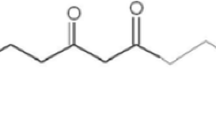
Abstract
Arsenic (As) is known for its carcinogenic and hepatorenal toxic effects causing serious health problems in human beings. Turmeric (Curcuma longa L.) extracted curcumin (Cur) is a polyphenolic antioxidant which has ability to combat hazardous environmental toxicants. This study (28 days) was carried out to investigate the therapeutic efficacy of different doses of Cur (Cur: 80, 160, 240 mg kg−1) against the oxidative damage in the liver and kidney of male rats caused by sodium arsenate (Na3AsO4) (10 mg L−1). As exposure significantly elevated the values of organ index, markers of hepatic injury (i.e., alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP)) and renal functions (i.e., total bilirubin, urea and creatinine, total cholesterol, total triglycerides, and lipid peroxidation malondialdehyde (MDA)). Moreover, different antioxidant markers such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) activities in the liver and kidney tissues were reduced after As-induced toxicity. However, Na3AsO4 induced histopathological changes in various organs were minimized after the treatment with Cur. The alleviation effect of Cur was dosage dependent with an order of 240>160>80 mg kg−1. The oral administration of Cur prominently alleviated the As-induced toxicity in liver and kidney tissues by reducing lipid peroxidation, ALT, AST, ALP, total bilirubin, urea, creatinine, total cholesterol, total triglycerides, and low-density lipoproteins (LDL). In addition, Cur being an antioxidant improved defense system by enhancing activities of SOD, CAT, GPx, and GR. Overall, the findings explain the capability of Cur to counteract the oxidative alterations as well as hepatorenal injuries due to As intoxication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution and abiotic stress cast detrimental effects on the quality of soil, water, and crops (Akman et al. 2019; Bilen et al. 2019; Sayyed et al. 2019; Tauqeer et al. 2021a, b; Fahad et al. 2021a, b, c, d Sonmez et al. 2016; Turan et al. 2019; Iftikhar et al. 2021). Arsenic (As) existing in four oxidation states, such as −3, 0, +3, and +5, is known as one of the most hazardous toxicants causing serious threats to human health around the globe (Jiang et al. 2013) including in Pakistan (Rabbani and Fatmi 2020). The major factor of As exposure to human beings is the consumption of As-contaminated groundwater through agriculture, drinking, and cooking (Celik et al. 2008). Undoubtedly, As contamination of groundwater takes place via extensive use of insecticides in agriculture, industrial and mining activities, and natural deposits of As-ores. The exposure of As higher than the permitted limit of 10 μg L−1 can cause severe malfunctioning of multiple body organs especially the liver and kidney (Mehrzadi et al. 2018). In addition, As-induced toxicity can cause cancer and fatal tumors (Brown and Ross 2002), skin problems such as hyper-pigmentation, hypo-pigmentation, and hyperkeratosis (Sun et al. 2019), and dysfunctioning of the central nervous system (Flora and Mehta 2009). After ingestion, As can be bound to red blood cells and accumulated in various body tissues through the blood circulatory system (Guo et al. 2020). In the near past, it has been reported that chronic exposure to inorganic As (>50 μg L−1) may induce hypertension, ischemic heart disease, and atherosclerosis (Hossain et al. 2017; Moon et al. 2012; Navas-Acien et al. 2005).
Though the molecular mechanisms involved in As-induced hepato- and nephrotoxicity have not been fully understood, various studies suggested that the production of reactive metabolites causing protein modifications, tissue injury, and oxidative damages is the major mechanism underlying As toxicity (Mershiba et al. 2013; Rees et al. 2019). It has been largely accepted that exposure of toxic As and other metals to rats and human generates various reactive oxygen species (ROS) such as superoxides (O2–), hydroxyls (OH−), hydroperoxyls (HO2), hydrogen peroxide (H2O2), lipoperoxidation (MDA), and nitric oxide (NO), which ultimately causes organ impairment especially fatty liver and hepatic fibrosis (Allen and Rana 2007; Ghosh et al. 2010; Kaya et al. 2013; Mittal and Flora 2007; Turan 2020, 2019). As has also been found to deplete the expressions of antioxidant enzyme activities, i.e., catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), and glutathione peroxidase (GPx) (Akcura et al. 2019; Sonmez et al. 2009; Ghosh et al. 2010; Guo et al. 2020; Yadav et al. 2012; Turan 2021a, b). Regardless of huge achievements and developments in synthetic pharmaceutical industry, various side effects have been reported (Tripoli et al. 2007). On contrary, recent findings still have suggested that natural therapies such as supply of phytochemicals could exert beneficial effects against various diseases (Hossen et al. 2017; Mershiba et al. 2013).
The supply of antioxidants, chelators, and herbal extracts is a major approach which has been employed to recover the toxic effects caused by arsenicosis (Flora and Pachauri 2010; Hossen et al. 2017). Among various herbal therapies, turmeric (Curcuma longa L.), a perennial species belonging to the Zingiberaceae family, which has also been recognized as “queen of plant species,” is used as a traditional ingredient in food (Gupta et al. 2013; Hossen et al. 2017) and herbal medicine against anorexia and gynecological, gastric, hepatic, and renal disorders (Hashish and Elgaml 2016; Soliman et al. 2015). Curcumin (Cur), a yellow-colored polyphenol [1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], is obtained from the rhizome of turmeric plant (Bahrami et al. 2020; Yu et al. 2014). Cur has no side effects (Conterato et al. 2007) and proved for its broader pharmacological applications, for example, immunomodulatory, anti-inflammation, and anti-microbial activities, anti-parasitic and anti-proliferative agent, and anti-carcinogenic compound (Mohamed et al. 2020; Sankar et al. 2015). The beneficial effects of Cur against the injuries caused by hazardous chemicals are well described (Bahrami et al. 2020; Saber et al. 2019). Recently, Cur has been found to alleviate paracetamol-induced organ injuries (Yousef et al. 2010), copper (Cu) - and lead (Pb)-based hepato- and nephrotoxicity (Hashish and Elgaml 2016; Soliman et al. 2015), mancozeb-caused hepatotoxicity (Saber et al. 2019), bisphenol A–induced heart and liver damages (Apaydin et al. 2019; Uzunhisarcikli and Aslanturk 2019), carbofuran-induced hematological changes (Hossen et al. 2017), and As-induced toxicity (Bahrami et al. 2020). The potential mechanisms involved in Cur-assisted alleviation of metal-induced toxicity are the Cur-based reductive metabolites (Pandey et al. 2020) and inhibition of ROS production due to its oxygen-free radical scavenging property (Uzunhisarcikli and Aslanturk 2019), and upregulation of detoxifying enzymatic including CAT, SOD, POD, APX, GR, GPx, and GST because of its antioxidative capability (Hashish and Elgaml 2016; Trujillo et al. 2013). Moreover, Cur has been reported to enhance the production of intracellular glutathione concentration which resultantly reduces the lipid peroxidation (Yadav et al. 2012). Despite its validated beneficial effects, its hepato-reno-protective role against As-induced biochemical modifications, oxidative injuries, and impaired organ functions has not yet been evaluated either at physiological or molecular levels. Considering the present scenario, this investigation was aimed to access the role of turmeric (Curcuma longa L.)-extracted Cur against As-induced hepatorenal injury in orally administrated rats. The ameliorative effects of Cur were evaluated by measuring (i) several biochemical assays, (ii) organ index, (iii) oxidative injuries, and (iv) activities of antioxidant enzymes in liver and kidney tissues of rats.
Material and methods
Chemical reagents and materials collection
Fresh rhizomes of turmeric (Curcuma longa L.) were purchased from the commercial market of Faisalabad. Sodium arsenate (Na3AsO4) and all other chemicals used in the research were of >99% purity and imported from Merck Chemicals Limited. All the reagents used in this experiment were of analytical grade. The selection of the dose of arsenic (As) (Na3AsO4) was based on previous investigations (Mehrzadi et al. 2018; Yadav et al. 2012). The rats were purchased from the research-purposed animal room of the Faculty of Veterinary Science, University of Agriculture, Faisalabad.
Experimental animals
In the present study, 10-week-old 35 male rats having a weight between 220 and 250 g were purchased from the animal room of the Faculty of Veterinary Science, University of Agriculture, Faisalabad, Pakistan. All the rats were kept in quarantine by distributing in separate groups for 1 week before the beginning of the oral As treatment to adapt to the environment under controlled conditions, i.e., temperature of 20±2°C, 45–50% relative humidity, and light control of 12-h dark:12-h light cycle. During the process, the rats were fed with standard rat diet and given water and libitum during the experimental period. Compliance of this study with ethical rules was approved by the ethical committee of the university for animal experiments and standards procedures and protocols were followed.
Extraction of curcumin from rhizomes of turmeric
Fresh rhizomes of turmeric plant were collected, weighed, and dried in a hot air oven at a temperature of 110°C for 24 h. For extraction of curcumin (Cur) by a Soxhlet apparatus, 120 mL of hexane was poured into 20 g of powdered turmeric rhizome in a cellulose-made thimble which was put into a Soxhlet apparatus at 60°C for 20 h. After that, hexane was evaporated in a rotary evaporator and organic extract was condensed by the method of distillation. This condensed solution of a sample of Cur was further dried on a hot plate at 45°C and then weighed the final sample of Cur. The crude yield of the extracted Cur was approximately 6.7% on w/w basis. Amount of Cur extracted from a sample of turmeric plant (w/w) was measured by using the equation given by Sahne et al. (2016):
Experimental design
Research work was performed in the National Institute of Food Science and Technology, University of Agriculture, Faisalabad. A dosage of 10 mg L−1 of Na3AsO4 was added in the normal saline solution for oral intoxication of rats and hence chronic toxicity was initiated. The doses of Cur (80, 160, and 240 mg kg−1) were selected to promise the defense against As toxicity. All the rats were erratically alienated into five groups with 7 rats in each group (n = 7) and kept in different cages in an animal room. A detailed description of each group and experimental duration is given in Table 1. There were two phases of the experimental design, i.e., completely randomized design (CRD). In the first induction phase, all rat groups were orally administrated with As-contaminated saline solution for 4 weeks (28 days) continuously with a gap of 1 day, except the negative control group (normal saline). In the second treatment phase, as the symptoms of As toxicity were obvious, the application of Cur was initiated. All the groups were guarded on a regular basis for the next 28 days following these administrations: 1st group, rats were given normal saline (neither As nor Cur supply); 2nd group, containing arsenate-treated rats (ATR) which were not provided with Cur (positive control); 3rd group, Cur at a quantity of 80 mg kg−1 was received by rats; 4th group, Cur at a quantity of 160 mg kg−1 was received by rats; 5th group, Cur at a quantity of 240 mg kg−1 was received by rats.
Sample collection and preparation
After the experimental period was over, cervical execution of animals was done under anesthesia with xylazine (100 mg kg−1) and ketamine (100 mg kg−1), and blood samples were collected and positioned on ice. For the determination of various biochemical assays in the serum, the blood samples were centrifuged at 4°C under 3000 rpm for 15 min and serum was obtained which was stored at −20°C for further analysis. After the serum collection, kidneys and liver were excised from euthanized animals, washed, and rinsed in 0.2 M sucrose solution. Slice fractions from the liver and kidneys were stocked at −20°C. For homogenate collection, 10% (w/v) phosphate (P) buffer (pH 7) was used for homogenization process and subsequent centrifugation at 4°C for 60 min (Oyedemi et al. 2010) to collect the tissue supernatant. This supernatant was stored at −80°C for oxidative stress and enzymatic antioxidant analysis.
Evaluation of rat organ index
Organ index including the liver, pancreas, and kidney of the normal and As-administered rats was recorded. The rats were scrutinized under anesthesia. The liver, pancreas, and kidney tissues were segregated, weighed, and recorded. The organ index in terms of relative weight of the liver, kidney, and pancreas was calculated by applying the formula given by Yang et al. (2005).
Liver and kidney function analysis
The liver and kidney function enzymes were examined with commercially available kits and marketable reagents by adopting excellent laboratory observations for the following parameters. The serum liver function was evaluated in terms of aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) according to the methods described by Reitman and Frankel (1957), while alkaline phosphatase (ALP) activity was also examined following the procedure of Belfield and Goldberg (1971). On the other hand, various kidney functions were also determined as creatinine level was assessed by the method explained by Chromý et al. (2008). Urea was determined according to the standard method (Wybenga et al. 1971). Total bilirubin was calculated by utilizing the method taken from Garber (1981).
Assessment of serum lipid profile
Serum lipid profile was evaluated by measuring the total contents of triglycerides, cholesterol level, low-density lipoproteins (LDL), and high-density lipoproteins (HDL) by using the methods as explained in Kim et al. (2011) and Seo et al. (2012).
Evaluation of malondialdehyde and antioxidant activities in organ homogenates
The extracted liver and kidney homogenates were used to determine the lipid peroxidation parameter such as malondialdehyde (MDA) level using the Ohkawa method (Ohkawa et al. 1979). The MDA contents were measured using thiobarbituric acid (TBA) and a subsequent spectrophotometric observation was done at 532 nm. Moreover, various antioxidant enzyme activity levels were also carried out in the liver and kidney homogenates to evaluate the Cur-induced defense system in rats. The catalase (CAT) enzyme activity level was calculated by Aebi method (Aebi 1984). CAT enzyme activity was measured spectrophotometrically at 240 nm by measuring the rate of degradation of hydrogen peroxide (H2O2). The procedure outlined by Misra and Fridovich (1972) was used to assay superoxide dismutase (SOD) activity, while the protocol developed by Lawrence was adopted to estimate the glutathione peroxidase (GPx) level in rat organ homogenates (Lawrence and Burk 1976). The activity of glutathione reductase (GR) was determined by following the procedure as explained in Moron et al. (1979).
Statistical evaluations
A single-sided analysis of variance was performed to determine the statistically significant differences among experimental treatments using the least significant difference (LSD) comparison test on the SPSS-16 package at a P < 0.05 significance level. OriginPro 17 was used to carry out all graphical analyses. The data acquired were described as mean ± standard deviation (SD) of the mean values. A multivariate analysis, i.e., principal component analysis (PCA), was carried out to measure the successful distribution of experimental treatments using the RStudio software.
Results
Effect of curcumin on rat organ index under arsenic toxicity
The results regarding the effects of curcumin (Cur) on relative weight of various organs of arsenic (As) administrated rate are presented in Table 2. The values of rat organ index including the liver, pancreas, and kidney showed a significant increase when exposed to As treatment, compared to the control. In brief, the As toxicity significantly increased liver, pancreas, and kidney index by 4.2%, 76.6%, and 17.2% respectively as compared to control treatment. However, the application of different doses of Cur (Cur80, Cur160, and Cur240) significantly alleviated the As-induced toxicity in rats. The highest alleviation rate in liver, pancreas, and kidney index was recorded at 240 mg kg−1 Cur which decreased the organ index of the liver by 2.7%, the pancreas by 34.5%, and the kidney by 8.8%, in comparison with As-treated rats.
Effect of curcumin on liver and kidney functions under arsenic toxicity
The results showed that As toxicity aggravated the liver and kidney functions in rats when compared to control (Table 2). As-treated rats showed significant elevation in serum activities of alanine aminotransaminase (ALT), aspartate aminotransaminase (AST), and alkaline phosphatase (ALP) in the liver and total bilirubin, urea, and creatinine in the kidney. Exposure to As intensified serum activities of ALT, AST, and ALP in the liver by 94.5%,40.4%, and 65.8% respectively over control treatment while the elevation of serum activities by As in total bilirubin, urea, and creatinine was recorded as 109.7%, 32.6%, and 95.6% respectively in kidney functions. However, the highest restoration of the biochemical alteration parameters was observed at As-Cur240 to counter As toxicity in serum activities of ALT, AST, and ALP in the liver by 42.1%, 22.9%, and 34.3% respectively and total bilirubin, urea, and creatinine in the kidney by 36.0%, 15.5%, and 42.2% respectively.
Effect of curcumin on serum lipid profile under arsenic toxicity
The obtained findings regarding the serum lipid profile containing total cholesterol contents, total triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) under As toxicity are presented in Fig. 1. As treatment significantly elevated the contents of total cholesterol, total triglycerides, HDL, and LDL by 68.2%, 66.6%, 31.0%, and 74.5% respectively over control treatment. However, different dosages of Cur significantly alleviated the As toxicity on the serum lipid profile of As-administrated rats. The As-Cur160 and As-Cur240 treatments exhibited the highest alleviation rate than other Cur dosages by reducing the total cholesterol contents (11.0% and 25.7%), total triglycerides (14.5% and 26.2%), and LDL (19.9% and 29.6%) respectively when compared to As-administrated rats.
Effects of different dosages of curcumin (Cur) on serum lipid profile comprising (a) total cholesterol contents, (b) total triglyceride contents, (c) high-density lipoprotein (HDL), and (d) low-density lipoprotein (LDL) in the normal and arsenic-administered rats. The given values represent mean ± SD of seven animals per group (n = 7). The error bars with different lowercase letters designate statistically significant variances among the tested treatments based on one-sided ANOVA results through LSD comparison test
Effect of curcumin on oxidative stress (MDA) in organ homogenates under arsenic toxicity
The results revealed that the As-treated rat group exhibited a significant increase in malondialdehyde (MDA) contents in the liver and kidney homogenates, in comparison to the control (Fig. 2). As-treated rats increased MDA contents by 37.8% in liver tissues and 42.4% in kidney tissues respectively over the control treatment. While various dosages of Cur significantly reduced MDA contents in liver and kidney homogenates of As-administrated rates, Cur dosages such as 160 and 240 mg kg−1 caused a prominent reduction in MDA contents in the liver (14.5% and 22.7%) and kidney tissues (12.6% and 27.7%) respectively as compared to the As treatment.
Effects of different dosages of curcumin (Cur) on oxidative stress indicator in terms of malondialdehyde (MDA) contents in the supernatant of tissue such as liver (a) and kidney (b) of normal and arsenic-administered rats. The given values represent mean ± SD of seven animals per group (n = 7). The error bars with different lowercase letters designate statistically significant variances among the tested treatments based on one-sided ANOVA results through LSD comparison test
Effect of curcumin on antioxidant markers in organ homogenates under arsenic toxicity
As-treated rats showed a significant reduction in activities of antioxidant markers such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxide (GPx), and glutathione reductase (GR) in the liver and kidney tissue homogenates (Figs. 3 and 4). A prominent reduction was noticed in activities of SOD by 17.2%, CAT by 79.3%, GPx by 39.2%, and GR by 35.6% respectively as compared to the control treatment. However, different dosages of Cur significantly improved antioxidant activities in the liver tissues of As-administrated rats. The highest activities of antioxidant enzymes were recorded at the dose 240 mg kg−1 of Cur as it improved the activities of SOD, CAT, GPx, and GR by 12.8%, 25.0%, 27.8%, and 22.0% accordingly, over As treatment alone (Fig. 3).
Effects of different dosages of curcumin (Cur) on the antioxidant enzyme activities such as (a) SOD: superoxide dismutase, (b) CAT: catalase, (c) GPx: glutathione peroxidase, and (d) GR: glutathione reductase measured in the supernatant of liver tissues of normal and arsenic-administered rats. The given values represent mean ± SD of seven animals per group (n = 7). The error bars with different lowercase letters designate statistically significant variances among the tested treatments based on one-sided ANOVA results through LSD comparison test
Effects of different dosages of curcumin (Cur) on the antioxidant enzyme activities such as (a) SOD: superoxide dismutase, (b) CAT: catalase, (c) GPx: glutathione peroxidase, and (d) GR: glutathione reductase measured in the supernatant of kidney tissues of normal and arsenic-administered rats. The given values represent mean ± SD of seven animals per group (n = 7). The error bars with different lowercase letters designate statistically significant variances among the tested treatments based on one-sided ANOVA results through the LSD comparison test
Like liver tissues, antioxidant enzyme activities in the kidney tissues of As-administrated rats were also significantly decreased when As-treated rats were compared over control treatment (Fig. 4). As-treated rats showed a reduction of 29.1%, 53.8%, 61.0%, and 65.0% in the activities of SOD, CAT, GPx, and GR respectively when compared with the control treatment. However, As-Cur160 and As-Cur240 treatments significantly improved the activities of SOD, CAT, GPx, and GR by 26.7%, 16.7%, 39.1%, 69.9%, and 32.9%, 50%, 80.1%, 133.3% respectively in comparison to As-treated rats.
Principal component analysis
The biplot graph (scores and loading plots) resulting from the principal component analysis (PCA) illustrating the relationship between measured variables (oxidative stress markers with biochemical assays, and liver and kidney enzymes) as well as representing separation of all treatments among the first two principal components, i.e., PC1 and PC2, is presented in Fig. 5. Among all principal components, PC1 and PC2 accounted for 96.5% of the total variance of whole dataset. The PC1 contributed 87.4%, while the PC2 component contributed 9.1% of the total variance. All the five treatments were successfully distinguished from each other through the principal components. The treatments of different dosages of Cur on As-administrated rats (As-Cur80, As-Cur160, As-Cur240) were clustered and displaced away from control and As treatments. This may indicate that the Cur had more therapeutic effects against As intoxication in rats. The measured variables such as AST, ALT, ALP, TB, urea, creatinine, cholesterol, triglycerides, LDL, liver index, MDA. L, and MDA. K were categorized in PC1 which showed an inverse correlation with the variables occurred in the PC2 component, i.e., HDL, SOD. L, CAT. L, GPx. L, GR. L, SOD. K, CAT. K, GPx. K, and GR. K.
The biplot graph showing principal component analysis (PCA) score and loadings of different studied variables of the normal and arsenic-administered rats supplemented with different dosages of curcumin (Cur). The score and loading plots represent the dispersion of all five treatments and studied variables around two principal components. The abbreviations of presented variables are as follows: AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; MDA-L, malondialdehyde contents in the liver; MDA-K, malondialdehyde contents in the kidney; HDL, high-density lipoprotein; TB, total bilirubin; LDL, low-density lipoprotein; GPx, glutathione peroxidase; CAT, catalase; SOD, superoxidase dismutase; GR, glutathione reductase
Discussion
In the present study, antioxidative, anti-inflammatory, and anti-fibrogenic properties of curcumin (Cur) were examined histopathologically and biochemically against arsenic (As)-induced liver and kidney toxicity in rats. It is well known that As is present in surface water through industrial effluents, dissolution of rocks, mining processes, and atmospheric deposition (El-Demerdash et al. 2009). The kidney and liver are the main organs of As metabolism which are highly sensitive to exposures of As (Mershiba et al. 2013). After oral ingestion, As is consumed by the gastrointestinal tract and affects the kidney, liver, and spleen (Brunati et al. 2010). Metal-induced oxidative injury is the major trigger for severe toxicity by ingestion, inhalation, and accumulation (Seif et al. 2019). Moreover, As-induced rats revealed a marked increase in the body weight (Table. 2) might be due to the tissue necrosis convoyed by fat depositions on the kidney and liver causing abnormal swelling of these organs. Alkaline phosphatase (ALP), alanine-amino transaminase (ALT), and aspartate-amino transaminase (AST) are key liver enzymes for several metabolic pathways and are widely used to evaluate liver function (Hashish and Elgaml 2016), which were significantly enhanced under As toxicity (Table 2). An increment in the value of ALP, ALT, and AST in hepatic cells is the most prominent sign of hepatitis, mononucleosis, cirrhosis, or other liver disorders and the most vital biomarker in hepatocellular inflammation, impairment, and injury (Mershiba et al. 2013). The stress-induced modification in the permeability of injured liver cells causes leakage of ALT and AST into the blood vessels of liver cells; thus, the outcome is the elevation of serum activity levels. A higher blood level of these enzymes indicates non-alcoholic fatty liver disease (Lakhani et al. 2018). ALP is found in many body tissues, including the liver, bones, intestine, and placenta. ALT and AST are mainly found in the liver, and smaller amounts are present in kidneys and many other organs. ALT converts alanine into pyruvate (intermediate in cellular energy synthesis) while aspartate aminotransferase (AST) is an enzyme that contributes to the metabolism of some amino acids (Mahdavinia et al. 2019). The results of the current investigation showed that As toxicity elevated the levels of liver enzymes such as ALT, ALP, and AST, and ir-regularized the liver functions (Table 2). However, the severity of liver damage and hepatic toxicity was relieved by administration and treatment with Cur. The application of Cur showed a significant decline in the levels of hepatic enzymes near to the normal. The results were in accordance with the previous findings of Mershiba et al. (2013), Mailafiya et al. (2020), and Hossen et al. (2017). These Cur-induced improvements might be attributed to the presence of phenolic and flavonoid compounds in turmeric-extracted curcumin which resultantly could inhibit lipid peroxidation (Farkhondeh and Samarghandian 2016; Namgyal et al. 2020).
Elevated levels of bilirubin in urine and bile may designate certain diseases such as jaundice (Shirzadfar et al. 2019). Stercobilin and urobilin which are breakdown products of bilirubin can cause the brown color of feces and straw-yellow color of urine. In addition, elevated creatinine level may also induce kidney-related diseases or abnormal kidney functions (Jagadeesan and Bharathi 2014). Urea is manufactured by hepatocytes from ammonia which is a by-product of catabolism of amino acids and excreted from the kidney, saliva, colon, and sweat. The elevated level of bilirubin, creatinine, and urea in the serum is reflected to be one of the key indicators of metal-induced damage (Apaydin et al. 2019; Uzunhisarcikli and Aslanturk 2019). In the current study, serum bilirubin, creatinine, and urea levels in the group with As-treated rats was prominently increased as compared to the control group. It was noticed that Cur has dose-dependent protective and defensive effects against serum bilirubin, urea, and creatinine levels increased by As-induced toxicity (Table. 2). Similar findings were also observed by Hashish and Elgaml (2016) where bilirubin, urea, and creatinine levels were significantly alleviated after the supplementation of Cur against copper-induced toxicity in rat models.
Meanwhile, a number of serum lipid profile tests are usually conducted to trace the problems that relate to cardiac diseases. Abnormal high level of lipids in the serum is an indication of serious problems regarding increased level of low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol, and total triglycerides in the liver tissues (Hossen et al. 2017; Vahouny et al. 1987). Cholesterol helps to regularize a huge number of body functions such as production of vitamin D, bile, and androgens. While a high level of blood cholesterol can cause serious problems such as obesity and cardiovascular diseases in human beings, on the other hand, elevated level of triglycerides is often a signal of other situations that intensify the risk of heart disorders and metabolic syndrome (Anderson and Borlak 2008). The result of the current study revealed that As toxicity significantly enhanced the contents of total cholesterol, total triglycerides, and low-density lipoprotein in arsenic intoxicated rats. However, Cur-treated rats showed that levels of LDL, total triglyceride, and total cholesterol were dropped gradually with an increasing amount of Cur (Fig. 1). The application of Cur might have alleviated As toxicity on rat lipid profile by inhibiting the cholesterol production and adsorption, reducing the synthesis of LDL, and enhancing the excretion of bile acids with the help of antioxidative compounds present in Cur (Vahouny et al. 1987; Yoon et al. 2011).
The increased level of malondialdehyde (MDA) (produced after the peroxidation of polyunsaturated fatty acids and lipids) is the most vital indicator of oxidative damage in response to As-induced stress to cells that indirectly reflects the degree of tissue and cell injury (Kamran et al. 2020). Previously, it was examined that reactive oxygen species (ROS), hydrogen peroxide (H2O2), and MDA contents were significantly increased in the tissue homogenates of kidney and liver of As-intoxicated rats (Mehrzadi et al. 2018; Ogun et al. 2016; Sankar et al. 2015). These ROS radicles can damage cellular proteins, DNA structure which ultimately blocks cellular defense systems such as the antioxidant defense system against As toxicity (Visnagri et al. 2015). Similarly, the findings of the current study also proved that acute exposure to As has elevated the generation of ROS in various organs (Fig. 2). However, Cur treatment has inhibited the production of MDA contents in liver and kidney tissues which is in accordance with the previous results where Cur reduced ROS generation by alleviating the toxicity of As (Yadav et al. 2012), paracetamol (Yousef et al. 2010), bisphenol A (Apaydin et al. 2019), Cu (Hashish and Elgaml 2016), and aluminum chloride (Cheraghi and Roshanaei 2019). This Cur-induced alleviation of ROS toxicity in rat organs might be due to the several mechanisms such as Cur has an ability to enhance hepatorenal contents of polyphenolic compounds, ROS quenching, and sodium arsenate chelation (Ercal et al. 2000; Uzunhisarcikli and Aslanturk 2019; Yadav et al. 2009).
Moreover, various studies have revealed that several factors may help to protect and defend the body cells by removing radicle species to reduce oxidative stress (Hossen et al. 2017; Kamran et al. 2021). Among these factors, antioxidant markers such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxide (GPx), and glutathione reductase (GR) play a main role in alleviating ROS-induced oxidative stress (Parveen et al. 2020; Uzunhisarcikli et al. 2016). It was previously examined that CAT and SOD activities showed an As-induced depletion which downregulated the antioxidant defense system showing an organ-dependent variability (Mishra et al. 2008). In the present work, the liver and kidney organs were chosen to evaluate the changes in GPx and GR activities because they possess higher contents of glutathione (GSH) protein which may indicate that As-induced toxicity is involved in changing the cellular redox status. Our findings suggested that As toxicity has downregulated the antioxidant activities (Figs. 3 and 4), and hence, it can be assumed that the decline in As-induced GSH concentration might cause the effectiveness of GR and GPx activities to be constrained (Czeczot et al. 2006). Our observations are in agreement with the previous findings where Cur proved its beneficial role in inducing antioxidative defense against various abiotic stresses in rats as well as human beings (Apaydin et al. 2019; Soliman et al. 2015; Yousef et al. 2010). It is well recognized that Cur has exhibited many therapeutic characteristics and antioxidant behavior against various environmental stressors (Strimpakos and Sharma 2008). In addition, the presence of various polyphenol compounds in the Cur has made it feasible to scavenge ROS which ultimately can boost up the body defense system in terms of antioxidative enzyme activities (especially GSH synthesis) in a dose-dependent manner (Apaydin et al. 2019; Sharma et al. 2001; Sreejayan and Rao 1994). PCA has also strengthened our results and indicated that As toxicity has enhanced the organ damage and altered biochemical properties in the serum of As-intoxicated rats, which were, however, overcome after the application of Cur (Fig. 5). Despite the therapeutic effect of Cur against liver and kidney toxicity induced from As and other metals, further research work is needed to assess the detailed cellular and molecular mechanisms which are involved in the hepatorenal protective effect of Cur (Fig. 6).
Conclusion
In this study, turmeric-extracted curcumin (Cur) effectively restored kidney and liver functions as well as normalized the oxidative injury that occurred after the intoxication of male rats with sodium arsenate (Na3AsO4). The Cur also minimized the lethal effects of arsenic on the organs’ relative weight and serum lipid profile including total cholesterol, total triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) in As-administered rats. The antioxidative role of Cur also alleviated the As-induced oxidative injury in live and kidney tissues and therefore enhanced the enzymatic defense system. Conclusively, the concurrent consumption of Cur can diminish the harmful effects of As. Still, future investigations should be carried out to evaluate the role of Cur under As-induced alterations in human beings at a physio-molecular level.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aebi H (1984) Methods in enzymology, Volume. 105, Catalase in vitro. Chance, Britt. Acta Chem Scand 1:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Akcura M, Turan V, Kokten K, Kaplan M (2019) Fatty acid and some micro element compositions of cluster bean (Cyamopsis tetragonoloba) genotype seeds growing under Mediterranean climate. Ind Crop Prod 128:140–146. https://doi.org/10.1016/j.indcrop.2018.10.062
Akman F, Turan V, Sayyed MI, Akdemir F, Kaçal MR, Durak R, Zaid MHM (2019) Comprehensive study on evaluation of shielding parameters of selected soils by gamma and X-rays transmission in the range 13.94–88.04 keV using WinXCom and FFAST programs. Results Phys 15:102751. https://doi.org/10.1016/j.rinp.2019.102751
Allen T, Rana SVS (2007) Effect of n-propylthiouracil or thyroxine on arsenic trioxide toxicity in the liver of rat. J Trace Elem Med Biol 21:194–203. https://doi.org/10.1016/j.jtemb.2007.04.004
Anderson N, Borlak J (2008) Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev 60:311–357. https://doi.org/10.1124/pr.108.00001
Apaydin FG, Aslanturk A, Uzunhisarcikli M, Bas H, Kalender S, Kalender Y (2019) Histopathological and biochemical studies on the effect of curcumin and taurine against bisphenol A toxicity in male rats. Environ Sci Pollut Res 26:12302–12310. https://doi.org/10.1007/s11356-019-04578-4
Bahrami A, Sathyapalan T, Moallem SA, Sahebkar A (2020) Counteracting arsenic toxicity: curcumin to the rescue? J Hazard Mater 400:123160
Belfield A, Goldberg DM (1971) Normal ranges and diagnostic value of serum 5′nucleotidase and alkaline phosphatase activities in infancy. Arch Dis Child 46:842–846. https://doi.org/10.1136/adc.46.250.842
Bilen S, Bilen M, Turan V (2019) Relationships between cement dust emissionsand soil properties. Pol J Environ Stud 28:3089–3098. https://doi.org/10.15244/pjoes/92521
Brown KG, Ross GL (2002) Arsenic, drinking water, and health: a position paper of the American Council on Science and Health. Regul Toxicol Pharmacol 36:162–174. https://doi.org/10.1006/rtph.2002.1573
Brunati AM, Pagano MA, Bindoli A, Rigobello MP (2010) Thiol redox systems and protein kinases in hepatic stellate cell regulatory processes. Free Radic Res 44:363–378. https://doi.org/10.3109/10715760903555836
Celik I, Gallicchio L, Boyd K, Lam TK, Matanoski G, Tao X, Shiels M, Hammond E, Chen L, Robinson KA, Caulfield LE, Herman JG, Guallar E, Alberg AJ (2008) Arsenic in drinking water and lung cancer: a systematic review. Environ Res 108:48–55. https://doi.org/10.1016/j.envres.2008.04.001
Cheraghi E, Roshanaei K (2019) The protective effect of curcumin against aluminum chloride-induced oxidative stress and hepatotoxicity in rats. Pharm Biomed Res. https://doi.org/10.18502/pbr.v5i1.761
Chromý V, Rozkošná K, Sedlák P (2008) Determination of serum creatinine by Jaffe method and how to calibrate to eliminate matrix interference problems. Clin Chem Lab Med 46:1127–1133. https://doi.org/10.1515/CCLM.2008.224
Conterato GMM, Augusti PR, Somacal S, Einsfeld L, Sobieski R, Torres JRV, Emanuelli T (2007) Effect of lead acetate on cytosolic thioredoxin reductase activity and oxidative stress parameters in rat kidneys. Basic Clin Pharmacol Toxicol 101:96–100. https://doi.org/10.1111/j.1742-7843.2007.00084.x
Czeczot H, Ścibior D, Skrzycki M, Podsiad M (2006) Glutathione and GSH-dependent enzymes in patients with liver cirrhosis and hepatocellular carcinoma. Acta Biochim Pol 53:237–241. https://doi.org/10.18388/abp.2006_3384
El-Demerdash FM, Yousef MI, Radwan FME (2009) Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol 47:249–254. https://doi.org/10.1016/j.fct.2008.11.013
Ercal N, Neal R, Treeratphan P, Lutz PM, Hammond TC, Dennery PA, Spitz DR (2000) A role for oxidative stress in suppressing serum immunoglobulin levels in lead-exposed fisher 344 rats. Arch Environ Contam Toxicol 39:251–256. https://doi.org/10.1007/s002440010102
Fahad S, Sönmez O, Saud S, Wang D, Wu C, Adnan M, Turan V (2021a) Plant growth regulators for climate-smart agriculture. In: Footprints of climate variability on plant diversity, 1st edn. CRC Press, Boca Raton
Fahad S, Sonmez O, Saud S, Wang D, Wu C, Adnan M, Turan V (2021b) Climate change and plants: biodiversity, growth and interactions. In: Footprints of climate variability on plant diversity, 1st edn. CRC Press, Boca Raton
Fahad S, Sonmez O, Saud S, Wang D, Wu C, Adnan M, Turan V (2021c) Developing climate resilient crops: improving global food security and safety. In: Footprints of climate variability on plant diversity, 1st edn. CRC Press, Boca Raton
Fahad S, Sönmez O, Turan V, Adnan M, Saud S, Wu C, Wang D (2021d) Sustainable soil and land management and climate change. In: Footprints of climate variability on plant diversity, 1st edn. CRC Press, Boca Raton
Farkhondeh T, Samarghandian S (2016) The hepatoprotective effects of curcumin against drugs and toxic agents: an updated review. Toxin Rev 35:133–140. https://doi.org/10.1080/15569543.2016.1215333
Flora SJS, Mehta A (2009) Monoisoamyl dimercaptosuccinic acid abrogates arsenic-induced developmental toxicity in human embryonic stem cell-derived embryoid bodies: comparison with in vivo studies. Biochem Pharmacol 78:1340–1349. https://doi.org/10.1016/j.bcp.2009.07.003
Flora SJS, Pachauri V (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7:2745–2788. https://doi.org/10.3390/ijerph7072745
Garber CC (1981) Jendrassik-Grof analysis for total and direct bilirubin in serum with a centrifugal analyzer. Clin Chem 27:1410–1416. https://doi.org/10.1093/clinchem/27.8.1410
Ghosh D, Ghosh S, Sarkar S, Ghosh A, Das N, Saha KD, Mandal AK (2010) Quercetin in vesicular delivery systems: evaluation in combating arsenic-induced acute liver toxicity associated gene expression in rat model. Chem Biol Interact 186:61–71. https://doi.org/10.1016/j.cbi.2010.03.048
Guo X, Liu X, Wang J, Fu X, Yao J, Zhang X, Jackson S, Li J, Zhang W, Sun D (2020) Pigment epithelium-derived factor (PEDF) ameliorates arsenic-induced vascular endothelial dysfunction in rats and toxicity in endothelial EA.hy926 cells. Environ Res 186:109506. https://doi.org/10.1016/j.envres.2020.109506
Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB (2013) Multitargeting by turmeric, the golden spice: from kitchen to clinic. Mol Nutr Food Res 57:1510–1528. https://doi.org/10.1002/mnfr.201100741
Hashish EA, Elgaml SA (2016) Hepatoprotective and nephroprotective effect of curcumin against copper toxicity in rats. Indian J Clin Biochem 31:270–277. https://doi.org/10.1007/s12291-015-0527-8
Hossain K, Suzuki T, Hasibuzzaman MM, Islam MS, Rahman A, Paul SK, Tanu T, Hossain S, Saud ZA, Rahman M, Nikkon F, Miyataka H, Himeno S, Nohara K (2017) Chronic exposure to arsenic, LINE-1 hypomethylation, and blood pressure: a cross-sectional study in Bangladesh. Environ Health A Glob Access Sci Source 16:20. https://doi.org/10.1186/s12940-017-0231-7
Hossen MS, Tanvir EM, Prince MB, Paul S, Saha M, Ali MY, Gan SH, Khalil MI, Karim N (2017) Protective mechanism of turmeric (Curcuma longa) on carbofuran-induced hematological and hepatic toxicities in a rat model. Pharm Biol 55:1937–1945. https://doi.org/10.1080/13880209.2017.1345951
Iftikhar S, Turan V, Tauqeer HM, Rasool B, Zubair M, Mahmood-ur-Rahman, Khan MA, Akhtar S, Khan SA, Basharat Z, Zulfiqar I, Iqbal J, Iqbal M, Ramzani PMA (2021) Phytomanagement of As-contaminated matrix: physiological and molecular basis. In: Handbook of Bioremediation. Elsevier, Amsterdam, pp 61–79. https://doi.org/10.1016/B978-0-12-819382-2.00005-3
Jagadeesan G, Bharathi E (2014) In vivo restoration of hepatic and nephro protective potential of hesperidin and ellagic acid against mercuric chloride intoxicated rats. Biomed Aging Pathol 4:219–222. https://doi.org/10.1016/j.biomag.2014.01.008
Jiang JQ, Ashekuzzaman SM, Jiang A, Sharifuzzaman SM, Chowdhury SR (2013) Arsenic contaminated groundwater and its treatment options in bangladesh. Int J Environ Res Public Health 10:18–46. https://doi.org/10.3390/ijerph10010018
Kamran M, Malik Z, Parveen A, Huang L, Riaz M, Bashir S, Mustafa A, Abbasi GH, Xue B, Ali U (2020) Ameliorative effects of biochar on rapeseed (Brassica napus L.) growth and heavy metal immobilization in soil irrigated with untreated wastewater. J Plant Growth Regul 39:266–281. https://doi.org/10.1007/s00344-019-09980-3
Kamran M, Danish M, Saleem MH, Malik Z, Parveen A, Abbasi GH, Jamil M, Ali S, Afzal S, Riaz M, Rizwan M, Ali M, Zhou Y (2021) Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 263:128169. https://doi.org/10.1016/j.chemosphere.2020.128169
Kaya C, Aydemir S, Sonmez O, Ashraf M, Dikilitas M (2013) Regulation of growth and some key physiological processes in salt-stressed maize (Zea mays L.) plants by exogenous application of asparagine and glycerol. Acta Bot Croat 72(1):157–168
Kim JY, Paik JK, Kim OY, Park HW, Lee JH, Jang Y, Lee JH (2011) Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis 215:189–195. https://doi.org/10.1016/j.atherosclerosis.2010.11.036
Lakhani HV, Sharma D, Dodrill MW, Nawab A, Sharma N, Cottrill CL, Shapiro JI, Sodhi K (2018) Phenotypic alteration of hepatocytes in non-alcoholic fatty liver disease. Int J Med Sci 15:1591–1599. https://doi.org/10.7150/ijms.27953
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958. https://doi.org/10.1016/0006-291X(76)90747-6
Mahdavinia M, Alizadeh S, Vanani AR, Dehghani MA, Shirani M, Alipour M, Shahmohammadi HA, Asl SR (2019) Effects of quercetin on bisphenol A-induced mitochondrial toxicity in rat liver. Iran J Basic Med Sci 22:499–505. https://doi.org/10.22038/ijbms.2019.32486.7952
Mailafiya MM, Abubakar K, Chiroma SM, Danmaigoro A, Rahim EBA, Mohd Moklas MA, Zakaria ZAB (2020) Curcumin-loaded cockle shell-derived calcium carbonate nanoparticles: a novel strategy for the treatment of lead-induced hepato-renal toxicity in rats. Saudi J Biol Sci 27:1538–1552. https://doi.org/10.1016/j.sjbs.2020.03.009
Mehrzadi S, Fatemi I, Malayeri AR, Khodadadi A, Mohammadi F, Mansouri E, Rashno M, Goudarzi M (2018) Ellagic acid mitigates sodium arsenite-induced renal and hepatic toxicity in male Wistar rats. Pharmacol Rep 70:712–719. https://doi.org/10.1016/j.pharep.2018.02.007
Mershiba SD, Dassprakash MV, Saraswathy SD (2013) Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol Biol Rep 40:3681–3691. https://doi.org/10.1007/s11033-012-2444-8
Mishra D, Mehta A, Flora SJS (2008) Reversal of arsenic-induced hepatic apoptosis with combined administration of DMSA and its analogues in guinea pigs: role of glutathione and linked enzymes. Chem Res Toxicol 21:400–407. https://doi.org/10.1021/tx700315a
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175. https://doi.org/10.1016/S0021-9258(19)45228-9
Mittal M, Flora SJS (2007) Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug Chem Toxicol 30:263–281. https://doi.org/10.1080/01480540701380075
Mohamed AA-R, El-Houseiny W, Abd Elhakeem E-M, Ebraheim LLM, Ahmed AI, Abd El-Hakim YM (2020) Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol Environ Saf 188:109890
Moon K, Guallar E, Navas-Acien A (2012) Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep 14:542–555. https://doi.org/10.1007/s11883-012-0280-x
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta Gen Subj 582:67–78. https://doi.org/10.1016/0304-4165(79)90289-7
Namgyal D, Ali S, Mehta R, Sarwat M (2020) The neuroprotective effect of curcumin against Cd-induced neurotoxicity and hippocampal neurogenesis promotion through CREB-BDNF signaling pathway. Toxicology 442:152542
Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, Guallar E (2005) Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol 162:1037–1049. https://doi.org/10.1093/aje/kwi330
Ogun M, Ozcan A, Karaman M, Merhan O, Ozen H, Kukurt A, Karapehlivan M (2016) Oleuropein ameliorates arsenic induced oxidative stress in mice. J Trace Elem Med Biol 36:1–6. https://doi.org/10.1016/j.jtemb.2016.03.006
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Oyedemi SO, Bradley G, Afolayan AJ (2010) In -vitro and -vivo antioxidant activities of aqueous extract of Strychnos henningsii Gilg. Afr J Pharm Pharmacol 4:070–078
Pandey A, Chaturvedi M, Mishra S, Kumar P, Somvanshi P, Chaturvedi R (2020) Reductive metabolites of curcumin and their therapeutic effects. Heliyon 6:e05469
Parveen A, Hamzah Saleem M, Kamran M, Zulqurnain Haider M, Chen JT, Malik Z, Shoaib Rana M, Hassan A, Hur G, Tariq Javed M, Azeem M (2020) Effect of citric acid on growth, ecophysiology, chloroplast ultrastructure, and phytoremediation potential of jute (Corchorus capsularis l.) seedlings exposed to copper stress. Biomolecules 10. https://doi.org/10.3390/biom10040592
Rabbani U, Fatmi Z (2020) Arsenic contamination of drinking water and mitigation in Pakistan: a case of Indus river basin, pp 273–296. https://doi.org/10.1007/978-3-030-21258-2_12
Rees H, Duncan S, Gould P, Wells R, Greenwood M, Brabbs T, Hall A (2019) A high-throughput delayed fluorescence method reveals underlying differences in the control of circadian rhythms in Triticum aestivum and Brassica napus. Plant Methods 15:1–14. https://doi.org/10.1186/s13007-019-0436-6
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63. https://doi.org/10.1093/ajcp/28.1.56
Saber TM, Abo-Elmaaty AMA, Abdel-Ghany HM (2019) Curcumin mitigates mancozeb-induced hepatotoxicity and genotoxicity in rats. Ecotoxicol Environ Saf 183:109467
Sahne F, Mohammadi M, Najafpour GD, Moghadamnia AA (2016) Extraction of bioactive compound curcumin from turmeric (Curcuma longa L.) via different routes: a comparative study. Pakistan J. Biotechnol. 13:173–180
Sankar P, Gopal Telang A, Kalaivanan R, Karunakaran V, Manikam K, Sarkar SN (2015) Effects of nanoparticle-encapsulated curcumin on arsenic-induced liver toxicity in rats. Environ Toxicol 30:628–637. https://doi.org/10.1002/tox.21940
Sayyed MI, Akman F, Turan V, Araz A (2019) Evaluation of radiation absorption capacity of some soil samples. Radiochim Acta 107:83–93. https://doi.org/10.1515/ract-2018-2996
Seif MM, Madboli AN, Marrez DA, Aboulthana WMK (2019) Hepato-renal protective effects of Egyptian purslane extract against experimental cadmium toxicity in rats with special emphasis on the functional and histopathological changes. Toxicol Rep 6:625–631. https://doi.org/10.1016/j.toxrep.2019.06.013
Seo EY, Ha AW, Kim WK (2012) α-lipoic acid reduced weight gain and improved the lipid profile in rats fed with high fat diet. Nutr Res Pract 6:195–200. https://doi.org/10.4162/nrp.2012.6.3.195
Sharma RA, Hill KA, Williams ML, Steward WP, Ireson CR, Verschoyle RD, Leuratti C, Manson MM, Gescher A, Marnett LJ (2001) Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: Relationship with drug levels. Clin Cancer Res 7:1452–1458
Shirzadfar H, Amirzadeh P, Hajinoroozi MH (2019) A comprehensive study over the jaundice causes and effects on newborns and reviewing the treatment effects. Int J Biosens Bioelectron 5:107–112
Soliman MM, Baiomy AA, Yassin MH (2015) Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatotoxicity and nephrototoxicity in Wistar rats. Biol Trace Elem Res 167:91–102. https://doi.org/10.1007/s12011-015-0280-0
Sonmez O, Aydemir S, Kaya C (2009) Mitigation effects of mycorrhiza on boron toxicity in wheat (Triticum durum) plants. N Z J Crop Hortic Sci 37(2):99–104
Sonmez O, Turan V, Kaya C (2016) The effects of sulfur, cattle, and poultry manure addition on soil phosphorus. Turk J Agric For 40:536–541. https://doi.org/10.3906/tar-1601-41
Sreejayan N, Rao MNA (1994) Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol 46:1013–1016. https://doi.org/10.1111/j.2042-7158.1994.tb03258.x
Strimpakos AS, Sharma RA (2008) Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10:511–546. https://doi.org/10.1089/ars.2007.1769
Sun Q, Yang Q, Xu H, Xue J, Chen C, Yang X, Gao X, Liu Q (2019) MiR-149 negative regulation of mafA is involved in the arsenite-induced dysfunction of insulin synthesis and secretion in pancreatic beta cells. Toxicol Sci 167:4–125. https://doi.org/10.1093/toxsci/kfy150
Tauqeer HM, Fatima M, Rashid A, Shahbaz AK, Ramzani PMA, Farhad M, Basharat Z, Turan V, Iqbal M (2021a) The current scenario and prospects of immobilization remediation technique for the management of heavy metals contaminated soils. In: Hasanuzzaman M (ed) Approaches to the Remediation of Inorganic Pollutants. Springer Singapore, Singapore, pp 155–185. https://doi.org/10.1007/978-981-15-6221-1_8
Tauqeer HM, Karczewska A, Lewińska K, Fatima M, Khan SA, Farhad M, Turan V, Ramzani PMA, Iqbal M (2021b) Environmental concerns associated with explosives (HMX, TNT, and RDX), heavy metals and metalloids from shooting range soils: Prevailing issues, leading management practices, and future perspectives. In: Handbook of Bioremediation. Elsevier, Amsterdam, pp 569–590. https://doi.org/10.1016/B978-0-12-819382-2.00036-3
Tripoli E, La Guardia M, Giammanco S, Di Majo D, Giammanco M (2007) Citrus flavonoids: Molecular structure, biological activity and nutritional properties: a review. Food Chem 104:466–479. https://doi.org/10.1016/j.foodchem.2006.11.054
Trujillo J, Chirino YI, Molina-Jijón E, Andérica-Romero AC, Tapia E, Pedraza-Chaverrí J (2013) Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol 1:448–456. https://doi.org/10.1016/j.redox.2013.09.003
Turan V (2019) Confident performance of chitosan and pistachio shell biochar on reducing Ni bioavailability in soil and plant plus improved the soil enzymatic activities, antioxidant defense system and nutritional quality of lettuce. Ecotoxicol Environ Saf 183:109594. https://doi.org/10.1016/j.ecoenv.2019.109594
Turan V (2020) Potential of pistachio shell biochar and dicalcium phosphate combination to reduce Pb speciation in spinach, improved soil enzymatic activities, plant nutritional quality, and antioxidant defense system. Chemosphere 245:125611. https://doi.org/10.1016/j.chemosphere.2019.125611
Turan V (2021a) Calcite in combination with olive pulp biochar reduces Ni mobility in soil and its distribution in chili plant. Int J Phytoremediation:1–11. https://doi.org/10.1080/15226514.2021.1929826
Turan V (2021b) Arbuscular mycorrhizal fungi and pistachio husk biochar combination reduces Ni distribution in mungbean plant and improves plant antioxidants and soil enzymes. Physiol Plant. https://doi.org/10.1111/ppl.13490
Turan V, Schröder P, Bilen S, Insam H, Fernández-Delgado Juárez M (2019) Co-inoculation effect of Rhizobium and Achillea millefolium L. oil extracts on growth of common bean (Phaseolus vulgaris L.) and soil microbial-chemical properties. Sci Rep 9:15178. https://doi.org/10.1038/s41598-019-51587-x
Uzunhisarcikli M, Aslanturk A (2019) Hepatoprotective effects of curcumin and taurine against bisphenol A-induced liver injury in rats. Environ Sci Pollut Res 26:37242–37253. https://doi.org/10.1007/s11356-019-06615-8
Uzunhisarcikli M, Aslanturk A, Kalender S, Apaydin FG, Bas H (2016) Mercuric chloride induced hepatotoxic and hematologic changes in rats: the protective effects of sodium selenite and vitamin E. Toxicol Ind Health 32:1651–1662
Vahouny GV, Khalafi R, Satchithanandam S, Watkins DW, Story JA, Cassidy MM, Kritchevsky D (1987) Dietary fiber supplementation and fecal bile acids, neutral steroids and divalent cations in rats. J Nutr 117:2009–2015. https://doi.org/10.1093/jn/117.12.2009
Visnagri A, Adil M, Kandhare AD, Bodhankar SL (2015) Effect of naringin on hemodynamic changes and left ventricular function in renal artery occluded renovascular hypertension in rats. J Pharm Bioallied Sci 7:121–127. https://doi.org/10.4103/0975-7406.154437
Wybenga DR, Di Giorgio J, Pileggi VJ (1971) Manual and automated methods for urea nitrogen measurement in whole serum. Clin Chem 17:891–895. https://doi.org/10.1093/clinchem/17.9.891
Yadav RS, Sankhwar ML, Shukla RK, Chandra R, Pant AB, Islam F, Khanna VK (2009) Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol Appl Pharmacol 240:367–376. https://doi.org/10.1016/j.taap.2009.07.017
Yadav A, Lomash V, Samim M, Flora SJS (2012) Curcumin encapsulated in chitosan nanoparticles: a novel strategy for the treatment of arsenic toxicity. Chem Biol Interact 199:49–61. https://doi.org/10.1016/j.cbi.2012.05.011
Yang Q, Xie RJ, Geng XX, Luo XH, Han B, Cheng ML (2005) Effect of Danshao Huaxian capsule on expression of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in fibrotic liver of rats. World J Gastroenterol 11:4953–4956. https://doi.org/10.3748/wjg.v11.i32.4953
Yoon KN, Alam N, Lee JS, Cho HJ, Kim HY, Shim MJ, Lee MW, Lee TS (2011) Antihyperlipidemic effect of dietary Lentinus edodes on plasma, feces and hepatic tissues in hypercholesterolemic rats. Mycobiology 39:96–102. https://doi.org/10.4489/MYCO.2011.39.2.096
Yousef MI, Omar SAM, El-Guendi MI, Abdelmegid LA (2010) Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol 48:3246–3261. https://doi.org/10.1016/j.fct.2010.08.034
Yu C, Mei XT, Zheng YP, Xu DH (2014) Zn(II)-curcumin protects against hemorheological alterations, oxidative stress and liver injury in a rat model of acute alcoholism. Environ Toxicol Pharmacol 37:729–737. https://doi.org/10.1016/j.etap.2014.02.011
Acknowledgements
The authors highly acknowledge the Department of Chemistry and Faculty of Veterinary Sciences at The University of Agriculture, Faisalabad, for providing experimental materials and analyzing the samples.
Author information
Authors and Affiliations
Contributions
Conceptualization: Ali Hassan, Muhammad Kamran, Shah Fahad; data curation: Anam Ishaq, Muhammad Riaz, Aasma Parveen; formal analysis: Anam Ishaq, Huma Gulzar; Muhammad Sohaib Chattha, Noman Walayat; investigation: Anam Ishaq, Huma Gulzar; methodology: Anam Ishaq, Sana Fatima; resources: Muhammad Riaz, Ali Hassan, Muhammad Kamran; writing—original draft: Anam Ishaq, Huma Gulzar, Muhammad Kamran; writing—review and editing: Shah Fahad, Muhammad Sohaib Chattha, Sobia Afzal, Muhammad Kamran; supervision: Shah Fahad, Ali Hassan
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
We all declare this manuscript reporting studies does not involve any human participants, human data, or human tissue. So ethics approval and consent to participate is not applicable.
Consent for publication
Our manuscript does not contain data from any individual person, so consent for publication is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishaq, A., Gulzar, H., Hassan, A. et al. Ameliorative mechanisms of turmeric-extracted curcumin on arsenic (As)-induced biochemical alterations, oxidative damage, and impaired organ functions in rats. Environ Sci Pollut Res 28, 66313–66326 (2021). https://doi.org/10.1007/s11356-021-15695-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15695-4