Abstract
The present study aimed to examine individual nutritional and ameliorative effects of silica nanoparticles (SiO2NPs) and natural zeolite nanoparticles (ZeNPs) and their potential role as carriers to alter the bioavailability of curcumin. Common carps (Cyprinus carpio) were fed during 60 days with a control diet, and curcumin, turmeric, SiO2NPs, curcumin-loaded SiO2NPs, ZeNPs, and curcumin-loaded ZeNPs each at 1, 50, 6.15, 7.15, 39, and 40 g/kg diet, respectively. The highest weight gain (WG) and specific growth rate (SGR) were observed in fish fed with turmeric (P < 0.05). Moreover, dietary curcumin and ZeNPs increased the content of monounsaturated fatty acids (P < 0.05). After exposure to silver nanoparticles (AgNPs), the lowest amount of aspartate aminotransferase (AST) was obtained in fish fed with curcumin (P < 0.05). In addition, alanine aminotransferase (ALT) decreased significantly in the negative control, curcumin, and curcumin-loaded SiO2NPs treatments in comparison to the positive control group (P < 0.05). The lowest silver accumulation was observed in the negative control and SiO2NPs groups (P < 0.05). This experiment demonstrated that while the nanoencapsulation of curcumin on SiO2NPs and ZeNPs did not enhanced the impact of curcumin on the growth and biochemical factors of carps, it can still be considered a potential dietary supplement for enhancing growth and antioxidant indices when added individually to the diet.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The application of bioactive compounds extracted from plants has rapidly increased in the aquaculture industry during the past 25 years. These phytochemicals, due to the high quantity and variety, can be extracted and used as feed additives in the formulation of diets in aquaculture. Since feed represents the most high-cost section of fisheries, developing new strategies improves feed efficiency and allows us to use sustainable additives sources (Hasan 2001; Shi et al. 2020).
Recently, curcumin, a polyphenol micronutrient obtained from C. longa, has been one of the main focus of attention due to its various biological activities (Akdemir et al. 2017; Alagawany et al. 2021). Curcumin has been used in the diet of grass carp (Ctenopharyngodon idella) (Ming et al. 2020), juvenile largemouth bass (Micropterus salmoides) (Bao et al. 2022), Nile tilapia (Oreochromis niloticus) (Mahmoud et al. 2017), Pacific white shrimp (Penaeus vannamei) (Moghadam et al. 2021), and common carp (Cyprinus carpio) (Pirani et al. 2021). Although the administration of curcumin in the diet has shown potential benefits, it has some crucial challenges associated with lower solubility and bioavailability (Yang et al. 2007). To overcome these poor characteristics, new approaches, such as nanoencapsulation and nanoformulation, have been developed to increase the accessibility of Curcumin (Bao et al. 2022; Li et al. 2017; Moniruzzaman and Min 2020). Nanoscale chemicals can be used in drug delivery systems to improve drug targeting and stabilization of bioactive compounds during storage (Choudhury et al. 2017).
Natural zeolite (i.e., Clinoptilolite), due to its numerous advantages such as nontoxicity, dual functional surface, and easy encapsulation, has been used as a career in drug delivery systems (Petushkov et al. 2010). As far as we know, the application of curcumin-loaded zeolite has not been well investigated; however, it has been shown that zeolite alone as an additive in the diet can improve growth performances (Eya et al. 2008; Kanyılmaz et al. 2015; Sheikhzadeh et al. 2017) and histological structures (Hamidian et al. 2018).
Silica nanoparticles have also been used in encapsulation techniques because of their unique features, such as high surface area and thermal and chemical stability (Trewyn et al. 2008). These properties enable silica nanoparticles to be used in drug and biogenic molecule delivery (Gangwar et al. 2013; Jambhrunkar et al. 2014). Furthermore, a new study showed that silica nanoparticles are potent aqueous additives for the adsorption of heavy metals from aqueous media (Mahboub et al. 2022). In this study, we tried to determine whether zeolite and silica nanoparticles can be used as a potential nanoencapsulation method to increase the bioavailability of dietary curcumin in the common carp.
Materials and methods
Silica nanoparticle and synthesis of natural zeolite nanoparticles
In this study, silica nanoparticles (SiO2NPs) were purchased from US Research Nanomaterials Co., Inc., Houston, Texas. The particle size of nano-silica, according to the manufacturing company, ranged from 20 to 30 nm. Natural zeolite nanoparticles (ZeNPs) were synthesized by physical grinding and a top-down approach. Natural zeolite granules (Clinoptilolite, 2–3 mm) were washed with water, dried at (70° C) and milled using a planetary ball mill apparatus (MARYA, Amin Asia Fanavar Pars Co., Iran) for 4 h to minimize the diameters in the range of nanometer size. The shape and size of produced ZeNPs were characterized by scanning electron microscope (SEM, FEI Quanta200 ESEM) and transmission electron microscopy (TEM, FE-TEM, JEM2100F, JEOL, Tokyo, Japan) equipped with an energy-dispersive X-ray (EDX) detector.
Curcumin loading on SiO2NPs and ZeNPs
Curcumin was loaded on nano-silica and nano-zeolite according to the method of Kotcherlakota et al. (2016) with some modifications. To functionalization, silica and zeolite nanoparticles were first dehydrated for 6 h at 50° C. Then, 5.8 ml of 3-aminopropyltriethoxysilane (22.6 mM) was dissolved in 100 ml of toluene and added to 5 g of SiO2NPs or ZeNPs. The mixture was stirred under reflux conditions for 48 h at 100° C. Next, the mixed solution was centrifuged (5000 RPM) for 30 min at 26° C, and solid residues were washed with 40 ml of each toluene (×2), methanol (×2), and ethanol (×2) for 5 min. The washing process was repeated, and solid residues were vacuum-dried to form entirely white (SiO2NPs) and gray (ZeNPs) powders. Finally, a solution of curcumin (0.63 g, 2.4 mM) was made in 100 ml ethanol and mixed with 2.5 g of each functionalized nanoparticle under reflux conditions for 48 h. Solid residues were separated by centrifugation and vacuum-dried at room temperature for 24 h to obtain orange curcumin-loaded nanoparticles of silica and zeolite. To determine the amount of curcumin loaded on the nanoparticles, the thermogravimetric/differential thermal analysis (TG/DTA, Perkin Elmer Diamond) was used under a controlled gas atmosphere and temperature (30–950° C).
Preparation of the diet
In this study, the commercial basal diet for carp was purchased and had the following proximate composition: protein (36.28±0.22%), lipid (15.75±0.04%), ash (9.03±0.21%), and moisture (10±0.33%). Then, seven experimental diets were prepared by adding each ingredient to the basal diet and mixing with water until a smooth paste was obtained (Table 1). A manual meat grinder was used to pellet the diets, then dried them at 40° C for 24 h and stored them at −20° C until used.
Raring condition
A total of 250 juvenile carps were purchased from a reproduction center (Qasr-e Shirin, Kermanshah, Iran) and transferred to the Aquatic Nanobiotechnology Lab (University of Kurdistan, Sanandaj, Iran). Fish were acclimated in 600 L holding tanks for 1 month and fed three times daily with a basal diet at 3% of their body weight (Ashouri et al. 2015). One-third of rearing water was replaced daily with aerated and dechlorinated tap water. At the end of the acclimation period, a total of 210 healthy carps with an initial average weight of 26.35±4.36 g (mean ± SD) were randomly transferred to 21 glass aquaria (150 L; 10 fish/tank), and they were acclimated for 1 more week in aquaria before the feeding trial. During the feeding trial, physicochemical parameters, including the photoperiod, temperature, pH, and dissolved oxygen (DO), were maintained at 16L: 8D, 17.3–21.6 ° C, 7.6–8.0, and 6.5–7.2 mg L−1, respectively. The feeding rate was set at 3% of the body weight during the first 40 days and 4% for the next 20 days. No mortality was observed during the feeding experiment.
Growth performances
At the end of the feeding experiment, and after 24 h of starvation, fish were anesthetized by MS-222 (tricaine methanesulfonate, 200 mg L−1), and growth performance indices were measured by using the following equations:
Collection and analysis of blood and plasma
After the feeding experiment, blood samples were collected from the caudal vein, centrifuged (4000 rpm, 15 min, 4 °C), and plasma separated and held at −80 °C until use. Anesthetized fish were sacrificed by rapid cervical section, eviscerated, and stored at −80 °C to measure the proximate composition and fatty acid profile. The biochemical parameters of plasma, including glucose (mg dL−1), cholesterol (mg dL−1), and total protein (mg dL−1), were measured using commercial kits (Pars-Azmun Chemical Company, Tehran, Iran) according to the manufacturer’s instructions.
Diet and body proximate and fatty acid composition
The composition of moisture, protein, lipid, and ash in basal diet and whole body were determined according to the Association of Official Agricultural Chemists (AOAC 1990). The fatty acid composition of the samples was determined according to the method described in our previous study (Pirani et al. 2021).
Silver nanoparticle exposure experiments
The well-characterized colloidal silver nanoparticles (AgNPs) were purchased from Pars Nano-Nasb (Tehran, Iran). Detailed characterization of this product is reported in our recent paper (Babaei et al. 2022). After the feeding trial, five starved fish from each replication of experimental groups were transferred to the 40 L aquaria (n=24) filled with dechlorinated tap water. Here, the fish of the control group (which received the basal diet) were distributed randomly into two groups (in triplicates), one of which was exposed to silver nanoparticles, and the other group was kept in normal water (without nanoparticles). Fish were exposed for 96 h to 0.5 mg L−1 AgNPs, which were selected by a series of preliminary range-finding tests (Pirani et al. 2021). After exposure, plasma samples (see the “Collection and analysis of blood and plasma” section) and liver tissues were taken from all of the fish and stored at −80 °C for measurement of silver accumulation and antioxidant status.
The levels of plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by an automated analyzer (Mindray BS-200, China) using commercial kits (Pars-Azmun Chemical Company, Tehran, Iran) and according to the manufacturer’s instructions.
Three fish from each replicate were used to determine silver accumulation in the liver. For this purpose, samples were freeze dried (24 h), weighed, and digested in nitric acid. Acid digestion was performed by adding 3 ml of concentrated nitric acid to 0.2 mg of liver samples and left for 1 h in a water bath at 100 °C and cooled at room temperature. Finally, the samples were filtered and made up to 10 mL with ultrapure water. Silver concentration was measured with the Perkin-Elmer pinAAcle 900T atomic absorption spectrophotometer in the Institute of Nanoproduct Safety Research (Hoseo University, South Korea) and expressed as μg g−1 dry weight.
Samples (livers) from two fish were used to determine antioxidant enzyme activity. For this, 100 mg of samples were thawed and homogenized with 1 mL ice-cold phosphate-buffered saline (PBS; pH 7.4) for 2 min. The samples were centrifuged (10,000 RPM, 15 min, 4 °C), and the supernatant was obtained and stored at −80 °C. Catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), and malondialdehyde (MDA) activities were measured using commercial kits (ZellBio, Germany) and according to the instructions from the manufacturer.
Statistical analysis
The normality of data was evaluated by the Kolmogorov-Smirnov test. One-way analysis of variance (ANOVA) followed by the Duncan multiple range test was performed to determine the significant differences between groups. The SPSS software (version 16) was used for statistical analysis. In all cases, the significance level was set at P < 0.05. Also, values are presented as mean ± standard deviation.
Results
Characterization of zeolite and silica nanoparticles
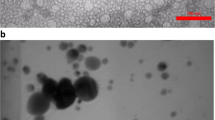
TEM and SEM images of zeolite nanoparticles (ZeNPs, Fig. 1a, b) showed that zeolite particles had been reduced in nanoscale sizes (∼50 nm). Furthermore, EDX analysis (Fig. 1c) showed that synthesized nano-zeolites contained iron, silicon, aluminum, oxygen, potassium, and sodium. Similarly, the characterization of silica nanoparticles (SiO2NPs, Fig. 2) shows a size range of 20 to 30 nm and silicon and oxygen as the main components.
Curcumin loading on zeolite and silica nanoparticles
The TG/DTA showed that the amounts of curcumin loaded on SiO2NPs and ZeNPs were 14% and 2.5%, respectively. As a result, 1 g of curcumin was loaded on each 7.15 g of SiO2NPs and for each 40 g of ZeNPs (as a result, the numbers mentioned in Table 1 have been chosen for this reason).
Growth performances
The results of growth performances after 60 days of the feeding trial are shown in Fig. 3. Body weight gain index was significantly different between different treatments (P < 0.05), so the highest weight gain was observed in the fish fed with turmeric powder, and the lowest weight gain was observed in the experimental group fed with zeolite nanoparticles and zeolite nanoparticles containing curcumin. There was no significant difference between other treatments and the control group. There was a significant difference in terms of specific growth rate between different treatments (P < 0.05), so the highest SGR was observed in the groups fed with turmeric powder, and the lowest SGR was observed in the treatment provided with zeolite nanoparticles, and there was no significant difference between other treatments and the control group. There was no significant difference between the hepatosomatic index and condition factor between different dietary treatments (P > 0.05).
Growth indices of common carp, C. carpio, after 60 days of feeding experiment with basal diet (Ctrl), turmeric (Tur), curcumin (Cur), SiO2NPs (Si), curcumin-loaded SiO2NPs (Cur-Si), ZeNPs (Ze), and curcumin-loaded ZeNPs (Cur-Ze). Bars with different letters differ significantly (mean ± SD, Duncan, P < 0.05).
Proximate composition of the body
The results of the proximate body composition after 60 days of feeding trial are presented in Table 2. The protein content in fish fed with ZeNPs had the lowest amount compared to the other experimental groups (P < 0.05). Supplementation with ZeNPs also increased (P < 0.05) the lipid content of the whole body in comparison to fish that fed on turmeric. The ash content remained unchanged; however, the lowest amount was observed in fish fed with ZeNPs and turmeric powder. The moisture was unaffected by diets between experimental groups.
Fatty acid composition
The fatty acid profile of the whole body is summarized in Table 3. The results showed that the level of saturated fatty acid (SFA) was affected by different dietary supplementation, such that the highest amounts (P < 0.05) were found in fish fed with turmeric, curcumin, ZeNPs, and curcumin-loaded ZeNPs. Significant higher amounts of total monounsaturated fatty acids (MUFA) were found in fish fed with Curcumin and ZeNPs diets (48.42% and 49.01%); meanwhile, no significant difference was observed between fish fed with curcumin-loaded SiO2NPs and control group. The amount of total polyunsaturated fatty acids (PUFA) in fish fed with dietary supplementations was significantly (P < 0.05) lower than that found in the control group (33.53%).
Plasma biochemical factors
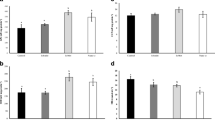
The results of plasma indices after the 60-day feeding trial are shown in Fig. 4. As can be seen, the levels of glucose, cholesterol, and total protein remained almost constant, without any significant changes, in comparison to the control group (P > 0.05).
Changes in cholesterol, glucose, and total protein of common carp, C. carpio after 60 days of feeding experiment with basal diet (Ctrl), turmeric (Tur), curcumin (Cur), SiO2NPs (Si), curcumin-loaded SiO2NPs (Cur-Si), ZeNPs (Ze), and curcumin-loaded ZeNPs (Cur-Ze). Bars with different letters differ significantly (mean ± SD, Duncan, P < 0.05).
Effects of exposure of fish to silver nanoparticles
The plasma ALT and AST levels after the exposure experiment are shown in Fig. 5. The results showed that fish fed with Curcumin had the lowest AST activity than the other groups, but no significant changes were observed compared to the control group (P > 0.05). The ALT activity decreased significantly (P < 0.05) in AgNPs-exposed fish that were fed with basal diet (positive control), curcumin, and curcumin-loaded SiO2NPs when compared to the negative control (non-exposed fish fed with basal diet).
Changes in liver enzymes of common carp, C. carpio after 60 days of feeding experiment with basal diet (Ctrl), turmeric (Tur), curcumin (Cur), SiO2NPs (Si), curcumin-loaded SiO2NPs (Cur-Si), ZeNPs (Ze), and curcumin-loaded ZeNPs (Cur-Ze) and 96-h exposure to silver nanoparticles (AgNPs, 0.5 mg L−1). The “Ctrl” is the negative control group that fed with the control basal diet and was not exposed to AgNPs, while the “AgNPs” is the positive control group that fed with the control basal diet and was exposed to AgNPs. Bars with different letters differ significantly (mean ± SD, one-way ANOVA, P < 0.05).
The antioxidant enzyme activities in the liver of fish were evaluated after the exposure experiment and are presented in Fig. 6. There were no significant changes in GPx and SOD activities after the exposure experiment (P > 0.05). The results showed that the negative control group had the lowest activity of CAT in comparison to the other exposure groups. Furthermore, liver MDA levels in the positive control group increased significantly (P > 0.05) compared to the other groups.
Changes in antioxidants biomarkers of common carp, C. carpio after 60 days of feeding experiment with basal diet (Ctrl), turmeric (Tur), curcumin (Cur), SiO2NPs (Si), curcumin-loaded SiO2NPs (Cur-Si), ZeNPs (Ze), and curcumin-loaded ZeNPs (Cur-Ze) and 96-h exposure to silver nanoparticles (AgNPs, 0.5 mg L−1). The “Ctrl” is the negative control group that fed with the control basal diet and was not exposed to AgNPs, while the “AgNPs” is the positive control group that fed with the control basal diet and was exposed to AgNPs. Bars with different letters differ significantly (mean ± SD, one-way ANOVA, P < 0.05).
The results of silver accumulation in fish liver after 96-h exposure to AgNPs are shown in Fig. 7. The highest concentration of silver was detected in the positive control group, followed by the turmeric group (P < 0.05). On the other hand, the lowest amounts of silver were observed in the negative control and SiO2NPs groups (P < 0.05).
Bioaccumulation of silver in the liver of common carp, C. carpio, after 60 days of feeding experiment with basal diet (Ctrl), turmeric (Tur), curcumin (Cur), SiO2NPs (Si), curcumin-loaded SiO2NPs (Cur-Si), ZeNPs (Ze), and curcumin-loaded ZeNPs (Cur-Ze) and 96-h exposure to silver nanoparticles (AgNPs, 0.5 mg L−1). The “Ctrl” is the negative control group that fed with the control basal diet and was not exposed to AgNPs, while the “AgNPs” is the positive control group that fed with the control basal diet and was exposed to AgNPs. Bars with different letters differ significantly (mean ± SD, one-way ANOVA, P < 0.05).
Discussion
The present study evaluated the potential role of silica and zeolite nanoparticles to increase the bioavailability of curcumin and its impact on the performance of juvenile common carp. A significant increase was observed in weight gain and SGR in fish fed with turmeric (50 g kg−1 diet) following our last paper (Pirani et al. 2021), where we showed that feeding carps for 60 days with turmeric caused the highest WG in comparison to the other experimental groups. Previous studies have shown that growth performances in fish fed with turmeric were quite different and depended pretty on the amount of turmeric and the species that were provided (Abdel-Tawwab and Abbass 2017; Abdelrazek et al. 2017; Sahu et al. 2008).
The current study showed that the proximate composition of fish, including crude protein, lipid, and ash, was not significantly changed compared to the control group. The protein content in fish fed with ZeNPs was lower than the other experimental groups, which might be related to lower feed intake and, consequently, lower protein digestion capacity. However, studies on ZeNPs as a feed additive in aquaculture, are still ongoing and more works are required to identify their potential roles in fish nutrition (Alinezhad et al. 2017; Ismael et al. 2021). On the other hand, the highest lipid content was observed in the ZeNPs feeding group. This might be explained by lipid peroxidation due to the high dosage of ZeNPs, which led to higher lipid levels. Following our results, Ismael et al. (2021) reported that the amount of crude fat was significantly influenced by dietary zeolites. However, Kanyılmaz et al. (2015) showed that the lipid content in juvenile gilthead sea bream (Sparus aurata) fed with different dietary zeolites up to 40 g kg−1 diet remained unchanged. Furthermore, the ash content in fish fed with curcumin-loaded ZeNPs was significantly higher than that of the ZeNPs group. This may be due to the higher bioavailability of curcumin when it is loaded on zeolite nanoparticles. Additionally, it has been shown that the antioxidant properties of additives could enhance digestion and absorption (Liu et al. 2019), which can improve the ash content.
Fatty acid composition is usually related to environmental conditions and can be improved by different dietary additives (Danabas 2011). In the present study, the highest amount of saturated fatty acids (SFA) was observed in fish fed with dietary turmeric, curcumin, ZeNPs, and curcumin-loaded ZeNPs. Furthermore, curcumin and ZeNPs significantly increased the amount of MUFA in the whole body of common carp. The highest levels of PUFA were observed in the control group. The existence of high amounts of PUFA in fish may increase the susceptibility of different fish tissues to oxidative stress and consequently increases the demand for antioxidant supplements in the diet (Dalle Zotte 2002; Nakano et al. 1999). In the present study, however, the higher amount of SFAs and MUFAs could be associated with the inhibitory effects of curcumin on fatty acid desaturase activity such as Δ5 desaturase and Δ6 desaturase (Nakano et al. 2000). In the current study, SiO2NPs and curcumin-loaded SiO2NPs supplementation increased the amount of PUFAs such as EPA (C20:5n3), DTA (C22:4n6), and DPA (C22:5n3). This could be associated with a better nanocarrier system of SiO2NPs to improve curcumin bioavailability.
Typically, liver tissue is one of the main targets of oxidative damage induced by metal nanoparticles (Ansari et al. 2016; Sun et al. 2016; Tang et al. 2019; Xiong et al. 2011). Liver enzymes such as AST and ALT are frequently used to assess the general health condition of fish. These enzymes can be leaked through the plasma due to liver injuries and subsequently elevate the serum concentrations (Abdel-Latif et al. 2020; Cao et al. 2015). In the present study, biochemical indices such as AST and ALT after 96-h exposure to AgNPs were found in the lowest levels in the plasma of fish fed with curcumin and curcumin-loaded SiO2NPs. This can be explained by the protective effects of curcumin and its capacity to neutralize the free radicals caused by AgNPs (Yusuf et al. 2017).
After the exposure experiment, the peroxidation index (MDA) and antioxidant enzymes were evaluated in the fish’s liver. Silver nanoparticles can induce oxidative stress by generating reactive oxygen species (ROS), which in turn causes several injuries in the liver. On the other hand, antioxidant enzymes play a protective role against ROS-mediated oxidative damage. Furthermore, it has been shown that the oxidative stress of silver nanoparticles increases in a concentration-dependent manner (Khan et al. 2017).
In the present study, the highest level of MDA was observed in the positive control group. Also, the highest level of CAT was detected in fish fed with curcumin, turmeric, and curcumin-loaded ZeNPs. Previous studies have shown that dietary supplementation with curcumin can reduce the MDA level in Nile tilapia and Jian carp (Cyprinus carpio var. Jian) (Cao et al. 2015; Mahmoud et al. 2017). Our findings confirm the antioxidant-enhancing activity of curcumin by scavenging free radicals and inhabitation of lipid peroxidation (Somparn et al. 2007).
In the present study, we also tried to determine the silver accumulation in the liver of AgNPs-exposed fish. Interestingly, the lowest silver concentration was detected in the liver of fish fed SiO2NPs. Previous studies have shown that silver ions released from AgNPs can potentially pose aquatic organisms with various hazardous threats (Levard et al. 2012). This mainly occurs due to the interaction of Ag+ with protein (AshaRani et al. 2009). The lower silver bioaccumulation in the liver of fish fed with SiO2NPs could be explained by the chelating activity of these nanoparticles against Ag+ ions. According to earlier studies, it has been shown that SiO2NPs can mediate the absorption of heavy metals and eventually can reduce their concentration (Akhter et al. 2022).
In conclusion, we have supplemented curcumin in the diet of juvenile carp with two different nanocarriers. This study showed that the loaded curcumin on silica and zeolite nanoparticles did not differ from curcumin when it was supplemented alone. Furthermore, the present study showed that silica and zeolite nanocarriers might be responsible for reducing acute toxicity and liver bioaccumulation of AgNPs at 0.5 mg L−1. This study provides insight into the encapsulation of curcumin with silica and zeolite nanoparticles, but still requires more investigation before any conclusion can be drawn.
Data availability
All data and materials are included in this published article.
References
Abdel-Latif HM, Abdel-Tawwab M, Khafaga AF, Dawood MA (2020) Dietary oregano essential oil improved the growth performance via enhancing the intestinal morphometry and hepato-renal functions of common carp (Cyprinus carpio L.) fingerlings. Aquaculture 526:735432
Abdelrazek HMA, Tag HM, Kilany OE, Reddy PG, Hassan AM (2017) Immuomodulatory effect of dietary turmeric supplementation on Nile tilapia (Oreochromis niloticus). Aquacult Nutr 23:1048–1054
Abdel-Tawwab M, Abbass FE (2017) Turmeric powder, Curcuma longa L., in common carp, Cyprinus carpio L., diets: growth performance, innate immunity, and challenge against pathogenic Aeromonas hydrophila infection. J W Aquacult Soc 48:303–312
Akdemir F, Orhan C, Tuzcu M, Sahin N, Juturu V, Sahin K (2017) The efficacy of dietary curcumin on growth performance, lipid peroxidation and hepatic transcription factors in rainbow trout Oncorhynchus mykiss (Walbaum) reared under different stocking densities. Aquacult Res 48:4012–4021
Akhter F, Rao AA, Abbasi MN, Wahocho SA, Mallah MA, Anees-ur-Rehman H, Chandio ZA (2022) A comprehensive review of synthesis, applications and future prospects for silica nanoparticles (SNPs). Silicon:1–16
Alagawany M, Farag MR, Abdelnour SA, Dawood MA, Elnesr SS, Dhama K (2021) Curcumin and its different forms: a review on fish nutrition. Aquaculture 532:736030
Alinezhad S, Faridi M, Falahatkar B, Nabizadeh R, Davoodi D (2017) Effects of nanostructured zeolite and aflatoxin B1 in growth performance, immune parameters and pathological conditions of rainbow trout Oncorhynchus mykiss. Fish Shellfish Immunol 70:648–655
Ansari MA, Shukla AK, Oves M, Khan HM (2016) Electron microscopic ultrastructural study on the toxicological effects of AgNPs on the liver, kidney and spleen tissues of albino mice. Environ Toxicol Pharmacol 44:30–43
AOAC (1990) Official methods of analysis. AOAC, Washington DC
AshaRani P, Low Kah Mun G, Hande MP, Valiyaveettil S (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3:279–290
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 446:25–29
Babaei M, Behzadi Tayemeh M, Jo MS, Yu IJ, Johari SA (2022) Trophic transfer and toxicity of silver nanoparticles along a phytoplankton-zooplankton-fish food chain. Sci Total Environ 842:156807. https://doi.org/10.1016/j.scitotenv.2022.156807
Bao X, Chen M, Yue Y, Liu H, Yang Y, Yu H, Yu Y, Duan N (2022) Effects of dietary nano-curcumin supplementation on growth performance, glucose metabolism and endoplasmic reticulum stress of juvenile largemouth bass, Micropterus salmoides. Front Mar Sci 9:924569
Cao L, Ding W, Du J, Jia R, Liu Y, Zhao C, Shen Y, Yin G (2015) Effects of curcumin on antioxidative activities and cytokine production in Jian carp (Cyprinus carpio var. Jian) with CCl4-induced liver damage. Fish Shellfish Immunol 43:150–157
Choudhury SR, Ordaz J, Lo C-L, Damayanti NP, Zhou F, Irudayaraj J (2017) From the cover: zinc oxide nanoparticles-induced reactive oxygen species promotes multimodal cyto-and epigenetic toxicity. Toxicol Sci 156:261–274
Dalle Zotte A (2002) Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livestock Prod Sci 75:11–32
Danabas D (2011) Fatty acids profiles of rainbow trout (Oncorhynchus mykiss Walbaum, 1792), fed with zeolite (Clinoptilolite). J Animal Plant Sci 21:561–565
Eya JC, Parsons A, Haile I, Jagidi P (2008) Effects of dietary zeolites (bentonite and mordenite) on the performance juvenile rainbow trout Onchorhynchus myskis. Aust J Basic Appl Sci 2:961–967
Gangwar RK, Tomar GB, Dhumale VA, Zinjarde S, Sharma RB, Datar S (2013) Curcumin conjugated silica nanoparticles for improving bioavailability and its anticancer applications. J Agri Food Chem 61:9632–9637
Hamidian G, Zirak K, Sheikhzadeh N, Khani Oushani A, Shabanzadeh S, Divband B (2018) Intestinal histology and stereology in rainbow trout (Oncorhynchus mykiss) administrated with nanochitosan/zeolite and chitosan/zeolite composites. Aquacult Res 49:1803–1815
Ismael NE, El-hameed A, Samah A, Salama AM, Naiel MA, Abdel-Latif HM (2021) The effects of dietary clinoptilolite and chitosan nanoparticles on growth, body composition, haemato-biochemical parameters, immune responses, and antioxidative status of Nile tilapia exposed to imidacloprid. Environ Sci Pollut Res 28:29535–29550
Jambhrunkar S, Karmakar S, Popat A, Yu M, Yu C (2014) Mesoporous silica nanoparticles enhance the cytotoxicity of Curcumin. RSC Adv 4:709–712
Kanyılmaz M, Tekelioğlu N, Sevgili H, Uysal R, Aksoy A (2015) Effects of dietary zeolite (clinoptilolite) levels on growth performance, feed utilization and waste excretions by gilthead sea bream juveniles (Sparus aurata). Animal Feed Sci Technol 200:66–75
Khan MS, Qureshi NA, Jabeen F, Asghar MS, Shakeel M, Fakhar-e-Alam M (2017) Eco-friendly synthesis of silver nanoparticles through economical methods and assessment of toxicity through oxidative stress analysis in the Labeo rohita. Biol Trace Element Res 176:416–428
Kotcherlakota R, Barui AK, Prashar S, Fajardo M, Briones D, Rodríguez-Diéguez A, Patra CR, Gómez-Ruiz S (2016) Curcumin loaded mesoporous silica: an effective drug delivery system for cancer treatment. Biomater Sci 4:448–459
Levard C, Hotze EM, Lowry GV, Brown GE Jr (2012) Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol 46:6900–6914
Li M, Xin M, Guo C, Lin G, Wu X (2017) New nanomicelle curcumin formulation for ocular delivery: improved stability, solubility, and ocular anti-inflammatory treatment. Drug Dev Ind Pharm 43:1846–1857
Liu HP, Wen B, Chen ZZ, Gao JZ, Liu Y, Zhang YC, Wang ZX, Peng Y (2019) Effects of dietary vitamin C and vitamin E on the growth, antioxidant defence and digestive enzyme activities of juvenile discus fish (Symphysodon haraldi). AquacultNutr 25:176–183
Mahboub HH, Shahin K, Mahmoud SM, Altohamy DE, Husseiny WA, Mansour DA, Shalaby SI, Gaballa MM, Shaalan M, Alkafafy M (2022) Silica nanoparticles are novel aqueous additive mitigating heavy metals toxicity and improving the health of African catfish Clarias gariepinus. Aquat Toxicol 249:106238
Mahmoud HK, Al-Sagheer AA, Reda FM, Mahgoub SA, Ayyat MS (2017) Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 475:16–23
Ming J, Ye J, Zhang Y, Xu Q, Yang X, Shao X, Qiang J, Xu P (2020) Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-κB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish Shellfish Immunol 97:540–553
Moghadam H, Sourinejad I, Johari SA (2021) Growth performance, haemato-immunological responses and antioxidant status of Pacific white shrimp Penaeus vannamei fed with turmeric powder, curcumin and curcumin nanomicelles. Aquacult Nutr 27:2294–2306
Moniruzzaman M, Min T (2020) Curcumin, curcumin nanoparticles and curcumin nanospheres: a review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics 12:447
Nakano T, Kanmuri T, Sato M, Takeuchi M (1999) Effect of astaxanthin rich red yeast (Phaffia rhodozyma) on oxidative stress in rainbow trout. Biochim et Biophys Acta (BBA)-Gen Subj 1426:119–125
Nakano N, Shirasaka N, Koyama H, Hino M, Murakami T, Shimizu S, Yoshizumi H (2000) C19 odd-chain polyunsaturated fatty acids (PUFAs) are metabolized to C21-PUFAs in a rat liver cell line, and curcumin, gallic acid, and their related compounds inhibit their desaturation. Biosci, Biotechnol Biochem 64:1641–1650
Nickum JG, Bart HL Jr, Bowser PR, Greer IE, Hubbs C, Jenkins JA, MacMillan JR, Rachlin JW, Rose JD, Sorensen PW, Tomasso JR (2004) Guidelines for the use of fishes in research. American Fisheries Society, Bethesda, Maryland, p 54
Petushkov A, Ndiege N, Salem AK, Larsen SC (2010) Toxicity of silica nanomaterials: zeolites, mesoporous silica, and amorphous silica nanoparticles. Adv Mol Toxicol 4:223–266
Pirani F, Moradi S, Ashouri S, Johari SA, Ghaderi E, Kim HP, Yu IJ (2021) Dietary supplementation with curcumin nanomicelles, curcumin, and turmeric affects growth performance and silver nanoparticle toxicity in Cyprinus carpio. Environ Sci Pollut Res 28:64706–64718
Sahu S, Das BK, Mishra BK, Pradhan J, Samal SK, Sarangi N (2008) Effect of dietary Curcuma longa on enzymatic and immunological profiles of rohu, Labeo rohita (Ham.), infected with Aeromonas hydrophila. Aquacult Res 39:1720–1730
Sheikhzadeh N, Kouchaki M, Mehregan M, Tayefi-Nasrabadi H, Divband B, Khataminan M, Khani Oushani A, Shabanzadeh S (2017) Influence of nanochitosan/zeolite composite on growth performance, digestive enzymes and serum biochemical parameters in rainbow trout (Oncorhynchus mykiss). Aquacult Res 48:5955–5964
Somparn P, Phisalaphong C, Nakornchai S, Unchern S, Morales NP (2007) Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharmaceut Bull 30:74–78
Sun Y, Zhang G, He Z, Wang Y, Cui J, Li Y (2016) Effects of copper oxide nanoparticles on developing zebrafish embryos and larvae. Int J Nanomed 11:905
Tang T, Zhang Z, Zhu X (2019) Toxic effects of TiO2 NPs on zebrafish. Int J Environ Res Public Health 16:523
Trewyn BG, Nieweg JA, Zhao Y, Lin VS-Y (2008) Biocompatible mesoporous silica nanoparticles with different morphologies for animal cell membrane penetration. Chem Eng J 137:23–29
Xiong D, Fang T, Yu L, Sima X, Zhu W (2011) Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ 409:1444–1452
Yang K-Y, Lin L-C, Tseng T-Y, Wang S-C, Tsai T-H (2007) Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. JChromatogr B 853:183–189
Yusuf M, Hassan MA, Tag HM, Sarivistava K, Reddy P, Hassan A (2017) Influence of turmeric (Curcuma longa) on performance, histomorphology and microbiota of intestine in juvenile tilapia (Oreochromis niloticus). Int J Agr Sci Vet Med 5:7–16
Code availability
Not applicable
Funding
This study was financially granted by the University of Kurdistan (UOK, Iran) under research grant No. GRC97-06503-1.
Author information
Authors and Affiliations
Contributions
Sh. M. and F. P.: Investigation, data curation, formal analysis. S. A.: Data curation, writing — original draft. S. A. J.: Conceptualization, supervision, funding acquisition, project administration, validation, writing — review and editing. H. P. K. and E. Gh.: Investigation. I. J. Y.: Project administration
Corresponding author
Ethics declarations
Ethics approval
All the animals were treated humanely as regards the alleviation of suffering, and all the laboratory procedures involving the animals were reviewed and approved by an Animal Care and Use Committee following the Animal Welfare Act and Interagency Research Animal Committee guidelines (Nickum et al. 2004).
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• SiO2NPs and ZeNPs were used as nanocarriers of curcumin in fish diets.
• Growth indices, biochemical factors, and fatty acids were measured after feeding trial.
• Liver enzymes, antioxidant status, and Ag accumulation were analyzed after AgNPs exposure.
• Curcumin was more effective when it was supplemented without nanocarriers.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moradi, S., Ashouri, S., Pirani, F. et al. Nutritional and ameliorative effects of dietary curcumin and its nano-silica and nano-zeolite encapsulated forms on growth, biochemical and fatty acid profile of common carp (Cyprinus carpio). Fish Physiol Biochem 49, 599–612 (2023). https://doi.org/10.1007/s10695-023-01209-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01209-1