Abstract
An 8-week feeding experiment was conducted to investigate the effects of curcumin nanomicelle, curcumin, and turmeric (Curcuma longa) on growth performances, body composition, fatty acid profile, and biochemical parameters of common carp (Cyprinus carpio), and their ameliorative effects against toxicity of silver nanoparticles (AgNPs). A total of 120 healthy carps were randomly distributed into four equal treatments. Curcumin nanomicelle, curcumin, and turmeric were each added separately to the basal diet. After the feeding trials, all treatments were exposed to a non-lethal concentration of AgNPs (0.5 mg L−1) for 96 h. Fish fed dietary turmeric showed a significantly higher weight gain. The body protein content was significantly increased in all feeding groups, while the lipid content showed a significant decrease in the turmeric-treated group. Dietary turmeric improved the concentration of saturated fatty acids (SFA) and monounsaturated fatty acid (MUFA). AgNP exposure led to increases in liver catalase (CAT) activity of carps fed with turmeric and curcumin. The lowest amount of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was obtained in fish fed with nanomicelle curcumin and curcumin diets. The lowest amount of silver accumulation in the liver of carps was found in fish fed with dietary curcumin nanomicelle. This experiment suggests that supplementation of turmeric (50 g kg−1) or curcumin (1000 mg kg−1) may play an important role in enhancing growth performances and fatty acid composition of the common carp. Moreover, administration of curcumin nanomicelle in the diet may have a potential ameliorative effect against toxicity of AgNPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is being used to produce different products in the global market, some of which are based on silver nanoparticles (AgNPs). The unique properties of AgNPs, including surface functionalization, chemical stability, and catalytic activity (Buzea et al. 2007; Krutyakov et al. 2008), make them valuable materials for different industries such as cosmetics, medicine, household, etc. (De et al. 2008; Raj et al. 2012). However, widespread use of AgNPs is not free from undesirable side effects (Yu et al. 2013). Nowadays, many experimental studies have been conducted to evaluate the negative consequences of releasing nanoresidues into the aquatic ecosystems (Asadi Dokht Lish et al. 2019; Haghighat et al. 2021; Zeumer et al. 2020). In nano-ecotoxicological studies, focus was set on the bioavailability and bioaccumulation of AgNPs at the whole-organism level, followed by the route of absorption and physiological changes (McGillicuddy et al. 2017). It has been shown that the toxicity of AgNPs is dependent on their particle sizes (Ivask et al. 2014). In rainbow trout (Oncorhynchus mykiss) exposed to different forms of AgNPs, smaller sizes of AgNPs were detected in the liver, while larger sizes were mainly accumulated in the intestine (Johari et al. 2015). Also, Joo et al. (2018) showed that the vulnerability of rainbow trout to AgNPs is concentration-dependent, irrespective of water salinity.
Encapsulation of nutrients using nanotechnology is another aspect that has received much attention. Availability and accessibility of nutrients would be elevated using this method by loading a nutrient (Moniruzzaman and Min 2020; Veisi et al. 2021). Nanomicelles are one of the nanocarriers that consist of an external hydrophilic region and an internal hydrophobic core (Vaishya et al. 2014) that has been used in drug delivery systems to improve permeability and drug bioavailability (Vadlapudi and Mitra 2013).
Two grand overarching strategies of aquaculture are increasing the growth rate and improving the meat quality (Baldissera et al. 2020). In this regard, previous findings have shown that medicinal plants as feed additives in the diet not only minimize the production cost, but also reduce negative side effects of synthetic drugs in both fish and environment (Flück and Jaspersen-Schib 1976). Turmeric, Curcuma longa, that belongs to the Zingiberaceae family possesses unique properties such as anti-inflammatory (Nonose et al. 2014), antimicrobial (Abdel-Tawwab and Abbass 2017; Sahu et al. 2008), antioxidant (Manju et al. 2012; Rajabiesterabadi et al. 2020), and immunomodulatory (Abdelrazek et al. 2017; Sahu et al. 2008). Curcumin, which is a polyphenol obtained from C. longa, is the main biological active component found in turmeric and has a wide range of physiological activities such as antistress (Akdemir et al. 2017) and immunostimulant (Kohshahi et al. 2019). Curcumin nanomicelles, hereafter called as “curcumin-NM,” has been recently developed as a new nano-based structure to enhance physiochemical properties of curcumin such as stability and solubility (Li et al. 2017). Considering the importance of turmeric and curcumin on the immune system quality, it is important to address whether curcumin-NM supplementation may influence the growth performances and biochemical properties. As far as the authors are aware, the impact of dietary curcumin-NM on fish species has not been investigated; therefore, curcumin-NM as the novel form of curcumin was used as a feed additive in the diet of common carp to assess its nutritional benefits.
The common carp, Cyprinus carpio, is one of the major freshwater species produced in the world that in 2016 it ranked third in terms of worldwide finfish production (FAO 2018). Taken together, the present study was carried out to investigate the effects of curcumin-NM, curcumin, and turmeric on the growth performances, body composition, and fatty acid profile, and also to determine ameliorative effects of experimental diets on common carp exposed to AgNPs.
Materials and methods
Preparation of experimental diet
The commercial feed for carps was purchased (Dan-e-Rose. Co., Iran) and had the following proximate composition: protein (36.0 ± 2.2), lipid (9.5 ± 1.4), ash (9.0 ± 1.2), and moisture (10.1 ± 1.3). Curcumin nanomicelles (SinaCurcumin®, 9.3 ± 0.2 nm) were provided by Exir Nano Sina company (transmission electron microscopy, TEM; images are also shown in Fig. 1a), Tehran, Iran, and contained 6.65% curcuminoids (72% curcumin, 25% desmethoxycurcumin, and 3% bisdemethoxycurcumin). The curcumin powder (C21H20O6, ≥ 75.0% curcumin, ≤ 5.0% bisdemethoxycurcumin, ≤ 20.0% demethoxycurcumin) was purchased from Merck and freshly powdered turmeric (contained 2% curcumin) was purchased from a local market. First, the food was completely powdered by mill and then freshly powdered turmeric (50,000 mg kg−1, equivalent to 1000 mg kg−1 curcumin), curcumin powder (1000 mg kg−1), and curcumin-NM (15 mL kg−1, equivalent to 1000 mg kg−1 curcumin) were added and mixed to the experimental basal diet. Experimental diets then were dried at 40 °C for 24 h and stored in − 20 °C until use. The control group was fed with basal diet. The level of incorporated turmeric to the diet was chosen according to the intermediated amount reported by (Ferreira et al. 2017).
A total of 120 healthy carps with the initial average weight of 26.32 ± 4.37 g (mean ± SD) were purchased from a local hatchery (Qasr-e Shirin, Kermanshah, Iran) and were acclimated to laboratory conditions (Aquatic Nanobiotechnology Lab, University of Kurdistan, Sanandaj, Iran) for 1 week; during that time, they were kept in 500-L tanks and fed on basal diet. After that, the fish were randomly distributed to 12 glass aquaria (150 L), resulting in ten fish per aquarium, and they were acclimated for one more week before the feeding trial in aquaria. During the experiment, the photoperiod, temperature, pH, and dissolved oxygen (DO) were maintained at 16L:8D, 19.40 ± 3.08 °C, 7.8 ± 0.2, and 6.9 ± 0.5 mg L−1, respectively. About 70% of water changes were done every day. Fish were fed three times per day at 3% of their body weight (Ashouri et al. 2015) for the first 40 days, and 4% for the next 20 days. No mortality was observed during the feeding experiment.
Growth performance
At the end of the feeding experiment, fish were anesthetized by MS-222 (tricaine methanesulfonate) and growth performance parameters were calculated by using the following equations:
Blood collection
After the feeding trial, blood samples were collected from the caudal vein by using a 1-mL plastic syringe and centrifuged (4000 rpm) for 15 min at 4 °C. The plasma sample then was separated and stored at − 80 °C until use. Anesthetized fish then were sacrificed by rapid pithing, eviscerated, and stored at − 80 °C to measure the proximate composition and fatty acid profile.
Proximate composition and fatty acid profile
Five fish from each replicate were used to perform proximate composition analysis. Crude protein, lipid, ash, and moisture were determined according to AOAC (1990). Crude protein and crude lipid were measured using the Kjeldahl and Soxhlet methods, respectively. Moisture was determined by oven drying at 105 °C to a constant weight, and ash was determined by combusting the dry samples at 550 °C for 24 h.
To determine fatty acid composition, total lipid was extracted from samples according to Folch et al. (1957). Briefly, tissues (max 1 g) were homogenized in a solvent mixture of chloroform:methanol (2:1) and kept for 24 h. The solvent was removed from samples under nitrogen gas by using a water bath. For sample esterification, 5 mL of sodium hydroxide 2% was added to extracted lipid, vigorously shaken, and kept for 10 min in the water bath. Then, 3 mL of 20% boron trifluoride-methanol (BF3) was added to the samples and kept at room temperature for 3 min. The samples were recovered by adding 1 mL hexane and 1 mL of sodium chloride 30% (AOCS 1998). Gas chromatography analysis was conducted using a Philips gas chromatograph equipped with a flame ionization detector (FID) and silica capillary column (30 m × 0.25 mm, ID × 0.22 μm, SGE BPX70). Helium was used as the carrier gas. The temperature was set at 160 °C for 5 min, followed by an increase to 180 °C at a rate of 20 °C/min and then to 200 °C at 1 °C/min and then again to 230 °C at 30 °C/min. The final temperature was maintained for 5 min using the retention time of standard mixtures which allowed the identification of fatty acids.
Plasma biochemical parameters
The plasma biochemical parameters including glucose (mg dL−1), cholesterol (mg dL−1), and total protein (g dL−1) were measured after the feeding trial using commercial kits (Pars-Azmun Chemical Company, Tehran, Iran) according to the manufacturer’s instructions.
AgNP exposure experiment
The AgNPs used in this study were from exactly the same stock that was well characterized, and their properties were reported in our recently published work (Tayemeh et al. 2020). In addition, TEM analyses of AgNPs were performed using a Carl Zeiss AG - Zeiss EM900 transmission electron microscope (Fig. 1b). After the feeding trial, the remaining fish (n = 5) in each aquarium were distributed to the exposure aquaria. The exposure trial consisted of three experimental groups (fed by curcumin-NM-, curcumin-, and turmeric-supplemented diets and exposed to AgNPs), a positive control group (fed by a basal diet and exposed to AgNPs), and a negative control group (fed by a basal diet and were not exposed to AgNPs). Fifteen aquaria (40 L capacity) were filled with fresh-aerated water up to 30 L. Fish were exposed for 96 h to one non-lethal concentration of AgNPs (0.5 mg L−1) which was selected by a series of preliminary range finding tests. At the end of the exposure experiment, all of the fish were anesthetized, blood samples were collected, and plasma were separated and kept at − 80 °C until use. Also, the livers were taken from all of the fish for measurement of silver accumulation and stored at − 80 °C until use.
Hepatic enzyme activity
For the livers of two individual fish, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured calorimetrically using commercial kits supplied by Pars-Azmun Chemical Company, Tehran, Iran, according to the manufacturer’s instructions.
Antioxidant status
The livers of 3 individual fish for measurement of antioxidant enzyme activity were thawed, and 100 mg of each was homogenized with 1 mL ice-cold phosphate-buffered saline (PBS; pH 7.4) for 2 min. Then, the samples were centrifuged (10,000 rpm) for 15 min at 4 °C and the supernatant was obtained and stored at − 80 °C. Plasma catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), and malondialdehyde (MDA) activities were measured using commercial kits (Zellbio, Germany) according to the instructions of the manufacturing company.
Accumulation of silver in liver
Liver samples were kept at − 20 °C for 48 h, freeze-dried for 24 h, weighed, and digested by concentrated nitric acid. For 0.2 g of sample, 3 mL of concentrated nitric acid (Supelco™) was added and kept for 1 h in a water bath at 100 °C. After cooling, the samples were filtered through a paper filter and made up to 10 mL volume with ultrapure water. Silver concentration was measured with the Perkin-Elmer pinAAcle 900T atomic absorption spectrophotometer in the Institute of Nanoproduct Safety Research (University of Hoseo, South Korea).
Statistical analysis
The normal distribution of data was evaluated by the Kolmogorov-Smirnov test. The significant differences between groups were detected by Duncan’s multiple range test followed by one-way analysis of variance (ANOVA). The software SPSS version 16 was used for statistical analysis. In all cases, the significance level was set at P < 0.05.
Results
Growth performances
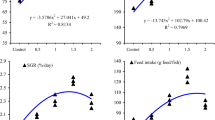
Results obtained for growth performances are shown in Fig. 2. Fish fed 50 g kg−1 turmeric diet showed a significantly higher WG (P < 0.05) in comparison to other experimental groups, but no significant difference was observed between the curcumin and curcumin-NM groups. The mean HSI of the control was significantly higher than that of the other trials except for that of the curcumin group. The values of CF were significantly lower (P < 0.05) in the turmeric group than in the other experimental groups. However, no significant difference was observed in SGR among the experimental groups.
Proximate composition
The effect of different dietary supplements on the body composition of carps after 2 months of feeding is presented in Table 1. The content of protein in fish fed turmeric, curcumin, and curcumin-NM was significantly higher (P < 0.05) than that of the control group. Also, the lipid content after the 8-week feeding trial was decreased significantly (P < 0.05) by dietary turmeric supplementation. Ash content was significantly increased in fish fed curcumin compared to other experimental groups. No significant difference was observed in moisture content among the treatment groups.
Fatty acid profile
The fatty acid composition of carps after the 8-week feeding trial is shown in Table 2. Fish fed dietary supplemented with turmeric and curcumin showed a significantly higher concentration of saturated fatty acids (P < 0.05). Among the SFAs, palmitic acid (C16:0) and stearic acid (C18:0) contents were significantly higher in fish fed curcumin. Similarly, the mean concentration of MUFA was markedly higher (P < 0.05) in fish fed curcumin followed by turmeric. However, no significant difference was observed in the content of MUFA between the curcumin-NM and control groups. Palmitoleic acid (C16:1) and oleic acid (C18:1(n-9)) were the predominant MUFA in carp muscles fed with curcumin. On the other hand, the lowest level (P < 0.05) of PUFA was observed in fish fed diet supplemented with curcumin followed by the turmeric group. The highest concentration of PUFA was found in the control group. The PUFA, EPA, and DHA levels of the group supplemented with 1000 mg kg−1 curcumin-NM were found to be higher (P < 0.05) than those of the other groups.
Biochemical indices
Biochemical blood parameters after the 8-week feeding trial are shown in Fig. 3. The levels of glucose, cholesterol, and total protein at the end of the 8-week feeding trial showed no significant differences between the experimental groups (P > 0.05).
The levels of glucose, cholesterol, and total protein in the plasma of common carp (Cyprinus carpio) following a 2-month feeding trial with control diet (Ctrl), turmeric (Tur), curcumin (Cur), and curcumin nanomicelle (Cur-NM). Bars with different letters are significantly different (mean ± SD, Duncan, P < 0.05)
The activities of AST and ALT after the exposure experiment are presented in Fig. 4. After the 96-h nanosilver exposure, the highest and lowest levels of AST were observed in the negative control and curcumin groups, respectively. Similarly, the negative control group showed the highest level of ALT (P < 0.05), but no significant differences were observed between ALT levels of the other experimental groups following AgNP exposure.
The levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in the plasma of common carp (Cyprinus carpio) following a 2-month feeding trial with control diet, turmeric, curcumin, and curcumin nanomicelle and 96-h exposure to silver nanoparticles (AgNPs, 0.5 mg L−1). Ctrl, fed on control diet and was not exposed to AgNPs; AgNPs, fed on control diet and exposed to AgNPs; Tur + AgNPs, fed on diet containing turmeric and exposed to AgNPs; Cur + AgNPs, fed on diet containing curcumin and exposed to AgNPs; Cur-NM + AgNPs, fed on diet containing curcumin nanomicelle and exposed to AgNPs. Bars with different letters are significantly different (mean ± SD, one-way ANOVA, P < 0.05)
Antioxidant status
Antioxidant responses of carps after exposure to 0.5 mg L−1 AgNPs are shown in Fig. 5. Exposure to AgNPs caused a decrease of the liver CAT in fish that fed on basal diet (P < 0.05), but the levels of CAT activity in fish fed dietary turmeric and curcumin were higher than those of the negative control group (P < 0.05). On the other hand, the concentration of MDA increased significantly (P < 0.05) in the negative control group, but no differences were found among other experimental groups. The liver SOD and GPx activities, however, remained unchanged over the exposure between test groups.
The levels of catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), and malondialdehyde (MDA) in the liver of common carp (Cyprinus carpio) following a 2-month feeding trial with control diet, turmeric, curcumin, and curcumin nanomicelle and 96-h exposure to silver nanoparticles (AgNPs, 0.5 mg L−1). Ctrl, fed on control diet and was not exposed to AgNPs; AgNPs, fed on control diet and exposed to AgNPs; Tur + AgNPs, fed on diet containing turmeric and exposed to AgNPs; Cur + AgNPs, fed on diet containing curcumin and exposed to AgNPs; Cur-NM + AgNPs, fed on diet containing curcumin nanomicelle and exposed to AgNPs. Bars with different letters are significantly different (mean ± SD, one-way ANOVA, P < 0.05)
Silver accumulation in liver
The changes of silver content in the liver of carps after the 96-h exposure are shown in Fig. 6. Exposure to AgNPs caused severe accumulation of silver in the liver tissue of fish fed basal diet and dietary turmeric (P < 0.05). Moreover, silver accumulation in the liver of carp fed dietary curcumin was higher than that of carp fed curcumin-NM, although statistically it was not significant (P > 0.05).
Silver accumulation in the liver tissue of common carp (Cyprinus carpio) following a 2-month feeding trial with control diet, turmeric, curcumin, and curcumin nanomicelle and 96-h exposure to silver nanoparticles (AgNPs, 0.5 mg L−1). Ctrl, fed on control diet and was not exposed to AgNPs; AgNPs, fed on control diet and exposed to AgNPs; Tur + AgNPs, fed on diet containing turmeric and exposed to AgNPs; Cur + AgNPs, fed on diet containing curcumin and exposed to AgNPs; Cur-NM + AgNPs, fed on diet containing curcumin nanomicelle and exposed to AgNPs. Bars with different letters are significantly different (mean ± SD, one-way ANOVA, P < 0.05)
Discussion
Recently, large number of experiments were conducted to investigate the effects of turmeric and curcumin as feed additives on important farmed fish species. Moreover, the potential effects of dietary nanoencapsulated curcumin have received less attention (Shah and Mraz 2020). Thus, the present study was designed to evaluate the effects of turmeric, curcumin, and nanoencapsulated curcumin individually over an 8-week feeding trial followed by a 96-h nanosilver exposure.
In the present study, fish fed with the 50 g kg−1 turmeric diet showed a higher rate of WG than the other experimental groups. It has been shown that administration of turmeric up to 5 mg kg−1 diet could improve the final weight of rohu (Labeo rohita) and common carp (Abdel-Tawwab and Abbass 2017; Sahu et al. 2008). In contrast, Abdelrazek et al. (2017) reported a negative effect in the growth performances of Nile tilapia (Oreochromis niloticus) fed more than 2 g kg−1 turmeric diet. Also, no differences were found in juvenile characin, Astyanax aff. Bimaculatus, fed with different levels of turmeric (Ferreira et al. 2017). Fish species have responded differently to dietary turmeric that might be related to the species-specific digestive function. However, turmeric has been reported as a potential stimulant for the activity of digestive enzymes (Prasad and Aggarwal 2011).
The present study showed that curcumin and curcumin-NM did not improve the WG in comparison with the control group. These findings are in agreement with our previous results in rainbow trout (Kohshahi et al. 2019). The absence of a marked difference between fish fed curcumin and curcumin-NM in comparison with the control group may be due to the taste of curcumin which may be a factor causing lower feed intake and growth rate (Hosseini-Vashan et al. 2012). Recently, a quantitative review evaluated the results of 13 studies derived from turmeric and curcumin and showed that higher levels of curcumin in the diet as the main bioactive substance of turmeric can potentially cause inflammation and lower body weight (Fagnon et al. 2020; Yusuf et al. 2017)
In the present study, HSI decreased significantly in fish fed dietary turmeric and curcumin-NM supplementation. HSI is a good indicator of feeding activity and provides information about the liver, and the lower HSI could be attributed to decreased liver glycogen and fat deposition as described by Liu et al. (2020). In contrast to our results, however, no significant change was observed in HSI of African catfish, crucian carp, Astyanax aff. bimaculatus, and genetically improved farmed tilapia (GIFT) (Cui et al. 2013; Ferreira et al. 2017; Jiang et al. 2016; Rawung et al. 2020). Normally, the body composition is affected by the diet and feeding rate, genetics, and rearing conditions (Dumas et al. 2007). Proximate analysis could be a good indicator of fish growth (Ali et al. 2005). Supplementation of turmeric, curcumin, and curcumin-NM caused a significant increase in the crude protein. Similar to our results, Hwang et al. (2013) found that dietary turmeric produced a higher protein content in the body composition of fish that were fed with a higher level of turmeric. Moreover, Mahmoud et al. (2014) reported that dietary turmeric resulted in a significantly higher protein content, but produced a lower total lipid with the increase of the turmeric level in the diet. In the present study, the levels of protein and lipid could be affected by the level of turmeric which consequently stimulates synthesis of muscle protein and lipid contents. However, some other studies have shown that the dietary turmeric had no effects on the proximate composition of common carp and rainbow trout (Abdel-Tawwab and Abbass 2017; Kohshahi et al. 2019). The increasing level of crude protein could be related to the stimulation effect of curcumin and curcumin-NM on digestive enzymes such as trypsin and lipase (Jiang et al. 2016). Similarly, previous studies showed that dietary curcumin (50 mg kg−1) and turmeric (0.5%) could effectively increase the protein content of the body (Mahmoud et al. 2017; Mahmoud et al. 2014). The level of ash was found significantly higher in the curcumin group (2.4 ± 0.29%) than fish fed curcumin-NM (1.98 ± 0.04%). This may be related to the ability of curcumin to bind with metal ions to offer antioxidant activity (Began et al. 1999).
The mean concentrations of SFA and MUFA have increased following 2 months feeding trial with turmeric and curcumin. Furthermore, dietary curcumin showed an ability to reduce the muscular PUFA content. Generally, the fatty acid composition depends on the species and their diets (Ackman 2007). A higher concentration of PUFA in the fish meat can cause oxidation as well as oxidative stress which consequently a higher amount of antioxidant must be added into diets to prevent the effects of oxidative stress. Several studies have reported that curcumin might be involved in the biosynthesis of fatty acids, and its antioxidant property indirectly can affect the meat fatty acid profile (Rukkumani et al. 2003; Salah et al. 2019). However, there are few studies focused on consumption of curcumin in fish and its mechanism of action on the fatty acid profile.
It has been shown that dietary turmeric reduces lipid peroxidation by increasing the activities of antioxidant enzymes (Reddy and Lokesh 1994). Also, curcumin can potentially increase the content of PUFA by blocking lipid peroxidation. Our results showed that the total SFA content of fish fed turmeric and curcumin increased significantly compared to the control group. This might be related to the action of curcumin in stimulating the activity of enzymes involved in the production of SFA from unsaturated fatty acids (Kaul and Krishnakantha 1997). In other vertebrates, the effects of dietary turmeric on fatty acid composition have also been investigated. Daneshyar et al. (2011) reported that supplementation of 0.75% turmeric in the diet of broiler chicken significantly decreased the total SFA and trans-vaccenic acid (C18:1n7) of thigh muscles, but total unsaturated fatty acids in contrast to our results, remained unchanged.
Also, Peiretti et al. (2011) showed that adding 3 g kg−1 turmeric diet led to higher levels of α-linolenic acid and PUFA n-3 in the meat of the rabbits, and therefore improved the meat quality.
The biochemical parameters (i.e., AST and ALT) after the exposure experiment showed a remarkable reduction in fish with pre-feeding trial experience in comparison to the positive control group. The serum levels of AST and ALT provide vital information about the liver disorders. It has been shown that higher concentrations of AST and ALT may reflect stress-based tissue injuries (Huang et al. 2006). Previous studies have demonstrated that AgNPs have direct effects on the elevated level of AST and ALT (Kumar et al. 2018; Ramachandran et al. 2018). This might be due do the release of these enzymes to the bloodstream, after the interaction of hepatocytes by AgNPs derived free radicals (Martínez-Gutierrez et al. 2012). However, the decreased levels of AST and ALT during the exposure experiment may be a sign of antioxidant activity of turmeric powder and its derivatives (Saccol et al. 2017). Our results are similar to that reported by El-Houseiny et al. (2019) who found African catfish (Clarias gariepinus) fed on 5% turmeric showed significantly lower values of ALT and ALP activities.
Awasthi et al. (2019) showed the protective role of curcumin against Cr6+-induced hepatotoxicity, genotoxicity, and oxidative stress in spotted snakehead (Channa punctatus) fish. Based on our results, no significant differences were observed in activity of GPx and SOD after 96-h exposure to AgNPs. Previous studies have also reported similar finding (Ale et al. 2018; Choi et al. 2010; Ferreira et al. 2017; Kumar et al. 2018). On the other hand, the activity of CAT following 96-h exposure to AgNPs was significantly lower in fish fed on basal diet and exposed to AgNPs. The reduced level of CAT activity may be influenced by the environmental stressors, and liver’s failure to provide an efficient antioxidant defense system (Wu and Zhou 2013). Similar to our results, Govindasamy and Rahuman (2012) reported that CAT activity was significantly decreased in liver tissues of Mozambique tilapia (Oreochromis mossambicus) fed on 0.5 mg kg−1 Se, and the lowest MDA level was observed in the 0.48 mg kg−1 Se group. Similarly, Choi et al. (2010) has also reported dose-dependent decrease in CAT activity in zebrafish liver following exposure to AgNPs. The results obtained from the CAT activity is in agreement with those obtained by El-Houseiny et al. (2019), who reported that the value of CAT activity in the serum African catfish increased remarkably in fish fed diets supplemented with 0.5% turmeric. However, Ferreira et al. (2017) showed that dietary turmeric did not affect the CAT activity in Astyanax aff. bimaculatus.
In this study, the highest level of MDA content was observed in fish fed on basal diet and exposed to AgNPs. MDA is one the final products of lipid peroxidation and its measurement as an index of lipid peroxidation provides vital information about the meat quality (Gatta et al. 2000; Tsikas 2017). In the present study, the level of MDA in the liver was significantly decreased in fish fed on turmeric, curcumin and curcumin-NM after exposure to AgNPs. The decrease in the MDA levels seems to be due to the ability of curcumin to scavenge free radicals and enhance the antioxidant system against oxidative stress (Somparn et al. 2007). Our results are in agreement with those obtained by El-Houseiny et al. (2019) who reported that 0.5% turmeric in diet of cadmium-induced fish caused a significant decrease in serum MDA level. Meanwhile, Ferreira et al. (2017) reported no significant changes in liver MDA level of Astyanax aff. bimaculatus fed with dietary turmeric. Recently, Rajabiesterabadi et al. (2020) showed that administration of 10 g kg−1 turmeric diet would be effective to protect the common carps against oxidative damage cause by copper exposure.
Silver accumulation in the liver after 96-h of exposure showed significant changes between the AgNP-exposed fish. Curcumin-NM and curcumin groups exhibited significant decreases in liver Ag accumulation. However, slightly higher accumulation occurred in the curcumin group. Environmental pollutants are generally accumulated in the fish organs, causing several health hazards such as hepatotoxicity (Costa et al. 2010) and oxidative damages (Gagné et al. 2012). It has been shown that AgNPs are mainly accumulated in the liver (Wu and Zhou 2013). This is most likely to be dependent on chemical structure of AgNPs (Navarro et al. 2008), different uptake pathways (Wu and Zhou 2013) and species-specific factors. Although it has been shown that supplementation of turmeric powder in the diet reduced the amount of cadmium in the liver (El-Houseiny et al. 2019). However, in the present study, turmeric could not prevent Ag accumulation in the liver of exposed fish in compare to the curcumin-NM and curcumin group. It has been shown that the protective activity of turmeric in ingesting the toxic metals could be associated with the presence of curcuminoids and their different biding sites such as carboxyl, phenolic, and amines/hydroxyl groups (Qayoom et al. 2017). Furthermore, previous studies have been suggested that these groups are all involved in metal-ligand interface which results in lower bioaccumulation of metals (Anayurt et al. 2009; Veglio et al. 1997).
Conclusion
In summary, the present study shows that supplementation of turmeric (50 g kg−1) or curcumin (1000 mg kg−1) in diet resulted in a positive effect on weight gain and fatty acid composition in the common carp. However, these findings rejected our initial hypothesis that the nanoencapsulated form of curcumin is more effective than free curcumin as the growth and biochemical parameters did not improve in the curcumin-NM group. Although the exposure experiment showed that AgNP induces oxidative stress and hepatotoxicity in carps, the biochemical parameters after exposure experiment demonstrated that dietary turmeric and curcumin might be effective to protect the fish against AgNPs. This study provides interesting evidence that supplementation of curcumin-NM to the diet can potentially protect the liver against the bioaccumulation of AgNPs. However, further work is needed to understand the mechanism of dietary curcumin-NM on growth performances in different fish species. Thus, the results of the present study suggest adding 50 g kg−1 turmeric or 1000 mg kg−1 curcumin diet to enhance growth performances, health, and meat quality of juvenile carps.
References
Abdelrazek HMA, Tag HM, Kilany OE, Reddy PG, Hassan AM (2017) Immuomodulatory effect of dietary turmeric supplementation on Nile tilapia (Oreochromis niloticus). Aquac Nutr 23:1048–1054
Abdel-Tawwab M, Abbass FE (2017) Turmeric Powder, Curcuma longa L., in Common carp, Cyprinus carpio L., diets: growth performance, innate immunity, and challenge against pathogenic Aeromonas hydrophila infection. J World Aquacult Soc 48:303–312
Ackman RG (2007) Fatty acids in fish and shellfish. In: Chow CK (ed) Fatty acids in foods and their health implications, 3rd edn. CRC Press, pp 155–185
Akdemir F, Orhan C, Tuzcu M, Sahin N, Juturu V, Sahin K (2017) The efficacy of dietary curcumin on growth performance, lipid peroxidation and hepatic transcription factors in rainbow trout Oncorhynchus mykiss (Walbaum) reared under different stocking densities. Aquac Res 48:4012–4021
Ale A, Rossi AS, Bacchetta C, Gervasio S, de la Torre FR, Cazenave J (2018) Integrative assessment of silver nanoparticles toxicity in Prochilodus lineatus fish. Ecol Indic 93:1190–1198
Ali M, Iqbal F, Salam A, Iram S, Athar M (2005) Comparative study of body composition of different fish species from brackish water pond. Int J Environ Sci Technol 2:229–232
Anayurt RA, Sari A, Tuzen M (2009) Equilibrium, thermodynamic and kinetic studies on biosorption of Pb (II) and Cd (II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem Eng J 151:255–261
AOAC (1990) Official methods of analysis. Assoc Off Anal Chem 1:684
AOCS (1998) Official methods and recommended practices of the American Oil Chemists’ Society. AOCS 5:2–93
Asadi Dokht Lish R, Johari SA, Sarkheil M, Yu IJ (2019) On how environmental and experimental conditions affect the results of aquatic nanotoxicology on brine shrimp (Artemia salina): a case of silver nanoparticles toxicity. Environ Pollut 255:113358
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 446:25–29
Awasthi Y, Ratn A, Prasad R, Kumar M, Trivedi A, Shukla JP, Trivedi SP (2019) A protective study of curcumin associated with Cr6+ induced oxidative stress, genetic damage, transcription of genes related to apoptosis and histopathology of fish, Channa punctatus (Bloch, 1793). Environ Toxicol Pharmacol 71:103209
Baldissera MD, Souza CF, Zeppenfeld CC, Velho MC, Klein B, Abbad LB, Ourique AF, Wagner R, Da Silva AS, Baldisserotto B (2020) Dietary supplementation with nerolidol nanospheres improves growth, antioxidant status and fillet fatty acid profiles in Nile tilapia: benefits of nanotechnology for fish health and meat quality. Aquaculture 516:734635
Began G, Sudharshan E, Udaya Sankar K, Appu Rao A (1999) Interaction of curcumin with phosphatidylcholine: a spectrofluorometric study. J Agric Food Chem 47:4992–4997
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2:MR17–MR71
Choi JE, Kim S, Ahn JH, Youn P, Kang JS, Park K, Yi J, Ryu D-Y (2010) Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol 100:151–159
Costa P, Chicano-Gálvez E, Barea JL, DelValls T, Costa M (2010) Alterations to proteome and tissue recovery responses in fish liver caused by a short-term combination treatment with cadmium and benzo [a] pyrene. Environ Pollut 158:3338–3346
Cui H, Liu B, X-p G, XiE J, Xu P, L-h M, Sun S, Liao Y, Chen R, Ren M (2013) Effects of dietary curcumin on growth performance, biochemical parameters, HSP70 gene expression and resistance to Streptococcus iniae of juvenile Gift Tilapia, Oreochromis niloticus. Isr J Aquac 66:986–996
Daneshyar M, Ghandkanlo MA, Bayeghra FS, Farhangpajhoh F, Aghaei M (2011) Effects of dietary turmeric supplementation on plasma lipoproteins, meat quality and fatty acid composition in broilers. South Afr J Animal Sci 41:420–428
De M, Ghosh PS, Rotello VM (2008) Applications of Nanoparticles in Biology. Adv Mater 20:4225–4241
Dumas A, De Lange CF, France J, Bureau DP (2007) Quantitative description of body composition and rates of nutrient deposition in rainbow trout (Oncorhynchus mykiss). Aquaculture 273:165–181
El-Houseiny W, Khalil AA, Abd-Elhakim YM, Badr HA (2019) The potential role of turmeric and black pepper powder diet supplements in reversing cadmium-induced growth retardation, ATP depletion, hepatorenal damage, and testicular toxicity in Clarias gariepinus. Aquaculture 510:109–121
Fagnon MS, Thorin C, Calvez S (2020) Meta-analysis of dietary supplementation effect of turmeric and curcumin on growth performance in fish. Rev Aquac 12:2268–2283
FAO (2018) The state of world fisheries and aquaculture 2018-meeting the sustainable development goals. License CC BY-NC-SA 3.0 IGO
Ferreira PMF, Martins MTS, Caldas DW, Gomes JR, de Oliveira JM, Salaro AL, Rocha JS, Zuanon JAS (2017) Curcuma longa as additive in the diet for Astyanax aff. bimaculatus. Fish Physiol Biochem 43:691–702
Flück H, Jaspersen-Schib R (1976) Medicinal plants and their uses: medicinal plants, simply described and illustrated with notes on their constituents, actions and uses, their collection, cultivation and preparations. W. Foulsham
Folch J, Lees M, Stanley GS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gagné F, André C, Skirrow R, Gélinas M, Auclair J, Van Aggelen G, Turcotte P, Gagnon C (2012) Toxicity of silver nanoparticles to rainbow trout: a toxicogenomic approach. Chemosphere 89:615–622
Gatta P, Pirini M, Testi S, Vignola G, Monetti P (2000) The influence of different levels of dietary vitamin E on sea bass Dicentrarchus labrax flesh quality. Aquac Nutr 6:47–52
Govindasamy R, Rahuman AA (2012) Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus). J Environ Sci 24:1091–1098
Haghighat F, Kim Y, Sourinejad I, Yu IJ, Johari SA (2021) Titanium dioxide nanoparticles affect the toxicity of silver nanoparticles in common carp (Cyprinus carpio). Chemosphere 262:127805
Hosseini-Vashan S, Golian A, Yaghobfar A, Zarban A, Afzali N, Esmaeilinasab P (2012) Antioxidant status, immune system, blood metabolites, and carcass characteristic of broiler chickens fed turmeric rhizome powder under heat stress. Afr J Biotechnol 11:16118–16125
Huang X-J, Choi Y-K, Im H-S, Yarimaga O, Yoon E, Kim H-S (2006) Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors 6:756–782
Hwang J-H, Rha S-J, Han K-H, Kim S-J (2013) Body composition of black rockfish Sebastes schlegeli fed on diets containing different levels of turmeric Curcuma longa L. Kor J Fish Aqua Sci 46:540–545
Ivask A, Kurvet I, Kasemets K, Blinova I, Aruoja V, Suppi S, Vija H, Käkinen A, Titma T, Heinlaan M (2014) Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS One 9:e102108
Jiang J, Wu X-Y, Zhou X-Q, Feng L, Liu Y, Jiang W-D, Wu P, Zhao Y (2016) Effects of dietary curcumin supplementation on growth performance, intestinal digestive enzyme activities and antioxidant capacity of crucian carp Carassius auratus. Aquaculture 463:174–180
Johari SA, Kalbassi MR, Yu IJ, Lee JH (2015) Chronic effect of waterborne silver nanoparticles on rainbow trout (Oncorhynchus mykiss): histopathology and bioaccumulation. Comp Clin Pathol 24:995–1007
Joo HS, Kalbassi MR, Johari SA (2018) Hematological and histopathological effects of silver nanoparticles in rainbow trout (Oncorhynchus mykiss)—how about increase of salinity? Environ Sci Pollut Res 25:15449–15461
Kaul S, Krishnakantha T (1997) Influence of retinol deficiency and curcumin/turmeric feeding on tissue microsomal membrane lipid peroxidation and fatty acids in rats. Mol Cell Biochem 175:43–48
Kohshahi AJ, Sourinejad I, Sarkheil M, Johari SA (2019) Dietary cosupplementation with curcumin and different selenium sources (nanoparticulate, organic, and inorganic selenium): influence on growth performance, body composition, immune responses, and glutathione peroxidase activity of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 45:793–804
Krutyakov YA, Kudrinskiy AA, Olenin AY, Lisichkin GV (2008) Synthesis and properties of silver nanoparticles: advances and prospects. Russ Chem Rev 77:233–257
Kumar N, Krishnani KK, Gupta SK, Singh NP (2018) Effects of silver nanoparticles on stress biomarkers of Channa striatus: immuno-protective or toxic? Environ Sci Pollut Res 25:14813–14826
Li M, Xin M, Guo C, Lin G, Wu X (2017) New nanomicelle curcumin formulation for ocular delivery: improved stability, solubility, and ocular anti-inflammatory treatment. Drug Dev Ind Pharm 43:1846–1857
Liu W, Lu X, Jiang M, Wu F, Tian J, Yu L, Wen H (2020) Effects of dietary niacin on liver health in genetically improved farmed tilapia (Oreochromis niloticus). Aquacult Rep 16:100243
Mahmoud MM, El-Lamie MM, Dessouki AA, Yusuf MS (2014) Effect of turmeric (Curcuma longa) supplementation on growth performance, feed utilization, and resistance of Nile tilapia (Oreochromis niloticus) to Pseudomonas fluorescens challenge. Global Res J Fish Sci Aquacult 1:26–33
Mahmoud HK, Al-Sagheer AA, Reda FM, Mahgoub SA, Ayyat MS (2017) Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 475:16–23
Manju M, Akbarsha MA, Oommen OV (2012) In vivo protective effect of dietary curcumin in fish Anabas testudineus (Bloch). Fish Physiol Biochem 38:309–318
Martínez-Gutierrez F, Thi EP, Silverman JM, de Oliveira CC, Svensson SL, Hoek AV, Sánchez EM, Reiner NE, Gaynor EC, Pryzdial EL (2012) Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomedicine 8:328–336
McGillicuddy E, Murray I, Kavanagh S, Morrison L, Fogarty A, Cormican M, Dockery P, Prendergast M, Rowan N, Morris D (2017) Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci Total Environ 575:231–246
Moniruzzaman M, Min T (2020) Curcumin, curcumin nanoparticles and curcumin nanospheres: a review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics 12:447
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao A-J, Quigg A, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386
Nickum J, Bart H Jr, Bowser P, Greer I, Hubbs C, Jenkins JA, MacMillan J, Rachlin J, Rose J, Sorensen P (2004) Guidelines for the use of fishes in research. Fisheries-Bethesda 29:26–26
Nonose N, Pereira JA, Machado PRM, Rodrigues MR, Sato DT, Martinez CAR (2014) Oral administration of curcumin (Curcuma longa) can attenuate the neutrophil inflammatory response in zymosan-induced arthritis in rats. Acta Cir Bras 29:727–734
Peiretti P, Masoero G, Meineri G (2011) Effects of replacing palm oil with maize oil and Curcuma longa supplementation on the performance, carcass characteristics, meat quality and fatty acid profile of the perirenal fat and muscle of growing rabbits. Animal 5:795–801
Prasad S, Aggarwal BB (2011) Turmeric, the golden spice. In: Herbal medicine: biomolecular and clinical aspects, 2nd edn
Qayoom A, Kazmi SA, Ali SN (2017) Turmeric powder as a natural heavy metal chelating agent: Surface characterisation. Pak J Scient Industr Res Ser A Phys Sci 60:1–8
Raj S, Jose S, Sumod U, Sabitha M (2012) Nanotechnology in cosmetics: opportunities and challenges. J Pharm Bioallied Sci 4:186–193
Rajabiesterabadi H, Hoseini SM, Fazelan Z, Hoseinifar SH, Doan HV (2020) Effects of dietary turmeric administration on stress, immune, antioxidant and inflammatory responses of common carp (Cyprinus carpio) during copper exposure. Aquac Nutr 26:1143–1153
Ramachandran R, Krishnaraj C, Kumar VA, Harper SL, Kalaichelvan TP, Yun S-I (2018) In vivo toxicity evaluation of biologically synthesized silver nanoparticles and gold nanoparticles on adult zebrafish: a comparative study. 3 Biotech 8:1–12
Rawung LD, Ekastuti DR, Junior MZ, Rahminiwati M, Sunarma A, Manalu W (2020) Reproductive performances and egg qualities in African catfish (Clarias gariepinus) broodstocks supplemented with curcumin and thyroxine hormone. Omni-Akuatika 16:32–47
Reddy ACP, Lokesh B (1994) Effect of dietary turmeric (Curcuma longa) on iron-induced lipid peroxidation in the rat liver. Food Chem Toxicol 32:279–283
Rukkumani R, Balasubashini MS, Menon VP (2003) Protective effects of curcumin and photo-irradiated curcumin on circulatory lipids and lipid peroxidation products in alcohol and polyunsaturated fatty acid-induced toxicity. Phytother Res 17:925–929
Saccol EMH, Toni C, Pês TS, Ourique GM, Gressler LT, Silva LVF, Mourão RHV, Oliveira RB, Baldisserotto B, Pavanato MA (2017) Anaesthetic and antioxidant effects of Myrcia sylvatica (G. Mey.) DC. and Curcuma longa L. essential oils on tambaqui (Colossoma macropomum). Aquac Res 48:2012–2031
Sahu S, Das BK, Mishra BK, Pradhan J, Samal SK, Sarangi N (2008) Effect of dietary Curcuma longa on enzymatic and immunological profiles of rohu, Labeo rohita (Ham.), infected with Aeromonas hydrophila. Aquac Res 39:1720–1730
Salah AS, Mahmoud MA, Ahmed-Farid OA, El-Tarabany MS (2019) Effects of dietary curcumin and acetylsalicylic acid supplements on performance, muscle amino acid and fatty acid profiles, antioxidant biomarkers and blood chemistry of heat-stressed broiler chickens. J Therm Biol 84:259–265
Shah BR, Mraz J (2020) Advances in nanotechnology for sustainable aquaculture and fisheries. Rev Aquac 12:925–942
Somparn P, Phisalaphong C, Nakornchai S, Unchern S, Morales NP (2007) Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull 30:74–78
Tayemeh MB, Esmailbeigi M, Shirdel I, Joo HS, Johari SA, Banan A, Nourani H, Mashhadi H, Jami MJ, Tabarrok M (2020) Perturbation of fatty acid composition, pigments, and growth indices of Chlorella vulgaris in response to silver ions and nanoparticles: A new holistic understanding of hidden ecotoxicological aspect of pollutants. Chemosphere 238:124576
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem 524:13–30
Vadlapudi AD, Mitra AK (2013) Nanomicelles: an emerging platform for drug delivery to the eye. Ther Deliv 4:1–3
Vaishya RD, Khurana V, Patel S, Mitra AK (2014) Controlled ocular drug delivery with nanomicelles. WIREs Nanomed Nanobiotechnol 6:422–437
Veglio F, Beolchini F, Gasbarro A (1997) Biosorption of toxic metals: an equilibrium study using free cells of Arthrobacter sp. Process Biochem 32:99–105
Veisi S, Johari SA, Tyler CR, Mansouri B, Esmaeilbeigi M (2021) Antioxidant properties of dietary supplements of free and nanoencapsulated silymarin and their ameliorative effects on silver nanoparticles induced oxidative stress in Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res 28:26055–26063
Wu Y, Zhou Q (2013) Silver nanoparticles cause oxidative damage and histological changes in medaka (Oryzias latipes) after 14 days of exposure. Environ Toxicol Chem 32:165–173
Yu S-j, Y-g Y, J-f L (2013) Silver nanoparticles in the environment. Environ Sci Process Impacts 15:78–92
Yusuf M, Hassan MA, Tag HM, Sarivistava K, Reddy P, Hassan A (2017) Influence of turmeric (Curcuma longa) on performance, histomorphology and microbiota of intestine in juvenile tilapia (Oreochromis niloticus). Int J Agric Sci Vet Med 5:7–16
Zeumer R, Hermsen L, Kaegi R, Kühr S, Knopf B, Schlechtriem C (2020) Bioavailability of silver from wastewater and planktonic food borne silver nanoparticles in the rainbow trout Oncorhynchus mykiss. Sci Total Environ 706:135695
Acknowledgements
The authors are grateful to Exir Nano Sina Co. (Iran) for donating nanomicelles encapsulated curcumin (SinaCurcumin®).
Availability of data and materials
All data and materials are included in this published article.
Funding
This work was supported by the University of Kurdistan (Grant number: GRC97-06503-1).
Author information
Authors and Affiliations
Contributions
FP: investigation, data curation; SM: investigation; SA: investigation, data curation, writing—original draft preparation; SAJ: supervision, conceptualization, methodology, funding acquisition, project administration, writing—review and editing; EG: investigation; HPK: investigation; IJY: conceptualization, methodology
Corresponding author
Ethics declarations
Ethics approval
All animal manipulation procedures were done based on Animal Welfare Act and Interagency Research Animal Committee guidelines (Nickum et al. 2004).
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pirani, F., Moradi, S., Ashouri, S. et al. Dietary supplementation with curcumin nanomicelles, curcumin, and turmeric affects growth performance and silver nanoparticle toxicity in Cyprinus carpio. Environ Sci Pollut Res 28, 64706–64718 (2021). https://doi.org/10.1007/s11356-021-15538-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15538-2