Abstract
Use of microneedles as drug delivery systems provides a number of benefits to patients. Easier and simple drug use, reduced side effects, and incontinence are some of them. Microstock patch has been developing for the last few decades and they represent minimally invasive way for administration drugs in organism through the skin (transdermal administration). One of the approaches ‘‘poke with patch’’ uses microneedles to make holes (Hollow microneedles) and then apply a transdermal (TD) patch to the skin surface. TD drug delivery can improve compliance, minimize peaks, provide continuous drug administration and troughs in plasma levels during the day. These systems can take the place of more risky and invasive injection-based drug delivery (such as insulin injection). Objective of this paper is to research and present the application of hollow microneedles in treating diabetes and to compare it with conventional application of the drug. The drugs that we processed are metformin, insulin and exendin. Metformin is effective for treating diabetic patients therapeutically and preventively, maximizing the use of the current diabetes patch device. The main lack of conventional application of insulin is the fact that the application itself isn’t optimal for a patient. TDl showed significant improvement at level of absorption and bioavailability of the drug. New findings indicate that Exendin-4 diabetes microneedles can potentially replace the currently used subcutaneous (SC) injections because they can effectively reduce blood-glucose levels in patients with type 2 diabetes and are convenient to use.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Diabetes is a chronic disease characterized by low pancreas ability to produce enough insulin (a hormone regulating blood sugar or glucose) or when the body can not effectively use the insulin it produces. According to the World Health Organization report, the global prevalence of diabetes has almost doubled since 1980, with an increase of 4.7–8.5% in the adult population [1].
Ogurtsova et al. in one global study included data sources from 111 countries and results showed that there were 415 million people with diabetes aged 20–79 years. That number was predicted to rise to 642 million by 2040 [2].
Oral ingestion and hypodermic injections as the most common forms of drug administration possess several limitations (e.g. pain, absorption and metabolism issues and side effects).
Microneedles can enable collection of the same information or drug delivery with less trauma to the tissue (or even eliminating it). Microneedles represent a unique technological approach to enhance drug permeation across the stratum corneum [3].
Microneedle technologies have been subject to intensive research and development efforts. The number of publications describing microneedles as minimal invasive devices for DD has grown exponentially [4].
In form of the patent, the first concept to make micropores in the skin came by Gerstel and Place from Alza Research in the early 1970s. It took about 25 years for Microchip fabrication technology to converge with newer ways to make the possibilities for the mass production of microneedle arrays. With the onset of new microfabrication techniques available in the microchip industry, came various three-dimensional designs with greater aspect ratios of solid and hollow microneedles [5].
Current applications of microneedles include the delivery of macromolecules such as vaccines, proteins, and peptides including insulin for diabetics [6].
2 Transdermal Application: Hollow Microneedles
Transdermal drug delivery (TDD) has proven to be of great therapeutic use. It can improve compliance and provide continuous drug administration. This system can take place of more risky and invasive injection-based DD (such as insulin injection) [7].
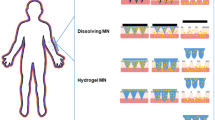
Microneedles represent a unique technological approach to enhance drug permeation across the stratum corneum. There are specific strategies and designs of microneedles for TDD. One of the approaches ‘‘poke with patch’’ uses microneedles to make holes (Hollow microneedles) and then apply a transdermal patch to the skin surface. Hollow microneedles are traditionally used to allow liquid formulations through the SC and act like micron scale syringes. Compared to solid, the hollow microneedles have an added advantage as they can permit the administration of a larger drug dose [4].
They facilitate active fluid flow through the needle bore and into the skin. This can lead to much faster rates of delivery that can be modulated over time. These microneedles offer the possibility of transporting drugs for more rapid rates of delivery by pressure-driven flow or diffusion. There are only a few hollow microneedles that have been fabricated and limited work has been published on their possible use to deliver compounds into the skin [8].
Microneedles technologies have been subject to intensive research and development efforts. Their usage improves the surface contact with the skin and facilitates penetration of therapeutic molecules into the skin. There are many advantages of microneedle technologies, such as pain-free delivery; minimal introduction of pathogens through microneedle-induced holes; they do not cause bleeding; eliminate transdermal dosing variability of small molecules; potential for self-administration; the potential to overcome and reduce instances of accidental needle-stick injuries and the risk of transmitting infections. Microneedles are easy and safe to use and they can be produced with high precision, accuracy, and low cost. They also avoid the first-pass effect. Some studies, in addition to the ease of microneedles waste disposal, have combined the microneedle systems with a pump or pressurized gas.
Microneedles sharp tips are short enough to limit contact with skin nerves and in that way prevent pain sensation. Because they are narrow enough, microneedles do not induce minimal trauma and also reduce the opportunities for infections to develop the following insertion. This method is minimizing disadvantages of conventional injection needles and transdermal patches. Microneedles can be manufactured using polymers (poly-L-lactic acid, polyglycolic acid, polycarbonate, ect.), metal or silicon. Biocompatible and biodegradable polymers can be safely applied to the skin and are generally cost-effective. Microneedles can deliver a wide range of drugs ranging from small molecular weight (e.g. ibuprofen) to high molecular weight (e.g. ovalbumin compounds).
A number of challenges with microneedles include scaleup manufacture to industrial levels which will require considerable planning and standardization. The issues which should be considered are issues surrounding product sterility; the potential for accidental reuse of certain microneedle modalities; appropriate packaging and manufacturing aspects and the potential for undesired immunological effects. The choice of appropriate biomaterials for preparation of microneedle is limited due to lack of mechanical strength, poor control of DD, and limitation of drug loading dose [4].
Microneedles can only be inserted into the skin if they have the correct shape and adequate physical properties. They need to be applied with the required force to avoid breaking or bending before insertion, because some fragments can be left in the skin. Microneedles can cause skin irritation and in some cases allergy [9].
3 Transdermal Delivery of Metformin
Metformin is used for treating and protecting people at a prediabetes stage as one of the most effective drugs. Commonly administered orally, microneedles loaded with metfromin distribute it directly into the metabolic circulation. This prevents some of the complications with the slow absorption in the digestive system [10].
Metformin that is directly introduced through the skin reaches metabolic circulation more than when it is metabolized through digestion. TDD systems require more studies on human patients in the future [11].
The hydrophobic layer prevents moisture from damaging the microneedles after insertion into the skin, as well as preventing premature release of metformin. The heater embedded in the patch triggers when it detects elevated glucose levels, warming the microneedles then the transitional temperature of tridecanoic acid of ~41 °C is exceeded, exposing the underlying polymer to interstitial water thus releasing the drug. Adjusting the quantity of metformin being liberated in this way can be done stepwise in a titratable fashion by adjusting the number of microneedles in the device. Experiments on genetically diabetic mice demonstrate without a doubt the drug release on thermal activation of dissoluting polymers results in substantial lowering of blood sugar levels in the test subjects over the next few hours.
The present design makes it unlikely to deliver the daily adult dose of metformin without the use of an unacceptably large patch and/or unfeasibly large amounts of microneedles. An essential criterion as with all TDD applications, passive or facilitated in some way, is that the potency of the drug is high and the daily dosage no more than a few milligrams [12].
Hydrogel-forming microneedles swell in the skin allowing diffusion of metformin contained in a reservoir layer. Containing no drug themselves this process enables microneedles to deliver the drug to the dermal microcirculation. Migdadi et al. study showed that hydrogel-forming microneedles could find use to enhance TDD of a great range of therapeutic substances. This could be a technology that could be used for TDD of many drugs with high oral doses.
In Table 1 is shown a comparison of plasma profiles (μg/mL/h) after oral administration of metformin Cl to TD microneedles administration at different periods on the rat model. Laboratory rats of an average weight of 224.5 g (±16.57 g) were administered with 100 g of metformin HCL transdermally.
Plasma concentrations were lower after TDD in comparison to oral administration of metformin HCl. Metformin HCl in the rat model yielded targeted plasma concentration reaching human therapeutic concentration after being delivered [13].
The relative TD bioavailability of metformin HCl when using hydrogel-forming microneedles was estimated as 0.6 of its oral bioavailability. The TD bioavailability of metformin HCl when using hydrogel-forming microneedles was estimated as 0.3 meaning that 30% of the drug loading would be delivered within 24 h. The dose of metformin HCl used in this study is much lower than the oral human dose. Steady-state concentration was achieved in the plasma profile of metformin HCl within 24 h. Results of the in vivo experiment using hydrogel-forming microneedles arrays suggest potentially successful TDD of metformin HCl [13].
4 Transdermal Delivery of Insulin
Insulin is widely used to control blood glucose levels in people with diabetes since its extraction and identification in 1921 by Banting and associates. From the moment of insulin discovery, so far it has been continuously working on the improvement of insulin production, purification, the creation of new pharmaceutical formulations and methods of delivery, with a view to the patient’s compliance [14].
Non-invasive insulin delivery systems have the potential to overcome the most important problems with regard to the effective treatment of diabetic patients, replacing traditional treatment. Pain in the application site, needle phobia, and therapeutic compliance [15]. It is also a worrying statistic that nearly 65% of patients with type 1 or 2 diabetes are not sure of their ability to administer insulin on their own [16]. In order to overcome this disadvantage, noninvasive routes such as oral, buccal, pulmonary, nasal, and TD are suggested. TDD is an attractive alternative to the SC delivery route for different drugs and vaccines but is usually limited by an extremely low throughput of the outer layer of the skin, the stratum corneum (SC) of 10–15 m. The Microneedle Field (MNA) has shown tremendous potential to effectively deliver the drugs intradermally and transdermally, especially when relatively small drug volume is required [17, 18].
In the past decade, many studies have been conducted aimed at assessing pharmacokinetic and pharmacodynamic variability in insulin behavior in relation to the use of various insulin preparations [19,20,21,22,23,24,25,26,27]. Rigorously controlled conditions, identical doses of the same preparations may have been shown to result in different pharmacokinetic and pharmacodynamic responses in the same patients [28].
Gupta et al. performed the first reported clinical study that was based on comparing TD drug use and use of microsystems with SC drug administration. The results of this study showed significant differences in insulin absorption, with hollow micro-administration being superior. The results also suggest that the use of insulin by microneedles can increase patient compliance, especially in children and adolescents who often miss insulin injections due to fear, pain, anxiety, and discomfort associated with SC needles and catheters. It has been concluded that the TD route of use is much more successful than the traditional way of applying insulin, as increased patient compliance and improved health outcomes as well as pharmacokinetics itself [28].
Kochba et al. performed a clinical study in 14 patients, this study shows a superior pharmacological profile of the hollow microneedles compared to the SC injection of the same insulin using a conventional needle. Insulin delivery showed a shorter absorption time, worsened exposure and reduced interpatient variability in time absorption. In addition, the time to reach maximum concentrations, by 50%, is considerably shorter after using hollow microneedles compared to SC injection. Finally, late AUC levels of glucose were higher in TD applications, which potentially limited the late hypoglycemic event [29].
Review of insulin delivery systems published in 2018, reports improvement in developing glucose responsive systems to constitute smart insulin patch. Creating forms based on a microgel, micelles and microcapsules with core-shell structures, which are thermo- and hypoxia-sensitive, possibly could be borrowed to microneedles system for accurate insulin delivery. It’s believed that those forms act like glucose sensitive, so it’s suspected that they could imitate the function of β-cell [30].
5 Transdermal Delivery of Exendin
Exendin-4 is the first glucagon-like peptide-1 (GLP-1) receptor agonist to be approved for therapeutic use in humans. This peptide has 39 amino acids originally isolated from the saliva of the Gila monster. It shares approximately 53% sequence homology with the mammalian gut hormone, GLP-1 [31]. Due to changes in the amino acid sequence, exendin-4 is resistant to degradation against the enzyme dipeptidyl peptidase-4 (DPP-4) and has a longer half-life than native GLP-1 [32]. As a GLP-1 receptor agonist, exendin-4 shows numerous anti-diabetic actions, including glucose-dependent simulation of insulin secretion, suppression of glucagon secretion, reduction of motility and food intake and improvement in pancreatic endocrine function [33].
Ex-4 is generally administered by SC injection on a daily basis, which may result in pain, needle phobia, infection, and inconvenience to the patient. Daily injections create a large amount of needle waste, which may result in needle-stick injury, blood-borne virus transmission, and needle recycling costs [34].
Microneedles fabricated from hyaluronic acid (HA) were developed, evaluated their characteristics and assessed the improvement on TDD of relatively high molecular weight drugs. The novel microneedles fabricated from hyaluronic acid is found to have several advantages over previous microneedles. Firstly, as a major component of skin, hyaluronic acid reasonably expected to overcome the safety issues when applied to silicon and metal microneedles. Moreover, its high water-soluble characteristic makes it easy and suitable for mass production, in contrast to other published methods that require more complex multistep fabrication schemes.
The exendin-4 tip-loaded microneedles provided rapid exendin-4 administration. They showed comparable acute effects on glucose tolerance, insulin secretion, and plasma concentration profiles, as compared with subcutaneous injection, in type 2 diabetic GK/Slc rats. To sum up, HA microneedles are a useful alternative method to improve the TDD, especially drug with relatively high molecular weight without seriously damaging the skin. They might be effective and safe dosage form for transdermal delivery in the clinical setting.
In conclusion, these findings indicate that the novel soluble microneedles fabricated with HA were very useful alternative method to DD from the skin to the systemic circulation without serious skin damage. Therefore, the HA microneedles might be effective and safe dosage form for TDD of insulin and exendin-4 in clinical applications for the treatment of diabetes [35].
In a study conducted by Lahiji, Jang et al. they used the most recent technique for dissolving microneedles fabrication, in which the activity of encapsulated compounds is highly conserved during the fabrication process. They analyzed the thermal, chemical, and physical factors that can affect the activity of Ex-4 dissolving microneedles. They optimized the fabrication conditions for Ex-4 dissolving microneedles for fabrication of Ex-4 dissolving microneedles. The in vivo delivery comparison of SC injection and Ex-4 dissolving microneedles suggested that they possess efficiency similar to that of SC in reducing the levels of blood glucose. In conclusion, they have successfully developed and fabricated Ex-4 dissolving microneedles by optimizing the thermal, chemical, and physical factors involved in their fabrication; these factors (temperature during fabrication, pH, and concentration of the polymer) were highly instrumental in maintaining the activity of encapsulated Ex-4. Their findings indicate that Ex-4 dissolving microneedles can potentially replace the currently used SC injections because they can effectively reduce blood-glucose levels in patients with type 2 diabetes and are convenient to use [36].
6 Conclusion
The use of microneedles for TDD is still a relatively new concept since it’s only been in use since the 1990s. So far this method of delivery has been studied in drugs that otherwise have a significant risk of adverse reactions by their usual methods. Since fluctuation of blood glucose can have dangerous effects and controlling the doses of medicines that regulate glucose levels can be tricky, antidiabetic medication has been the subject of many such studies. Studies in the early 2000s on diabetic rats showed promise for an applicable microchip patch for insulin delivery. More recent studies have confirmed this and have shown that the main benefit is the faster onset of the insulin’s effect. It is also possible to formulate hydrogel-forming microneedles for the delivery of metformin. The dose required to get the same blood concentrations as with the oral application is significantly lower. Tests for metformin delivery have been promising in rats and show a potential for a future micro patch formulation that could maintain constant therapeutic doses for 24 h. Recent studies have also shown that exendin-4 can also be delivered transdermally using microneedles fabricated from hyaluronic acid. Studies show that this delivery method could be just as effective and less costly than the conventional subcutaneous injections. Overall, the method of using microneedles for TDD of antidiabetic medication has been shown to have numerous advantages for patients. The greatest benefits are realized where subcutaneous injections could be replaced. Although some further research is needed to determine the conditions under which some of the medications could best be used in humans, this method of drug distribution is promising to significantly reduce medical costs and the incidence of adverse effects in patients.
Reference
Global report on diabetes [Internet]: World Health Organization. Available from http://www.who.int/diabetes/global-report/en/. 7 Nov 2018
Ogurtsova, K., da Rocha Fernandes, J., Huang, Y., Linnenkamp, U., Guariguata, L., Cho, N., Cavan, D., Shaw, J., Makaroff, L.: IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128, 40–50 (2017)
Khanna, P., Strom, J.A., Malone, J.I., Bhansali, S.: Microneedle-based automated therapy for diabetes mellitus. J. Diabetes Sci. Technol. 2(6), 1122–1129 (2008)
Alkilani, A., McCrudden, M., Donnelly, R.: Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 7(4), 438–470 (2015)
Cleary, G.W.: Microneedles for drug delivery. Pharm. Res. 28(1), 1–6 (2011)
Cheung, K., Das, D.: Microneedles for drug delivery: trends and progress. Drug Deliv. 23(7), 2338–2354 (2014)
Wermeling, D., Banks, S., Hudson, D., Gill, H., Gupta, J., Prausnitz, M., Stinchcomb, A.: Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc. Natl. Acad. Sci. 105(6), 2058–2063 (2008)
Prausnitz, M.: Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 56(5), 581587 (2004)
Serrano-Castañeda, P., Escobar-Chavez, J., Rodriguez-cruz, I., Melgoza, L., Martinez-Hernandez, J.: Microneedles as enhancer of drug absorption through the skin and applications in medicine and cosmetology. J. Pharm. Pharm. Sci. 21(1), 73 (2018)
Yu, W., Jiang, G., Zhang, Y., Liu, D., Xu, B., Zhou, J.: Nearinfrared light triggered and separable microneedles for transdermal delivery of metformin in diabetic rats. J. Mater. Chem. B 5, 9507–9513 (2017)
Lee, H., Choi, T., Lee, Y., Cho, H., Ghaffari, R., Wang, L., Choi, H., Chung, T., Lu, N., Hyeon, T., Choi, S., Kim, D.: A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016)
Guy, R.: Managing diabetes through the skin. Nat. Nanotechnol. 11, 493–494 (2016)
Migdadi, E.M., Courtenay, A.J., Tekko, I.A., McCrudden, M.T.C., Kearney, M.C., McAlister, E., McCarthy, H.O., Donnelly, R.F.: Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Control. Release 285, 142–151 (2018)
Roxhed, N., Samel, B., Nordquist, L., Griss, P., Stemme, G.: Painless drug delivery through microneedle-based transdermal patches featuring active infusion. IEEE Trans. Biomed. Eng. 55, 1063–1071 (2008)
Pearson, T.: Practical aspects of insulin pen devices. J. Diabetes Sci. Technol. 4, 522–531 (2010)
Ross, S., Scoutaris, N., Lamprou, D., Mallinson, D., Douroumis, D.: Inkjet printing of insulin microneedles for transdermal delivery. Drug Deliv. Transl. Res. 5, 451–461 (2015)
Sousa, F., Castro, P., Fonte, P., Sarmento, B.: How to overcome the limitations of current insulin administration with new noninvasive delivery systems. Ther. Deliv. 6, 8394 (2015)
Tucak, A.: Mikroiglama posredovana dostava lijekova kroz kožu- formulacijski aspekti [Završni rad integriranog studija prvog i drugog ciklusa]. Univerzitet u Sarajevu (2017)
Resnik, D., Možek, M., Pečar, B., Janež, A., Urbančič, V., Iliescu, C., Vrtačnik, D.: In vivo experimental study of noninvasive insulin microinjection through hollow si microneedle array. Micromachines 9, 40 (2018)
Zhou, C., Liu, Y., Wang, H., Zhang, P., Zhang, J.: Transdermal delivery of insulin using microneedle rollers in vivo. Int. J. Pharm. 392, 127133 (2010)
Norman, J., Brown, M., Raviele, N., Prausnitz, M., Felner, E.: Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes. Pediatr. Diabetes 14, 459–465 (2013)
Ito, Y., Nakahigashi, T., Yoshimoto, N., Ueda, Y., Hamasaki, N., Takada, K.: Transdermal insulin application system with dissolving microneedles. Diabetes Technol. Ther. 14, 891–899 (2012)
Gill, H., Denson, D., Burris, A., Prausnitz, M.: Effect of microneedle design on pain in human volunteers. Clin. J. Pain 24, 585–594 (2008)
Pettis, R., Harvey, A.: Microneedle delivery: clinical studies and emerging medical applications. Ther. Delivery 3, 357–371 (2012)
Jung, H.: Microneedle: the future of pharmaceutical and cosmeceutical delivery systems. J. Phar. Drug Deliv. Res. 06 (2017)
Lee, J., Prausnitz, M.: Drug delivery using microneedle patches: not just for skin. Expert Opin. Drug Deliv. 15, 541–543 (2018)
Ma, G., Wu, C.: Microneedle, bio-microneedle and bioinspired microneedle: a review. J. Control. Release 251, 11–23 (2017)
Gupta, J., Felner, E., Prausnitz, M.: Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol. Ther. 13, 451–456 (2011)
Kochba, E., Levin, Y., Raz, I., Cahn, A.: Improved insulin pharmacokinetics using a novel microneedle device for intradermal delivery in patients with type 2 diabetes. Diabetes Technol. Ther. 18, 525–531 (2016)
Jin, X., Zhu, D., Chen, B., Ashfaq, M., Guo, X.: Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 127, 119–137 (2018)
Doyle, M.E., Egan, J.M.: Mechanisms of action of glucagonlike peptide 1 in the pancreas. Pharmacol. Ther. 113, 546–593 (2007)
Gallwitz, B.: New therapeutic strategies for the treatment of type 2 diabetes mellitus based on incretins. Rev. Diabetic Stud. 2, 61–69 (2005)
Kolterman, O.G., Buse, B., Fineman, M.S., Gaines, E., Heintz, S., Bicsak, T.A., Taylor, K., Kim, D., Aispoma, M., Wang, Y., Baron, A.D.: Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J. Clin. Endocrinol. Metab. 88, 3082–3089 (2013)
Li, J., Zeng, M., Shan, H., Tong, C.: Microneedle patches as drugs and vaccine delivery platform. Curr. Med. Chem. 24, 2413–2422 (2017)
Liu, S.: The development of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of diabetes drugs. Mol. Pharm. 13, 272–279 (2016)
Lahiji, S., Jang, Y., Huh, I., Yang, H., Jang, M., Jung, H.: Exendin-4–encapsulated dissolving microneedle arrays for efficient treatment of type 2 diabetes. Sci. Rep. 8 (2018)
Conflict of Interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Sušić, A. et al. (2020). Use of Hollow Microneedle Drug Delivery Systems in Treatment of Diabetes Mellitus. In: Badnjevic, A., Škrbić, R., Gurbeta Pokvić, L. (eds) CMBEBIH 2019. CMBEBIH 2019. IFMBE Proceedings, vol 73. Springer, Cham. https://doi.org/10.1007/978-3-030-17971-7_87
Download citation
DOI: https://doi.org/10.1007/978-3-030-17971-7_87
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-17970-0
Online ISBN: 978-3-030-17971-7
eBook Packages: EngineeringEngineering (R0)