment group (600 mg/kg) and the control group was not significant (p > 0.05). A low concentration of Zn had no great influence on POD content. As the Zn concentration increased, the POD content significantly increased reaching its maximum (273% higher than that of the control group) in the highest Zn treatment group (800 mg/kg). The POD content under combined Cd+Cu+Zn treatment first increased and then decreased. It reached its maximum in the M4-3 treatment group, which increased by 244%, and then reached its minimum in the M4-5 treatment group, which decreased by 32.6%.
Effects of Cd, Cu, and Zn stress on CAT content in leaves of R. communis
Changes in the CAT content of R. communis leaves with heavy metal concentrations are shown in Fig. 5. Heavy metals had no obvious effect on CAT when compared with SOD and POD. A low concentration of Cd (1 mg/kg) treatment led to no significant difference in the CAT content in R. communis leaves when compared with that in the control group (p > 0.05). The CAT content slightly increased as Cd increased, which increased by 24.7% in the highest treatment group (40 mg/kg) when compared with that of the control group. Cu treatments showed similar trends with Cd, and the CAT content for the highest Cu treatment group (600 mg/kg) increased by 19.8% when compared with that of the control group. Zn did not greatly affect CAT content for all treatment groups (p > 0.05). Under the combined pollution, CAT content slightly increased when compared with that of the control group.
Effects of Cd, Cu, and Zn stress on root exudate of R. communis
GC-MS chromatogram of the root exudate compounds under single or combined heavy metal contamination is shown in Fig. 6. Under control treatment, 17 different compounds were identified in the root exudate of R. communis, mainly ester (37.8%), alcohol (23.8%), ethers (18.8%), and other amides (13.3%), acid (3.27%), phenol (2.48%), and paraffin (0.57%). The three compounds with the highest content were 1,2-benzenedicarboxylic acid-bis(2-methylpropyl) ester (28.6%), (2S,3S)-(+)-2,3-butanediol (18.6%), and 1-(2-butoxyethoxy) ethanol (9.78%).
Under single Cu treatment, 18 different compounds were identified in the root exudate of R. communis, mainly ester (40.2%), alcohol (20.2%), amide (18.2%), and other alkanes (15.9%), aromatic hydrocarbon (3.10%), phenol (1.66%), and ketone (0.70%). The three compounds with the highest content were 1,2-benzenedicarboxylic acid-bis(2-methylpropyl) ester (29.6%), 9-octadecenamide (15.1%), and 1-(2-butoxyethoxy) ethanol (14.2%).
Under single Zn treatment, 18 different compounds were identified in the root exudate of R. communis, mainly ester (38.6%), alcohol (27.1%), amide (20.0%), and other alkanes (9.71%), aromatic hydrocarbon (2.39%), phenol (1.74%), and ketone (0.42%). The three compounds with the highest content were 1,2-benzenedicarboxylic acid-bis(2-methylpropyl) ester (28.1%), 9-octadecenamide (22.5%), and 1-(2-butoxyethoxy) ethanol (14.6%).
Under single Cd treatment, the compounds in the root exudate of R. communis increased obviously and 29 different substances were identified. Overall, 90.4% of the components were dominated by alkanes and the rest of the components were amide (3.46%), acid (3.19%), esters (1.39%), ketone (0.95%), ether (0.49%), aromatic hydrocarbon (0.23%), and alcohol (0.14%). The relative content of the top three secretions was all alkanes: p-dioxane-2,3-diol (45.4%), 1,3-dioxolane, 2-(1-bromoethyl)- (25.9%), and 3-hexene, 1-(1-ethoxyethoxy)-, (Z)- (7.66%).
Under combined Cd, Cu, and Zn treatment, 33 different compounds were identified in the root exudate of R. communis. The dominant components were alkanes (88.8%) and the other components were ester (5.56%), amide (3.05%), alcohol (1.33%), ketone (0.55%), acid (0.44%), aromatics (0.17%), and nitrile (0.07%). The relative content of the top three secretions was also all alkanes: p-dioxane-2,3-diol (73.4%), 1,1-diethoxy-ethane (7.17%), and 2-(2-bromoethyl)-1,3- dioxolane (5.28%).
Discussion
Chlorophyll is the material basis of photosynthesis in plants. The destruction and degradation of chlorophyll will directly lead to a reduction in the efficiency of photosynthesis. As a result, plants will grow slowly, accompanied by a reduction in biomass, and some plants may even stop growing (Hou et al. 2007). Under injury by stress, plant tissues will invoke membrane lipid peroxidation. MDA is the decomposition product of membrane lipid peroxidation, which can react with proteins, amino acid residues, or nucleic acid after being released from the membrane, and thus lowering membrane stability. Moreover, MDA can also lead to the relaxation of bridging bonds between cellulose molecules and thus promote membrane leakage (Chaudhry 1998). Therefore, determining the MDA content can help us to understand the degree of membrane lipid peroxidation and stress injury in plants, which can indirectly show the degree of injury of the membrane system and stress resistance in plants under certain conditions. The literature has suggested that under heavy metal stress, numerous plants, such as Spirulina platensis-S5 (Chaudhry 1998), Solanum nigrum (Sun et al. 2007), and Lemna minor (Hou et al. 2007), will decrease chlorophyll content and increase MDA content.
The results of this study showed that heavy metals had little effect on the chlorophyll b when compared with the control group (p > 0.05) except for the highest Cd and Cd+Cu+Zn treatment group. The differences in chlorophyll a content and MDA content in R. communis leaves between the low-level Cd, Cu, and Zn treatment groups and the control group were not statistically significant (p > 0.05). However, with an increase of heavy metals, the chlorophyll a content and the total chlorophyll content in leaves decreased and the MDA content increased accordingly (p < 0.05). These results demonstrate that the toxic effects of heavy metals on growth, development, and photosynthesis of plants were largely associated with heavy metal concentration. Heavy metal at low concentrations would not completely destroy the chlorophyll structure of R. communis and no membrane lipid peroxidation damage was caused. Heavy metal at high concentrations had toxic effects on R. communis by suppressing photosynthesis and aggravating membrane lipid peroxidation. The toxic effects of the three heavy metals at high concentrations in R. communis followed the order of Cd > Zn > Cu. Compared with pollution by a single heavy metal, combined pollutants at high concentrations resulted in a greater decrease in chlorophyll content and increase in MDA content, which demonstrated that combined pollutants at high concentrations exerted greater toxic effects on plants than a single heavy metal.
ROS and the antioxidant system in plants maintain a dynamic balance under normal conditions. However, under the stress of heavy metals, ROS will accumulate excessively in plants and result in oxidative stress. Therefore, the antioxidant system in plants will be activated to defend against the oxidative stress. The enzyme system is complicated, and enzyme activities in different plants show different responses under heavy metal stress: enhanced or reduced. Under the combined influence of Cd, Pb, and Hg, the POD and SOD activities in Bruguiera gymnorrhiza leaves increase continuously as the heavy metal content increases, reaching maximal levels in the 10 HM treatment group, and subsequently, they decreased and returned to the control group level in the 15 HM treatment group. However, the CAT content was not greatly changed with the increase in heavy metals (Zhang et al. 2007). Wang et al. (2008) compared changes in the antioxidase systems between hyperaccumulator plants (Thlaspi caerulescens and Brassica) and the non-hyperaccumulator plant (Nicotiana tabacum) under Cd stress. The results suggested that the SOD and CAT contents in T. caerulescens and Brassica were distinctly increased but the POD content did not significantly change. In contrast, the SOD content of Nicotiana tabacum slightly increased, CAT content markedly decreased, and POD content showed no obvious change. The authors believed that Cd hyperaccumulator plants displayed a greater ability to tolerate Cd stress-induced oxidative stress than did non-hyperaccumulator plants. The SOD content in L. minor was significantly increased under the single stress of Cd and Cu, whereas the POD and CAT contents first increased and then decreased (Hou et al. 2007). Under Zn stress, both SOD and POD contents in Jatropha curcas leaves showed an increasing trend, whereas the CAT content first increased and then decreased with increasing of Zn concentration (Luo et al. 2010). Under Zn stress, both the SOD and the CAT contents in Brassica napus showed a decreasing trend, whereas the POD content first increased and then decreased with Zn concentration (Abedi and Pakniyat 2010). The authors thought the antioxidant defense capacity and the increase of individual enzymatic activities during stress were thereby dependent on plant genotype.
The results of this study revealed that under Cd stress, Cd at a low concentration (1 mg/kg) had no great influence on the enzyme system in R. communis leaves (p > 0.05). With the increase in Cd, the SOD content in R. communis leaves markedly increased when compared with that of the control group, whereas the CAT content only increased slightly. The POD content first increased (10 mg/kg treatment group) and then decreased (40 mg/kg treatment group); however, it was still higher than that of the control group. As the first line in defense against ROS, SOD in the antioxidant defense system gradually increases with Cd concentrations, which helps to alleviate excessive oxidative damage. Increases in POD and CAT contents under low and moderate Cd concentrations further protect R. communis from peroxidation damage. The POD activity at the highest Cd concentration decreases, which might be due to the high level of Cd inhibiting enzyme synthesis or changes in the subunit assembly of the enzyme. However, the POD activity at the highest Cd concentration was still higher than that for the control group, which demonstrated that the enzyme system of Cd was not catastrophically destroyed under the experimental concentration (1–40 mg/kg). The antioxidant system in R. communis might play an important role in Cd tolerance and detoxification.
Under Zn stress, Zn at low concentrations (100 mg/kg) also had no great influence on the enzyme system when compared with the control group (p > 0.05). SOD and POD contents in R. communis leaves increased distinctly with the increase in Zn concentration (with the highest treatment concentration of 800 mg/kg), while the CAT content did not change significantly. These results suggest that the enzyme system in R. communis might have become a powerful antioxidant barrier for the plant under Zn stress. The enzyme system of R. communis was not destroyed under the Zn concentrations studied (100–800 mg/kg), while SOD and POD exerted high activities to enhance the high-level Zn tolerance of R. communis.
Compared with the control group, Cu did not exert a great influence on SOD, while the CAT content slightly increased as the Cu concentration increased. Cu at a low concentration (50 mg/kg) slightly increased the POD content, which subsequently decreased slowly. Among the three heavy metals, Cu had the least influence on enzyme activity. This might be attributed to the low absorption of Cu in R. communis and the weak ability to transport Cu from the root to the parts of the plant above ground. The accumulation of Cu in the leaf is not high; therefore, Cu has little influence on enzyme systems in the leaf. Certainly, the antioxidant system in the plant is extremely complicated, and antioxidase might not be the main pathway for R. communis to defend against Cu-induced antioxidant responses. The antioxidant system in the plant might be carried out through non-enzyme-dependent or other pathways.
Under multi-contaminated conditions, the SOD and POD contents in R. communis leaves increased at low heavy metal concentration treatments and decreased as heavy metal concentrations increased. The CAT content was slightly elevated; however, it was not significantly different from the level found in the control group (p > 0.05). Among all treatment groups, the lowest SOD and POD contents were found in the highest heavy metal concentration group with co-contaminated treatment (M4-5). This finding indicates that a high level of combined heavy metal pollution can destroy the antioxidase system in the leaves of R. communis, which leads to a boost of free radical content in the plant, and thus affects normal growth and gives rise to toxic symptoms.
The plant root can secrete substances involved in allelopathy, and the species and quantity of the root exudate are affected by many factors such as species, growth stage, nutritional status, illumination, and temperature. Notably, compositions secreted by different plant roots greatly differ from each other. The allelochemicals of root exudates can suppress or stimulate the germination and growth of the plant. Among the root exudates of Medicago sativa L., triacontanol, saponin, canavanine, and phenol acids are plant growth regulators that can favorably promote plant growth (Khanh et al. 2007). In comparison, p-hydroxybenzoic acid, vanillic acid, and trans-ferulic acid among the root exudates of Lolium rigidum can suppress root growth (Wu et al. 2000). Moreover, some studies have found that exudate production and accumulation could be increased in plants in the presence of metal poisoning. Root exudates of wheat grown in water culture and sand culture conditions will be notably changed in terms of quality and quantity under Cd stress (Zhang et al. 2002). Root exudates of Spinacia oleracea L. and Lycopersicon esculentum L. can enhance Cu and Zn absorption (Degryse et al. 2008). Moreover, mesoxalic acid, together with an unknown organic acid with a peak flowing out time of about 6.5 min, among root exudates of Elsholtzia splendens and Commelina communis can partly activate Cu in polluted soil (Shi et al. 2004).
The results of this study indicate that the root exudates of R. communis grown in uncontaminated soils are dominated by esters, alcohols, and ethers, with compound 1,2-benzenedicarboxylic acid-bis(2-methylpropyl) ester having the highest content. 1,2-Benzenedicarboxylic acid-bis(2-methylpropyl) ester could also be detected from root exudates of maize (Qi et al. 2015) and hot pepper (Xu 2011). It is a substance involved in allelopathy that can distinctly promote plant seed germination and seedling growth, and showed certain antibacterial activity. The compound with the highest content in the root exudate of R. communis under the single stress of Cu or Zn was 1,2-benzenedicarboxylic acid-bis(2-methylpropyl) ester. This suggests that the major allelochemicals in root exudates of R. communis were not affected by the single stress of Cu or Zn. However, for Cd and Cd+Cu+Zn treatment groups, the composition of the exudates was evidently changed, with an apparent increase in exudate species. The level of 1,2-benzenedicarboxylic acid-bis(2-methylpropyl) ester in exudate was reduced outstandingly, while alkane occupied over 90% of the composition. Reports have indicated that nonacosane, cetyl chloride, icosane, hexadecane, and nonadecane in plant root exudates can partly inhibit plant growth (Lan et al. 2016), but their allelopathy remains to be further investigated.
It is noteworthy that there are several different research methods to analyze root exudates. The diverse methods for collection, isolation, and identification have greatly affected the research results. Consequently, the research methods for root exudates remain one of the difficulties in the field. On the one hand, rhizosphere research methods should be improved and innovated in future research: for instance, designing a simulated rhizosphere environment that is closer to the natural growth status of the root; alternatively, developing more effective in situ collection methods, which will not disturb the natural growth of root or destroy the rhizosphere; and in addition, constructing more accurate techniques for root exudate isolation, purification, and determination by taking advantage of the modern detecting instruments and analytical techniques that are being increasingly developed. On the other hand, the range of investigated root exudates should be enlarged, so as to comprehensively reveal the relations of a variety of root exudates with pollution stress as well as the ecological effect, and thus obtain an in-depth and profound understanding of the mechanism (Bertin et al. 2003).
Conclusion
Single Cd, Cu, or Zn stress did not completely destroy the enzyme system of R. communis under experimental concentrations (Cd < 40 mg/kg, Cu < 600 mg/kg, or Zn < 800 mg/kg). The antioxidant system in R. communis might play an important role in heavy metal tolerance and detoxification. Among the three heavy metals, Cu has the least influence on enzyme activity. The antioxidase system in the leaves of R. communis was seriously destroyed under high levels of combined heavy metal pollution and the lowest SOD and POD contents were found in the highest heavy metal concentration group with co-contaminated treatment (M4-5). The major allelochemicals in root exudates of R. communis were not affected by the single stress of Cu or Zn. However, for Cd and Cd+Cu+Zn treatment groups, the composition of exudates was evidently changed.
References
Abedi T, Pakniyat H (2010) Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Genet Plant Breed 46(1):27–34
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53(372):1331–1341
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bauddh K, Singh RP (2012) Cadmium tolerance and its phytoremediation by two oil yielding plants Ricinus communis (L.) and Brassica juncea (L.) from the contaminated soil. Int J Phytoremediation 14(8):772–785
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Bergmeyer H U, Bergmeyer J, Grassl M (1974) Methods of enzymatic analysis. Vol. 2, Samples, reagents, assessment of results[M]. Verlag Chemie
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256(1):67–83
Chaudhry TM (1998) Phytoremediation-focusing on accumulator plants that remediate metal contaminated soils. Aust J Ecotoxicol 4:37–51
Cheng S (2003) Heavy metals in plants and phytoremediation. Environ Sci Pollut Res 10(5):335–340
Dat J, Vandenabeele S, Vranová E et al (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57(5):779–795
Degryse F, Verma VK, Smolders E (2008) Mobilization of Cu and Zn by root exudates of dicotyledonous plants in resin-buffered solutions and in soil. Plant Soil 306(1–2):69–84
Ekmekçi Y, Tanyolac D, Ayhan B (2008) Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J Plant Physiol 165(6):600–611
He H, Chen X, Lin R et al (2005) Chemical components of root exudates from allelopathic rice accession PI312777 seedlings. Ying yong sheng tai xue bao 16(12):2383–2388
Hou W, Chen X, Song G, Wang Q, Chi Chang C (2007) Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol Biochem 45(1):62–69
Khanh TD, Chung IM, Tawata S et al (2007) Allelopathy for weed management in sustainable agriculture. Health 1:2
Kosugi H, Kikugawa K (1985) Thiobarbituric acid reaction of aldehydes and oxidized lipids in glacial acetic acid. Lipids 20(12):915–921
Lan Y, Shen XF, Yan ZY et al (2016) GC-MS comparative analysis in root exudates of Salvia miltiorrhiza from different geographical provenances. Jiangsu Agric Sci 44(1):301–305 (In Chinese)
Luo ZB, He XJ, Chen L et al (2010) Effects of zinc on growth and antioxidant responses in Jatropha curcas seedlings. Int J Agric Biol 12:119–124
Nardi S, Tosoni M, Pizzeghello D, Provenzano MR, Cilenti A, Sturaro A, Rella R, Vianello A (2005) Chemical characteristics and biological activity of organic substances extracted from soils by root exudates. Soil Sci Soc Am J 69(6):2012–2019
Passonneau JV, Lowry OH (1993) Enzymatic analysis: a practical guide. Springer Science & Business Media, Berlin
Qi Y, Zhen W, Li H (2015) Allelopathy of decomposed maize straw products on three soil-born diseases of wheat and the analysis by GC-MS. J Integr Agric 14(1):88–97
Rice EL (1984) Allelopathy, vol 2004. Academicpress, New York
Sartory DP, Grobbelaar JU (1984) Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114(3):177–187
Scandalios JG (2005) Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 38(7):995–1014
Shi J, Chen Y, Tian G et al (2004) Copper uptake mechanism of Elsholtzia splendens and Commelina communis. Plant Nutr Fert Sci 10(6):642–646 (In Chinese)
Sun RL, Zhou QX, Sun FH, Jin CX (2007) Antioxidative defense and proline/phytochelatin accumulation in a newly discovered Cd-hyperaccumulator, Solanum nigrum L. Environ Exp Bot 60(3):468–476
Wang Z, Zhang Y, Huang Z, Huang L (2008) Antioxidative response of metal-accumulator and non-accumulator plants under cadmium stress. Plant Soil 310(1–2):137–149
Wang C, Zhang SH, Wang PF, Hou J, Zhang WJ, Li W, Lin ZP (2009) The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 75(11):1468–1476
Wang S, Zhao Y, Guo J, Zhou L (2016) Effects of Cd, Cu and Zn on Ricinus communis L. growth in single element or co-contaminated soils: pot experiments. Ecol Eng 90:347–351
Whittaker RH, Feeny PP (1971) Allelochemics: chemical interactions between species. Science 171(3973):757–770
Wu H, Haig T, Pratley J, Lemerle D, An M (2000) Distribution and exudation of allelochemicals in wheat Triticum aestivum. J Chem Ecol 26(9):2141–2154
Xu W (2011) Evaluation and GC: MS analysis of significant allelopathic components of hot pepper. J Changjiang Vege 18:46–49 (In Chinese)
Xu WH, Huang H, Wang AH et al (2006) Advance in studies on activation of heavy metal by root exudates and mechanism. Ecol Environ 15(1):184–189 (In Chinese)
Zhang G, Fukami M, Sekimoto H (2002) Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in cd tolerance at seedling stage. Field Crop Res 77(2):93–98 (In Chinese)
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67(1):44–50
Acknowledgements
We thank the International Science Editing for editing this manuscript.
Funding
This research was supported by the Special Research for Public Welfare of Ministry of Land and Resources of China (2015-02-02-05).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wang, S., Zhao, Y., Guo, J. et al. Antioxidative response in leaves and allelochemical changes in root exudates of Ricinus communis under Cu, Zn, and Cd stress. Environ Sci Pollut Res 25, 32747–32755 (2018). https://doi.org/10.1007/s11356-018-3283-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3283-5