Abstract
Diabetes mellitus is a severe condition in which the pancreas produces inadequate insulin or the insulin generated is ineffective for utilisation by the body; as a result, insulin therapy is required for control blood sugar levels in patients having type 1 diabetes and is widely recommended in advanced type 2 diabetes patients with uncontrolled diabetes despite dual oral therapy, while subcutaneous insulin administration using hypodermic injection or pump-mediated infusion is the traditional route of insulin delivery and causes discomfort, needle phobia, reduced adherence, and risk of infection. Therefore, transdermal insulin delivery has been extensively explored as an appealing alternative to subcutaneous approaches for diabetes management which not only is non-invasive and easy, but also avoids first-pass metabolism and prevents gastrointestinal degradation. Microneedles have been commonly investigated in human subjects for transdermal insulin administration because they are minimally invasive and painless. The different types of microneedles developed for the transdermal delivery of anti-diabetic drugs are discussed in this review, including solid, dissolving, hydrogel, coated, and hollow microneedles. Numerous microneedle products have entered the market in recent years. But, before the microneedles can be effectively launched into the market, a significant amount of investigation is required to address the numerous challenges. In conclusion, the use of microneedles in the transdermal system is an area worth investigating because of its significant benefits over the oral route in the delivery of anti-diabetic medications and biosensing of blood sugar levels to assure improved clinical outcomes in diabetes management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is regarded as one of the world’s most complex health issues in the twenty-first century. In reality, it has been dubbed the “Black Death of the Twenty-First Century” because of its striking resemblance to the fourteenth century Plague in aspects of prevalence morbidity, as well as mortality (Jain 2015). Diabetes mellitus affects an approximately 20.8 million people in the USA, as per the Centre for Disease Control and Prevention (Jain and Joshi 2013). In 2010, 285 million and, in 2019, 463 million adults (20–79 years) worldwide were reported to have diabetes, and these cases are anticipated to increase to 578 million by 2030 and 700 million by 2045 according to International Diabetes Federation (https://diabetesatlas.org/data/en/world/; Zhang et al. 2019a). While there are many forms of diabetes mellitus, the most common are type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), and gestational diabetes (Fonseca et al. 2020). Insulin-dependent diabetes mellitus, commonly known as T1DM, is induced by the autoimmune disruption of pancreatic beta cells, which leads to a decrease or elimination of biological insulin production (Jana and Wadhwani 2019; Galderisi and Sherr 2019). The resulting absolute insulin deficiency causes high glucose levels termed as hyperglycaemia, as well as changes in protein and lipid metabolism (Wolkowicz et al. 2021). Hyperglycaemia can cause a number of symptoms, including cardiovascular and neurological issues, whereas hypoglycaemia causes fatigue and eventually death. Although current therapeutic alternatives can regulate short-term glycaemia, none of the existing anti-diabetic medications can restore functional β-cell mass (Dong and Wu 2018; Alejandro et al. 2015). At present, the management of T2DM focuses on glucose control via lowering of fasting/postprandial blood glucose and hemoglobin A1c (HbA1c). The shortages of existing oral drugs for the treatment of diabetes include that these medications do not address the key driver of type 2 diabetes i.e., loss of functional beta-cell mass and the majority of patients do not achieve glycated haemoglobin targets (Giugliano et al. 2009; Loretelli et al. 2020). As a result, treatment failure causes a long time in controlling glycaemia, and ultimately leads to disease progression, disability, infection risks, and eventually early mortality (Gotfredsen et al. 2020). Therefore, the objective of therapy should be delay of disease progression and should specifically target the newly identified pathogenic targets of disease. Recently, sodium glucose co-transport 2 inhibitors are approved by the Food and Drug Administration (FDA) in 2013 as a new class of antidiabetic medicines but post-marketing data indicated that the use of SGLT2 inhibitor is associated with several adverse drug reactions such as diabetic ketoacidosis, cancer, bone fracture, genital and urinary tract infection, and foot and leg amputation (Singh and Kumar 2018; Singh et al. 2019). In 2014, FDA has approved dulaglutide (GLP-1 analog) for the treatment of T2DM; however, various risks associated with the use of this drug include septicaemia, malignant neoplasm, coronary artery disease, and pancreatic cancer (Garg and Kumar 2018).
Patients with insulin-dependent diabetes mellitus lose their ability to produce endogenous insulin, which can lead to blood glucose instability and ketosis without the use of exogenous insulin. The insulin-dependent diabetes mellitus treatment entails delivering exogenous insulin by injection or pump to achieve a plasma glucose level that is close to average, i.e. below 8.0 mmol/L prior to large meals for adult diabetes patients. Blood glucose levels should not drop below the normal range, i.e. 70–140 mg/dL which describes hypoglycaemia condition, leading to increased morbidity and mortality (Jana and Wadhwani 2019; Zong et al. 2021). The most common methods for treating and controlling diabetes consist of multiple regular insulin injections, as well as continuous and precise monitoring of blood glucose levels (BGLs) in order to keep their normal blood glucose levels between 70 and 140 mg/dL (Primavera et al. 2020; Liu et al. 2016; Raval et al. 2021). Because of its low oral bioavailability, insulin is normally given subcutaneously (SC); however, SC injections are linked to greater inflammation and infection danger, and also poor patient compliance. People having diabetes are frequently encouraged to subcutaneously self-administer insulin on multiple occasions per day; this necessitates both intensive self-management and training, including regular dose modifications by patients depending on glucose monitoring. Furthermore, repeated injections in the same place can cause thickening of skin and inadequate glycaemia regulation, leading to poor diabetes management. Several other new and minimally invasive delivery mechanisms, like buccal, oral, transdermal, and nasal systems, are being studied to ascertain their efficacy and improved patient compliance in order to mitigate these limitations; however, such technologies are mostly still in preclinical development (Fonseca et al. 2020; Ross and Neville 2019; Tucak et al. 2020).
One of the most notable aspects of current efforts of researchers is the invention of the microneedle (MN) patch, which can successfully overcome the implicit barriers to insulin absorption through the skin and therefore facilitate transdermal drug delivery despite the use of complex systems or external energy sources (Chen et al. 2020a; Hultström et al. 2014; Thuillier et al. 2018). Without causing pain, the micro-scaled needles can penetrate the outermost keratinous stratum corneum layer and enter the epidermal and dermal layers of the skin for drug release (Alimardani et al. 2021; Dharadhar et al. 2019). MN creates temporary micro-channels for drug transport, but they immediately heal after MN is removed, preventing long-term skin tissue injury (Jin et al. 2018).
Diabetes is one of the most prevailing health issues in recent times due to highly busy scheduled lifestyle of the modern era people, as the people are not having enough time to go for the exercises to burn their calories and use their body glucose as a source of energy which leads to the accumulation of glucose in the muscles and blood and increases the glucose levels in the blood above the normal range, giving rise to diabetes, which leads to serious health problems. Therefore, this needs immediate care as well as treatment. In this review, we give an overview of several types of diabetes with an emphasis on pathophysiology and causes. This article discusses the several types of MNs available and their drug release patterns in the skin after insertion, as well as glucose monitoring in diabetic patients using blood or interstitial fluid samples. This review describes the various potential and applications of the MNs and also includes a brief summary of recent patents and the current clinical status of MN use in diabetes. The primary search engines employed throughout the paper search strategy were PubMed, Google Scholar, ScienceDirect databases, and Web of Science. Literature review was done using publications published in peer-reviewed journals from the year 2004 to year 2021.
Pathophysiology of diabetes mellitus
Type 1 diabetes mellitus
T1DM is now widely accepted as an autoimmune disease caused by the destruction of insulin-producing pancreatic cells (Zaccardi et al. 2016). As a result of this process, insulin deficiency develops, eventually leading to full dependence on exogenous insulin (Brinkman 2017). Beta cells regulate and generate insulin as well as acting as glucose sensors (Bluestone et al. 2010). As the number of beta cells in the body decreases, less insulin is produced to maintain blood glucose homeostasis, leading to a rise in blood glucose levels (Cnop et al. 2005). The individual with diabetes can no longer control their blood glucose levels due to the loss of beta cell mass. If left untreated, this can cause a person to become sick in a short period of time, with the risk of developing diabetic ketoacidosis (Devendra et al. 2004). Further consequences on this condition could end up with diabetic coma as sequentially represented in Fig. 1.
Type 2 diabetes mellitus
The steady decline in ß-cell function that occurs against a background of insulin resistance leads to changes in glucose metabolism. Insulin secretion and insulin sensitivity are the two most important aspects of the blood glucose control mechanism (D’Adamo and Caprio 2011). Insulin resistance is a defining characteristic of T2DM, and it affects more than 90% of patients (Imam 2013). A reduction in the metabolic response of insulin-responsive cells to insulin or, at a systemic level, an inadequate/decreased response to circulating insulin by blood glucose levels is referred to as insulin resistance (Galicia-Garcia et al. 2020). The liver and muscles have long been known to have a role in systemic insulin resistance. During fasting, the liver generates glucose from non-glucose substrates via gluconeogenesis process to assure that a carbohydrate energy source is always available. Several investigations have found that people with T2DM have enhanced gluconeogenesis despite having hyperinsulinemia, implying that hepatic insulin resistance is a major factor in fasting hyperglycaemia. The causes of decreased hepatic insulin sensitivity are unknown; however, a deposition of fat in the liver (steatosis) is thought to be a major factor (Koufakis et al. 2021; Zaccardi et al. 2016). The second and as important pathogenic factor is a reduction in β-cell dysfunction. Insulin is generally released in two stages in response to increased glucose levels: first, a rapid first-phase release (0–10 min), then by a longer second phase (10–120 min), which lasts as long as essential to sustain euglycaemia. First-phase insulin production is lost after fasting glucose levels reach 115–120 mg/dL. The β-cell function has already been lowered by 60–70% by the moment poor glucose tolerance develops with glucose levels of 141–199 mg/dL 12 h after the challenge (Imam 2013). Insulin secretory failure, the fundamental cause of β-cell dysfunction and the base of T2DM, can be caused by deficiencies in the production of insulin intermediates or insulin itself, and also a disruption in the secretion process (Hoang Do and Thorn 2015). The sequential illustration of pathophysiology of T1DM and T2DM is described in Fig. 1.
Assessment of diabetes risk factors for type 1 diabetes mellitus
Genetic and environmental factors
Genetic mutations account for about one-third of disease sensitivity while environmental factors account for the other two-thirds. About 40% of the genetic risk is attributed to genes connected to the human leukocyte antigen (HLA) locus. HLA-DR3 or HLA-DR4 is found in around 95% of T1DM patients. The other significant gene, located in the 5' polymorphic region of the insulin gene, provides nearly 10% of the genetic risk (Imam 2013; Kerner and Brückel 2014). HLA genes, which encode cell surface proteins implicated in antigen presentation and self-tolerance, are crucial in controlling the immune response. As a result, genetically controlled changes in the amino acid sequence of these proteins can modify the repertoire of peptides given, leading to the loss of self-tolerance. These findings, together with current understanding of a link between HLA and other autoimmune disorders, as well as evidence of the efficacy of immunosuppressive medications on T1DM disease progression, greatly supported the notion that “insulin-dependent” diabetes was an immune-mediated disease implicating the pancreatic islets of Langerhans (Zaccardi et al. 2016; Von Herrath et al. 2016). Vitamin D deficiency has long been believed to be a risk factor for developing T1DM. Consumption of meat preservatives and alcohol are some other factors that may contribute to the development of type 1 diabetes (Mayo 2016).
Co-existent autoimmunity
Immune-mediated diseases such as thyroid disease and celiac disease have been related to T1DM. However, it is unknown whether they constitute risk factors for the disease. Thyroid auto-antibodies are found in about 25% of children with T1DM when they are diagnosed, and thyroid dysfunction is more common in people with T1DM than in those without the disease. T1DM patients are more likely to develop celiac disease than non-diabetic patients. Thyroid disease and celiac disease affect metabolic regulation; if left untreated, they can increase the risk of hypoglycaemia in people with T1DM (Chiang et al. 2014, 2018). Once a person is diagnosed with T1DM, the only way that allows them to live is to replace the missing endogenous insulin by subcutaneous insulin injections at periodic intervals every day for the rest of their lives (Bluestone et al. 2010).
Assessment of diabetes risk factors for type 2 diabetes mellitus
Multiple factors, like β-cell mass and secretory capacity, which are affected by genetic and environmental variables, influence the ability of the β-cell to release adequate insulin to effectively respond to the peripheral insulin resistance condition. In fact, various metabolic derangements (insulin resistance, lipotoxicity) could cause progressive loss of β-cell function.
Reduction in β-cell mass
The reduced β-cell mass may play a role in explaining lower maximal secretory capability for insulin secretion in people with T2DM. This decrease in mass, however, cannot account for the complete pattern of functional alterations seen in T2DM. As a result of the altered metabolic state, like elevated glucose and free fatty acids, as well as amyloid deposits, a rise in programmed cell death, also called apoptosis, may occur (Pozzilli et al. 2011; Weir et al. 2020).
Nutritional factors
The high-calorie Western diet comprises considerable quantities of carbohydrates and fats, which raise glucose levels of blood and circulating triglyceride-rich chylomicrons, and very-low-density lipoproteins. This causes an increase in the levels of reactive oxygen species (ROS), which results in aberrant inflammatory molecule production. Since oxidative stress is a renowned inducer of inflammation, the two processes interact synergistically after a large meal, increasing the negative postprandial consequences. The pathogenesis of T2DM is aided greatly by a prolonged and considerable rise in steady-state ROS levels (DeFronzo et al. 2015).
Western lifestyle
A Western lifestyle is typically connected with high-energy foods and less physical activity. The broad availability and intake of high-fat, high-sugar, energy-dense processed convenience meals add considerably to the obesity epidemic that has gripped developed countries. Also, these diets are often lacking in vitamin D, vitamin B12, and folic acid, all of which have been associated to the development of T2DM (Nolan et al. 2011; Kahn et al. 2014).
Endocrine-disrupting chemicals
Chemicals that affect the endocrine system function and induce severe health effects like T2DM have been identified. Pesticides, cosmetic preservatives and food, components and compounds used in the plastics sector, consumer products, and waste incineration by-products are all examples of endocrine-disrupting chemicals. These chemicals are all around us, and they are impossible to avoid (Chevalier and Fénichel 2015).
Microneedle technology
The use of MN technology for drug delivery via and into the skin and other target tissues has advanced significantly over the last decade (Sharma et al. 2019; Mdanda et al. 2021). MNs were first suggested as a drug delivery tool in the 1970s, and since then, they have been produced using a range of technologies, materials, and geometries (He et al. 2019). MNs have been thoroughly researched in the production of insulin patches (Ng and Gupta 2020). Microneedles are classified as solid or hollow cannulas with an external diameter of less than 300 mm and a length of 50–900 mm (Queiroz et al. 2020). The MN system is focused on the painless piercing of the skin by several needles inside a patch that are micrometres in size (less than 1 mm in length) to administer insulin in a minimally invasive and targeted manner (Chen et al. 2020a). Whenever the patch is applied to the skin whether manually or by an applicator, MNs with lengths varying between 100 to 1500 µm puncture the outermost layer stratum corneum having a width of 10–20 µm and penetrate via the skin epidermis to a level of 70 to 200 µm. Microchannels produced in this way serve as temporary hydrophilic pathways in the skin, allowing small drugs like alendronate, macromolecules, and nanoparticles to be transmitted to the skin. The dermis’s dense capillary bed allows the medication to be absorbed quickly. Skin integrity is restored, as determined by transepidermal water loss, and microchannels reclose within hours (El-Khordagui 2012).
Silicon, glass, metal, polymers, and less conventional materials such as carbohydrates have all been used to make MNs. The large numbers of silicon-based MNs for clinical use are engraved perpendicular to the silicon wafer surface and are called “out-of-plane” designs (Yang et al. 2020). Strong and hollow MNs are the two broad types of MNs. Non-dissolving and dissolving/degradable MNs are also solid MNs. Drugs can be administered through MN-treated skin in a number of ways, including (a) “poke and patch”, which involves skin pre-treatment using solid MNs accompanied by topical application of drug preparation or patch on microporated skin; (b) “coat and poke”, which involves coating solid MNs with the drug and inserting them into the skin, allowing accurate dosing and skin administration of unstable drugs; (c) “poke and release” using drug-loaded solid MNs composed of dissolving/biodegradable polymers or polysaccharides enables concurrent skin microporation and drug release in one step. There is no need for a patch or a micropump, and there is no dangerous sharp waste left; (d) “poke and flow”, which involves injecting a liquid drug preparation into the skin using hollow MNs. Hollow MNs may also be utilized to take a sample of dermal interstitial fluid for glucose testing (El-Khordagui 2012).
Microneedles as an emerging therapy for diabetes mellitus
Diabetes mellitus (DM) has been a global health problem for many decades and is now the fifth leading cause of death. DM is a metabolic disorder with several aetiologies that affects several organs and contributes to a number of cardio-vascular and neuropathic complications. In clinical terms, DM is defined as an increase in blood glucose levels and a decrease in plasma insulin levels (Zaric et al. 2019). Type 1 and type 2 diabetes mellitus are the two main types of diabetes mellitus (T1DM and T2DM). T1DM is caused by an absolute lack of insulin, while T2DM is caused by a combination of insulin resistance, impaired insulin secretion, and increased glucose production. T1DM is divided into two types, i.e. type 1A (autoimmune destruction of ß-cells) and type 1B (idiopathic insulin deficiency) (Mohsen 2019). The new normal treatment for type 1 diabetics with inadequate insulin secretion is to keep BGLs under tight control via regular exogenous insulin subcutaneous injection. Type 2 diabetes is characterised by insulin resistance that can be controlled by exercise, a healthy diet, or oral anti-diabetic medicines. Insulin administration is also necessary to effectively control BGLs in people with advanced type 2 diabetes. In order to achieve effective glucose regulation, many insulin formulations are currently available on the market. Owing to the increasing degradation of insulin in the gastrointestinal tract, numerous routes of insulin administration have been studied, including subcutaneous injection, nasal, pulmonary, and transdermal delivery. Because of their high absorption capacity and distribution performance, subcutaneous insulin administration through hypodermic injection or pump infusion is still the preferred method, but SC injections are linked to higher inflammation and infection risk, as well as poor patient compliance. Minimally intrusive alternative routes such as oral, pulmonary, nasal, buccal, peritoneal, and transdermal administration have been studied to address these disadvantages. Transdermal insulin administration, in particular, has gained popularity in recent decades due to its ease of use and increased patient adherence. MNs have recently gained popularity as a convenient and minimally invasive way to self-administer this medication. Since the stratum papillare of the skin is rich in small vessels, MNs have the potential to speed up insulin absorption (Chen et al. 2020b; Vora et al. 2020; Wang et al. 2020a). MN system is produced by arranging hundreds of MNs in arrays on a tiny patch (similar to a commercially available transdermal patch) to deliver enough medication to produce the desired therapeutic response (Waghule et al. 2019; Jung and Jin 2021). For drug release, the micro-scaled needles will penetrate the stratum corneum and enter the epidermal and dermal layers without causing pain. MN creates temporary micro-channels for drug delivery, but they rapidly heal after MN is removed, preventing long-term skin tissue damage (Zhang et al. 2019b). The dermis has a lot of blood capillaries and is relatively hydrophilic. Insulin can swiftly circulate throughout the dermis and then be absorbed into systemic circulation through blood capillaries, resulting in a therapeutic reaction when it reaches the site of action (Shen et al. 2020; Bilal et al. 2021). MNs have a base width or diameter of around 200 to 300 μm, which is substantially greater than the diameter of a follicle of approximately 100 μm. As a result, MNs aid macromolecule dispersion through the skin by forming drug-permeating channels. MN tips may penetrate the nerve-distributed skin layer; their scale is so minute that they cause minimal damage or activation to nerves (Wang et al. 2020b).
Types of microneedles
Solid microneedles
Metals, silicon, and polymers, such as polycarbonate, have all been used to make solid MNs. The first solid MNs were fabricated from silicon using microfabrication technology (Puri et al. 2021). Solid silicon MNs emerged as the most common method due to their high biocompatibility (Agrawal et al. 2020). Solid MNs are stronger and have a better mechanical strength than hollow MNs (Yadav et al. 2020). MN-assisted transdermal administration using solid MN is also referred to as the “poke with patch” method as depicted in Fig. 2 (Xie et al. 2015). Solid MNs may be used as a skin pre-treatment to create large pores for drug delivery. Topical formulations like lotion, gel, and ointment required to cure skin can be delivered into the dermis via the pores once they have created. They can then be dispersed across the body via systemic circulation (Duarah et al. 2019).
Coated microneedles
The use of coated MNs in transdermal delivery of MNs is an appealing process (Xie et al. 2015). Coated MNs may be made of silicon or metal, and the medicine is packed onto the individual needles of the MN array as a coating layer in a dry state (Tarbox et al. 2018). The drug delivery pattern from coated MNs via skin layers is depicted in Fig. 2. Coated MNs serve two functions; the first is to penetrate the skin, and the second is to add required drugs to the surface of the MNs. Regrettably, the maximum drug dosage is less than 1 mg; therefore, the production of coated MNs is limited (Ingrole and Gill 2019).
Hollow microneedle patch
Microelectromechanical system techniques have been used to create hollow MNs in a variety of heights and shapes, primarily out of silicon and metal. MNs made of polymeric materials, hollow glass, and ceramics have also been produced (Cárcamo-Martínez et al. 2021). Hollow MNPs are made up of hollow needles that allow for continuous insulin delivery into the skin (Tarbox et al. 2018). After the needle is injected into the tissue, the medicine is permitted to pass via the hole and then into the systemic circulation (Fig. 3). Some of the benefits of this form of transdermal delivery include drug distribution rates can be controlled with a pump; drug administration amounts are far greater; and accurate dosage led to very effective delivery (Xie et al. 2015).
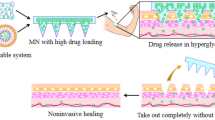
Dissolving microneedles
Insulin is encapsulated in the polymeric matrices of a dissolving/degradable MN patch composed of soluble/degradable polymer materials. Insulin is released when a polymer dissolves or degrades, and the rate at which insulin is released is regulated by the rate at which matrices dissolve or degrade (Wang et al. 2020c). Since the MN is not withdrawn after injection like in other situations, there is only one step to the procedure (Fig. 3). Within the skin, the polymer degrades and regulates drug release. The bio-acceptability of the polymer and its breakdown within the skin make it one of the best options for long-term therapy with better patient compliance. When designing dissolving microneedles, efficient needle drug delivery is a critical component that confronts challenges. As a result, mixing of polymer and the drug is an essential stage in the manufacturing process (Waghule et al. 2019). Numerous dissolving MNs composed of sugar glass polymers, like maltose and trehalose, have been identified to date. After insertion, sugar glass MNs typically dissolve rapidly in human skin. The production of these MNs, however, necessitates an elevated temperature of over 100 °C to cause rubber to glass transitions of sugar glasses, which can impair the bioactivity of biomolecules such as insulin (Jeong et al. 2021).
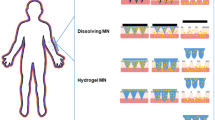
Hydrogel-forming or phase transition microneedles (PTMs)
Microneedles that form hydrogels are made of cross-linked hydrophilic polymers. Phase transition microneedles (PTMs) are strong enough in their dry glassy condition to pierce the epidermis and transform to a water-swollen hydrogel via absorbing interstitial fluid in the dermis layer. The preloaded insulin in the MNs diffuses quickly into the skin via hydrogel network. The cross-linking between the molecular chains allows the PTMs to retain their hardness when hydrated, ensuring that they are completely removed from the skin following application (Shen et al. 2020). In a nutshell, Table 1 summarizes differentiating features between various types of earlier explained microneedles (Fig. 4).
Application of microneedles in glucose monitoring
The diagnosis of all forms of diabetes, at an early stage, is important for the management of the disease to slow down potential complications such as retinopathy, diabetic nephropathy, cardiovascular diseases, neuropathy, diabetic foot ulcer, and viral infections (Szunerits et al. 2021; Baghban et al. 2019). The advantages of strict glycaemic regulation towards the management of blood glucose levels in diabetic patients have long been known. Continuous glucose monitoring can significantly minimize the incidence of diabetes-related diseases, allowing diabetics to maintain a healthier lifestyle while avoiding expensive and life-threatening late-stage diabetic complications (Teymourian et al. 2020; Juska and Pemble 2020). Microneedles can be explored as a glucose-sensing component in glucose monitoring. Glucose sensing may be performed with blood or interstitial fluid (ISF) samples. The biofluid to be sampled is the most important factor in MN design. Several studies have been carried out on the relationship between blood glucose levels in blood and ISF. It has been widely reported that there exists a time lag in the distribution of glucose from blood to ISF. The lag time is estimated to be between 0 and 45 min (Mathur et al. 2010). Blood and ISF glucose levels, on the other hand, are strongly correlated once equilibrium is achieved. It essential to understand the physiological dissimilarities between blood and ISF in order to better understand design differences between blood and ISF extracting microneedles (Khanna et al. 2008; Bariya et al. 2012).
Microneedles for interstitial fluid (ISF) sampling
For a variety of analytes, MN-mediated sampling of interstitial fluid is evolving as a promising alternative to blood sampling, with glucose being a major target. Because of their short length (less than 1000 m), MNs can penetrate the stratum corneum and enter the ISF in the viable epidermis and top layers of the dermis without stimulating nociceptors or touching blood vessels, making them a minimally invasive method of extraction (Wang et al. 2019; Kap et al. 2021; Jendrike et al. 2017). Fracture and buckling are two possible failure scenarios of MNs. Shorter needles with the same diameter and material can generally tolerate greater pressure without breaking. As a result, needles made of lower-strength materials, such as silicon dioxide, can be utilized for ISF sampling. Another benefit of silicon dioxide is that it is highly biocompatible. Because of the lower height, smaller needle diameters may be used without causing buckling (Friedl 2005; Sivamani et al. 2009; Davis et al. 2004). With a narrower tip diameter, the ratio of fracture force to insertion force into skin is much greater (Ranamukhaarachchi et al. 2019). This improves the safety margin for using MNs without failure. For ISF sampling, MN lumen diameters can be as small as 10 µm in most cases. Extremely high capillary forces are produced by a small MN diameter combined with a low density of ISF. Capillary forces significantly increase as the hydrophilicity of the MN material raises. And without a pumping system, this makes fluid extraction easier. Unfortunately, as the diameter of the MNs decreases, the flow rate also decreases. As a result, before the MNs are loaded with ISF, there is an initial latent time. The majority of commercial ISF glucose sensors only need 0.5–2 µl of fluid, and this figure is steadily decreasing. An array of MNs is employed to accomplish the necessary flow rates in order to improve flow rates. In humans, vacuum pump-assisted ISF sampling with MNs has been established and proved to monitor changing glucose levels with a time lag of less than 20 min following insulin injection (Kolluru et al. 2019; Jiang and Lillehoj 2020; Samant and Prausnitz 2018; Miller et al. 2018).
Microneedles for blood sampling
Blood capillaries are found deep under the epidermis. Commonly, blood microcapillaries are located at 400-µm penetration depths. In the same depth, the nerve tips can also be found. As a result, some of the MNs in the array can only scratch the nerve cells at the top. The narrow diameters and regulated shank length, on the other hand, minimise the chances of encountering a nerve or stimulating it sufficiently to cause significant pain. In a study of the impact of MN design on pain in humans, researchers discovered that needles varying in length ranging from 480 to 1450 µm resulted in pain scores of 5 to 40% of a 26-gauge hypodermic needle. MN shank lengths of 400–900 µm are required to extract blood without causing severe pain. The MNs must be made of stronger materials like metal or silicon at these lengths. The size of a female mosquito proboscis is a popular model used by researchers (Li et al. 2013). The diameter of the MNs must be large enough to allow easy access to the largest blood cells. Also, the longer length requires greater diameters to avoid needle failure via buckling. Typical MNs diameters must be at least 50 µm in width. And although capillary action alone can be sufficient for blood extraction, factors like greater fluid density, greater conduit diameter, and material of choice can all help to minimize the impact. In these circumstances, a microfluidic pumping device is required to produce negative pressure (Lisi et al. 2020; Zhang et al. 2019c).
Recent advancements in microneedle-based treatment modalities for management of diabetes mellitus
The MN technique is being used for various medicines, but it must overcome a number of obstacles before being commercially available. It will take a lot of research to get it clinically authorized. Skin allergies, redness, and irritation are the most common concerns connected with MN technology. The MNs can only hold a small quantity of medicines. It is extremely difficult to pass hydrophilic and big substances through the skin. In order to fabricate these needles, the ideal material must be used that has sufficient mechanical toughness and insertion force. The basic goal is to enhance permeability without creating discomfort. A patient may find it extremely challenging to poke with a needle and then put the patch. If the skin pores do not seal following application, there is a risk of infection (Ita 2015). In spite of these challenges, the MN-based method appears to be the most widely studied field, serving as a foundation for drug penetration and dermal delivery. Most studies have revealed that it can provide sustained release of drugs over a long period of time while avoiding a rapid drop in blood glucose in the early phase to avoid hypoglycaemic side effects. However, we are unable to manage drug release depending on variable glucose levels using simply MN administration without additional features like biosensors which would be beneficial in this regard, as the release of drug would be triggered by the glucose level. One of the challenging issues encountered while using MNs is achieving precision of measurement and correlation between results acquired from interstitial fluid or sweat and plasma or blood glucose (Tarbox et al. 2018; Sharma et al. 2019; Puri et al. 2021). Table 2 summarizes the various recent advancements in MN-based treatment modalities for management of diabetes mellitus.
Patent literature focussing application of microneedles in treatment of diabetes mellitus
Patent literature searches were performed from the official website of World Intellectual Property Organization (WIPO) with analytics to ensure and categorize the current research about the applications of MNs in diabetes mellitus from the period of 2014 to date (Table 3). The keywords entered in search strategy were “insulin”, “microneedle”, “diabetes mellitus”, “therapy”, and “delivery”, “in various combinations”. This literature would increase understanding and prospective for research scientists to comprehend better outlook in the research and development of MNs systems for treatment and monitoring of diabetes.
Current clinical status spotlighting expedient role of microneedles in diabetes mellitus
Various pre-clinical studies on MNs have been conducted and proved to be beneficial in several areas, but only a few have undergone success in human patients (https://clinicaltrials.gov/). Numerous clinical trials based on applications of MNs for monitoring and treatments of diabetes conditions are currently under different phases carried by several universities and industries (Table 4).
Conclusions
MNs are emerging as essential physical enhancers in transdermal drug delivery and fluid extraction systems, and their importance will grow as a result of the benefits they provide and it has the potential to replace traditional drug delivery approaches, most notably the transdermal approach. MN devices have shown tremendous promise in enhancing insulin penetration by breaking the skin barrier, as contrasted to passive transport via the skin. Unlike traditional hypodermic injections, insulin delivery through MNs has the advantages of requiring little training and painless insertion. However, MN-based delivery has a number of drawbacks, such as less accurate dosage accuracy than hypodermic needles, variances in skin layer thickness and skin hydration among individuals, drug delivery issues related with non-vertical insertion of the MNs to the skin, harm to veins with repeated use, and probable breaking of the MNs tip or the entire MNs within the skin. A greater understanding of the pharmacokinetics of insulin delivered intradermally using MNs may also be beneficial. Furthermore, the effectiveness of increasing skin penetration, which is one of the most significant challenges in transdermal drug delivery, has expanded the reach of MN drug delivery in the coming years.
Current status and future prospects
Silicon was used to create the first MNs and an analysis was carried to see if MNs might be utilized to more effectively administer medications via the skin. First, permeation studies on cadaver skin were conducted to investigate if big molecules such as albumin and insulin could pass via the skin when MNs were used. Microneedles were found to deliver big molecules more effectively in subsequent investigations. Many new fascinating MN concepts are now being developed that will be extremely beneficial in the future (Christensen and Gannon 2019; Gastaldelli 2011; Marselli et al. 2014). MNs, as a new device, have distinct benefits (painless and quick delivery) over previous systemic administration methods, and they offer a variety of biomedical applications (Rao et al. 2014). The MN-based method appears to be the most widely studied field, serving as a foundation for drug penetration and dermal delivery. Most studies have revealed that it can provide sustained release of drugs over a long period of time while avoiding a rapid drop in blood glucose in the early phase to avoid hypoglycaemic side effects (Rojas et al. 2018).
In microneedle research, there is more than enough space for advancement. For example, novel materials for microneedle fabrication can be used, and manufacturing approaches can be upgraded. These materials must have appropriate mechanical strength and skin adherence, as well as be free of harmful degradation products. Materials generated from nature are also excellent possibilities. Another research area is to equip microneedles with unique features that allow them to adapt to increasingly complex functional prerequisites. Multi-responsive microneedles, for example, can deliver medications in a controlled manner whereas breathable microneedles can enhance skin comfort. In addition to wound-healing patches and 3D cell culture chips, microneedles can also be used in a variety of different biomedical applications. Furthermore, microneedles are frequently overlooked when compared to popular therapeutic technology. The results of biomarker detection obtained using microneedles should be compared to those obtained using conventional methods to assess the accuracy and efficiency of microneedle-based detections. Despite the increasing scientific advances in the field of microneedles, there is still a significant gap between academic research and industrial products. This is reflected in the restricted number of microneedle products available, all of which have basic characteristics (Zhang et al. 2020a, b). Several novel and fascinating MN concepts have recently been developed, all of which have the potential to be very useful in the future. Biodegradable polymer MNs, for example, have recently been developed and characterised. Polymer needles have the benefit of being far less expensive to manufacture than silicon needles, and they should not cause any harm if they break in the skin because they are biodegradable. This research focuses on biocompatible and biodegradable polymer MNs, which are intended to enhance safety and manufacturing efficiency (Chatterjee and Davies 2015). With the increasing variety of MNs, a comprehensive set of tests that can be used to examine all needles should be suggested. Preclinical testing (in vivo studies in animal models), clinical tests (to assess pain, inflammation), mechanical testing (to assess characteristics such as margin of safety), and fluidic flow testing (e.g. fluid pressure needed for particular flow rate) should all be included in this list. This would aid in not only objectively comparing MNs, but also in selecting the best MNs for each application (Teo et al. 2006; Liu et al. 2012). Conclusively, it has been manifested that MNs have a lot of potential in biomedical applications.
Data availability
Not applicable.
Abbreviations
- Arg:
-
Arginine
- BGLs:
-
Blood glucose levels
- CMC:
-
Carboxymethyl cellulose
- CMCS:
-
Carboxymethyl chitosan
- CLA:
-
Conjugated linoleic acid
- CCA:
-
CLA-CMCS-Arg polymer
- DM:
-
Diabetes mellitus
- EE-ASI-1:
-
Enhanced epidermal antigen-specific immunotherapy trial-1
- GUMP:
-
Glucose measurement using microneedle patches
- HbA1c:
-
Hemoglobin A1c
- HLA:
-
Human leukocyte antigen
- ISF:
-
Interstitial fluid
- LA:
-
Lauric acid
- MBGs:
-
Mesoporous bioactive glasses
- MSN:
-
Mesoporous silica nanoparticle
- MNs:
-
Microneedles
- PTMs:
-
Phase transition microneedles
- PVPMAA:
-
Poly (vinylpyrrolidone-co-methacrylic) acid
- PDA:
-
Polydopamine
- PGA:
-
Polyglycolic acid
- PLA:
-
Polylactic acid
- LGA:
-
Polylactic-co glycolic acid
- PVP:
-
Polyvinylpyrrolidone
- RS-PGC-MNs:
-
Rapidly separating genepin-crosslinked gelatin (MNs) mounted on polyvinyl alcohol-coated polylactic acid MNs
- ROS:
-
Reactive oxygen species
- SC:
-
Subcutaneous
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- WIPO:
-
World Intellectual Property Organization
- ZnO QDs:
-
Zinc oxide quantum dots
- ZP:
-
Zosano Pharma
References
Agrawal S, Gandhi SN, Gurjar P, Saraswathy N (2020) Microneedles: an advancement to transdermal drug delivery system approach. J Appl Pharm Sci 10:149–159. https://doi.org/10.7324/JAPS.2020.103019
Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Méneur C, Bernal-Mizrachi E (2015) Natural history of β-cell adaptation and failure in type 2 diabetes. Mol Asp Med 42:19–41. https://doi.org/10.1016/j.mam.2014.12.002
Alimardani V, Abolmaali SS, Yousefi G, Rahiminezhad Z, Abedi M, Tamaddon A, Ahadian S (2021) Microneedle arrays combined with nanomedicine approaches for transdermal delivery of therapeutics. J Clin Med 10:181. https://doi.org/10.3390/jcm10020181
Arikat F, Hanna SJ, Singh RK, Vilela L, Wong FS, Dayan CM, Coulman SA, Birchall JC (2020) Targeting proinsulin to local immune cells using an intradermal microneedle delivery system; a potential antigen-specific immunotherapy for type 1 diabetes. J Control Release 322:593–601. https://doi.org/10.1016/j.jconrel.2020.02.031
Baghban TZ, Imani R, Mohabatpour F (2019) A review on bioengineering approaches to insulin delivery: a pharmaceutical and engineering perspective. Macromol Biosci 19:1800458. https://doi.org/10.1002/mabi.201800458
Bai X (2015) Built-in non-verbal compact instructional device integratable to applicator. JP2015171546.
Bariya SH, Gohel MC, Mehta TA, Sharma OP (2012) Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol 64:11–29. https://doi.org/10.1111/j.2042-7158.2011.01369.x
Bilal M, Mehmood S, Raza A, Hayat U, Rasheed T, Iqbal HM (2021) Microneedles in smart drug delivery. Adv Wound Caref 10:204–219. https://doi.org/10.1089/wound.2019.1122
Bluestone JA, Herold K, Eisenbarth G (2010) Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464:1293–1300. https://doi.org/10.1038/nature08933
Brinkman AK (2017) Management of type 1 diabetes. Nurs Clin North Am 52:499–511. https://doi.org/10.1016/j.cnur.2017.07.001
Cárcamo-Martínez Á, Mallon B, Domínguez-Robles J, Vora LK, Anjani QK, Donnelly RF (2021) Hollow microneedles: a perspective in biomedical applications. Int J Pharm 599:120455. https://doi.org/10.1016/j.ijpharm.2021.120455
Chatterjee S, Davies MJ (2015) Current management of diabetes mellitus and future directions in care. Postgrad Med J 91:612–621. https://doi.org/10.1136/postgradmedj-2014-133200
Chen H, Zhu H, Zheng J, Mou D, Wan J, Zhang J, Shi T, Zhao Y, Xu H, Yang X (2009) Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J Controlled Release 139:63–72. https://doi.org/10.1016/j.jconrel.2009.05.031
Chen MC, Ling MH, Kusuma SJ (2015) Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater 24:106–116. https://doi.org/10.1016/j.actbio.2015.06.021
Chen BZ, Ashfaq M, Zhu DD, Zhang XP, Guo XD (2018a) Controlled delivery of insulin using rapidly separating microneedles fabricated from genipin-crosslinked gelatin. Macromol Rapid Commun 39:1800075. https://doi.org/10.1002/marc.201800075
Chen CH, Shyu VB, Chen CT (2018b) Dissolving microneedle patches for transdermal insulin delivery in diabetic mice: potential for clinical applications. Materials 11:1625. https://doi.org/10.3390/ma11091625
Chen BZ, Zhang LQ, Xia YY, Zhang XP, Guo XD (2020) A basal-bolus insulin regimen integrated microneedle patch for intraday postprandial glucose control. Sci Adv 6:eaba7260. https://doi.org/10.1126/sciadv.aba7260
Chen M, Quan G, Sun Y, Yang D, Pan X, Wu C (2020b) Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery. J Control Release 325:163–175. https://doi.org/10.1016/j.jconrel.2020.06.039
Chenggang Z, Mengfan D, Xiaolei W, Shanshan W (2019) Prussian blue microneedle electrode for blood glucose monitoring, preparation method thereof, blood glucose monitoring patch and preparation method thereof. CN110558993.
Chevalier N, Fénichel P (2015) Endocrine disruptors: new players in the pathophysiology of type 2 diabetes? Diabetes Metab 41:107–115. https://doi.org/10.1016/j.diabet.2014.09.005
Chiang JL, Kirkman MS, Laffel LM, Peters AL (2014) Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 37:2034–2054. https://doi.org/10.2337/dc14-1140
Chiang JL, Maahs DM, Garvey KC, Hood KK, Laffel LM, Weinzimer SA, Wolfsdorf JI, Schatz D (2018) Type 1 diabetes in children and adolescents: a position statement by the American Diabetes Association. Diabetes Care 41:2026–2044. https://doi.org/10.2337/dci18-0023
Christensen AA, Gannon M (2019) The beta cell in type 2 diabetes. Curr Diabetes Rep 19:1–8. https://doi.org/10.1007/s11892-019-1196-4
Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL (2005) Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54:S97-107. https://doi.org/10.2337/diabetes.54.suppl_2.S97
D’Adamo E, Caprio S (2011) Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care 34:S161–S165. https://doi.org/10.2337/dc11-s212
Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR (2004) Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J Biomech 37:1155–1163. https://doi.org/10.1016/j.jbiomech.2003.12.010
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC (2015) Type 2 diabetes mellitus. Nat Rev Dis Primers 1:1–22. https://doi.org/10.1038/nrdp.2015.19
Devendra D, Liu E, Eisenbarth GS (2004) Type 1 diabetes: recent developments. BMJ 328:750–754. https://doi.org/10.1136/bmj.328.7442.750
Dharadhar S, Majumdar A, Dhoble S, Patravale V (2019) Microneedles for transdermal drug delivery: a systematic review. Drug Dev Ind Pharm 45:188–201. https://doi.org/10.1080/03639045.2018.1539497
Dong S, Wu H (2018) Regenerating β cells of the pancreas–potential developments in diabetes treatment. Expert Opin Biol Ther 18:175–185. https://doi.org/10.1080/14712598.2018.1402885
Duarah S, Sharma M, Wen J (2019) Recent advances in microneedle-based drug delivery: special emphasis on its use in paediatric population. Eur J Pharm Biopharm 136:48–69. https://doi.org/10.1016/j.ejpb.2019.01.005
El-Khordagui LK (2012) Microneedles: an emerging approach for active transdermal delivery of insulin. J Bioequiv Availab 4:7. https://doi.org/10.4172/jbb.10000e24
Fonseca DF, Costa PC, Almeida IF, Dias-Pereira P, Correia-Sá I, Bastos V, Oliveira H, Duarte-Araújo M, Morato M, Vilela C, Silvestre AJ (2020) Pullulan microneedle patches for the efficient transdermal administration of insulin envisioning diabetes treatment. Carbohydr Polym 30:116314. https://doi.org/10.1016/j.carbpol.2020.116314
Friedl CK (2005) Analysis: optimizing microneedles for epidermal access. Diabetes Technol Ther 7:546–548. https://doi.org/10.1089/dia.2005.7.546
Galderisi A, Sherr JL (2019) A technological revolution: the integration of new treatments to manage type 1 diabetes. Pediatr Ann 48:e311-318. https://doi.org/10.3928/19382359-20190725-03
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C (2020) Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci 21:6275. https://doi.org/10.3390/ijms21176275
Garg A, Kumar A (2018) Risk and benefit profile of dulaglutide in established therapeutic indication. Curr Drug Saf 13:165–170. https://doi.org/10.2174/1574886313666180601082412
Gastaldelli A (2011) Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Diabetes Res Clin Pract 93:S60–S65. https://doi.org/10.1016/S0168-8227(11)70015-8
Giugliano D, Standl E, Vilsbøll T, Betteridge J, Bonadonna R, Campbell IW, Schernthaner GH, Staels B, Trichopoulou A, Farinaro E (2009) Is the current therapeutic armamentarium in diabetes enough to control the epidemic and its consequences? What are the current shortcomings? Acta Diabetol 46:173–181. https://doi.org/10.1007/s00592-009-0134-3
Gotfredsen DR, Vinther S, Petersen TS, Cortes R, Jensen TB, Jimenez-Solem E, Christensen MB (2020) Glycemic control and use of glucose-lowering medications in hospital-admitted type 2 diabetes patients over 80 years. Sci Rep 10:1–8. https://doi.org/10.1038/s41598-020-60818-5
He X, Sun J, Zhuang J, Xu H, Liu Y, Wu D (2019) Microneedle system for transdermal drug and vaccine delivery: devices, safety, and prospects. Dose-Response 17:1559325819878585
Hoang Do O, Thorn P (2015) Insulin secretion from beta cells within intact islets: location matters. Clin Exp Pharmacol Physiol 42:406–414. https://doi.org/10.1111/1440-1681.12368
https://clinicaltrials.gov; Accessed 25 July 2021
https://diabetesatlas.org/data/en/world/; Accessed 28 July 2021
Hultström M, Roxhed N, Nordquist L (2014) Intradermal insulin delivery: a promising future for diabetes management. J Diabetes Sci Technol 8:453–457. https://doi.org/10.1177/1932296814530060
Imam K (2013) Clinical features, diagnostic criteria and pathogenesis of diabetes mellitus. In: Ahmad SI (ed) diabetes, 1st edn. Springer, New York, pp 340–355. https://doi.org/10.1007/978-1-4614-5441-0_25
Ingrole RS, Gill HS (2019) Microneedle coating methods: a review with a perspective. J Pharmacol Exp Ther 370:555–569. https://doi.org/10.1124/jpet.119.258707
Ita K (2015) Transdermal delivery of drugs with microneedles-potential and challenges. Pharmaceutics 7:90–105. https://doi.org/10.3390/pharmaceutics7030090
Jain AKC (2015) A simple new classification for diabetic foot ulcers. Med-Science 4:2109–2120. https://doi.org/10.5455/medscience.2014.03.8215
Jain AKC, Joshi S (2013) Diabetic foot classifications: review of literature. Med-Science 2:715–721. https://doi.org/10.5455/medscience.2013.02.8069
Jana BA, Wadhwani AD (2019) Microneedle–future prospect for efficient drug delivery in diabetes management. Indian J Pharmacol 51:4–10. https://doi.org/10.4103/ijp.IJP_16_18
Jendrike N, Baumstark A, Chen CH, Rittmeyer D, Haug C, Freckmann G (2017) Introduction of a novel smartphone-coupled blood glucose monitoring system. J Diabetes Sci Technol 11:1231–1233. https://doi.org/10.1177/1932296817706594
Jeong WY, Kwon M, Choi HE, Kim KS (2021) Recent advances in transdermal drug delivery systems: a review. Biomater Res 25:1–5. https://doi.org/10.1186/s40824-021-00226-6
Jiang X, Lillehoj PB (2020) Microneedle-based skin patch for blood-free rapid diagnostic testing. Microsyst Nanoeng 6:1–11. https://doi.org/10.1038/s41378-020-00206-1
Jin X, Zhu DD, Chen BZ, Ashfaq M, Guo XD (2018) Insulin delivery systems combined with microneedle technology. Adv Drug Deliv 127:119–137. https://doi.org/10.1016/j.addr.2018.03.011
Jung JH, Jin SG (2021) Microneedle for transdermal drug delivery: current trends and fabrication. J Pharm Investig 4:1–5. https://doi.org/10.1007/s40005-021-00512-4
Juska VB, Pemble ME (2020) A critical review of electrochemical glucose sensing: evolution of biosensor platforms based on advanced nanosystems. Sensors 20:6013. https://doi.org/10.3390/s20216013
Kahn SE, Cooper ME, Del Prato S (2014) Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. The Lancet 383:1068–1083. https://doi.org/10.1016/S0140-6736(13)62154-6
Kap Ö, Kilic V, Hardy JG, Horzum N (2021) Smartphone-based colorimetric detection systems for glucose monitoring in the diagnosis and management of diabetes. Analyst. https://doi.org/10.1039/D0AN02031A
Kerner W, Brückel J (2014) Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 122:384–386. https://doi.org/10.1055/s-0034-1366278
Khanna P, Strom JA, Malone JI, Bhansali S (2008) Microneedle-based automated therapy for diabetes mellitus. J Diabetes Sci Technol 2:1122–1129. https://doi.org/10.1177/193229680800200621
Kolluru C, Williams M, Chae J, Prausnitz MR (2019) Recruitment and collection of dermal interstitial fluid using a microneedle patch. Adv Healthc Mater 8:1801262. https://doi.org/10.1002/adhm.201801262
Koufakis T, Dimitriadis G, Metallidis S, Zebekakis P, Kotsa K (2021) The role of autoimmunity in the pathophysiology of type 2 diabetes: looking at the other side of the moon. Obes Rev 22:e13231. https://doi.org/10.1111/obr.13231
Lee IC, Lin WM, Shu JC, Tsai SW, Chen CH, Tsai MT (2017a) Formulation of two-layer dissolving polymeric microneedle patches for insulin transdermal delivery in diabetic mice. J Biomed Mater Res A 105:84–93. https://doi.org/10.1002/jbm.a.35869
Lee IC, Wu YC, Tsai SW, Chen CH, Wu MH (2017b) Fabrication of two-layer dissolving polyvinylpyrrolidone microneedles with different molecular weights for in vivo insulin transdermal delivery. RSC Adv 7:5067–5075. https://doi.org/10.1039/C6RA27476E
Levin Y (2014) Systems and methods for intradermal delivery of therapeutics using microneedles. US20140350514.
Li CG, Lee CY, Lee K, Jung H (2013) An optimized hollow microneedle for minimally invasive blood extraction. Biomed Microdevices 15:17–25. https://doi.org/10.1007/s10544-012-9683-2
Ling MH, Chen MC (2013) Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater 9:8952–8961. https://doi.org/10.1016/j.actbio.2013.06.029
Lisi F, Peterson JR, Gooding JJ (2020) The application of personal glucose meters as universal point-of-care diagnostic tools. Biosens Bioelectron 148:111835. https://doi.org/10.1016/j.bios.2019.111835
Liu S, Jin MN, Quan YS, Kamiyama F, Katsumi H, Sakane T, Yamamoto A (2012) The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J Control Release 161:933–941. https://doi.org/10.1016/j.jconrel.2012.05.030
Liu X, Li X, Zhang N, Zhao Z, Wen X (2016) Bioengineering strategies for the treatment of type I diabetes. J Biomed Nanotech 12:581–601. https://doi.org/10.1166/jbn.2016.2176
Liu D, Yu B, Jiang G, Yu W, Zhang Y, Xu B (2018a) Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater Sci Eng C 90:180–188. https://doi.org/10.1016/j.msec.2018.04.055
Liu D, Zhang Y, Jiang G, Yu W, Xu B, Zhu J (2018b) Fabrication of dissolving microneedles with thermal-responsive coating for NIR-triggered transdermal delivery of metformin on diabetic rats. ACS Biomater Sci Eng 4:1687–1695. https://doi.org/10.1021/acsbiomaterials.8b00159
Liu T, Jiang G, Song G, Zhu J, Yang Y (2020) Fabrication of separable microneedles with phase change coating for NIR-triggered transdermal delivery of metformin on diabetic rats. Biomed Microdevices 22:1–11. https://doi.org/10.1007/s10544-019-0468-8
Loretelli C, Assi E, Seelam AJ, Ben Nasr M, Fiorina P (2020) Cell therapy for type 1 diabetes. Expert Opin Biol Ther 20:887–897. https://doi.org/10.1080/14712598.2020.1748596
Marselli L, Suleiman M, Masini M, Campani D, Bugliani M, Syed F, Martino L, Focosi D, Scatena F, Olimpico F, Filipponi F (2014) Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia 57:362–365. https://doi.org/10.1007/s00125-013-3098-3
Mathur V, Satrawala Y, Rajput MS (2010) Emerging field of microneedle technology in transdermal delivery system and diabetes mellitus. Drug Invent Today 2:7
Mayo P (2016) An overview of diabetes. Nurs Stand 30:53–60. https://doi.org/10.7748/ns.2016.e10386
Mdanda S, Ubanako P, Kondiah PP, Kumar P, Choonara YE (2021) Recent advances in microneedle platforms for transdermal drug delivery technologies. Polymers 13:2405. https://doi.org/10.3390/polym13152405
Migalska K, Morrow DI, Garland MJ, Thakur R, Woolfson AD, Donnelly RF (2011) Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm Res 28:1919–1930. https://doi.org/10.1007/s11095-011-0419-4
Migdadi EM, Courtenay AJ, Tekko IA, McCrudden MT, Kearney MC, McAlister E, McCarthy HO, Donnelly RF (2018) Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J Control Release 285:142–151. https://doi.org/10.1016/j.jconrel.2018.07.009
Miller PR, Taylor RM, Tran BQ, Boyd G, Glaros T, Chavez VH, Krishnakumar R, Sinha A, Poorey K, Williams KP, Branda SS (2018) Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Commun Biol 1:1–11. https://doi.org/10.1038/s42003-018-0170-z
Mohsen AM (2019) Nanotechnology advanced strategies for the management of diabetes mellitus. Curr Drug Targets 20:995–1007. https://doi.org/10.2174/1389450120666190307101642
Ng LC, Gupta M (2020) Transdermal drug delivery systems in diabetes management: a review. Asian J Pharm Sci 15:13–25. https://doi.org/10.1016/j.ajps.2019.04.006
Nolan CJ, Damm P, Prentki M (2011) Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 378:169–181. https://doi.org/10.1016/S0140-6736(11)60614-4
Pozzilli P, Guglielmi C, Caprio S, Buzzetti R (2011) Obesity, autoimmunity, and double diabetes in youth. Diabetes Care 34:S166-170. https://doi.org/10.2337/dc11-s213
Primavera R, Kevadiya BD, Swaminathan G, Wilson RJ, De Pascale A, Decuzzi P, Thakor AS (2020) Emerging nano-and micro-technologies used in the treatment of type-1 diabetes. Nanomaterials 10:789. https://doi.org/10.3390/nano10040789
Puri A, Nguyen HX, Tijani AO, Banga AK (2021) Characterization of microneedles and microchannels for enhanced transdermal drug delivery. Ther Deliv 12:77–103. https://doi.org/10.4155/tde-2020-0096
Qiu Y, Qin G, Zhang S, Wu Y, Xu B, Gao Y (2012) Novel lyophilized hydrogel patches for convenient and effective administration of microneedle-mediated insulin delivery. Int J Pharm 437:51–56. https://doi.org/10.1016/j.ijpharm.2012.07.035
Queiroz ML, Shanmugam S, Santos LN, Campos CD, Santos AM, Batista MS, Araújo AA, Serafini MR (2020) Microneedles as an alternative technology for transdermal drug delivery systems: a patent review. Expert Opin Ther Pat 30:433–452. https://doi.org/10.1080/13543776.2020.1742324
Ranamukhaarachchi SA, Stoeber B (2019) Determining the factors affecting dynamic insertion of microneedles into skin. Biomed Microdevices 21:1–8. https://doi.org/10.1007/s10544-019-0449-y
Rao R, Mahant S, Chhabra L, Nanda S (2014) Transdermal innovations in diabetes management. Curr Diabetes Rev 10:343–359. https://doi.org/10.2174/1573399810666141124110836
Raval J, Trivedi R, Suman S, Kukrety A, Prajapati P (2021) Nano-biotechnology and its innovative perspective in diabetes management. Mini-Rev Med Chem. https://doi.org/10.2174/1389557521666210623164052
Rojas J, Bermudez V, Palmar J, Martínez MS, Olivar LC, Nava M, Tomey D, Rojas M, Salazar J, Garicano C, Velasco M (2018) Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. J Diabetes Res. https://doi.org/10.1155/2018/9601801
Ross LJ, Neville KA (2019) Continuous subcutaneous insulin infusion versus multiple daily injections for type 1 diabetes. J Paediatr Child Health 55:718–722. https://doi.org/10.1111/jpc.14480
Samant PP, Prausnitz MR (2018) Mechanisms of sampling interstitial fluid from skin using a microneedle patch. PNAS 115:4583–4588. https://doi.org/10.1073/pnas.1716772115
Seong KY, Seo MS, Hwang DY, O’Cearbhaill ED, Sreenan S, Karp JM, Yang SY (2017) A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J Control Release 265:48–56. https://doi.org/10.1016/j.jconrel.2017.03.041
Sharma S, Hatware K, Bhadane P, Sindhikar S, Mishra DK (2019) Recent advances in microneedle composites for biomedical applications: advanced drug delivery technologies. Mater Sci Eng C 103:109717. https://doi.org/10.1016/j.msec.2019.05.002
Shen D, Yu H, Wang L, Khan A, Haq F, Chen X, Huang Q, Teng L (2020) Recent progress in design and preparation of glucose-responsive insulin delivery systems. J Control Release 321:236–258. https://doi.org/10.1016/j.jconrel.2020.02.014
Singh M, Kumar A (2018) Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf 13:84–91. https://doi.org/10.2174/1574886313666180226103408
Singh M, Sharma R, Kumar A (2019) Safety of SGLT2 inhibitors in patients with diabetes mellitus. Curr Drug Saf 14:87–93. https://doi.org/10.2174/1574886314666190206164647
Sivamani RK, Stoeber B, Liepmann D, Maibach HI (2009) Microneedle penetration and injection past the stratum corneum in humans. JDT 20:156–159. https://doi.org/10.1080/09546630802512679
Szunerits S, Melinte S, Barras A, Pagneux Q, Voronova A, Abderrahmani A, Boukherroub R (2021) The impact of chemical engineering and technological advances on managing diabetes: present and future concepts. Chem Soc Rev 50:2102–2146. https://doi.org/10.1039/C9CS00886A
Tarbox TN, Watts AB, Cui Z, Williams RO (2018) An update on coating/manufacturing techniques of microneedles. Drug Deliv Transl Res 8:1828–1843. https://doi.org/10.1007/s13346-017-0466-4
Teo AL, Shearwood C, Ng KC, Lu J, Moochhala S (2006) Transdermal microneedles for drug delivery applications. Mater Sci Eng B 132:151–154. https://doi.org/10.1016/j.mseb.2006.02.008
Teymourian H, Barfidokht A, Wang J (2020) Electrochemical glucose sensors in diabetes management: an updated review (2010–2020). Chem Soc Rev 49:7671–7709. https://doi.org/10.1039/D0CS00304B
Thuillier P, Sonnet E, Alavi Z, Roudaut N, Nowak E, Dion A, Kerlan V (2018) Comparison between preprandial vs. postprandial insulin aspart in patients with type 1 diabetes on insulin pump and real-timecontinuous glucose monitoring. Diabetes Metab Res Rev 34:e3019. https://doi.org/10.1002/dmrr.3019
Tucak A, Sirbubalo M, Hindija L, Rahić O, Hadžiabdić J, Muhamedagić K, Čekić A, Vranić E (2020) Microneedles: characteristics, materials, production methods and commercial development. Micromachines 11:961. https://doi.org/10.3390/mi11110961
Ullah A, Choi HJ, Jang M, An S, Kim GM (2020) Smart microneedles with porous polymer layer for glucose-responsive insulin delivery. Pharmaceutics 12:606. https://doi.org/10.3390/pharmaceutics12070606
Von Herrath MG, Korsgren O, Atkinson MA (2016) Factors impeding the discovery of an intervention-based treatment for type 1 diabetes. Clin Exp Immunol 183:1–7. https://doi.org/10.1111/cei.12656
Vora LK, Courtenay AJ, Tekko IA, Larrañeta E, Donnelly RF (2020) Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int J Biol Macromol 146:290–298. https://doi.org/10.1016/j.ijbiomac.2019.12.184
Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, Dua K (2019) Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother 109:1249–1258. https://doi.org/10.1016/j.biopha.2018.10.078
Wang HC, Chang FY, Tsai TM, Chen CH, Chen YY (2019) Design, fabrication, and feasibility analysis of a colorimetric detection system with a smartphone for self-monitoring blood glucose. J Biomed Opt 24:027002. https://doi.org/10.1117/1.JBO.24.2.027002
Wang J, Wang Z, Yu J, Kahkoska AR, Gu BJB, Z, (2020a) Glucose-responsive insulin and delivery systems: innovation and translation. Adv Mater 32:1902004. https://doi.org/10.1002/adma.201902004
Wang Y, Wang H, Zhu XX, Guan Y, Zhang Y (2020b) Smart microneedle patches for rapid, and painless transdermal insulin delivery. J Mater Chem B 8:9335–9342. https://doi.org/10.1039/D0TB01822H
Wang Z, Wang J, Kahkoska AR, Buse JB, Gu Z (2020c) Developing insulin delivery devices with glucose responsiveness. Trends Pharmacol Sci. https://doi.org/10.1016/j.tips.2020.11.002
Weir GC, Gaglia J, Bonner-Weir S (2020) Inadequate β-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet Diabetes Endocrinol 8:249–256. https://doi.org/10.1016/S2213-8587(20)30022-X
Wolkowicz KL, Aiello EM, Vargas E, Teymourian H, Tehrani F, Wang J, Pinsker JE, Doyle FJ III, Patti ME, Laffel LM, Dassau E (2021) A review of biomarkers in the context of type 1 diabetes: biological sensing for enhanced glucose control. Bioeng Transl Med 6:e10201. https://doi.org/10.1002/btm2.10201
Xie S, Li Z, Yu Z (2015) Microneedles for transdermal delivery of insulin. J Drug Deliv Sci Technol 28:11–17. https://doi.org/10.1016/j.jddst.2015.04.008
Xu B, Jiang G, Yu W, Liu D, Zhang Y, Zhou J, Sun S, Liu Y (2017) H 2 O 2-responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J Mater Chem B 5:8200–8208. https://doi.org/10.1039/C7TB02082A
Xu B, Cao Q, Zhang Y, Yu W, Zhu J, Liu D, Jiang G (2018) Microneedles integrated with ZnO quantum-dot-capped mesoporous bioactive glasses for glucose-mediated insulin delivery. ACS Biomater Sci Eng 4:2473–2483. https://doi.org/10.1021/acsbiomaterials.8b00626
Yadav PR, Han T, Olatunji O, Pattanayek SK, Das DB (2020) Mathematical modelling, simulation and optimisation of microneedles for transdermal drug delivery: trends and progress. Pharmaceutics 12:693. https://doi.org/10.3390/pharmaceutics12080693
Yang J, Liu X, Fu Y, Song Y (2020) Recent advances of microneedles for biomedical applications: drug delivery and beyond. Acta Pharm Sin B 9:469–483. https://doi.org/10.1016/j.apsb.2019.03.007
Ye R, Yang J, Li Y, Zheng Y, Yang J, Li Y, Liu B, Jiang L (2020) Fabrication of tip-hollow and tip-dissolvable microneedle arrays for transdermal drug delivery. ACS Biomater Sci Eng 6:2487–2494. https://doi.org/10.1021/acsbiomaterials.0c00120
Yu W, Jiang G, Liu D, Li L, Chen H, Liu Y, Huang Q, Tong Z, Yao J, Kong X (2017a) Fabrication of biodegradable composite microneedles based on calcium sulfate and gelatin for transdermal delivery of insulin. Mater Sci Eng C 71:725–734. https://doi.org/10.1016/j.msec.2016.10.063
Yu W, Jiang G, Liu D, Li L, Tong Z, Yao J, Kong X (2017b) Transdermal delivery of insulin with bioceramic composite microneedles fabricated by gelatin and hydroxyapatite. Mater Sci Eng C 73:425–428. https://doi.org/10.1016/j.msec.2016.12.111
Yu W, Jiang G, Zhang Y, Liu D, Xu B, Zhou J (2017c) Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Mater Sci Eng C 80:187–196. https://doi.org/10.1016/j.msec.2017.05.143
Zaccardi F, Webb DR, Yates T, Davies MJ (2016) Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J 92:63–69. https://doi.org/10.1136/postgradmedj-2015-133281
Zaric BL, Obradovic M, Sudar-Milovanovic E, Nedeljkovic J, Lazic V, Isenovic ER (2019) Drug delivery systems for diabetes treatment. Curr Pharm Des 25:166–173. https://doi.org/10.2174/1381612825666190306153838
Zhang Y, Jiang G, Hong W, Gao M, Xu B, Zhu J, Song G, Liu T (2018a) Polymeric microneedles integrated with metformin-loaded and PDA/LA-coated hollow mesoporous SiO2 for NIR-triggered transdermal delivery on diabetic rats. ACS Appl Bio Mater 1:1906–1917. https://doi.org/10.1021/acsabm.8b00470
Zhang Y, Jiang G, Yu W, Liu D, Xu B (2018b) Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats. Mater Sci Eng C 85:18–26. https://doi.org/10.1016/j.msec.2017.12.006
Zhang Y, Wang D, Gao M, Xu B, Zhu J, Yu W, Liu D, Jiang G (2018c) Separable microneedles for near-infrared light-triggered transdermal delivery of metformin in diabetic rats. ACS Biomater Sci Eng 4:2879–2888. https://doi.org/10.1021/acsbiomaterials.8b00642
Zhang L, Gu C, Ma H, Zhu L, Wen J, Xu H, Liu H, Li L (2019a) Portable glucose meter: trends in techniques and its potential application in analysis. Anal Bioanal Chem 411:21–36. https://doi.org/10.1007/s00216-018-1361-7
Zhang X, Sun D, Jiang GC (2019b) Comparative efficacy of nine different dressings in healing diabetic foot ulcer: a Bayesian network analysis. J Diabetes 11:418–426. https://doi.org/10.1111/1753-0407.12871
Zhang Y, Yu J, Kahkoska AR, Wang J, Buse JB, Gu Z (2019c) Advances in transdermal insulin delivery. Adv Drug Deliv Rev 139:51–70. https://doi.org/10.1016/j.addr.2018.12.006
Zhang P, Zhang Y, Liu CG (2020a) Polymeric nanoparticles based on carboxymethyl chitosan in combination with painless microneedle therapy systems for enhancing transdermal insulin delivery. RSC Adv 10:24319–24329. https://doi.org/10.1039/D0RA04460A
Zhang X, Wang Y, Chi J, Zhao Y (2020) Smart microneedles for therapy and diagnosis. Research 2020:7462915. https://doi.org/10.34133/2020/746291s5
Zhen G, Jicheng Y (2016) Glucose-responsive insulin delivery system using hyoxia-sensitive nanocomposites. WO2016172320.
Zhen G, Jicheng Y (2017) Glucose-responsive insulin delivery system using hyoxia-sensitive nanocomposites. NZ736578.
Zhen G, Jicheng Y (2018a) Glucose-responsive insulin delivery system using hyoxia-sensitive nanocomposites. ID2018/06279.
Zhen G, Jicheng Y (2018b) Patch loaded with dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. WO2018085809.
Zhen G, Jicheng Y (2018c) Glucose-responsive insulin delivery system using hyoxia-sensitive nanocomposites. US20180110841.
Zhen G, Jicheng Y (2018e) Glucose-responsive insulin delivery system using hyoxia-sensitive nanocomposites. PH1/2017/501910.
Zhen G, Jicheng Y (2018f) Glucose-responsive insulin delivery system using hyoxia-sensitive nanocomposites. IN201727037788.
Zhen G, Jicheng Y (2018g) Glucose-responsive insulin delivery system using hyoxia-sensitive nanocomposites. CN107530296.
Zhen G, Jicheng Y (2019) Glucose-responsive insulin delivery system using hyoxia-sensitive nanocomposites. TNP/2017/000439.
Zhen G, Jicheng Y (2020) Patch loaded with dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. US20200330562.
Zhu M, Liu Y, Jiang F, Cao J, Kundu SC, Lu S (2020) Combined silk fibroin microneedles for insulin delivery. ACS Biomater Sci Eng 6:3422–3429. https://doi.org/10.1021/acsbiomaterials.0c00273
Zong Q, Guo R, Dong N, Ling G, Zhang P (2021) Design and development of insulin microneedles for diabetes treatment. Drug Deliv Transl Res. https://doi.org/10.1007/s13346-021-00981-y
Acknowledgements
The authors would like to thank Chitkara College of Pharmacy, Chitkara University, Punjab, India, for providing facilities for completion of this review.
Author contribution.
IZ, SS, and TB: conceived the study and wrote the first draft of the paper; NS, TN, VS, and SF: data compilation; IZ, NKF, and SB: figure work; AAH, SNW, and CVDLA: editing; LA and SBU: proofread.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
All the authors have approved the manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zahoor, I., Singh, S., Behl, T. et al. Emergence of microneedles as a potential therapeutics in diabetes mellitus. Environ Sci Pollut Res 29, 3302–3322 (2022). https://doi.org/10.1007/s11356-021-17346-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17346-0