Abstract
Approximately, more than 200 million hectares are contaminated with heavy metals (HMs) having very high concentrations greater than the standard values worldwide. Thus, the urgent remediation of HMs contaminated soils is the need of the hour. In situ immobilization of HMs through organic, inorganic, and other stabilizing additives seems to be the most promising remediation technique in managing HMs pollution. The efficiency of different stabilizing agents has been previously tested for the rehabilitation of HMs contaminated soils with the immediate estimation of their leaching and availability from them. Among tested amendments, biochar and iron base amendments have shown their high efficiency in removing multi-HMs polluted soils. Thus, the immobilization technique seems to be a preferable alternative over other traditional remediation methods owing to its vast applicability, easy availability of raw materials, and wide acceptability. However, weathering activities may increase the risk of HMs remobilization due to the breakdown of organic amendments. Thus continuous monitoring of HMs soils is recommended.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The pollution of arable soils with heavy metals (HMs) is an emergent environmental issue worldwide. The term “HMs” refers to “the elements (metals and metalloids) with potential capability to harm living organisms (plants, animals, humans) in a very small amount.” These HMs consist of cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn) (Singh et al. 2011).
Both natural such as weathering and geological processes while in parallel, the additions of artificial fertilizers into agricultural lands, the use of organic and agricultural residues, tannery industry, improper dumping of Pb–acid as well as Ni–Cd batteries, electronic wastes, and military firing ranges are the most common anthropogenic source of HMs pollution (Tauqeer et al. 2019).
When the concentrations of HMs increased in the soil from their threshold limits, noteworthy toxic symptoms observed on soil quality, which severely affect the performance of major soil functions. Further, the additions of HMs in arable lands increase the risk of their accumulation in plants grown on them, which leads to dietary exposure through biomagnification and thus causing metal poisoning in animals and humans. Likewise, the presence of HMs in such soils also resulted in poor seed germination, which adversely affects the yield and nutritional quality of crops grown on them. Additionally, leaching and surface water runoff is the additional output pathways of HMs migration into other surrounding ecosystems (Kumar et al. 2019; Hembrom et al. 2020).
Keeping in mind the above-narrated issues, the remediation of HMs contaminated lands via adopting efficient remediation methods is necessary. Several conventional remediation methods such as soil replacement, soil washing, and thermal desorption have been reported in managing HMs pollution. However, these reclamation methods are difficult in operation, required high cost, and is destructive. Therefore, these remediation methods are not preferred for the remediation of HMs contaminated soils (Yao et al. 2012).
Recently, the (ex situ) immobilization remediation method for HMs contaminated soils through organic and inorganic amendments has attained the attention of the scientific community across the globe due to its several advantages over traditional methods such as (1) less site destructive, (2) easy to handle due to its simplicity, (3) required comparatively cheap and easily available raw materials for amendments, (4) improved soil quality and soil health through the provision of essential nutrients in degraded soils, and (5) controlled the dispersal risk of contamination (Peng et al. 2009; Wuana and Okieimen 2011).
The addition of stabilizing agents in contaminated soils reduced the bioavailability of HMs by forming precipitates, HMs complexes, and adsorption. Furthermore, the addition of organic amendments not only immobilize HMs but also have additional benefits such as (1) increase the soil biological activities and provide them habitat, (2) improve physicochemical properties of degraded soil by enhancing organic content, (3) enhance water-holding capacity of the soil, (4) support root proliferation and elongation, (5) provide essential nutrients to the plants, and (6) enhance cation exchange capacity (CEC) of the soil.
The objective of this chapter is to collect the latest information about modern trends in the immobilization technique deployed for the management of HMs contaminated soils especially focusing on the interaction of amendments with HMs in soil and their influence on soil quality.
2 Classification of HMs Contaminated Soils
Several anthropogenic sources of HMs have already been cited in the literature, but here we discuss major sources that contributed to high levels of HMs pollution.
2.1 Contamination of Agricultural Soils from Agrochemicals and Wastewater
A dramatic increase in population, food insecurity, and other environmental factors (drought, etc.) puts huge burdens on agricultural resources worldwide. Recently, the farmers having low socio−economic status from developing countries, the excessive use of agrochemicals (fertilizers, pesticides, herbicides, etc.) and irrigation with wastewater are still being a common practice to fulfill the needs of huge population. These practices resulted in HMs accumulation and their transfer from soil to crops, which pose a serious threat to humans due to their direct or indirect bioaccumulation and biomagnification through soil–plant and soil–plant–animal pathways (Turan et al. 2018).
The applications of phosphorus fertilizers are another source of HMs especially Cd pollution in agricultural soils. For example, Cd is the major constituent of phosphorus fertilizers, which contains Cd from smaller amounts up to 300 mg kg−1 (Selim 2018). Except for Cd, the concentrations of other HMs such as Pb and Cr in phosphorus fertilizers have also previously been reported (Atafar et al. 2010; Cheraghi et al. 2012; Nacke et al. 2013). The applications of phosphorus additives in agricultural soils resulted in HMs deposition, which negatively influences the soil quality and crop production (Cheraghi et al. 2012; Nacke et al. 2013).
The overexploitation and excessive use of groundwater for irrigation depleted fresh and groundwater resources worldwide, especially in developing countries. Unfortunately, the farmers from economically poor status countries are compelled to rely on municipal (MWW) and industrial wastewater (IWW) to irrigate their croplands for crop and fodder production. The continuous applications of wastewater may result in HMs pollution of the arable lands (Turan et al. 2018). For example, irrigation with MWW in an arable field has resulted in the contamination of Cd, Ni, Cr, and Pb (Turan et al. 2018). Likewise, the irrigational use of effluents discharged from Pb−acid batteries and electroplating industries resulted in the serious contamination of surrounding agricultural soils with Pb (Khan et al. 2020) and Ni (Shahbaz et al. 2018a, b, 2019), respectively. The continuous irrigation of arable lands with MWW in Marrakech, Morocco, increased the levels of Cd, Cu, Pb, and Zn loading in agricultural soils and crops grown on them (Chaoua et al. 2019).
2.2 Aerially Deposited HMs from Mining, Smelting, and Fuel Burning
The areas with long-term industrial activities and their nearby ecosystems in Europe, South Asia, Western, and South Africa were supposed to be highly contaminated with HMs. Mining and smelting actions were previously performed to extract metals from their natural ores and caused significant damage to the ecosystem. The mining of Ni from ferronickel mines resulted in Ni accumulation in the agricultural soils of the Sinú River in northwestern Colombia (Marrugo-Negrete et al. 2017). The production of acid mine drainage (AMD) is another challenging task associated with mining actions due to the presence of HMs in excessive amounts. When water is released from abandoned mines and tailings, it dissolves a significant amount of HMs, which contaminate freshwater resources, nearby agricultural lands through rainwater runoff, and groundwater via leaching (Naidu et al. 2019). The aerial depositions of HMs from the burning of Pb−based gasoline and dust from mining and metal smelters contributed to the HMs contamination of their surrounding environment (Walraven et al. 2014; Žibret et al. 2013; Huang et al. 2018). For instance, the aerial depositions from Pb–Zn smelters in Hungary, Czech Republic, Austria, and Slovakia (Iqbal et al. 2012; Muhammad et al. 2012) and across Europe have resulted in the accumulation of Pb, Cd, and Zn in surrounding soils (Ettler 2016). Likewise, significantly the higher concentrations of As, Sb, Hg, Zn, Cd, and Pb have been reported in the surrounding soil of a 60-year-old Pb–Zn smelter in the town near Yunnan province, China (Li et al. 2015). The HMs solubility and bioavailability increased under acid rain conditions, which probably increased the risk of human exposure through runoff in urban areas (Žibret et al. 2013). The intense industrial and agricultural practices and high traffic activities caused remarkable HMs accumulation in agricultural soils (Cai et al. 2019). The dissolution of smelter wastes also resulted in the release of Pb (up to 200 mg kg−1), Cu (4000 mg kg−1), As (300 mg kg−1), and Zn (1500 mg kg−1) (Kierczak et al. 2013).
2.3 Firing Range Soils
Military firing or sports shooting ranges also play a key role in the deterioration of the environment via the release of HMs from the bullets and their fragments. The extent of firing ranges depends upon several factors including range type, the type and composition of ammunition, frequency of shooting, soil chemistry, and geochemistry of pollutants. Fayiga (2019) and references therein reported very high concentrations of Zn, Sb, Pb, Cu, and other HMs (Cd, Cr, Ni, Co, etc.) in military range soils from Canada, South Korea, the USA, Spain, Pakistan, and the Czech Republic. The continuous dissolution of bullets and their fragments due to redox reactions in the soil increased the labile fractions of HMs in firing range soils. The excessive release of HMs from highly polluted firing ranges significantly caused the contamination of groundwater, nearby water bodies as well as agricultural lands through leaching and rainwater runoff. In Norway, the surface runoff from a small-arms shooting range resulted in the contamination of Stitjønn and Kyrtjønn lakes. This contamination increased up to 161 mg Pb kg−1DW in Salmo trutta and up to 2700 mg Pb kg−1 DW in bottom sediments of the respective lakes (Mariussen et al. 2017). Similarly, the contamination of agricultural land through runoff from 30-year-old firing range soil in the Czech Republic has previously been described (Chrastný et al. 2010). Reportedly, the decommissioned ranges were also often used as pastures for livestock in Switzerland (Tandy et al. 2017), which may increase the accumulation risk of HMs in animals grazing on them (Johnsen et al. 2019). On the contrary, the contamination of sports shooting ranges due to Pb deposition from Pb pellets has been reported worldwide causing the significant deaths of more than million birds including endangered species because of Pb poisoning (Pain et al. 2019).
2.4 Fishponds
Aquaculture regarded as the backbone for economic development in African and Asian countries, which increases the GDP through fish export (Muddassir et al. 2019). Generally, the fish ponds are prepared in specific areas with distinctive characteristics such as (1) flat or depression lands, (2) waterlogged soils, (3) degraded agricultural lands due to salinity, (4) perennial source of sufficient water supply, i.e., rivers, canals, streams, etc., and (5) high water retention rate due to high clay content. However, the conversion of fertile agricultural lands into fishponds is also a common practice being observed in various areas of Pakistan. Fish ponds established in saline soils are mostly provided with brackish groundwater, which creates a new challenging task for sustainable fish forming (Shaheen et al. 2020). Likewise, fertilizer additions to such ponds are a common practice to increase the growth of aquatic animals and plants (Muddassir et al. 2019). The contamination of ponds could occur due to irrigation with brackish water and applications of different fertilizers (Shaheen et al. 2020; Muddassir et al. 2019). The contamination of ponds with HMs has been reported (Shaheen et al. 2020; Adeyeye 1994).
3 Factors Influencing the Efficiency of Remediation Strategies
The stabilization of HMs in soil primarily depends upon chemical speciation and binding potential of HMs, which are controlled by physicochemical characteristics of the soil as well as several environmental factors. Geochemical processes occurring in the soil such as precipitation, adsorption, complexation, redox reactions, organic matter, and interaction of Al, Fe, and Mn with HMs play a key role in HMs mobility (Komárek et al. 2013). Several soil factors such as soil texture, particle size, bulk density, pH, electrical conductivity (EC), CEC, water-holding capacity organic matter, redox potential, and the presence of clay contents as well as oxides and hydroxides of Fe, Al, and Mn influenced the geochemical reactions such as precipitation, adsorption, complexation, and redox reactions, thereby increasing or decreasing the bioavailability of HMs (Beiyuan et al. 2017).
3.1 Soil pH
Soil pH not only influenced the bioavailability of HMs but also affect cation exchange, surface–complexation interactions of cations, and other several binding mechanisms. Generally, a rise in pH increased the concentrations of OH−, which promotes strong and preferential adsorption of HMs through forming carbonate, precipitate, and hydroxides, thereby reducing their bioavailability (Wu et al. 2016).
3.2 Cation Exchange Capacity (CEC)
The CEC refers to the ion binding ability of the soil, which also plays a key role in governing the mobility of HMs. The presence of organic matter, metal oxides, and clay minerals increased CEC and furnished large surfaces for the adsorption of HMs, which reduced their mobility in soil solution (Kelebemang et al. 2017; Finzgar et al. 2007).
3.3 Organic Matter
The breakdown of plants and animal residues through soil microbial communities increased the concentrations of carboxyl, phenols, amino, and carboxylate functional groups, which restricts HMs mobility in the soil. These functional groups provide binding sites to HMs, which form stable metal complexes with organic matter through adsorption, ion exchange, and complexation mechanisms resultantly reducing their bioavailability in the soil at higher pH levels (Zeng et al. 2011; Quenea et al. 2009).
3.4 Abundance of Oxides and Hydroxides
The oxides and hydroxides of Fe, Mn, and Al are considered as the key components for the stabilization of HMs in soil due to their majestic features such as specific affinity for metal ions, strong binding ability, inner surface complexation, and the formation of minerals via precipitation mechanism (Zeng et al. 2017 ). Additionally, the oxide concentrations found to be higher under oxidized conditions, which promote HMs immobilization by metal oxides (Komárek et al. 2013).
3.5 The Contents and Types of Clay Minerals
The presence of clay minerals such as kaolinite, chlorite, smectite, illite, vermiculite, bentonite, and zeolite plays a vital role in the geochemistry of HMs in the soil. Owing to partial and stable negative charges on clay minerals, the soils having high clay contents showed higher adsorption tendency, thereby reducing HMs bioavailability (Rieuwerts 2007).
3.6 Soil Biota
The presence or absence of key soil (micro)organisms may (im)mobilize HMs in the soil. Large quantities of HMs may alter the functioning and activities of essential soil (micro)organisms. These (micro)organisms tend to tolerate and resist HMs stress, which influences the efficiency of a remediation strategy (Rajkumar et al. 2012). The Azotobacter spp., arbuscular mycorrhizal fungi (AMF), and Cellulosimicrobium cellulans are known to reduce HMs bioavailability in the soil through complexation, sorption, and reduction mechanisms. These microorganisms released several extracellular polymeric substances such as, glomalin, and insoluble glycoprotein, which strongly bound HMs and reduced their mobility in the soil. These HMs were sorbed by melanin and chitin in fungal cell wall as well as accumulated in the vacuoles of mycorrhizal fungi, which restrict their mobility (González-Guerrero et al. 2008; Rajkumar et al. 2012; Khan et al. 2020).
4 Traditional Remediation Methods
This section covers different remediation approaches deployed for the management of HMs contaminated soils.
4.1 Soil Excavation and Replacement
Soil excavation or replacement is effectively insulating the soil and environment for minimizing the effects of HMs in the environment (Nejad et al. 2018). This technique works with the usage of clean soil to partially or completely replace the contaminated soil to dilute the concentrations of toxic pollutants (Yao et al. 2012). This technique effectively controls the mobility of HMs but not permanently removes them from the environment. This method comprises of three steps: In the first step, contaminated soil is replaced with clean soil by removing its contaminated surface. The second and third steps are soil spading and soil importing, associated with the digging of contaminated soil at higher depths and stored for further treatment (Yao et al. 2012; Nejad et al. 2018).
4.2 Soil Washing and Flushing
The soil washing is an easy, reliable method used for the removal of HMs present in the soil (Peng et al. 2018; Ou-Yang et al. 2010; Nejad et al. 2018). In this method, several ingredients such as organic and inorganic acids, chelating substances, and surfactants are used to dissolve HMs from the solid phase to soil solution (Akcil et al. 2015). Some treatments effectually leached down the HMs from the soil, such as EDTA for the leaching of Cd (Peng et al. 2018), sulfuric, oxalic, and citric acid for the removal of various HMs through washing from the soil (Beolchini et al. 2013). Interestingly, phosphoric acid also showed the highest extraction efficiency of HMs removal from the soil with a positive influence on soil and plant health (Nejad et al. 2018). Above all treatments, diluted sulfuric acid is best for soil washing as it has fewer negative impacts on the environment. Likewise, some washing agents may not effectively work when remediating contaminated calcareous soils, for the reason that calcite and carbonates neutralize the protons (Fonti et al. 2013; Akcil et al. 2015).
4.3 Vitrifying Methods
Vitrification enforced intense energy source to melt contaminated soil at 1600–2000 °C temperature (Nejad et al. 2018). The steam generated by high temperature was collected by the off-gas treatment system (Yao et al. 2012). Interestingly, this method immobilizes and fixes the HMs into a divine glass pattern at molecular levels. This powerful in situ technique is often known as joule-heating vitrification, used to remediate tons of HMs contaminated soils by melting and heating, thereby subsequently immobilizing them in the soil (Nejad et al. 2018; Li and Zhang 2013; Wuana and Okieimen 2011). Extremely the highest power produced glass stone binds the HMs and decreases their leaching, thus preventing groundwater contamination (Nejad et al. 2018).
4.4 Electro-Kinetic Methods
The new technique based on the principle of vitrification method is “Electro-kinetic/Electrochemical” in which electric field (AC/DC flow) is produced at both sides of contaminated soil (Akcil et al. 2015; Peng et al. 2018; Nejad et al. 2018). Several researchers investigated the successful immobilization of HMs in the contaminated sites via electro-kinetic technology (Juris et al. 2015; Ottosen et al. 2012; Nejad et al. 2018) via the movement of water, ions, and charged particles between both electrodes. This method is suitable for fine particles because of the higher adsorption of HMs due to the relatively higher flow of electric current (Pedersen et al. 2015; Peng et al. 2018). Likewise, HMs mobilization associated with oxides, carbonates, nitrate, and hydroxides can also be enhanced via the electro-kinetic method. Electromigration, electroosmosis, electrolysis, and electrophoresis are associated mechanisms involved in the removal of HMs from the contaminated soils (Peng et al. 2018). Co-application of several surfactants and desorbing agents with this technique can also be used to enhance the HMs removal efficiency (Peng et al. 2018).
5 Negative Consequences of Traditional Remediation Strategies
The speciation, labile–immobile fractions of HMs and pollution levels strongly affect the efficiency of the remediation method. Therefore, the prior knowledge about the selected site such as characteristics of the contaminated soil, level, and distribution of HMs and climatic conditions must be acquired to design the most precise remedial alternative.
The main features that affect the applicability and choice of remediation strategies are (1) alleviate HMs toxicity, (2) efficient in reducing HMs bioavailability, (3) effective in managing multicontaminated soils (such as shooting range, E-waste, mining as well as industrially contaminated soils), (4) applicable to highly HMs contaminated soils, (5) support plant establishment and growth, (6) environment-friendly, economically feasible, and commercially available, (7) widely acceptable by the community as well as stakeholders, and (8) long-lasting effects. Although traditional or harsh remediation strategies are efficient in removing HMs from the soil, they severely damage and alter soil properties, which demands additional improvement. This section discusses the major limitations of the above-mentioned harsh remediation methods.
Soil washing required (1) enough space, (2) sufficient amount of water for washing, (3) washing chemicals (such as redox as well as chelating agents, surfactants, acids–bases, and salts), and (4) off-site dumping of residual solids and associated risk of HMs leaching from them, which increased the overall cost. Additionally, this remediation method is not recommended for the soils having high clay as well as organic matter contents (Nejad et al. 2018; Sharma et al. 2018). The drawbacks of soil replacement and excavation remediation process increased the costs due to the requirements of (1) heavy machinery for transportation of both contaminated and uncontaminated soils, (2) skilled labor and technically sound experts, (3) dilution with the import of new excessive uncontaminated soils, (4) deep digging, (5) large working volume, (6) recommended for upper layer soils, and (7) severely destroy soil structure by the compaction through the movement of heavy machinery (Nejad et al. 2018; Yao et al. 2012; Zhou et al. 2004). Usually, prior information about soil properties, dissolution, and transport of HMs, stirring rate and time, cell set-up, current density are the key factors influencing the efficiency of electrochemical remediation method (Alshawabkeh 2009; Nejad et al. 2018; Pedersen et al. 2015; Yeung and Gu 2011). Additionally, other demerits of this remediation technique, which increased the overall cost and longer treatment times, are (1) the formation of stable precipitates due to higher contents of OH− around the anode, (2) need of desorbing substances such as surfactants and acidification, (3) only applicable for low permeable soils with relatively higher concentrations of HMs, (4) efficient in removing HMs when sufficient pore fluid is available in soil pores, which facilitates the transport of pollutants, (5) as well as electric current, (6) modifications in soil microstructure as well as geochemistry due to acidification at anodes, and (7) higher energy requirements (Nejad et al. 2018; Peng et al. 2018; Kuppusamy et al. 2016; Pedersen et al. 2015; Alshawabkeh 2009). Likewise, the key limitations of vitrification remediation approach are (1) only effective for a certain depth (approximately 2–7 m below) of soil, (2) unfit for soils with low clay contents, (3) dewatering is necessary to remove HMs from permeable aquifers, (4) required dynamic compaction to decrease large voids, (5) need of soil, sand, and clay as well as electric current to acquire certain features of vitrified material, (6) should be avoided for soils with higher alkaline contents (K2O, Na2O = 1.4 wt%) due to less conductance of current by the molten soil, (7) inefficient in removing HMs from the deeper layers, which increased energy requirements, (8) regular monitoring is required to ensure the stabilization of HMs, and (9) required skilled labor and technical expertise, which increased the overall cost (Nejad et al. 2018; Peng et al. 2018; Kuppusamy et al. 2016; Akcil et al. 2015).
Apart from this, the remediation strategies as mentioned earlier severely damaged microbial communities, destroyed organic matter, and altered micro- and macropores in the soil, which required additional cost of improvement to support plantation after HMs removal from contaminated soils.
6 Remediation of HMs Contaminated Soils with the Focus on Immobilization Techniques
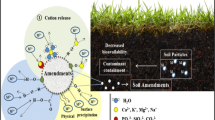
The process of diminishing the solubilization, mobilization, and bioavailability of HMs in the soils with the assistance of different organic and inorganic additives reagents is known as “Immobilization” (Lwin et al. 2018; Peng et al. 2018; Nejad et al. 2018). Different amendments such as organic (biochar, compost, agricultural wastes), inorganic (phosphates, calcium, iron-based additives), and clay (zeolite, bentonite, etc.) are being used for the effective fixation of HMs in the soil. The working principle of immobilization is to fix and bind the HMs via different mechanisms such as adsorption, oxidation, precipitation, and reduction instead of removing them from the soil (Akcil et al. 2015; Peng et al. 2018). The role of amendments is to transform the mobile phase into a more stable form (Lwin et al. 2018). Usually, this happened due to the higher CEC values of stabilizing additives, which strongly bind HMs, thereby reducing their bioavailability (Akcil et al. 2015). Interestingly, the applications of organic substances as stabilizing additives not only reduce the bioavailability of HMs but also provide essential mineral nutrients in the soil, which improved plant growth as well as microorganism (Lwin et al. 2018). Additionally, it is hard to understand the suitable amendment with its right dose for an efficient immobilization. Hence, exploration of new amendments due to the viability of different raw materials, their continuous testing, and performance monitoring as additives increase the interest of researchers toward immobilization during modern ages (Chiang et al. 2012; Peng et al. 2018; Nejad et al. 2018).
6.1 Immobilization Through Organic Soil Additives
Several organic compounds varying in properties supremely immobilize certain HMs in the environment. Generally, it includes different kinds of manures, biosolids, wood ash, biochar, different composts, and wood chips (Sabir et al. 2013; Peng et al. 2018). Organic amendments significantly bind HMs with the assistance of numerous functional groups present in humic acids, which reduced the bioavailability of HMs to some extent (Lwin et al. 2018). Moreover, organic-based additives not only bind HMs but also nourish the soil. However, the only drawback in the addition of organic amendments is the risk of HMs remobilization due to the decomposition of organic matter (Lwin et al. 2018).
6.1.1 Biochar
The permeable and carbonaceous material formed after pyrolyzing the organic feedstocks like manures, plant-wood biomass, and sludges is termed as “Biochar” (Nejad et al. 2018; Lwin et al. 2018). Biochar has distinctive attributes, i.e., higher CEC values, large surface area, alkaline nature, higher sorption capacity with many functional groups, and presence of humic and fulvic substances (Lin et al. 2012; Lwin et al. 2018). Interestingly, biochar has positively influenced the soil properties via enhancing its microbial activity and carbon sequestration (Paz-Ferreiro and Fu 2014; Nejad et al. 2018; Lwin et al. 2018). The co-effect of biochar with chitosan in the soil irrigated with wastewater containing excessive HMs was also tested to save the quality of brinjal. The combined application of biochar and chitosan significantly reduces the bioavailability of numerous HMs in the soil and promote the safe production of brinjal (Turan et al. 2018).
It acts as an excellent HMs adsorbent due to the presence of diverse functional groups such as hydroxyl, carboxyl, and phenolic hydroxyl, and presence of negative charge sites in its chemical structure, which helped in retaining more HMs in it (Mahar et al. 2015; Kammann et al. 2015; Lwin et al. 2018). Previous studies showed the remarkable immobilization of different HMs with biochar additions. For instance, biochar applications in HMs contaminated soils reduced the bioavailability of different HMs (Mahar et al. 2015; Huang et al. 2017). Likewise, the type of feedstock, as well as pyrolysis temperature, also influenced HMs immobilization in the soil (Igalavithana et al. 2017a, b; Khan et al. 2017, 2020). The application of biochar produced from different feedstock such as hardwood (Beesley et al. 2010), shell and cow bone (Ahmad et al. 2012), chicken manure, and green waste (Park et al. 2011; Lwin et al. 2018) in HMs contaminated soils resulted in the reduction of HMs bioavailability in soil. Likewise, the biochar produced from cottonseed hull feedstock at a higher temperature significantly adsorbs HMs due to change in the composition of oxygen-containing functional groups (Mahar et al. 2015). The reduction in HMs bioavailability could be due to the change in pH of the soil from acidic to alkaline, which increased the concentrations of OH− ions, thereby reducing HMs bioavailability (Cantrell et al. 2012; Joseph et al. 2015). Additionally, the applications of biochar not only reduced HMs bioavailability but also improved the hydro-infiltration, aeration, and aggregate stability of the soil (Lwin et al. 2018) as well as provide essential nutrients such as Mg, Na, K, S, and Ca linked with biochar (Nejad et al. 2018; Uchimiya et al. 2011; Lu et al. 2012). The biochar produced at low (Uchimiya et al. 2011) and high temperatures (Cao et al. 2011; Beesley et al. 2010) released available Ca, P, and K in the soil (Nejad et al. 2018), which improved overall plant health. Numerous studies reported that biochar could also augment microbial growth owing to its various functions of enhancing soil aeration, retaining nutrients, and providing micro-pores as habitats which increased nutrient cycling (Kong et al. 2018).
6.1.2 Mulching and Composting
The use of organic by-products “Mulching or Composting” for soil nourishment and remediation purposes is an effectually and economically viable option (Lwin et al. 2018; Mahar et al. 2015). Similarly, the peculiar nature of mulching is due to its makeup as it is derived from living waste (farmyard/swine manure, cow/pig/poultry waste, green waste). Exemplary mulching promotes nutritional quality and physical properties (particle size division, porosity, and cracking patterns) of the soil (Lwin et al. 2018; Mahar et al. 2015). Simultaneously, the application of mulching in soil stimulates the pH, boosts surface charges, and precipitation mechanisms for HMs immobiliztion. It can adsorb HMs through metal binders (phosphates or carbonates), but the performance of mulching in the soil completely relies on soil nature, pH, EC, humification, and CEC. Likewise, organic mulching significantly reduces the bioavailability of HMs and their uptake by the plants (Alamgir et al. 2011; Mahar et al. 2015; Lwin et al. 2018). Interestingly, the addition of compost/mulching in contaminated soil increases soil pH, encourages growth and nutrients uptake in plants via enhanced root development, and strengthens the activity of microbes as well as HMs immobilization. Furthermore, mulching also fertilizes the soil and plants by augmenting the concentrations of essential elements like N, P, Ca, Fe, and Mg, organic and microbial C, which influenced the soil respiration and enzymatic activities. The other side of mulching should also be considered because the organic amendments also have disease-causing bacteria and higher concentrations of dissolved salts, which can introduce new pathways of HMs in soil (Mahar et al. 2015; Lwin et al. 2018).
6.1.3 Agricultural Waste
Researchers have continuously discovered the cheap and accessible amendments for the remediation of HMs contaminated soils. Interestingly, agricultural waste is a powerful amendment for HMs immobilization in the soil. Agricultural waste includes rice−wheat husks and brans sawdust of several plants, the bark of trees, groundnut, and coconut shells, hazelnut shells, walnut shells, cotton seeds hull, waste tea leaves, maize corn cob, sugarcane bagasse, fruit and vegetable peels (apple, orange, and banana, etc.), sugar beet pulp, coffee beans, cotton stalks, sunflower stalks, grapes stalks, and Arjun nuts (Sud et al. 2008). The main components of agricultural waste are lignin, hemicellulose, extractives, proteins, sugar, starch, hydrocarbons, cellulose, and functional groups, which participated in the adsorption of HMs. The worthwhile agricultural waste is a highly efficient, nutrient-rich, eco-friendly amendment and used for the removal of HMs present in the soil. Interestingly, several HMs are removed from the environment by applying agro-waste. Under optimum pH conditions, agro-waste such as rice husk, sawdust of rubber, and Indian rosewood successfully stabilized higher Cr concentrations in polluted soils. Interestingly, the effective Pb immobilization was also reported with the assistance of different agro-waste (Sud et al. 2008).
6.2 Geo-Polymers as Stabilizing Additives
The geopolymers are also known as a promising ceramic adsorbent for HMs adsorption from domestic and industrial effluents. It is produced during the reaction between NaOH or KOH with Si or Al and has a great potential to clean the HMs contaminated sites. The divine and multitasking geopolymer are hard, heat and fire resistant, high water retention, and suitable shear stress as well as compressive strengths and used for the adsorption of HMs (Rasaki et al. 2019). Geopolymers comprising minerals, clay, slag, fly ash, and cement, which contains interlinked crossed bonds with cations having larger surface areas, substantially removed numerous HMs (Sturm et al. 2016; Rasaki et al. 2019; Cheng et al. 2012). Geopolymers show excellent adsorbing capabilities due to their larger surface area, high porosity, and strong adhesive forces because of mesopores that tightly bind HMs. Geopolymeric materials have proven a substantial HMs stabilization potential in metal-polluted soils owing to their strong adsorbing as well binding capabilities (Rasaki et al. 2019).
6.2.1 Cement-Based Stabilizing Agents
Cement is a strong binding agent to stabilize HMs in contaminated soils and significantly decreases their leaching as well as bioavailability due to the presence of Ca silicates, sulfates, aluminates, and alumino-ferrite minerals. Immobilization with cement is an interesting way of remediating metal-polluted soils, but its efficiency depends on its composition, chemical structure, temperature, particle size, etc. (Wuana and Okieimen 2011; Mahar et al. 2015).
The applications of Portland cement were being effective in reducing Pb and Cd concentrations in the leachates due to a reduction in their bioavailability (Voglar and Leštan 2011; Mahar et al. 2015). Likewise, magnesium phosphate cement has also outstanding features such as quick setting, strong binder, and excellent stabilization potential (Xu et al. 2015; Wang and Dai 2017; Wang et al. 2018). Magnesium phosphate cement is also used for remediation of Pb polluted soils by forming pyromorphite and lead phosphate complexes (Wang et al. 2018; Debela et al. 2013).
6.2.2 Clay Minerals
Remediation of metal contaminated soils with clay amendments is an innovative idea due to their several advantages such as universal availability, wide acceptability at commercial scale, high efficiency, and economic viability. Bentonite, attapulgite, and sepiolite are known to be the most common clay amendments recommended for remediating agricultural soils contaminated with HMs (Yi et al. 2017; Yuan et al. 2013). Bentonite mainly composed of montmorillonite, useful clay for reducing the bioavailability of HMs in the soil via providing adsorption sites (Yi et al. 2017; Sun et al. 2015). Likewise, attapulgite (palygorskite) is also effectively capable of immobilizing different HMs from contaminated sites. Attapulgite shows the quick and strong fixation of HMs in paddy soils by increasing its pH, thereby decreasing their leaching effect (Yi et al. 2017; Sheikhhosseini et al. 2013). Sepiolite proved to be the highest sorption capabilities of HMs immobilization due to its divine structure made up of one sheet of octahedral magnesium oxide or hydroxide between two sheets of tetrahedral silica (Yi et al. 2017). Previously, the additions of sepiolite not only stabilized various HMs but also reduced their uptake in different plants such as spinach, rice, alfalfa, and ryegrass (Sun et al. 2016; Liang et al. 2016; Wu et al. 2016; Abad-valle et al. 2016). Similarly, bentonite, palygorskite, and attapulgite effectively immobilized different HMs through immobilizing them, which resultantly restricted their uptake (Yi et al. 2017; Sun et al. 2015; Argiri et al. 2013; Houben et al. 2012; Zotiadis et al. 2012; Zhang et al. 2011). Correspondingly, biochar mixed with zeolite effectively reduced Ni mobility and its uptake in maize, sunflower, and red clover and improved the nutrient and health status of these plant species (Shahbaz et al. 2018a, b).
Although these clay minerals have the great immobilizing potential for remediation purposes, however, prior knowledge about their effective dose remarkably influenced the progress of remediation strategy.
6.3 Immobilization Through Inorganic Amendments
The stabilization of HMs with the assistance of cost-effective, energy-efficient, natural, and synthetic inorganic amendments is remarkable. Several inorganic stabilizers used for HMs immobilization are phosphates, calcium, silicon compounds, and mineral amendments (Peng et al. 2018).
6.3.1 Calcium Compounds
The particular calcium forms used for the remediation of HMs contaminated soils are gypsum and liming substances. The ability of gypsum for metal adsorption has already been extensively available in the literature. Gypsum was used for reducing the concentrations of different HMs (Cu, Pb, Cr, and Cd) in polluted soils due to the presence of excessive sulfide formation (Tsunematsu et al. 2012; Vink et al. 2010). Gypsum acts as a soil conditioner, provides nutrients, and enhances soil productivity and soil physical properties as well as improved sulfur and calcium uptake in plants. The HMs contaminated soils have poor structure as well as less aggregation, prone to erosion and infertility but the addition of Ca in gypsum form promote the development of soil structure (Smith 2011). The long-term effects of gypsum application to soil improved the Ca and P status in the soil, which strengthens the soil solution ionic system. On the other side, calcium addition in the form of lime ameliorated the acidity of soil and was effective for HMs immobilization. Lime applications in contaminated soil significantly reduce the DTPA extractable Zn, Fe, and Cu. Interestingly, lime materials induce high pH, which initiates hydrolysis reactions and produces metal precipitates. Interestingly, the lime effect in the soil causes nutrient bioavailability in soil. Although liming application induces better soil environment by fixing HMs, however, too much liming effect can inhibit the availability of beneficial micronutrients (Fe, Zn, and Mn) to plants and soil (Lwin et al. 2018).
6.3.2 Phosphorus Compounds
Both natural and synthetic phosphorous-based amendments effectively remediate metal-polluted soils (Lwin et al. 2018). The eminent nature of phosphorous compounds can be hydrophilic like diammonium phosphate and hydrophobic such as phosphate rocks for stabilizing HMs in soil (Nejad et al. 2018; Lwin et al. 2018; Mahar et al. 2015). The use of P-additives stabilized HMs by forming insoluble metal precipitates or by binding them through adsorption (Lwin et al. 2018; Bolan et al. 2014). Likewise, hydroxypyromorphite and chloropyromorphite as P-amendments were also useful in reducing Pb bioavailability in contaminated soils (Chen and Li 2010; Nejad et al. 2018). Although phosphate-based amendments are adequate for remediating the metal-polluted soils, however, the interaction of phosphate minerals with targeted pollutants is of a great concern due to their remobilization risk in the soil (Mahar et al. 2015; Nejad et al. 2018; Lwin et al. 2018). For example, the additions of single and triple superphosphates (SSP and TSP) enhance the Cd concentrations in soil (Lwin et al. 2018).
6.3.3 Iron Compounds
Immobilization of HMs in contaminated soils with iron-based materials has already been well examined in recent years. It was realized that the zero-valent iron (ZVI) and FeII tend to remove the HMs concentrations from the environment. Zero-valent iron binds HMs and other pollutants due to its strong oxidizing–reducing nature, which helps in reducing HMs bioavailability. For example, ferrous salt applications such as ferrous sulfate heptahydrate not only produced Cr precipitates but also transformed it into less toxic and more stable form (Hashim et al. 2011).
6.4 Metal Oxides
Metal oxides play an active role in controlling the chemistry of HMs in the soil. Several metal oxides such as Fe, Al, Ti, Ce, and Mn immobilize HMs in the soil due to large surface area, which makes them suitable for immobilization (Hua et al. 2012). These metal oxides bind HMs through co-precipitation, sorption, and forming metal complexes (Bolan et al. 2014). For example, the oxides of Mn such as todorokite, hausmannite, and cryptomelane have been previously used for reducing Pb bioavailability (Bolan et al. 2014). Likewise, iron oxides such as hematite, magnetite, and maghemite significantly reduced the concentrations of exchangeable Zn fractions as well as significantly decreased the DTPA-extractable Zn, Cu, and Cd.
6.5 Industrial By-products
The sustainable use of industrial waste products such as fly ash, red mud, iron and steel slag, sludge, and paper waste discharged from different industries has been well documented in recent years (Dang et al. 2019). Moreover, handling several hundred tons of wastes of such by-products required additional disposal sites; therefore, it is recommended to use them in a sustainable way rather deteriorating environmental quality (Lwin et al. 2018; Sengupta and Tarar 2014). These by-product additives are not only efficient in reducing HMs pollution but also cost effective (Ishak and Abdullah 2014). Previously, the applications of fly ash, slag, and red mud have been reported to reduce the bioavailability of several HMs by enhancing soil pH or by binding them via chemisorption onto the surfaces of oxides and hydroxides of Al and Fe (Lwin et al. 2018; Ning et al. 2016; Valerie 2015). Interestingly, both organic and inorganic industrial waste products also contain other essential nutrients such as P, S, K, B, Mn, Mo, Si, and Fe, which improved plant growth and soil enzymes (Lwin et al. 2018; Teresa and Valentina 2012). Soil structure, CEC, bulk density, water-holding capacity, and aggregate stability are known to be improved after the applications of these industrial waste products in HMs contaminated soils (Stanojković-Sebić et al. 2014). Besides these positive aspects of applying industrial waste products for remediation purposes, the presence of toxic hazardous substances in them should be considered carefully (Klebercz et al. 2012; Lwin et al. 2018).
6.6 Immobilization as a Group Technology
This section provides the recent trends in the field of immobilization coupled with other remediation techniques.
6.6.1 Immobilization Coupled with Phytomanagement
The remediation of HMs contaminated soils with the co-application of immobilization and phytoremediation was considered contradictory because the former reduced the bioavailability while the latter required higher HMs solubilization for plant uptake. Immobilizing additives not only reduced the excessive HMs leaching from the contaminated sites but also supported plant establishment by their limited uptake (Muhammad et al. 2012; Iqbal et al. 2012). For example, during the enhanced phytoextraction, the co-application of elemental sulfur with red mud and gravel sludge not only reduced Cd and Zn leaching but also enhanced their uptake by S. smithiana from Pb-Zn smelter contaminated soil (Iqbal et al. 2012). Like enhanced phytoextraction, aided phytostabilization coupled with organic and inorganic amendments has already been reported for the management of soils very highly contaminated with an individual (Lu et al. 2014) or multi-HMs such as military shooting ranges (Lago-Vila et al. 2019; Radziemska et al. 2019, 2020) and mining soils. However, this technique does not work well for such HMs contaminated soils with low pH and high salt contents (Gascó et al. 2019; Wang et al. 2012).
6.6.2 Immobilization Coupled with Microorganisms
The combined applications of amendments with (micro)organisms also attained the interest of researchers worldwide. Bioimmobilization remediation method reduces HMs bioavailability by bioprecipitation, bioaccumulation, and biosorption. For instance, phosphorous solubilizing microorganisms reduced Pb mobility by forming insoluble Pb–phosphate complexes by releasing phosphorus from its sources (Jalili et al. 2020; Khan et al. 2020; Nejad et al. 2018; Mahar et al. 2015). Likewise, the applications of arbuscular mycorrhizal fungi (AMF) with lignin-derived biochar in Pb-acid batteries contaminated soils significantly reduced Pb uptake and its translocation to barley grain. The mechanism associated with this reduction in Pb transport was due to restricted Pb accumulation in root cells, its adsorption with glomalin as well as immobilization in mycelia of AMF (Khan et al. 2020). Also, these microorganisms improved soil health owing to the presence of porous structure which served as a habitat for them as well as through solubilizing essential nutrients from the soil or organic matrix (Peng et al. 2018).
7 The Mechanism Involved in the Immobilization of HMs
Different mechanisms governed the chemistry and stabilization of HMs upon the addition of various organic and inorganic amendments in contaminated soils. Silicon dioxide plays a vital role in HMs immobilization, which strongly binds HMs onto the surfaces of SiO2, thereby reducing their mobility (Ricou-Hoeffer et al. 2000). The presence of SiO2 in both organic and inorganic stabilizing agents increased the formation of metal oxides as well as metal silicides. Likewise, the presence of Ca and Si complexes such as calcium silicates are known to have higher efficient adsorption capacity, which enhanced HMs immobilization by forming insoluble surface precipitates. In general, the presence of clay minerals, organic matter, the oxides and hydroxides of Fe and Mn, amorphous aluminosilicates, and calcium carbonates strongly influences the absorption of HMs in the soil. These substances provide phenolic, amino, carboxylic, and alcoholic functional groups in the soil, which strongly bind HMs (Nejad et al. 2018).
8 Recent Advances in Managing HMs Contaminated Soils Through Immobilization
This segment provides the necessary information about recent advancements in the immobilization remediation technique.
8.1 Modifications of Organic Additives
Recent advancements in research have proven that biochar has immense potential as an immobilizing amendment owing to comparatively its low cost and the availability of plenty of raw feedstock resources. Hence, biochar has developed as a practical alternative in remediating and managing different contaminants especially HMs. The physical and chemical characteristics of biochar depend upon several factors such as the type of feedstock as well as pyrolysis conditions such as temperature, residence time, and heating rate (Zhou et al. 2013). The modification and functionalization of biochar through different processes aimed to enhance its surface or interlayer spaces, pore size, molecular weight, CEC, and the type and amount of functional groups, which enhanced the HMs adsorption capacities of engineered biochars as compared to their native biochars (Nazari et al. 2019). Rajapaksha et al. (2016) and references therein comprehensively provided the essential information regarding different modification methods and associated mechanisms for the management of numerous contaminants present in the environment. The applications of magnetically modified poultry litter biochar in multi-HMs contaminated paddy soil collected from near Pb-Zn mine area remarkably increased plant growth while decreased HMs leaching (Lü et al. 2018). Similarly, the additions of modified coconut shell-derived biochar in multi-HMs contaminated soils not only resulted in the better immobilization of HMs but also increased soil biological properties as compared to native coconut shell biochar (Liu et al. 2018). The applications of modified biochar-chitosan-clay nano-amalgams and their applications in the simultaneous stabilization of HMs from the acid mine soil and water have been tested. Results revealed that HMs were efficiently immobilized by forming nanocomposites due to the presence of −NH2 groups (Arabyarmohammadi et al. 2018). Biochar chitosan and hematite composites also resulted in the efficient transformation of CrVI in contaminated soils. The additions of biochar-chitosan and biochar-hematite resulted in the Cr reduction up to 46% and 38% in contrast to biochar treatment (Zibaei et al. 2020). The application of biochar supported carboxymethyl cellulose nanoscale iron sulfide (FeS) composite has been evaluated for Cr stabilization in the soil. The additions of composite in the soil increased Cr immobilization up to 94% while decreased its concentration up to 95% in CaCl2 extract (Lyu et al. 2018).
8.2 Clay Modifications
Previously, the applications of clay minerals with other organic and inorganic amendments reported to efficiently reduce the bioavailability, mobility, and toxicity of HMs from the ecosystem (Sarkar et al. 2019; Yadav et al. 2019). Recently, the modifications of geomaterials through different methods have been reviewed in detail by Han et al. (2019), Sarkar et al. (2019), and citations therein for the removal of different HMs from the environment (Han et al. 2019; Sarkar et al. 2019). The attapulgite was modified with zero-valent iron and used for the immobilization of different HMs in the soil. Results suggested that the stabilizing additives remarkably reduced the extractable concentrations of HMs in the soil and promoted seed germination, root development as well as the activities of antioxidants by diminishing the contents of ROS (Xu et al. 2019). Sepiolite additions with phosphate fertilizer, limestone, and biochar resulted in the reduced bioavailability of HMs, which revealed its compatibility with other amendments. The successful immobilization of Cd in contaminated paddy soils was observed in sepiolite infused with limestone treatment (Yi et al. 2017). Likewise, the combined applications of bentonite with sodium, sepiolite with bentonite, limestone, and phosphate increased the immobilization of different HMs in the soil (Sun et al. 2016; Montenegro et al. 2015; Zhou et al. 2013; Liang et al. 2011). Although these clay composites are efficient in alleviating HMs toxicity from the soil, however, prior knowledge about their proper dose, the microbial status of the soil, and physicochemical features of the multi−contaminated matrix should be accounted for their better performance at the field scale.
9 Estimating the Efficacy of Immobilizing Agents Through Long-Term Monitoring at Field Scale
The success of immobilization remediation strategies depends upon numerous factors such as the extent and nature of soil contamination, soil pH, the presence of other mineral ions, land-use practices, as well as the economic feasibility of the remediation process. Therefore, sufficient knowledge about soil type taken into consideration before selecting a remediation plan for a specific site (Puschenreiter 2008; Sun et al. 2016). Also, immobilizing agents with strong HMs binding capabilities and long-term durability proven to be effective in immobilization, and the decision-makers allow to use them in field experiments (Xu et al. 2018; Sun et al. 2016). The significant valuable facts about the possibilities of ex-situ immobilization acquired from long-term field experiments in contrast to short-term pot and model experiments (Sun et al. 2016). The Cd contaminated soil was amended with different rates of sepiolite (0.1, 0.5, 1% w/w) to assess its toxicity to rice plants in a field experiment. The significant reduction of up to 49% as well as 50% of Cd concentrations in rice grain was observed during the first- and second-year experiments, respectively. The sepiolite additions for 2 successive years in a field restricted Cd translocation toward rice grain up to 75%, which promotes safe rice production for human consumption (Chen et al. 2020). The co-applications of biochar, as well as sepiolite, increased the growth, biomass, and grain quality of maize when grown in farmlands contaminated with HMs due to mining activity. This improvement in the growth of maize attributed to the reduction in Cd bioavailability, thereby restricting its contents in plants and grain (Zhan et al. 2019). The effectiveness of limestone and sepiolite amendments has been investigated to reduce exchangeable Pb and Cd in a 3-year in situ soil experiment. It was observed that the influences of limestone and sepiolite on exchangeable Cd remain relatively persistent, while a gradual decrease in exchangeable Pb was observed with time (Wu et al. 2016). Likewise, the long-term influence of sepiolite additives in Cd contaminated paddy soil was observed in a 2-year field experiment. A remarkable stabilization effect of sepiolite in Cd contaminated soil was observed, which also persisted during the next year. Results revealed that exchangeable as well as HCl–Cd labile fractions remain reduced, which showed the long-term stabilizing effect of sepiolite (Liang et al. 2016). The changes in the physicochemical properties of the soil due to weathering processes increase the risk of HMs remobilization. Likewise, the breakdown of organic matter also resulted in the release of organic-bound HMs, which increases the risk of their leaching especially from the soils with low pH values. Therefore, the long-term continuous monitoring of such sites is recommended to control other environmental hazards.
10 Environmental Concerns Associated with HMs After Their Fixation
The efficacy and potential dissolution of HMs from the amended soils could be assessed through different physicochemical and biological methods.
10.1 Biological Assessment Method
The phytotoxic assessment method is the most reliable, efficient, and widely acceptable method, performed to test the effectiveness of stabilizing agents. The improved plant height and biomass production was observed in plants grown on amended HMs contaminated soils (Lu et al. 2014). The reduced HMs uptake by the plants due to a reduction in bioavailability after their fixation on to the surfaces of immobilizing agents is another additional parameter of the phytotoxic assessment method. Presently, this trend is gaining the attention of scientists for the management of multicontaminated soils such as military firing range (Lago-Vila et al. 2019; Radziemska et al. 2019, 2020) as well as mining soils (Gascó et al. 2019). Moreover, soil (micro)organisms can also be used as indicators to assess the contamination level before and after the additions of immobilizing agents (Huang et al. 2020; Khan et al. 2020; Tu et al. 2020).
10.2 Physiochemical Assessment
Presently, different microdetection and fraction schemes were used to determine HMs fractions by taking soil water, DTPA, Ca(NO3)2 (Shahbaz et al. 2018a) as well as HCl extractants (Zeng et al. 2018). Likewise, the DGT (diffusive gradient in thin film) technique is also being used to determine HMs flux released by diffusion from the solid phase to soil solution (Muhammad et al. 2012). Additionally, single extractions through toxicity characteristic leaching procedure (TCLP), as well as sequential extraction methods, were also used to determine the behavior and speciation of HMs through a particular extractant (Guo et al. 2006). Among these methods, the sequential extraction developed by Tessier et al. (1979) has been widely used in soil experiments.
10.3 Release Assessment Approaches
The release of HMs from the immobilizing additives can never be avoided due to change in the soil properties as well as weathering processes. The mechanism associated with the release of HMs after fixation can be assessed by two empirical models.
10.3.1 Bulk Diffusion Model
This model describes the release of HMs from the cement-based stabilization matrix in HMs contaminated soils. The bulk diffusion is the main driving force that enhanced HMs release from monolith due to the breakdown of the outer shell of the stabilizing agent. The bulk diffusion model can be calculated according to the below equation of Fickian diffusion (Baker and Bishop 1997):
In the above expression, De represents the effective diffusion coefficient (cm2 S−1) associated with the porosity and tortuosity; C expresses HMs (g cm−3) concentrations; t expresses time (s), and x expresses the distance (cm). However, this model was considered failing in predicting the long-term leaching of HMs from contaminated soils under acidic conditions (Guo et al. 2006).
10.3.2 Shrinking Unreacted Core (SUC) Model
The SUC model well explains the release and dissolution mechanisms of HMs from the stabilizing agent under acidic conditions. This release was attributed to the disintegration of outer surfaces of immobilizing agents, which caused leaching of contaminants. The expression of potential release factor (PRF) evolved from the SUC model was proposed as a means of determining HMs mobilizing efficacy from the fixed surface (Baker and Bishop 1997).
PRF = \( \frac{\sqrt{2{D}_{\mathrm{e},\mathrm{s}}\ {f^2}_{\mathrm{m}\mathrm{o}}\ {C^2}_{\mathrm{m}}}}{\ {\beta}_{\mathrm{c}.}} \)
In this equation, PRF expresses potential release factor; De,s the coefficient of effective diffusion (under acidic environments); fmo expresses dimensionless leachable fractions; Cm denotes the concentrations of solid pollutant (mol cm−3), and βc acid neutralization capacity (kmol eq m−3).
11 Conclusion and Way Forward
The accumulation of HMs in soils due to their release from different anthropogenic sources significantly reduced productive agricultural lands for sustainable food production. Such poorly vegetated HMs contaminated soils are considered to be the continuous release of pollution via leaching or runoff affecting the other natural resources. Therefore, the remediation of HMs contaminated soils is necessary to prevent other additional hazards associated with them. The primary objective of remediation work is to reduce the risk of human exposure, limit the level of contamination, and prevent further deterioration of other environmental resources. In the past, different destructive remediation methods such as soil washing, soil excavation, soil replacement, vitrifying, and electrokinetic methods have been practiced for the management of HMs contaminated soil. Although, these methods are effective in limiting the concentrations of HMs from the soil, however, damaged soil fertility and organic matter alter physicochemical properties of the soil and produced residual wastes that required additional treatment cost. The success of the remediation technique entirely depends upon the speciation of HMs, soil factors as well as the level and depth of contamination. Therefore, the standards for the selection of suitable remediation methods are (1) long-term efficiency and durability of stabilizing agents in achieving the remediation objectives, (2) efficient reduction in the volume of HMs, (3) effective in promoting plant establishment via reducing HMs toxicity, and (4) cost effective. In situ stabilization of HMs in soils by using different organic, inorganic, and other stabilizing agents has gained the attention of scientists worldwide due to its economic feasibility and high efficiency. The additions of amendments in single or co-contaminated HMs not only reduced the mobility, toxicity as well as their labile fractions but also supported plant establishment with maximum production. Among different HMs immobilizing agents, the significant results were acquired in HMs contaminated soils (field and pot trials) when amended with clay minerals, rock phosphate, biochar, calcium hydroxide, phosphates, and hydroxylapatite. The dissolution of HMs due to climatic factors from stabilizing agents may result in their leaching. Therefore, the long-term monitoring of HMs contaminated sites is necessary to control such environmental hazards.
References
Abad-Valle P, Álvarez-Ayuso E, Murciego A, Pellitero E (2016) Assessment of the use of sepiolite amendment to restore heavy metal polluted mine soil. Geoderma 280:57–66
Adeyeye EI (1994) Determination of trace heavy metals in Illisha africana fish and in associated water and soil sediments from some fish ponds. Int J Environ Stud 45:231–238
Ahmad M, Lee SS, Yang JE, Ro HM, Lee YH, Ok YS (2012) Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol Environ Saf 79:225–231
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2015) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86:24–36
Alamgir M, Kibria MG, Islam M (2011) Effects of farm yard manure on cadmium and lead accumulation in Amaranth (Amaranthus oleracea L.). J Soil Sci Environ Manag 2(8):237–240
Alshawabkeh AN (2009) Electrokinetic soil remediation: challenges and opportunities. Sep Sci Technol 44(10):2171–2187
Arabyarmohammadi H, Darban AK, Abdollahy M, Yong R, Ayati B, Zirakjou A, van der Zee SE (2018) Utilization of a novel chitosan/clay/biochar nanobiocomposite for immobilization of heavy metals in acid soil environment. J Polym Environ 26(5):2107–2119
Argiri A, Ioannou Z, Dimirkou A (2013) Impact of new soil amendments on the uptake of lead by crops. Commun Soil Sci Plant 44:566–573
Atafar Z, Mesdaghinia A, Nouri J, Homaee M, Yunesian M, Ahmadimoghaddam M, Mahvi AH (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160(1–4):83
Baker PG, Bishop PL (1997) Prediction of metal leaching rates from solidified/stabilized wastes using the shrinking unreacted core leaching procedure. J Hazard Mater 52(2–3):311–333
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL (2010) Effects of biochar and green waste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158:2282–2287
Beiyuan J, Awad YM, Beckers F, Tsang DC, Ok YS, Rinklebe J (2017) Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 178:110–118
Beolchini F, Fonti V, Rocchetti L, Saraceni G, Pietrangeli B, Dell’Anno A (2013) Chemical and biological strategies for the mobilisation of metals/semi-metals in contaminated dredged sediments: experimental analysis and environmental impact assessment. Chem Ecol 29(5):415–426
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal(loid)s contaminated soils—to mobilize or to immobilize? J Hazard Mater 266:141–166
Cai LM, Wang QS, Wen HH, Luo J, Wang S (2019) Heavy metals in agricultural soils from a typical township in Guangdong Province, China: occurrences and spatial distribution. Ecotoxicol Environ Saf 168:184–191
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428
Cao XD, Ma L, Liang Y, Gao B, Harris W (2011) Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ Sci Technol 45:4884–4889
Chaoua S, Boussaa S, El Gharmali A, Boumezzough A (2019) Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J Saudi Soc Agric Sci 18(4):429–436
Chrastný V, Komárek M, Hájek T (2010) Lead contamination of an agricultural soil in the vicinity of a shooting range. Environ Monit Assess 162:37-46.
Chen YH, Li FA (2010) Kinetic study on removal of copper (II) using goethite and hematite nano-photocatalysts. J Colloid Interface Sci 347(2):277–281
Chen D, Ye X, Zhang Q, Xiao W, Ni Z, Yang L, Huang M (2020) The effect of sepiolite application on rice Cd uptake–a two-year field study in southern China. J Environ Manag 254:109788
Cheng TW, Lee ML, Ko MS, Ueng TH, Yang SF (2012) The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl Clay Sci 56:90–96
Cheraghi M, Lorestani B, Merrikhpour H (2012) Investigation of the effects of phosphate fertilizer application on the heavy metal content in agricultural soils with different cultivation patterns. Biol Trace Elem Res 145(1):87–92
Chiang YW, Santos RM, Ghyselbrecht K, Cappuyns V, Martens JA, Swennen R, Meesschaert B (2012) Strategic selection of an optimal sorbent mixture for in-situ remediation of heavy metal contaminated sediments: framework and case study. J Environ Manag 105:1–11
Dang VM, Joseph S, Van HT, Mai TLA, Duong TMH, Weldon S, Taherymoosavi S (2019) Immobilization of heavy metals in contaminated soil after mining activity by using biochar and other industrial by-products: the significant role of minerals on the biochar surfaces. Environ Technol 40(24):3200–3215
Debela F, Arocena JM, Thring RW, Whitcombe T (2013) Organic acids inhibit the formation of pyromorphite and Zn-phosphate in phosphorous amended Pb-and Zn-contaminated soil. J Environ Manag 116:156–162
Ettler V (2016) Soil contamination near non-ferrous metal smelters: a review. Appl Geochem 64:56–74
Fayiga AO (2019) Remediation of inorganic and organic contaminants in military ranges. Environ Chem 81–91
Finzgar N, Tlustos P, Lestan D (2007) Relationship of soil properties to fractionation, bioavailability and mobility of lead and zinc in soil. Plant Soil Environ 53(5):225
Fonti V, Dell’Anno A, Beolchini F (2013) Influence of biogeochemical interactions on metal bioleaching performance in contaminated marine sediment. Water Res 47(14):5139–5152
Gascó G, Álvarez ML, Paz-Ferreiro J, Méndez A (2019) Combining phytoextraction by Brassica napus and biochar amendment for the remediation of a mining soil in Riotinto (Spain). Chemosphere 231:562–570
González-Guerrero M, Melville LH, Ferrol N, Lott JN, Azcon-Aguilar C, Peterson RL (2008) Ultrastructural localization of heavy metals in the extraradical mycelium and spores of the arbuscular mycorrhizal fungus Glomus intraradices. Can J Microbiol 54(2):103–110
Guo G, Zhou Q, Ma LQ (2006) Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: a review. Environ Monit Assess 116:513–528
Han H, Rafiq MK, Zhou T, Xu R, Mašek O, Li X (2019) A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J Hazard Mater 369:780–796
Hashim MA, Mukhopadhyay S, Sahu JN, Sengupta B (2011) Remediation technologies for heavy metal contaminated groundwater. J Environ Manag 92(10):2355–2388
Hembrom S, Singh B, Gupta SK, Nema AK (2020) A comprehensive evaluation of heavy metal contamination in foodstuff and associated human health risk: a global perspective. In: Contemporary environmental issues and challenges in era of climate change, pp 33–63
Houben D, Pircar J, Sonnet P (2012) Heavy metal immobilization by cost-effective amendments in a contaminated soil: effects on metal leaching and phytoavailability. J Geochem Explor 123:87–94
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331
Huang D, Liu L, Zeng G, Xu P, Huang C, Deng L, Wang R, Wan J (2017) The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere 174:545–553
Huang Y, Chen Q, Deng M, Japenga J, Li T, Yang X, He Z (2018) Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in Southeast China. J Environ Manag 207:159–168
Huang C, Wang W, Yue S, Adeel M, Qiao Y (2020) Role of biochar and Eisenia fetida on metal bioavailability and biochar effects on earthworm fitness. Environ Pollut 114586
Igalavithana AD, Lee SE, Lee YH, Tsang DC, Rinklebe J, Kwon EE, Ok YS (2017a) Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 174:593–603
Igalavithana AD, Park J, Ryu C, Lee YH, Hashimoto Y, Huang L, Lee SS (2017b) Slow pyrolyzed biochars from crop residues for soil metal(loid) immobilization and microbial community abundance in contaminated agricultural soils. Chemosphere 177:157–166
Iqbal M, Puschenreiter M, Oburger E, Santner J, Wenzel WW (2012) Sulfur-aided phytoextraction of Cd and Zn by Salix smithiana combined with in situ metal immobilization by gravel sludge and red mud. Environ Pollut 170:222–231
Ishak CF, Abdullah R (2014) In-situ immobilization of selected heavy metals in soils using agricultural wastes and industrial by-products. In: Proceedings of MARCO-FFTC joint Int. seminar on management and remediation technologies of rural soils contaminated, pp 22–26
Jalili B, Sadegh-Zadeh F, Jabari-Giashi M, Emadi M (2020) Lead bioimmobilization in contaminated mine soil by Aspergillus Niger SANRU. J Hazard Mater 393:122375
Joseph S, Husson O, Graber ER, van Zwieten L, Taherymoosavi S, Thomas T (2015) The electrochemical properties of biochars and how they affect soil redox properties and processes. Agronomy 5(3):322–340
Johnsen IV, Mariussen E, Voie Ø (2019) Assessment of intake of copper and lead by sheep grazing on a shooting range for small arms: a case study. Environ Sci Pollut Res 26:7337–7346.
Juris B, Karina S, Ikrema H, Reinis J, Sandris L (2015) Removal of heavy metals from contaminated soils by electrokinetic remediation
Kammann CI, Schmidt HP, Messerschmidt N, Linsel S, Steffens D, Müller C, Koyro HW, Conte P, Joseph S (2015) Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci Rep 5:11080
Kelebemang R, Dinake P, Sehube N, Daniel B, Totolo O, Laetsang M (2017) Speciation and mobility of lead in shooting range soils. Chem Spec Bioavailab 29:143–152
Khan W, Ramzani PMA, Anjum S, Abbas F, Iqbal M, Yasar A, Ihsan MZ, Khan SA (2017) Potential of miscanthus biochar to improve sandy soil health, in situ nickel immobilization in soil and nutritional quality of spinach. Chemosphere 185:1144–1156
Khan MA, Ramzani PMA, Zubair M, Rasool B, Khan MK, Ahmed A, Iqbal M (2020) Associative effects of lignin-derived biochar and arbuscular mycorrhizal fungi applied to soil polluted from Pb-acid batteries effluents on barley grain safety. Sci Total Environ 710:136294
Kierczak J, Potysz A, Pietranik A, Tyszka R, Modelska M, Néel C, Mihaljevič M (2013) Environmental impact of the historical Cu smelting in the Rudawy Janowickie Mountains (South-Western Poland). J Geochem Explor 124:183–194
Klebercz O, Mayes WM, Anton AD, Feigl V, Jarvis AP, Gruiz K (2012) Ecotoxicity of fluvial sediments downstream of the Ajka red mud spill, Hungary. J Environ Monit 14:2063–2071
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides–a review. Environ Pollut 172:9–22
Kong L, Gao Y, Zhou Q, Zhao X, Sun Z (2018) Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. J. Hazard. Mater 343, 276-284.
Kumar V, Singh J, Kumar P (2019) Heavy metals accumulation in crop plants: sources, response mechanisms, stress tolerance and their effects. In: Contaminants in agriculture and environment: health risks and remediation 1, p 38
Kuppusamy S, Palanisami T, Megharaj M, Venkateswarlu K, Naidu R (2016) In-situ remediation approaches for the management of contaminated sites: a comprehensive overview. Rev Environ Contam Toxicol 236:1–115
Lago-Vila M, Rodríguez-Seijo A, Vega FA, Arenas-Lago D (2019) Phytotoxicity assays with hydroxyapatite nanoparticles lead the way to recover firing range soils. Sci Total Environ 690:1151–1161
Li Z, Zhang T (2013) Vitrification. The International Information Center for Geotechnical Engineers
Li P, Lin C, Cheng H, Duan X, Lei K (2015) Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol Environ Saf 113:391–399
Liang X, Xu Y, Wang L, Sun G, Qin X, Sun Y (2011) In-situ immobilization of cadmium and lead in a contaminated agricultural field by adding natural clays combined with phosphate fertilizer. Acta Sci Circum (in Chinese) 31:1011–1018
Liang X, Xu Y, Xu Y, Wang P, Wang L, Sun Y, Huang R (2016) Two-year stability of immobilization effect of sepiolite on Cd contaminants in paddy soil. Environ Sci Pollut Res 23(13):12922–12931
Lin Y, Munroe P, Joseph S, Henderson R, Ziolkowski A (2012) Water extractable organic carbon in untreated and chemical treated bio-chars. Chemosphere 87:151–157
Liu H, Xu F, Xie Y, Wang C, Zhang A, Li L, Xu H (2018) Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci Total Environ 645:702–709
Lu H, Zhang W, Yang Y, Huang X, Wang S, Qiu R (2012). Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res 46(3):854–862
Lu H, Li Z, Fu S, Méndez A, Gascó G, Paz-Ferreiro J (2014) Can biochar and phytoextractors be jointly used for cadmium remediation? PLoS One 9(4)
Lü J, Jiao WB, Qiu HY, Chen B, Huang XX, Kang B (2018) Origin and spatial distribution of heavy metals and carcinogenic risk assessment in mining areas at You’xi county Southeast China. Geoderma 310:99–106
Lwin CS, Seo BH, Kim HU, Owens G, Kim KR (2018) Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—a critical review. Soil Sci Plant Nutr 64(2):156–167
Lyu H, Zhao H, Tang J, Gong Y, Huang Y, Wu Q, Gao B (2018) Immobilization of hexavalent chromium in contaminated soils using biochar supported nanoscale iron sulfide composite. Chemosphere 194:360–369
Mariussen E, Heier LS, Teien HC, Pettersen MN, Holth TF, Salbu B, Rosseland BO (2017) Accumulation of lead (Pb) in brown trout (Salmo trutta) from a lake downstream a former shooting range. Ecotoxicol Environ Saf 135:327–336
Mahar A, Ping W, Ronghua LI, Zhang Z (2015) Immobilization of lead and cadmium in contaminated soil using amendments: a review. Pedosphere 25(4):555–568
Marrugo-Negrete J, Pinedo-Hernández J, Díez S (2017) Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ Res 154:380–388
Montenegro AC, Ferreyroa GV, Parolo ME, Tudino MB, Lavado RS, Molina FV (2015) Copper speciation in soil: time evolution and effect of clay amendment. Water Air Soil Pollut 226:1–10
Muddassir M, Noor MA, Ahmed A, Aldosari F, Waqas MA, Zia MA, Jalip MW (2019) Awareness and adoption level of fish farmers regarding recommended fish farming practices in Hafizabad, Pakistan. J Saudi Soc Agric Sci 18(1):41–48
Muhammad I, Puschenreiter M, Wenzel WW (2012) Cadmium and Zn availability as affected by pH manipulation and its assessment by soil extraction, DGT and indicator plants. Sci Total Environ 416:490–500
Nacke H, Gonçalves AC, Schwantes D, Nava IA, Strey L, Coelho GF (2013) Availability of heavy metals (Cd, Pb, and Cr) in agriculture from commercial fertilizers. Arch Environ Contam Toxicol 64(4):537–544
Naidu G, Ryu S, Thiruvenkatachari R, Choi Y, Jeong S, Vigneswaran S (2019) A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ Pollut 247:1110–1124
Nazari S, Rahimi G, Nezhad AKJ (2019) Effectiveness of native and citric acid-enriched biochar of Chickpea straw in Cd and Pb sorption in an acidic soil. J Environ Chem Eng 7(3):103064
Nejad ZD, Jung MC, Kim KH (2018) Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ Geochem Health 40(3):927–953
Ning D, Liang Y, Song A (2016) In situ stabilization of heavy metals in multiple-metal contaminated paddy soil using different steel slag-based silicon fertilizer. Environ Sci Pollut Res 23:23638
Ottosen LM, Jensena PE, Kirkelunda GM, Dias-Ferreirab C, Hansenc HK (2012) Electrodialytic remediation of heavy metal polluted soil–treatment of water saturated or suspended soil. Chem Eng 28:103–108
Ou-Yang X, Chen JW, Zhang XG (2010) Advance in supercritical CO2 fluid extraction of contaminants from soil. Geol Bull China 29(11):1655–1661
Park JH, Choppala G, Bolan NS, Chung JW, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–445
Pain DJ, Mateo R, Green RE (2019). Effects of lead from ammunition on birds and other wildlife: a review and update. Ambio 48:935–953
Paz-Ferreiro J, Fu S (2014) Biological indices for soil quality evaluation: perspectives and limitations. Land Degrad Dev
Pedersen KB, Kirkelund GM, Ottosen LM, Jensen PE, Lejon T (2015) Multivariate methods for evaluating the efficiency of electrodialytic removal of heavy metals from polluted harbour sediments. J Hazard Mater 283:712–720
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161(2–3):633–640
Peng W, Li X, Xiao S, Fan W (2018) Review of remediation technologies for sediments contaminated by heavy metals. J Soils Sediments 18(4):1701–1719
Puschenreiter M (2008) Final research report
Quenea K, Lamy I, Winterton P, Bermond A, Dumat C (2009) Interactions between metals and soil organic matter in various particle size fractions of soil contaminated with waste water. Geoderma 149(3–4):217–223
Radziemska M, Bęś A, Gusiatin ZM, Cerdà A, Mazur Z, Jeznach J, Brtnický M (2019) The combined effect of phytostabilization and different amendments on remediation of soils from post-military areas. Sci Total Environ 688:37–45
Radziemska M, Bęś A, Gusiatin ZM, Cerdà A, Jeznach J, Mazur Z, Brtnický M (2020) Assisted phytostabilization of soil from a former military area with mineral amendments. Ecotoxicol Environ Saf 188:109934
Rajapaksha AU, Chen SS, Tsang DC, Zhang M, Vithanage M, Mandal S, Ok YS (2016) Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere 148:276–291
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30(6):1562–1574
Rasaki SA, Bingxue Z, Guarecuco R, Thomas T, Minghui Y (2019) Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: a critical review. J Clean Prod 213:42–58
Ricou-Hoeffer P, Hequet V, Lecuyer I, Le Cloirec P (2000) Adsorption and stabilization of nickel ions on fly ash/lime mixing. Water Sci Technol 42:79–84
Rieuwerts JS (2007) The mobility and bioavailability of trace metals in tropical soils: a review. Chem Spec Bioavailab 19(2):75–85
Sabir M, Hanafi MM, Aziz T, Ahmad HR, Zia-Ur-Rehman M, Saifullah GM, Hakeem KR (2013) Comparative effect of activated carbon, pressmud and poultry manure on immobilization and concentration of metals in maize (Zea mays) grown on contaminated soil. Int J Agric Biol 15:559–564
Sarkar B, Rusmin R, Ugochukwu UC, Mukhopadhyay R, Manjaiah KM (2019) Modified clay minerals for environmental applications. In: Modified clay and zeolite nanocomposite materials. Elsevier, pp 113–127
Selim HM (2018) Phosphate in soils: interaction with micronutrients, radionuclides and heavy metals. CRC Press
Sengupta I, Tarar JL (2014) Use of fly ash as bio-pesticide for cotton plant. Int J Current Res Life Sci 3(12):087–090
Shahbaz AK, Iqbal M, Jabbar A, Hussain S, Ibrahim M (2018a) Assessment of nickel bioavailability through chemical extractants and red clover (Trifolium pratense L.) in an amended soil: related changes in various parameters of red clover. Ecotoxicol Environ Saf 149:116–127
Shahbaz AK, Lewińska K, Iqbal J, Ali Q, Iqbal M, Abbas F, Ramzani PMA (2018b) Improvement in productivity, nutritional quality, and antioxidative defense mechanisms of sunflower (Helianthus annuus L.) and maize (Zea mays L.) in nickel contaminated soil amended with different biochar and zeolite ratios. J Environ Manag 218:256–270
Shahbaz AK, Ramzani PMA, Saeed R, Turan V, Iqbal M, Lewińska K, Fatima M (2019) Effects of biochar and zeolite soil amendments with foliar proline spray on nickel immobilization, nutritional quality and nickel concentrations in wheat. Ecotoxicol Environ Saf 173:182–191
Shaheen SM, El-Naggar A, Antoniadis V, Moghanm FS, Zhang Z, Tsang DC, Rinklebe J (2020) Release of toxic elements in fishpond sediments under dynamic redox conditions: assessing the potential environmental risk for a safe management of fisheries systems and degraded waterlogged sediments. J Environ Manag 255:109778
Sharma S, Tiwari S, Hasan A, Saxena V, Pandey LM (2018) Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. Biotech 8(4):216
Sheikhhosseini A, Shirvani M, Shariatmadari H (2013) Competitive sorption of nickel, cadmium, zinc and copper on palygorskite and sepiolite silicate clay minerals. Geoderma 192:249–253
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43(3):246
Smith KL (2011) Gypsum as an agricultural amendment: general use guidelines. TDD no. 800-589-8292 (Ohio only)
Stanojković-Sebić A, Maksimović S, Jošić D, Pivić R (2014) The use of metallurgical slag as a by-product of the steel industry in chemical melioration of acid soils. Metall Mater Eng 20(3):191–198
Sturm P, Gluth GJG, Brouwers HJH, Kühne HC (2016) Synthesizing one-part geopolymers from rice husk ash. Constr Build Mater 124:961–966
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions–a review. Bioresour Technol 99(14):6017–6027
Sun Y, Li Y, Xu Y, Liang X, Wang L (2015) In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl Clay Sci 105:200–206
Sun Y, Xu Y, Xu Y, Wang L, Liang X, Li Y (2016) Reliability and stability of immobilization remediation of Cd polluted soils using sepiolite under pot and field trials. Environ Pollut 208:739–746
Tandy S, Meier N, Schulin R (2017) Use of soil amendments to immobilize antimony and lead in moderately contaminated shooting range soils. J Hazard Mater 324:617–625
Tauqeer HM, Hussain S, Abbas F, Iqbal M (2019) The potential of an energy crop “Conocarpus erectus” for lead phytoextraction and phytostabilization of chromium, nickel, and cadmium: an excellent option for the management of multi-metal contaminated soils. Ecotoxicol Environ Saf 173:273–284
Teresa AB, Valentina C (2012) Possible uses of steelmaking slag in agriculture: an overview, material recycling – trends and perspectives. ISBN: 978-953-51-0327-1
Tessier A, Campbell PG, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851
Tsunematsu S, Uematsu E, Saito K, Tamura H (2012) Immobilization of arsenic in natural soils by gypsum powder, mechanistic interpretations. Transactions of the Japanese Society of Irrigation. Drainage Rural Eng 80:141–150
Tu C, Wei J, Guan F, Liu Y, Sun Y, Luo Y (2020) Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ Int 137:105576
Turan V, Khan SA, Iqbal M, Ramzani PMA, Fatima M (2018) Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Ecotoxicol Environ Saf 161:409–419
Uchimiya M, Chang S, Klasson KT (2011) Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J Hazard Mater 190(1–3):432–444
Valerie C (2015) Use of red mud in soil remediation: review of applications and challenges. Bauxite Residue Valorization Best Practices 1(1):81–87
Vink JP, Harmsen J, Rijnaarts H (2010) Delayed immobilization of heavy metals in soils and sediments under reducing and anaerobic conditions; consequences for flooding and storage. J Soil Sediments 10(8):1633–1645
Voglar GE, Leštan D (2011) Efficiency modeling of solidification/stabilization of multi-metal contaminated industrial soil using cement and additives. J Hazard Mater 192(2):753–762
Walraven N, Van Os BJH, Klaver GT, Middelburg JJ, Davies GR (2014) The lead (Pb) isotope signature, behaviour and fate of traffic-related lead pollution in roadside soils in the Netherlands. Sci Total Environ 472:888–900
Wang YS, Dai JG (2017) Use of magnesia sand for optimal design of high performance magnesium potassium phosphate cement mortar. Construct Build Mater 153:385–392
Wang K, Zhang J, Zhu Z, Huang H, Li T, He Z, Yang X, Alva A (2012) Pig manure vermicompost (PMVC) can improve phytoremediation of Cd and PAHs co-contaminated soil by Sedum alfredii. J Soils Sediments 12:1089–1099
Wang YS, Dai JG, Wang L, Tsang DC, Poon CS (2018) Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere 190:90–96
Wu YJ, Zhou H, Zou ZJ, Zhu W, Yang WT, Peng PQ, Liao BH (2016) A three-year in-situ study on the persistence of a combined amendment (limestone+ sepiolite) for remedying paddy soil polluted with heavy metals. Ecotoxicol Environ Saf 130:163–170
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol 2011, Article ID 402647
Xu B, Ma H, Li Z (2015) Influence of magnesia-to-phosphate molar ratio on microstructures, mechanical properties and thermal conductivity of magnesium potassium phosphate cement paste with large water-to-solid ratio. Cem Concr Res 68:1–9
Xu Y, Liang X, Wang L, Sun Y, Huang Q (2018) Stability and universal applicability of immobilization effect of sepiolite on cadmium in acid paddy soil. In: Luo Y, Tu C (eds) Twenty years of research and development on soil pollution and remediation in China. Springer, pp 399–411
Xu C, Qi J, Yang W, Chen Y, Yang C, He Y, Lin A (2019) Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay. Sci Total Environ 686:476–483
Yadav VB, Gadi R, Kalra S (2019) Clay based nanocomposites for removal of heavy metals from water: a review. J Environ Manag 232:803–817
Yao Z, Li J, Xie H, Yu C (2012) Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ Sci 16:722–729
Yi X, Xuefeng L, Yingming X, Xu Q, Qingqing H, Lin W, Yuebing S (2017) Remediation of heavy metal-polluted agricultural soils using clay minerals: a review. Pedosphere 27(2):193–204
Yuan GD, Theng BKG, Churchman GJ, Gates WP (2013) Clays and clay minerals for pollution control. Dev Clay Sci 5:587–644
Yeung AT, Gu YY (2011) A review on techniques to enhance electrochemical remediation of contaminated soils. J Hazard Mater 195:11–29
Zeng F, Ali S, Zhang H, Ouyang Y, Qiu B, Wu F, Zhang G (2011) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut 159(1):84–91
Zeng G, Wan J, Huang D, Hu L, Huang C, Cheng M, Jiang D (2017) Precipitation, adsorption and rhizosphere effect: the mechanisms for phosphate-induced Pb immobilization in soils—a review. J Hazard Mater 339:354–367
Zeng X, Xiao Z, Zhang G, Wang A, Li Z, Liu Y, Zou D (2018) Speciation and bioavailability of heavy metals in pyrolytic biochar of swine and goat manures. J Anal Appl Pyrolysis 132:82–93
Zhan F, Zeng W, Yuan X, Li B, Li T, Zu Y, Li Y (2019) Field experiment on the effects of sepiolite and biochar on the remediation of cd-and Pb-polluted farmlands around a Pb–Zn mine in Yunnan Province, China. Environ Sci Pollut Res 26(8):7743–7751
Zhang G, Lin Y, Wang M (2011) Remediation of copper polluted red soils with clay materials. J Environ Sci 23:461–467
Zhou DM, Hao XZ, Xue Y, Cang L, Wang YJ, Chen HM (2004) Advances in remediation technologies of contaminated soils. Ecol Environ Sci 13(2):234–242
Zhou Y, Gao B, Zimmerman AR, Fang J, Sun Y, Cao X (2013) Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem Eng J 231:512–518
Zibaei Z, Ghasemi-Fasaei R, Ronaghi A, Zarei M, Zeinali S (2020) Improvement of biochar capability in Cr immobilization via modification with chitosan and hematite and inoculation with Pseudomonas putida. Commun Soil Sci Plant Anal:1–13
Žibret G, Van Tonder D, Žibret L (2013) Metal content in street dust as a reflection of atmospheric dust emissions from coal power plants, metal smelters, and traffic. Environ Sci Pollut Res 20(7):4455–4468
Zotiadis V, Argyraki A, Theologou E (2012) Pilot-scale application of attapulgitic clay for stabilization of toxic elements in contaminated soil. J Geotech Geoenviron 138:633–637
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tauqeer, H.M. et al. (2021). The Current Scenario and Prospects of Immobilization Remediation Technique for the Management of Heavy Metals Contaminated Soils. In: Hasanuzzaman, M. (eds) Approaches to the Remediation of Inorganic Pollutants. Springer, Singapore. https://doi.org/10.1007/978-981-15-6221-1_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-6221-1_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6220-4
Online ISBN: 978-981-15-6221-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)