Abstract
In excess, iron can induce the production and accumulation of reactive oxygen species (ROS), causing oxidative stress. The objective of this work was to evaluate the impact of toxic concentrations of iron (Fe) on the antioxidative metabolism of young Eugenia uniflora plants. Forty-five-day-old plants grown in Hoagland nutrient solution, pH 5.0, were treated with three Fe concentrations, in the form of FeEDTA, during three periods of time. At the end of the treatment, the plants were harvested and relative growth rate, iron content, lipid peroxidation and enzymes and metabolites of the antioxidative metabolism were determined. Iron-treated plants showed higher iron contents, reduced relative growth rates and iron toxicity symptoms in both leaves and roots. There was an increase in lipid peroxidation with increasing Fe, only in the leaves. The enzymatic activities of superoxide dismutase (SOD) and glutathione reductase (GR) increased with increasing Fe concentration and treatment exposure time. The activities of catalase (CAT), peroxidase (POX) and ascorbate peroxidase (APX) also increased with increasing Fe concentration but decreased with increasing treatment exposure time. Glutathione peroxidase activity (GPX) decreased with increasing Fe concentration and exposure time. The ascorbate (AA) and reduced glutathione (GSH) contents and the AA/DHA and GSH/GSSG ratios, in general, increased with increasing Fe concentration and treatment exposure time. The results indicate that under toxic levels of Fe, young E. uniflora plants suffer increased oxidative stress, which is ameliorated through changes in the activities of antioxidative enzymes and in the contents of the antioxidants AA and GSH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron is an essential micronutrient for plants that is involved in several fundamental processes, such as photosynthesis, respiration, nitrogen fixation and DNA and hormone synthesis (Briat and Lobréaux 1997; Becana et al. 1998; Schmidt 2003). Iron is also a major constituent of important proteins (ferredoxin and cytochromes) and antioxidative enzymes (catalase, peroxidase and superoxide dismutase). However, when absorbed in excess, this metal can displace the cell redox balance toward a pro-oxidant state, causing alterations in the morphologic, biochemical and physiological characteristics of the plants, generating oxidative stress (Briat and Lebrun 1999; Hell and Stephan 2003). The reactive oxygen species (ROS) produced might cause lipid peroxidation, protein oxidation, damage to nucleic acids, destruction of chloroplast pigments and/or other cell damages, leading to a programmed cell death (Moller et al. 2007).
To tolerate oxidative stress, plant cells have evolved an elaborate system of enzymatic and non-enzymatic antioxidants, which scavenge accumulated ROS (Becana et al. 1998; Briat and Lebrun 1999). The enzymatic system comprises enzymes, such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX), glutathione peroxidase (GPX) and glutathione reductase (GR), that act alone or in concert to eliminate accumulated ROS (Becana et al. 1998; Fang et al. 2001). The non-enzymatic defense system consists of low molecular weight metabolites, such as ascorbate (AA), glutathione (GSH), carotenoids, α-tocopherol, ubiquinol, uric acid and lipoic acid that act directly to eliminate free radicals from cell metabolism (Becana et al. 1998; Noctor and Foyer 1998; Mittler 2002) or serve as electron donors to enzymes of the ascorbate/glutathione cycle (Noctor and Foyer 1998).
Although most soils are rich in iron, the expression “iron toxicity” is typically associated with plant cultivation in flooded soils with high ferrous iron levels, particularly in lowland-rice production (Mehraban et al. 2008) or tea plantations in India (Hemalatha and Venkatesan 2011). Preliminary studies show that the native species Eugenia uniflora in a Restinga habitat near Guarapari, ES, Brazil, is affected by particulate and gaseous emissions from a nearby iron ore processing industry (Neves et al. 2009). The application of iron ore dust as iron solid particulate matter and simulated acid rain (pH 3.1) on E. uniflora plants results in high iron content in the leaves and the appearance of typical symptoms of iron toxicity. These plants also exhibit impaired photosynthesis and symptoms of oxidative stress (Neves et al. 2009).
Iron toxicity also potentially impacts vegetation in the “Iron Quadrangle” in Minas Gerais, Brazil, which is one of the most important iron mining regions in the world. Mining, iron ore processing and beneficiation factories in this region are producing and discharging iron residues and/or emitting iron particulate matter, contaminating water, soil and air. The plants growing in this region are certainly exposed to excess iron, which could change their metabolism, growth and development, affecting their occurrence in the vegetation, or even leading to their disappearance (Kuki et al. 2008).
E. uniflora is a shrub or small tree species native from Guyana, Surinam and French Guiana to southern Brazil, and to northern, eastern and central Uruguay. Recent studies have shown the combined effects of flooding and light intensity (Mielke et al. 2010; Mielke and Schaffer 2011) and exposure to simulated acid rain and iron ore dust deposition on these plants. However, there are few reports concerning the physiology of this species under iron toxicity conditions.
Therefore, the aim of this work is to evaluate the impact of toxic levels of iron on the oxidative metabolism in young E. uniflora plants.
Materials and methods
Seeds of Eugenia uniflora L. (Myrtaceae) were collected in Viçosa county, Minas Gerais, Brazil, from a single plant grown in soil that was not contaminated with iron. The seeds were selected for uniformity in size and form, surface sterilized with 3 % (v/v) sodium hypochlorite for 15 min, treated with a fungicide solution (0.2 % w/v Benlate) and germinated in paper towels submerged in a 0.5 mM CaCl2 solution under continuous aeration and a controlled temperature of 25 ± 1 °C.
Forty-five-day-old E. uniflora seedlings were selected for uniformity in size and form and transferred to polyethylene pots containing 1.6 L of Hoagland’s nutrient solution, pH 5.0, at half ionic strength (Hoagland and Arnon 1938). After an adaptation period of 7 days, under the controlled conditions of the growth room (room temperature: 25 ± 1 °C, irradiance: 230 μmol m−2 s−1 and photoperiod: 16 h), the plants were subjected to treatments with a factorial combination of three concentrations of 0.045 (control), 1.0 and 2.0 mM Fe in the form of FeEDTA. The plants were evaluated at 15, 30 and 45 days after the application of the treatments. The nutrient solution was continually aerated, and the pH was adjusted to 5.0 with HCl or NaOH daily. The nutrient solution was renewed every 7 days.
Plants taken at the begin of the experiment and harvested at the end of each treatment period were washed in running water and rinsed in deionized water, dried at 80 °C and the dry weight were determined for the relative growth rate (RGR) estimation. Samples from the roots and leaves were collected, frozen in liquid nitrogen and stored in an ultra-freezer at −80 °C until further analysis.
Determination of iron content
Oven-dried (80 °C) plant materials, which were finely ground in a stainless steel electric grinder, were digested in a nitric-perchloric acid mixture (2:1) and iron content in the extracts were determined by atomic absorption spectrophotometry (Shimadzu, model AA6701FG).
Determination of lipid peroxidation
The lipid peroxidation in the roots and leaves was estimated as the concentration of malondialdehyde (MDA) after a reaction with thiobarbituric acid (TBA) according to Cakmak and Horst (1991). The tissue was macerated in liquid nitrogen, homogenized in 0.1 % (w/v) trichloroacetic acid (TCA) and centrifuged at 12,000g for 15 min at 4 °C. Aliquots of the supernatant were added to 1.5 mL of 0.5 % (w/v) thiobarbituric acid (TBA) in 20 % TCA and incubated in a shaking water bath at 95 °C. The reaction was terminated after 30 min, and the tubes were centrifuged at 10,000g for 10 min. The absorbance of the supernatant was measured at 532 nm. The non-specific absorption at 600 nm was subtracted. The amount of MDA was calculated using a molar extinction coefficient of 155 mM−1 cm−1 and expressed as μmol (MDA) g−1 dry weight (Heath and Packer 1968).
Determination of the activities of antioxidative enzymes
To assess enzymatic activities, leaf and root fresh weight samples were ground in liquid nitrogen and homogenized in the corresponding buffer: (a) Superoxide dismutase (SOD, EC 1.15.1.1), peroxidase (POX, EC 1.11.1.7) and catalase (CAT, EC 1.11.1.6): 0.1 M potassium phosphate buffer (pH 6.8), 0.1 mM ethylenediamine tetraacetic acid (EDTA), 1 mM phenylmethanesulfonyl fluoride (PMSF) and 1 % (w/v) polyvinylpolypyrrolidone (PVPP) (Peixoto et al. 1999); (b) ascorbate peroxidase (APX, EC 1.11.1.11): 50 mM potassium phosphate buffer (pH 7.0), 1 mM EDTA, 1 mM ascorbic acid, 1 mM PMSF, 2 mM dithiothreitol (DTT) and 1 % (w/v) PVPP (Peixoto et al. 1999); (c) glutathione peroxidase (GPX, EC 1.11.1.9): 0.1 M Tris–HCl buffer, pH 7.5, 1 mM EDTA and 10 mM MgCl2 (Nagalakshmi and Prasad 2001); (d) glutathione reductase (GR, EC 1.6.4.2): 0.1 M potassium phosphate buffer (pH 7.0), 1 mM EDTA, 0.02 % (w/v) Triton X-100, 2 mM DTT, 1 mM PMSF and 1 % (w/v) PVPP (Carlberg and Mannervik 1985). In all cases, after filtration through four layers of cheesecloth, the homogenates were centrifuged at 12,000g for 15 min at 4 °C, and the supernatant was used as the source of crude enzyme.
The enzymatic activities were determined by adding 0.1 mL of the crude enzyme extract to the corresponding reaction mixture: (a) POX: 2.9 mL of 0.1 M potassium phosphate buffer (pH 6.8) containing 20 mM pyrogallol and 20 mM H2O2; (b) CAT: 2.9 mL of 50 mM potassium phosphate buffer (pH 7.0) containing 12.5 mM H2O2; (c) APX: 2.9 mL of 50 mM potassium phosphate buffer (pH 6.0) containing 0.8 mM ascorbic acid and 1 mM H2O2 (Cakmak and Horst 1991); (d) GPX: 0.9 mL of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA, 0.1 M NaCl, 1 mM GSH, 0.2 mM NADPH, 0.25 mM H2O2 and one unit of glutathione reductase (Nagalakshmi and Prasad 2001); (e) GR: 0.9 mL of 50 mM Tris–HCl buffer (pH 7.5) containing 1 mM oxidized glutathione (GSSG) and 0.1 mM NADPH (Carlberg and Mannervik 1985). In all cases, the mixtures were incubated at 30 °C, and the absorbances were measured during the first minute of the reaction. The enzymatic activities were estimated using the corresponding molar extinction coefficients: POX (420 nm; ε: 2.47 mM−1 cm−1); CAT (240 nm, ε: 36 M−1 cm−1); APX (290 nm, ε: 2.8 mM−1 cm−1); GPX (340 nm, ε: 9.6 mM−1 cm−1; and GR (340 nm, ε: 6.22 mM−1 cm−1).
The activity of SOD was determined by adding the root or leaf crude enzymatic extract to a reaction mixture of 50 mM potassium phosphate buffer (pH 7.8) containing 13 mM methionine, 0.1 mM EDTA, 75 μM nitroblue tetrazolium (NBT) and 2 μM riboflavin. The reaction was performed in a chamber with a 15-W fluorescent lamp at 25 °C. After 5 min of illumination, the blue formazan was measured at 560 nm (Giannopolitis and Ries 1977). All rates were corrected for non-enzymatic activity. One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition in the rate of NBT reduction.
Protein concentrations in the enzyme extracts were determined using Bradford method (Bradford 1976), and BSA was used as a standard.
Determination of antioxidative metabolite contents
To determine the ascorbate (AA) and dehydroascorbate (DHA) contents, leaf and root samples were ground in liquid nitrogen, homogenized in 2 mL of 6 % TCA (w/v), filtered and centrifuged at 15,000g for 5 min at 4 °C (Kampfenkel et al. 1995). The total ascorbate content (AA + DHA) was determined in aliquots of extracts treated with 0.5 mM DTT and 0.02 M sodium phosphate buffer, pH 7.4, at 42 °C for 15 min. Subsequently, 0.025 % N-ethylmaleimide, 2.5 % TCA, 8.4 % H3PO4, 0.8 % 2,2′-dipyridyl, and 0.3 % FeCl3 were added, and the extracts were incubated again at 42 °C for 40 min. After terminating the reaction on ice, the absorbance was measured at 525 nm. The AA content was determined as described above, omitting DTT and N-ethylmaleimide. The DHA content was calculated as the difference between the total ascorbate content (AA + DHA) and the reduced form content (AA).
To determine reduced (GSH) and oxidized (GSSG) glutathione contents, leaf and root samples were ground in liquid nitrogen and homogenized in 2 mL of 0.1 M HCl containing 1 mM EDTA. After filtration through four layers of cheesecloth, the homogenates were centrifuged at 12,000g, for 10 min at 4 °C. A 150 μL sample was used for the determination of total glutathione (GSH + GSSG) content. A volume of 7.5 μL of 2-vinilpiridina (2-VP) was added to an additional 150 μL sample, and the sample was derivatized at 30 °C for 1 h to determine the GSSG content. One hundred microliter aliquots of both samples were treated with 200 μL of 125 mM sodium phosphate buffer, pH 7.5, containing 6.3 mM EDTA, 500 μL of 0.3 mM NADPH, 100 μL of 0.1 M HCl containing 1 mM EDTA and 100 μL of 6 mM DTNB (Griffith 1980). After incubation at 30 °C for 5 min, 10 μL of glutathione reductase (50 U mL−1) was added to the mixture and the increase in absorbance at 412 nm was measured during the first minute of the reaction. The GSH content was calculated as the difference between the total glutathione content and the GSSG content. A standard curve was prepared with authentic standards of GSH and GSSG.
Statistical analysis
The treatments were arranged in a randomized factorial block design with three replicates. The results were subjected to analysis of variance, and the means were compared using the Duncan test at 5 % probability.
Results
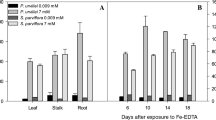
The relative growth rate (RGR) of the leaves and roots decreased with time and with increasing FeEDTA concentration (Fig. 1a, b). Forty-five days after treatment with 2.0 mM FeEDTA there was a reduction in RGR of about 28 % in both leaves and roots relative to control plants. Iron toxicity symptoms, characterized mainly by leaf chlorosis and/or bronzing and root darkening, were clearly visible after 45 days of iron treatment with 1.0 mM and especially with 2.0 mM FeEDTA (Fig. 2).
Relative growth rate (RGR) of leaf (a) and root (b) of Eugenia uniflora after exposure to FeEDTA treatment for different periods of time. Means followed by the same capital letter among days after FeEDTA treatment for each FeEDTA concentration and by the same small letter among FeEDTA concentrations for each exposure time do not differ (P < 5) using Duncan’s test. Bars represent standard deviations
Iron content in the roots was greater than in the leaves and increased with increasing FeEDTA concentration in the nutrient media and exposure time in both plant parts (Fig. 3a, b). The iron content at 45 days after treatment with 2.0 mM FeEDTA was 229.5 and 1,316.7 mg kg−1 dw in the leaf and root, respectively.
Iron content in the leaf (a) and root (b) of Eugenia uniflora after exposure to FeEDTA treatment for different periods of time. Means followed by the same capital letter among days after FeEDTA treatment for each FeEDTA concentration and by the same small letter among FeEDTA concentrations for each exposure time do not differ (P < 5) using Duncan’s test. Bars represent standard deviations
The lipid peroxidation in the leaves was greater than that in the roots and increased with increasing FeEDTA concentrations in the nutrient media and exposure time (Fig. 4a, b). The highest leaf lipid peroxidation activity was observed at 45 days after treatment with 2.0 mM FeEDTA. At this condition, there was an increase of >50 % in the MDA production relative to that observed in the control plants.
Effect of FeEDTA on lipid peroxidation in the leaf (a) and root (b) of Eugenia uniflora after exposure to iron treatment for different periods of time. Means followed by the same capital letter among days after FeEDTA treatment for each FeEDTA concentration and by the same small letter among FeEDTA concentrations for each exposure time do not differ (P < 5) using Duncan’s test. Bars represent standard deviations
In the roots, the lipid peroxidation intensity increased with increasing treatment exposure time, but it was reduced at higher Fe concentrations (Fig. 4b). In the 2.0 mM FeEDTA-treated plants, for example, we observed reductions in root lipid peroxidation of 8 and 32 % after 30 and 45 days of treatment, respectively, compared with their respective controls. At 1.0 mM FeEDTA, the lipid peroxidation intensity decreased only after 45 days of treatment.
In the leaves of young E. uniflora plants, no change in SOD activity was observed with increasing Fe concentration and exposure time to treatment, except at 2.0 mM FeEDTA after 45 days of treatment (Fig. 5a). At this iron concentration, a 30 % increase in SOD activity was observed compared to its respective control. The CAT activity was increased with increased exposure of the plants to high iron concentrations from the 15th to the 45th day of treatment, but no additional effect was observed doubling iron concentration (Fig. 5b). The highest CAT activity was observed at 30 days after iron treatment. The POX activity also increased with iron treatment, and an additional effect was observed after increasing the FeEDTA concentration from 1.0 to 2.0 mM (Fig. 5c). The highest POX activity was observed in plants exposed to 2.0 mM FeEDTA for 15 days. At this concentration, POX activity was approximately 112 % higher than in the control plants. The activity of APX increased with increasing iron concentration, but significant changes were not observed with increased exposure to iron treatment (Fig. 5d). The activity of this enzyme at the highest iron concentration was higher than in control plants and/or plants treated with 1.0 mM FeEDTA. The greatest enzymatic activity was observed after 30 days of treatment with 2.0 mM FeEDTA. In contrast with the other enzymes, the GPX activity decreased with increasing iron concentration, but it was not affected by increased exposure to iron (Fig. 5e). Forty-five days after treatment with 2.0 mM FeEDTA, we observed a 4.3-fold reduction in GPX activity relative to the control plants. The GR activity increased only with 2.0 mM FeEDTA treatment (Fig. 5f), and the highest GR activity was observed on the 30th day after treatment with 2.0 mM FeEDTA, showing an approximately 40 % higher activity than in control plants.
Effect of FeEDTA on the activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX), glutathione peroxidase (GPX) and glutathione reductase (GR) in the leaves of Eugenia uniflora after exposure to iron treatment for different periods of time. Means followed by the same capital letter among days after FeEDTA treatment for each FeEDTA concentration and by the same small letter among different FeEDTA concentrations for each exposure time do not differ (P < 5) using Duncan’s test. Bars represent standard deviations
The SOD activity in the roots increased after treatment with a high iron concentration (Fig. 6a). A significant increase in the SOD activity was detected after the 15th day of treatment and increased with increasing exposure and iron concentration in the nutrient solution. At 45 days after treatment, the SOD activity was 80 and 93 % higher in plants treated with 1.0 and 2.0 mM FeEDTA, respectively, compared with their respective control plants. No additional effect was observed with an iron concentration from 1.0 to 2.0 mM, except after the 30th day of treatment. The CAT activity increased only in plants treated with 2.0 mM FeEDTA (Fig. 6b). At 15 and 30 days after plant treatment, the CAT activity at this iron concentration was approximately 51 and 60 % higher, respectively, than in their controls. No significant change in enzyme activity was observed with increased exposure to iron treatment; however, a slight reduction in activity was observed after 45 days at the highest iron concentration. The POX activity at 2.0 mM FeEDTA did not increase with increasing exposure time, but was almost the same during time course. In the control or 1.0 mM FeEDTA-treated plants, however, there was a decrease in enzyme activity with increasing exposure time (Fig. 6c). At 45 days after treatment, the POX activity in plants treated with 2.0 mM FeEDTA was approximately 115 % higher than in the control plants. The APX activity increased with increasing iron concentration and exposure time (Fig. 6d). At 45 days after iron treatment, the APX activity at 2.0 mM FeEDTA was approximately 1.5 and 2.2-fold higher than at 1.0 mM FeEDTA and in control plants, respectively. The GPX activity was reduced with treatment at 2.0 mM FeEDTA for the duration of the exposure time tested (Fig. 6e). At 1.0 mM FeEDTA, a significant reduction was observed after 15 days of treatment compared with the control. The GR activity increased with increasing iron concentration and treatment time (Fig. 6f). At 45 days after treatment, the GR activity at 1.0 and 2.0 mM FeEDTA was approximately 116 and 143 % higher than that in the control plants, respectively. It is worth to note that the activities of SOD, POX, APX, and GR in the root were usually higher than in the leaves (Figs. 5, 6).
Effect of FeEDTA on the activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX), glutathione peroxidase (GPX) and glutathione reductase (GR) in the roots of Eugenia uniflora after exposure to iron treatment for different periods of time. Means followed by the same capital letter among days after FeEDTA treatment for each FeEDTA concentration and by the same small letter among the FeEDTA concentrations for each exposure time do not differ (P < 5) using Duncan’s test. Bars represent standard deviations
The ascorbate content in the leaves was approximately 2-fold higher than in the roots (Fig. 7a, b). The ascorbate contents in the leaves of treated plants at 1.0 mM FeEDTA were higher than at 2.0 mM FeEDTA and in the control plants until 30 days (Fig. 7a). On the 45th day, we observed an increase in the AA content in 2.0 mM FeEDTA-treated plants. Under this condition, the AA content was 68 % higher than in control or 1.0 mM FeEDTA-treated plants. The AA content increased significantly in the roots in 2.0 mM FeEDTA-treated plants after 15 days of treatment (Fig. 7b). After 30 days, there was a reduction in the AA content relative to control plants, but the differences among the treatments disappeared after 45 days. In general, the DHA content in the leaves was higher after 30 days of treatment, but it decreased with extended periods of treatment (Fig. 7c). Iron treatment increased the DHA content at the 15th day of exposure. Longer exposure times resulted in a reduction in DHA content, particularly with 2.0 mM FeEDTA. In the roots, the DHA content decreased with increasing iron concentration and time of application of the treatment (Fig. 7d). The reduction in DHA content with 2.0 mM FeEDTA after 45 days was approximately 240 % as compared with that in control plants. The AA/DHA ratio in the leaves did not change until 30 days after treatment with iron or with the exposure time (Fig. 7e). However, after 45 days of treatment, the AA/DHA ratio increased in the 2.0 mM FeEDTA-treated plants to 10.8, i.e., 2.9-fold higher than in control plants. In the roots, the AA/DHA ratio increased with iron treatment (Fig. 7f). After 45 days, the AA/DHA ratio in 2.0 mM FeEDTA-treated plants was 5.2, i.e., 3.6-fold higher than in control.
Effect of FeEDTA on the contents of ascorbate (AA) and dehydroascorbate (DHA) and the AA/DHA ratio in the leaves (a, c, e) and roots (b, d, f) of Eugenia uniflora after exposure to iron treatment for different periods of time. Means followed by the same capital letter among days after FeEDTA treatment for each FeEDTA concentration and by the same small letter among the FeEDTA concentrations for each exposure time do not differ (P < 5) using Duncan’s test. Bars represent standard deviations
The GSH contents increased in plants exposed to high levels of iron in both the leaves and the roots (Fig. 8a, b). In the leaves, both 1.0 and 2.0 mM FeEDTA treatments resulted in an increase in the GSH content relative to the control (Fig. 8a). The exposure time did not significantly affect the GSH content; however, a slight reduction in the GSH content was observed in plants treated with 1.0 mM FeEDTA after 45 days. In the roots, the GSH content was also enhanced with increasing iron concentration (Fig. 8b). The GSH content in the control plants continuously decreased with increasing exposure time. At 45 days after treatment, the GSH content in the control plants was 97 % less than that in plants treated with 2.0 mM FeEDTA. The GSSG content in the leaves and roots of plants treated with 1.0 mM FeEDTA was consistently higher than in plants treated with 2.0 mM FeEDTA or in control plants at all evaluation times (Fig. 8c, d). Treatment with 2.0 mM FeEDTA consistently resulted in a reduction of GSSG content relative to treatment with 1.0 mM FeEDTA and control plants. The GSH/GSSG ratio in both plant parts was higher at 2.0 mM FeEDTA, independent of the time of plant exposure to iron treatment (Fig. 8e, f). At 45 days after treatment, the GSH/GSSG ratio in plants exposed to 2.0 mM FeEDTA was approximately 103 and 137 % of the value, respectively, in the leaves and roots of the respective control plants.
FeEDTA effect on the contents of reduced glutathione (GSH) and oxidized glutathione (GSSG) and the GSH/GSSG ratio in the leaves (a, c, e) and roots (b, d, f) of Eugenia uniflora after exposure to iron treatment for different periods of time. Means followed by the same capital letter among days after FeEDTA treatment for each FeEDTA concentration and by the same small letter among the FeEDTA concentrations for each exposure time do not differ (P < 5) using Duncan’s test. Bars represent standard deviations
Discussion
Iron is an essential micronutrient for plants, involved in several fundamental metabolic processes. However, in excess, this element can displace the cell redox balance toward a pro-oxidant state, increasing the production and accumulation of reactive oxygen species (ROS) (Briat and Lebrun 1999; Hell and Stephan 2003), thereby establishing oxidative stress (Moller et al. 2007).
In this study, plants were exposed to 1.0 and 2.0 mM FeEDTA, iron concentrations of 22- and 44-fold the usual iron concentration observed in nutrient solution (0.045 mM). The plants exposed to these high iron concentrations showed reduced relative growth rate and typical foliar and root iron toxicity symptoms, especially at 2.0 mM FeEDTA. Nevertheless, the iron content in the leaves was maintained well below the established critical iron toxicity of 300 mg kg−1 dw (Marschner 1995). The iron concentration in the roots (1,316.7 mg kg−1 dw) was much higher than that in the leaves (229.5 mg kg−1 dw) in plants treated with 2.0 mM after 45 days. Apparently, E. uniflora is more sensitive to iron than the average plants used in previous studies (Marschner 1995).
Lipid peroxidation is an important metal toxicity indicator that is based on the production of malondialdehyde (Azevedo Neto et al. 2006). In this study, the level of lipid peroxidation increased with increasing iron concentration and exposure time to iron treatment, only in the leaves (Fig. 4). This was indicative of the production and accumulation of reactive oxygen species (ROS) induced by toxic concentrations of iron in this part of the plant. Enhanced lipid peroxidation under iron toxicity has been reported for different species (Sinhá et al. 1997; Souza-Santos et al. 2001; Sinhá and Saxena 2006; Kuki et al. 2008; Kumar et al. 2008; Stein et al. 2008; Xing et al. 2010) and has been associated with oxidative stress caused by this metal toxicity. The intensity of the oxidative damage, however, differs among species and/or genotypes, and the plant response depends on the duration and intensity of the applied stress (Sgherri et al. 2000). In the roots, however, lipid peroxidation was smaller than in the leaves and decreased with increasing iron concentration (Fig. 4). This reduction in lipid peroxidation may be the result of the higher activities of the antioxidative enzymes induced by higher iron content in this part of the plant (Fig. 6) or an effect of the EDTA left behind after iron uptake as suggested by González-Cuevas et al. (2011).
So, iron in excess may cause oxidative damage to the plants either directly or indirectly through the formation of ROS. To scavenge most of the produced ROS plants of Euglena uniflora L., similar to other species, exhibited a well-organized antioxidative defense system comprising antioxidative enzymes (e.g., SOD, POX, GR, GPX, CAT) and low molecular weight antioxidants such as AA and GSH (Reddy et al. 2005; Singh et al. 2006). The ability to activate these defense mechanisms is considered to be a key component in the process of plant tolerance, which involves the elimination of oxidative disturbances caused by biotic and abiotic stresses (Giannakoula et al. 2010).
Superoxide dismutase (SOD), a first line defense enzyme that catalyzes superoxide anion dismutation (Scandalios 1993) and considered as one of the most important enzymes in the cell defense against the oxidative stress (Foyer and Noctor 2000; Alscher et al. 2002; Apel and Hirt 2004) was enhanced by iron excess. Its activity increased with increasing iron concentration and exposure time, especially in the roots of E. uniflora (Fig. 6a). This higher SOD activity in the roots seems to be the result of increased iron content, which subsequently increases oxidative stress and the production of superoxide radicals. Increasing SOD activity under iron toxicity has also been observed in other plant species, such as Nicotiana plumbaginifolia (Kampfenkel et al. 1995), corn (Kumar et al. 2008), Clusia hilariana (Pereira et al. 2009), Hydrilla verticillata (Sinhá et al. 1997), and rice (Stein et al. 2008). However, Neves et al. (2009) observed a decrease in the activity of this enzyme in the leaves of E. uniflora exposed to iron dust deposition and/or acid rain and to treatment with 5.0 mM FeEDTA, but in this case the iron concentration was much higher than in the present experiment.
Hydrogen peroxide (H2O2) a by-product of the superoxide anion dismutation, however, is also toxic to plants and, therefore, requires other antioxidative enzymes to be eliminated. One of the most important enzymes involved in the elimination of hydrogen peroxide (H2O2) in plants is catalase (CAT). The activity of this enzyme increased with increasing iron concentration, especially in the leaves, indicating an increase in the production of H2O2 (Ghanati et al. 2005). The increase in CAT activity due to iron stress has been observed in other plant species (Kuki et al. 2008; Kumar et al. 2008; Stein et al. 2008; Xing et al. 2010). In a previous study, a decreased CAT activity was observed in E. uniflora after treatment with 5.0 mM FeEDTA for 2 months (Neves et al. 2009). Reductions in the activities of the enzymes SOD and CAT, as observed by Neves et al. (2009), are indicative of a much stronger oxidative stress, affecting enzyme biosynthesis and/or the assembly of enzyme subunits (Singh et al. 2006). Peroxidase (POX) and ascorbate peroxidase (APX) activities increased in both the leaves and, particularly, the roots of the plants with increasing iron concentration. In general, increased activity of these antioxidative enzymes is expected, implicating a role for these enzymes in the detoxification of ROS accumulated due to an oxidative stress. An increase in the activities of these enzymes in response to toxic levels of iron was observed in Solanum tuberosum (Chatterjee et al. 2006), Schinus terebinthifolius (Kuki et al. 2008), corn (Kumar et al. 2008), rice (Stein et al. 2008) and Elodea nuttallii (Plach) H. St. John (Xing et al. 2010). Due to its high activity in both the leaves and roots, the enzyme POX seems to have an important role in plant defense against oxidative stress in Eugenia uniflora.
Glutathione peroxidase (GPX) also detoxifies H2O2 to H2O but using GSH as reducing agent. The regeneration of GSH is made possible by the reduction of GSSG catalyzed by glutathione reductase (GR), a key-enzyme of the ascorbate/glutathione cycle essential to maintain cell homeostasis during oxidative stress (Noctor and Foyer 1998). In this study, GPX activity decreased in both plant parts with increasing iron concentration and exposure time. This reduction may be result of a direct attack by toxic reactive oxygen species as suggested by Wang et al. (2004) or a reduced supply of GSH. This last hypothesis seems to be improbable since the GR activity (Figs. 5, 6) and the GSH content (Fig. 8) were enhanced under increasing iron concentration. Interestingly, in the leaves, we observed lower GR activity and higher GSH content (Figs. 5f, 8a), while in the roots, we observed the opposite effect (Figs. 6f, 8b), indicating a higher consumption of GSH in the roots, probably to provide substrate for phytochelatin biosynthesis, a Cys-rich polypeptide involved in plant metal detoxification (Nagalakshmi and Prasad 2001) or other consuming –SH process.
To overcome oxidative stress, besides enzymatic mechanisms, plant can use low molecular weight antioxidants such as ascorbate (AA) and glutathione (GSH) (Noctor and Foyer 1998). Ascorbate (AA) is a major antioxidant metabolite involved in the detoxification of ROS during oxidative stress in plants (Noctor and Foyer 1998). Ascorbate can directly eliminate hydrogen peroxide or can be used as a reduced substrate by the enzyme APX. Cells, therefore, require the effective biosynthesis and/or regeneration of AA to prevent oxidative damage caused by excess metals (Sinhá and Saxena 2006). Apparently, the AA contents might vary depending on the degree of the stress and the capacity of the system to produce and/or regenerate AA. Here, the AA content varied from a slight decrease to a high increase in the leaves after plant treatment with 2.0 mM FeEDTA for 45 days. An increase in the AA content in plants treated with iron was observed in Bacopa monnieri (Sinhá and Saxena 2006) and in Spartia densiflora (Domínguez et al. 2009). We also observed an increase in APX at both 1.0 and 2.0 mM FeEDTA for the duration of the experimental period. This enzyme consumes AA to eliminate the H2O2 and produces monodehydroascorbate (MDHA) (Noctor and Foyer 1998); therefore, an increase in MDHA would be expected. Unfortunately, this metabolite was not determined in this work, but its non-enzymatic sub-product dehydroascorbate (DHA) was reduced, especially in the roots, with increasing iron concentration. Thus, enzymes such as dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDHAR) were probably activated to regenerate part of the AA consumed in H2O2 detoxification (Drazkiewicz et al. 2003; Aravind and Prasad 2005). At 45 days after the application of 2.0 mM FeEDTA, the AA/DHA ratio increased approximately 3-fold relative to the control. The AA/DHA ratio is considered to be an important redox state indicator in plant cells, functioning as a buffer system to supply antioxidant metabolites during oxidative stress (Potters et al. 2002). The increase in the AA/DHA ratio was due to an increase in AA and a reduction in DHA contents (Fig. 7). The total AA + DHA also increased with iron treatment and could potentially increase ascorbic acid synthesis.
GSH is another important antioxidant metabolite that exists interchangeably with the oxidized form, GSSG (Noctor and Foyer 1998). It can chemically react with some ROS, especially with hydrogen peroxide, or can be used as an electron donor for the enzyme GPX. GSH can also participate in AA regeneration in the AA-GSH cycle (Eshdat et al. 1997; Noctor and Foyer 1998). In this study, the GSH content in iron-treated plants was higher than in controls, especially in the leaf, indicating an adaptive response of the plants to the oxidative stress caused by high iron concentration. Increasing the time of exposure to iron treatment gradually reduced the GSH content, especially at 1.0 mM FeEDTA in the roots. Some authors suggest that this might be due to GSH utilization in the biosynthesis of phytochelatins or in the AA (Mendoza-Cózatl and Moreno-Sánchez 2006).
Usually, the oxidized GSSG form represents a small fraction of the total glutathione (<10 %) (Meister 1988). In our study, GSSG represented 25–30 % of the total glutathione. The GSSG content increased in plants treated with 1.0 mM but was reduced at higher iron concentrations, probably due its consumption by GR as observed in Figs. 5f, 6f. The GSH/GSSG ratio increased with increasing iron treatment, especially at the highest FeEDTA concentration, in the leaves and roots. This GSH/GSSG ratio is considered to be an important redox sensor in different signaling processes ROS produced through oxidative stress (Mittler 2002). The increase in the GSH/GSSG ratio represents a positive balance between GSH-producing enzymes, such as GR (Apel and Hirt 2004), γ-GCS and GSH-S (Ishikawa et al. 2006), and consumer enzymes, such as GPX, suggesting an important role for this system in the defense against oxidative stress caused by iron toxicity in Eugenia uniflora.
In conclusion, our results demonstrate that young Eugenia uniflora plants under high iron concentrations suffer strong oxidative stress with increase in lipid peroxidation. Under the experimental conditions applied in this study, Eugenia uniflora plants exhibited a complex defense mechanism composed of antioxidative enzymes and low molecular weight antioxidants that were capable of ameliorating the impact of the reactive oxygen species produced.
Author contribution
G.O. Jucoski performed all experiments and analyses and wrote the first manuscript; J. Cambraia designed and supervised the research and wrote the final manuscript; C. Ribeiro helped in the antioxidative enzymes and metabolites determinations; J.A. Oliveira, S.O. de Paula and M. A. Oliva supervised the research as co-advisers and reviewed the manuscript.
References
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Aravind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate-glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116
Azevedo Neto AD, Prisco JT, Enéas-Filho J, Abreu CEB, Gomes-Filho E (2006) Effects of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
Becana M, Moran JF, Ormaetxe I (1998) Iron–dependent oxygen free radical generation in plants subjected to environmental stress: toxicity and antioxidant protection. Plant Soil 201:137–147
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Briat JF, Lebrun M (1999) Plant responses to metal toxicity. C R Acad Sci 322:43–54
Briat JF, Lobréaux S (1997) Iron transport and storage in plants. Trends Plant Sci 2:187–193
Cakmak I, Horst WJ (1991) Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–495
Chatterjee C, Gopal R, Dube BK (2006) Impact of iron stress on biomass, yield, metabolism and quality of potato (Solanum tuberosum L.). Sci Hortic 108:1–6
Domínguez DM, Santiago RT, García FC (2009) Modulation of the antioxidative response of Spartina densiflora against iron exposure. Physiol Plant 136:169–179
Drazkiewicz M, Skorznska-Polit E, Krupa Z (2003) Response of the ascorbate-glutathione cycle to excess copper in Arabidopsis thaliana (L). Plant Sci 164:195–202
Eshdat Y, Holland D, Faltin Z, Bem-Hayyim G (1997) Plant glutathione peroxidases. Physiol Plant 100:234–240
Fang WC, Wang JW, Lin CC, Kao CH (2001) Iron induction of lipid peroxidation and effects on antioxidative enzyme activities in rice leaves. Plant Growth Regul 35:75–80
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signaling. New Phytol 146:359–388
Ghanati F, Morita A, Yokota H (2005) Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant Soil 276:133–141
Giannakoula A, Moustakas M, Syros T, Yupsanis T (2010) Aluminum stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line. Environ Exp Bot 67:487–494
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: occurrence in higher plants. Plant Physiol 59:309–314
González-Cuevas J, Navarro-Partida AL, Bueno-Topete MR, Beas-Zarate C, Armendáriz-Borunda J (2011) Ethylenediaminetetraacetic acid induces antioxidant and anti-inflammatory activities in experimental liver fibrosis. Redox Rep 16:62–70
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216:541–551
Hemalatha K, Venkatesan S (2011) Impact or iron toxicity on certain enzymes and biochemical parameters of tea. Asian J Biochem 6:384–394
Hoagland DR, Arnon DI (1938) The method for growing plants without soil: water-culture. Cal Agric Exp Station Circular 347:1–32
Ishikawa T, Dowdle J, Smirnoff N (2006) Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol Plant 126:343–355
Kampfenkel K, Van MV, Inzé D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Kuki KN, Oliva MA, Pereira EG, Costa AC, Cambraia J (2008) Effects of simulated deposition of acid mist and iron ore particulate matter on photosynthesis and the generation of oxidative stress in Schinus terebinthifolius Radii and Sophora tomentosa L. Sci Total Environ 403:207–214
Kumar P, Tewari RK, Sharma PN (2008) Modulation of copper toxicity-induced oxidative damage by excess supply of iron in maize plants. Plant Cell Rep 27:399–409
Marschner H (1995) Mineral Nutrition of Higher Plants. Academic Press, New York
Mehraban P, Zadeh AA, Sadeghipour HR (2008) Iron toxicity in rice (Oryza sativa L.), under different potassium nutrition. Asian J Plant Sci 3:251–259
Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263:17205–17208
Mendoza-Cózatl DG, Moreno-Sánchez R (2006) Control of glutathione and phytochelatin synthesis under cadmium stress. Pathway modeling for plants. J Theor Biol 238:919–936
Mielke MS, Schaffer B (2011) Effects of soil flooding and changes in light intensity on photosynthesis of Eugenia uniflora L. seedlings. Acta Physiol Plant 33:1661–1668
Mielke MS, Schaffer B, Li C (2010) Use of a SPAD meter to estimate chlorophyll content in Eugenia uniflora L. leaves as affected by contrasting light environments and soil flooding. Photosynthetica 48:332–338
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–408
Moller MI, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Neves NR, Oliva MA, Centeno DC, Costa AC, Ribas RF, Pereira EG (2009) Photosynthesis and oxidative stress in the resting plant species Eugenia uniflora L. exposed to simulated acid rain and iron ore dust deposition: potential use in environmental risk assessment. Sci Total Environ 407:3740–3745
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Peixoto PHP, Cambraia J, Sant’Anna R, Mosquim PR, Moreira MA (1999) Aluminum effects on lipid peroxidation and on the activities of enzymes of oxidative metabolism in sorghum. Rev Bras Fisiol Veg 11:137–143
Pereira EG, Oliva MA, Kuki KN, Cambraia J (2009) Photosynthetic changes caused by iron ore dust deposition in the tropical CAM tree Clusia hilariana. Trees- Struct Funct 23:277–285
Potters G, De Gara L, Asard H, Horemans N (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40:537–548
Reddy AM, Kumar SG, Jyothsnakumari G, Thimmanaik S, Sudhakar C (2005) Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and belgalgram (Cicer arietinum L.). Chemosphere 60:97–104
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7–12
Schmidt W (2003) Iron solutions: acquisition strategies and signaling pathways in plants. Trends Plant Sci 8:188–193
Sgherri CLM, Maffei M, Navari-izzo F (2000) Antioxidative enzymes in wheat subjected to increasing water deficit and rewatering. J Plant Physiol 157:273–279
Singh S, Eapen S, D`souza SF (2006) Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 62:233–246
Sinhá S, Saxena R (2006) Effect of iron on lipid peroxidation, and enzymatic and non-enzymatic antioxidants and bacoside-A content in medicinal plant Bacopa monnieri L. Chemosphere 62:1340–1350
Sinhá S, Gupta M, Chandra P (1997) Oxidative stress induced by iron in Hydrilla verticillata Royle: response of antioxidants. Ecotox Environ Safe 38:286–291
Souza-Santos P, Ramos RS, Ferreira ST, Carvalho-Alves PC (2001) Iron-induced oxidative damage of corn root plasma membrane H+-ATPase. Biochim Biophys Acta 1512:357–360
Stein RRJ, Duarte GL, Spohr MG, Lopes SIG, Fett JP (2008) Distinct physiological responses subjected to iron toxicity under field conditions. Ann Appl Biol 154:269–277
Wang H, Shan X, Wen B, Zhang S, Wang Z (2004) Responses of antioxidative enzymes to accumulation of copper in a copper hyperaccumulator of Commoelina communis. Arch Environ Contam Toxicol 47:185–192
Xing W, Li D, Liu G (2010) Antioxidative responses of Elodea nuttallii (Planch.) H. St. John to short-term iron exposure. Plant Physiol Biochem 48:873–878
Acknowledgments
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for financial support, and the Conselho Nacional de Desenvolvimento Científico Tecnológico (CNPq) for fellowships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Aroca.
Rights and permissions
About this article
Cite this article
de Oliveira Jucoski, G., Cambraia, J., Ribeiro, C. et al. Impact of iron toxicity on oxidative metabolism in young Eugenia uniflora L. plants. Acta Physiol Plant 35, 1645–1657 (2013). https://doi.org/10.1007/s11738-012-1207-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1207-4