Abstract
The present study describes, for the first time, the protective effect of natural curcumin in vivo in a lower vertebrate, a teleost, Anabas testudineus (Bloch). Two doses of curcumin 0.5 and 1% were supplemented in the 40% protein feed and fed to fish for the periods, 2 and 8 weeks. The antioxidant status, protein content, and the tissue structure in experimental fish were examined after the short-term and long-term feeding. In all the curcumin fed groups, the lipid peroxidation product, thiobarbituric acid reactive substances content either decreased or unaffected. The glutathione content increased while the antioxidant enzyme activity pattern varied with time and dose. The histological analysis also confirmed the safety of curcumin retaining the normal arrangement of hepatocytes, hepatopancreas, macrophage–melanocyte centers in Anabas. The improved antioxidant status and protein content suggest a favorable effect for curcumin in cultured fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Turmeric (the powdered rhizome of the plant Curcuma longa) has long been used as a food additive, preservative, and coloring agent in Asian countries, including China and Southeast Asia. Curcumin (diferuloylmethane) (3–4%) is responsible for the yellow color of turmeric and comprises curcumin I (94%), curcumin II (6%), and curcumin III (0.3%) (Ruby et al. 1995). A wide spectrum of biological activities is attributed to curcumin, which include anticancerous (Soudamini and Kuttan 1989; Kelloff et al. 2000), antioxidant (Elizabeth and Rao 1990), anti-inflammatory (Ruby et al. 1998), antibacterial (Allen et al. 1998), antiviral (Rasmussen et al. 2000), antifungal (Apisariyakul et al. 1995), antidiabetic (Eshrat and Ali Hussain 2002), antistress (Kato et al. 1998), hepatoprotective (Park et al. 2000), and gastro protective effects (Ramirez-Tortosa et al. 1999), based on studies conducted either in mammals or in mammalian cell lines.
Aquaculture, which uses water from the river, estuary, coastal area, is prone to external pollution and the produce (fish, prawns) can be a health risk if consumed. A wide range of chemicals is currently used in the aquaculture industry; mainly antibiotics, therapeutic chemicals (e.g., Ivermectin, Terramycin and Romet-30), copper containing anti-fouling agents etc. (Weston, 1996). Antibiotics added to water affect organisms for which they are not intended to. Thus, aquatic animals are more prone to pollution from chemicals or their own excrement. Pollutant-stimulated reactive oxygen species (ROS) production and resultant oxidative stress have been indicated as mechanisms of toxicity in aquatic organisms exposed to pollution (Livingstone 2003). ROS attack all biological molecules, especially PUFA (poly-unsaturated fatty acids) and lead to the formation of lipid peroxidation products, collectively, the thiobarbituric acid reactive substances (TBARS) content and conjugated dienes (CD), which are toxic to the body. In order to deal with the potential dangers of ROS, cells have developed a number of defense mechanisms. These include antioxidant enzymes like superoxide dismutase (SOD; EC.1.15.1.1, converts O2·− to H2O2), catalase (CAT; EC.1.11.1.6, converts H2O2 to water), glutathione peroxidase (GPx; EC. 1.11.1.9, detoxifies H2O2 and organic peroxides utilizing reduced glutathione—GSH), glutathione reductase (GR; EC.1.6.4.2), and nonenzymatic antioxidants like vitamin A, E, glutathione (GSH) etc. In the normal metabolic state of a cell, a balance exists between the generation of ROS and their quenching by antioxidants. Despite the presence of antioxidant defense systems, increased levels of oxidative damage to protein, lipid, and DNA occurs in fish with laboratory and field exposure to contaminants (Livingstone 2001). Transient increases in antioxidant enzyme activities and changes in free radical scavenger levels have been observed with exposure to contaminants, but overall, relatively little is known of the regulation of antioxidant system in fish. The same general situation of pollutant-stimulated ROS production, antioxidant defense, and oxidative damage as seen for mammals is indicated for aquatic animals. However, much less is known on many of these aspects, particularly with regard to in vivo events and the relationship of oxidative damage with disease (Livingstone 2001). Studies have shown that the consumption of food (fish, meat etc.) damaged by free radicals contains toxic peroxidation products. They can be absorbed through the gut into the systemic circulation. The accumulation of the lipid peroxidation products in the body is associated with various diseases like atherosclerosis, cancer, myopathy etc.
Artificial feeds, based mainly on feed stuffs of plant origin, are less expensive and can, with proper balance of nutrients, produce better yield. Recently, there was a report (AL-Sultan 2003) that turmeric as a feed additive enhanced the overall performance in broiler chickens. Studies on the effect of curcumin on fish tissue are scanty except for a recent report by D’Souza and Prabhu (2006) that turmeric in vitro inhibited lipid peroxidation in Somberus sombrus. Lately, in vitro hepatoprotective effect of various natural curcuminoids in fish Anabas testudineus (Manju et al. 2008a) and an in vivo and in vitro protective effect of a synthetic curcumin (salicylcurcumin) (Manju et al. 2008b) in the same fish were investigated in our laboratory. Despite reported benefits, in vitro studies in cultured cells have demonstrated that curcumin induces chromosomal and DNA damage (Antunes et al. 1999; Araujo et al. 1999a, b) detected by the comet assay (Kelly et al. 2001; Urbina-Cano et al. 2006). In view of above results, the present work was carried out to further ensure the long-term in vivo effects and safety of the doses of natural curcumin on liver antioxidant enzymes, lipid peroxidation, and also on histological structure of the liver in the freshwater teleost, Anabas testudineus.
Materials and methods
Experimental conditions
Adult fish, weighing 40 ± 5 g were collected from a local supplier captured from the rivers of Thiruvananthapuram district, Kerala, using fish nets. They were reared in large stock tanks with aerated well-water (28–30°C) and natural photoperiod 12L:12D for a month. The fish were fed ad libitum with 40% protein feed (basal feed) once daily, prepared in the laboratory (Hardy 1980). The basal feed was prepared following the square method of Hardy (1980). The components were weighed and mixed with 0.5 or 1% curcumin (powder) and made into a paste and steamed. Later, adequate amount of vitamins were added, made into pellet form, and dried under shade. Its components (Table 1) and proximate composition (Table 2) were determined earlier in our laboratory (Johnson 2004).
Experimental diet
Curcumin was a gift from Synthite Industrial Chemicals Limited, Kochi. The curcumin present in turmeric is in fact a mixture of three closely related linear diarylheptanoids. The interest of the study was to explore the beneficial effects of curcumin in aquaculture. Therefore, at first, in vitro studies were conducted using various doses and confirmed the protective effect (Manju et al. 2008a). Later on, in vivo studies were also conducted on fish fingerlings using different doses of curcumin of which the lower doses were not effective (unpublished observation). So for our long-term study, we selected 0.5 and 1% doses of curcumin and measured the activities of hepatic and renal marker enzymes in order to assess the toxicity of the doses. Since our interest was to investigate the beneficial effects, the safe doses were fixed and conducted the studies. The experimental feeds were prepared by supplementing two different doses viz. 0.5 and 1% curcumin (by weight) to the components of the basal feed and made into a paste and steamed. Later, adequate amount of vitamins were added, made into pellet form, and dried under shade. Curcumin has been extensively investigated for its antioxidant potential, and it is now well established that curcumin is a strong antioxidant in cell lines and in mammalian models. Its in vitro protective effects on fish hepatocytes (Manju et al. 2008a) were confirmed recently in our laboratory. Therefore, no known antioxidants were used as reference for the study.
Experimental design
At the end of acclimatization, the fish weighing 40 ± 5 g were transferred to aquarium tanks (61 × 30.5 × 30.5 cm) that maintained the conditions identical to the stock tanks. The tanks were labeled A1, A2, A3, B1 B2, B3, C1, C2, and C3, with eight fish each. Tank A series fish were fed with basal feed (control), tank B series with 0.5%, and tank C series with 1% curcumin supplemented feed in each set. Each tank received accurately weighed 10% BW feed once daily in the morning hours. A short-term study was conducted for a period of 2 weeks, and a long-term study for 8 weeks.
Sample preparation
At the end of experimentation period (2 and 8 weeks), fish were fasted overnight and eight fish were selected at random from each triplicate group. They were killed by decapitation. Liver was selected for the study since it constitutes the main organ involved in detoxification and metabolism of xenobiotics. Curcumin is entirely a new compound to fish system. Therefore, at first, it was planned to evaluate effect of curcumin on the physiological state especially on the antioxidant system of the fish. The effect of various doses of curcumin on Anabas liver was examined by biochemical methods in the short-term study and in the long-term feeding; the effect was analyzed by histological, biochemical, and electrophoretic methods. Liver was fixed immediately in buffered formalin fixative for histology. For biochemical analysis, the tissue was collected in ice-cold containers and washed with saline and the tissues (100 mg) were homogenized using MICRA-D8 homogenizer in ice-cold sucrose buffer and centrifuged at 5,000 rpm for 10 min. The supernatant was used for the enzyme assays. For the analysis of TBARS content, tissue was homogenized in the Tris–HCl buffer, centrifuged and the same fraction was taken.
Histological sampling
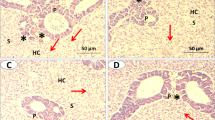
Liver from five randomly selected fish from each long-term fed fish were fixed in buffered formalin was washed in running water and dehydrated by passing through ethanol series (30–100%). The tissue was then cleaned and infiltrated for 2 h in molten paraffin wax for 24 h at 60°C. The blocks prepared were then sectioned in a Leica microtome at 3–4 μm thickness. The sections were taken on slides, dehydrated once again using the same alcohol series from 100 to 30% ethanol after which it was stained using Hematoxylin–Eosin (H & E) (Clark 1981). The stained sections were mounted in DPX. Light microscopic preparations were observed under Leica research microscope, and the images were captured in a Pentium IV computer using Qwin software (Leica, Jena, Germany). Ten microscopic fields (sorted randomly) of five slides with sections taken from five different fish in each group were examined and scored in a blinded fashion by a single pathologist, and histometric measurements were taken. Surface area of hepatocyte (μm2), surface area of hepatocyte nuclei (μm2), and the number of hepatocyte nuclei per μm2 of hepatic tissue [hepatocyte density (cells μm−2)] were determined using a Pentium IV computer using Qwin software (Leica, Jena, Germany). The hepatocellular evaluation was assessed according to Bernet et al. (1999).
Biochemical analyses
All the chemicals used were of analytical grade and purchased from SRL, Mumbai. The secondary lipid peroxidation products were determined as the thiobarbituric acid reactive substances (TBARS) content, by the method of Nichans and Samuelsson (1968). The activities of antioxidant enzymes, superoxide dismutase (SOD) (EC.1.15.1) using the method of Kakkar et al. (1984), catalase (CAT) (EC.1.11.1.6) by Maehly and Chance (1954), glutathione peroxidase (GPx) (EC. 1.11.1.9) by the method of Lawrence and Burk (1976), and glutathione reductase (GR) (EC.1.6.4.2) by the method of David and Richard (1983) were determined. Glutathione content (GSH) was assayed according to Benke and Cheevar (1974). Protein was estimated with bovine serum albumin (BSA) as standard (Bradford 1976). Absorbance was measured using a UV–visible spectrophotometer (UV-1601, Shimadzu, Japan).
Electrophoretic analyses—activity staining for SOD, CAT, GPX, and GR
Activity staining for SOD, CAT, GPx, and GR—Analytical polyacrylamide gel was performed according to a modified procedure. (Gabriel 1971). It was performed using a 10% native acrylamide slab gel in Tris–Glycine buffer, pH 8.3. The samples were mixed with 2× sample buffer without reducing agents. The concentration of protein loaded at each lane was same, and a constant voltage of 100 V was applied. Natural curcumin-treated samples (0.5%) were run along with the control for comparison. After electrophoresis, the SOD activity was visualized by the modified photochemical method of Beauchamp and Fridovich (1971). The gel was first soaked in 0.1% TEMED, 1.225 mM nitro blue tetrazolium solution and 28 μl riboflavin, and incubated in dark for 40 min. After incubation, the gel was transferred into 0.1% TEMED solution. After briefly washing, it was illuminated with a light box with an intensity of 30 μEs−lm−2 for 15 min to initiate photochemical reaction. After exposure, achromatic bands corresponding to SOD activity appeared on a dark blue back ground. For catalase activity staining (Sun et al. 1988), the gel was first rinsed in distilled water three times and then incubated in 0.003% hydrogen peroxide (H2O2) for 10 min. The gel was then stained with 2% ferric chloride and 2% potassium ferricyanide. When achromatic bands demonstrating catalase activity appeared in a green background, the staining solution was replaced and washed with distilled water. For GPx (Mishra and Fridovich 1977), the gels were stained in a solution containing 2 mM dianisidine, 10 mM potassium phosphate buffer at pH 7.2 for 1 h followed by 15 min incubation in 0.1 mM H2O2. Brown bands against pale yellow background were considered GPx. The gel was placed in a freshly prepared dye solution (3.4 mM GSSG, 0.36 mM NADPH, 0.052 mM dichlorophenol indophenol, 1.1 mM MTT, prepared in 250 mM Tris, pH 8) for GR activity. GR activity appeared as purple precipitate in the gel. All the activity gels were scanned, and densities were determined using Bio-Rad gel quantitation system and quantity one software.
Statistical analyses
The enzyme and the histometric data were statistically analyzed by one-way analysis of variance (ANOVA), and the protein, MDA, and GSH were analyzed by two-way ANOVA at 3, 2, and 8 weeks using the SPSS setup. The results were expressed as mean ± SE of eight animals. The significant difference among means was determined by Duncan’s multiple range test (Duncan 1955) at the level, P < 0.05.
Results
Histological analyses
Histopathological investigations have proved to be a sensitive tool to detect direct effects of chemical compounds within target organs of animals in laboratory experiments and field animals. The present study demonstrated that the liver of both the control and experimental fish exhibited a normal structure, and there were no pathological abnormalities. The liver of control fish was formed of compactly arranged hepatocytes, a few blood capillaries, and hepatopancreas. Bile canaliculi were prominently seen. Melano-Macrophage (MM) is a special category of macrophage also present in the hepatic parenchyma, usually seen in the vicinity of hepatic arteries, portal veins, or bile ducts and concentrated as melanomacrophage centers (MMC). Numerous such patches were seen in both the control and the treated groups. Exocrine pancreatic tissue occurs around the major portal vessels and collectively called the hepatopancreas were seen abundantly. MMCs mainly occur in the vicinity of hepatopancreas. In the curcumin-treated fish, there were no pathological abnormalities with hepatocytes presenting a homogeneous cytoplasm and a large central or subcentral spherical nucleus irrespective of the dose of curcumin (Fig. 1). The hepatocyte density, hepatocyte area, and hepatocyte nuclear area were also unchanged after curcumin treatment (Fig. 2).
a Effect of curcumin on surface area of hepatocyte (μm2) b Effect of curcumin on surface area of hepatocyte nuclei (μm2) c Effect of curcumin on hepatocyte density (cells μm−2). Values are mean ± SE of 10 values. Values with same lowercase letters are not statistically different (P < 0.05) as determined by one-way ANOVA followed by Duncan’s multiple range test using the SPSS setup
Biochemical analyses
Short-term study
Total protein increased in all the curcumin fed groups. SOD activity increased in the 1% curcumin fed group. The activity of CAT increased in 1%-treated group. GPx and GR activity were unaffected. Glutathione was lowered in the 1% group. TBARS decreased in all treated groups (Table 3; Fig. 3).
a Effect of curcumin on protein content (mg ml−1) after 2 and 8 weeks of treatment b Effect of curcumin on GSH content [mmol (100 g tissue)−1] after 2 and 8 weeks c Effect of curcumin on TBARS content [μmol MDA (g tissue)−1] after 2 and 8 weeks of treatment. Results expressed as mean ± SE of 8 animals. The significant difference between groups was analyzed by two-way ANOVA. *P < 0.05 compared with respective controls
Long-term study
Protein content and glutathione content increased in all the groups. TBARS content was unaffected (Fig. 3). Two-way ANOVA of protein (Fig. 3a) revealed that there was a significant effect of time on the protein content of the fish (F = 1007.5, P < 0.001). As the time period increased, the protein content also increased. Similarly, there was a dose-dependent effect of curcumin on protein concentration (F = 111.7, P < 0.001). Time and dose interaction was also significant with an F value 58 and p value 0.0001. Similarly, the effect of GSH (Fig. 3b) and MDA (Fig. 3c) was also time (F = 70.1, P < 0.001 for GSH and F = 50.3, P < 0.001for MDA) and dose (F = 23.6, P < 0.001 for GSH and F = 8.2, P < 0.001 for MDA) dependent. The time and dose interaction on GSH was significant with F value 24.2, p value 0.000, whereas that of MDA was not significant (F = 2.17, P < 0.131).
Electrophoretic analyses of antioxidant enzymes
Long-term study
Liver SOD increased in density in native gel analysis at the 0.5% (Fig. 3a). Catalase increased in the 1% curcumin-treated group when compared to the 0.5% treatment group (Fig. 3b). GPx was inhibited in the 1% treatment group (Fig. 3c), whereas GR activity was unaffected in the curcumin-treated groups (Fig. 3d).
Discussion
Curcuma longa extracts have been traditionally used for centuries as a hepatoprotective agent for liver disorders. In the present study also, the normal liver histology was unaffected after the two doses of curcumin feeding, confirming an in vivo protective effect. The results were also consistent with observations by some authors in rats, guinea pigs, monkeys, and pigs (Wahlstrom and Blennow 1978; Bhavanishankar et al. 1980; Bille et al. 1985). However, there are studies which revealed that administration of turmeric extract induced hepatotoxic effects in mice and rats (Deshpande et al. 1998; Kandarkar et al. 1998). Curcumin and its analogs regained the normal histology of CCl4-treated rat liver and caused mild sinusoidal dilation (Kamalakkannan et al. 2005). Feeding of turmeric added to diet to chicken induced dilation of bile ducts, hyperplasia of biliary epithelium, and periportal hepatocytes degeneration, which were not time and dose dependent (AL-Sultan 2003). Therefore, it can be concluded that effects of curcumin are dependent on time and dose of treatment chosen for the study (Fig. 4).
a Effect of curcumin on superoxide dismutase activity [density (mm−2)] in the liver of A. testudineus b Effect of curcumin on catalase activity [density (mm−2)] in the liver of A. testudineus c Effect of curcumin on glutathione peroxidase activity [density (mm−2)] in the liver of A. testudineus d Effect of curcumin on glutathione reductase activity [density (mm−2)] in the liver of A. testudineus. Results expressed as mean ± SE of 8 animals. The significant difference between groups was analyzed by one-way ANOVA. Mean values of different letter headings (a and b) are significantly different (P < 0.05) as determined by Duncan’s multiple range test, using the SPSS set up
In the short-term in vivo study, lipid peroxidation product decreased in all the curcumin-treated groups, assuring their protective role in vivo too. The increase in protein in curcumin-fed group in the present study is consistent with an earlier report by AL-Sulthan (2003) in broiler chickens. SOD activity increased in the 1% group and unaffected in the 0.5% group indicating that curcumin does not always depend on the enzymatic pathway to mediate their antioxidant effect. The GPx and GR activity remain unaffected. This may be due to the increased catalase activity in the treated group, which might have depleted the substrate (H2O2). It has been suggested that curcumin exerts its action by maintaining the activities of antioxidant enzymes like SOD, CAT, and GPx (Pulla Reddy and Lokesh 1992). GSH, the primary intracellular free thiol fulfills many intracellular critical roles, including scavenging ROS to maintain protein bound thiols in their reduced active forms. It also serves as a cofactor for the phase II enzymes including peroxidases. The decrease in GSH content in the 1% group may be due to the presence of high dose curcumin, which could also function as a nonenzymatic antioxidant similar to GSH. Even though there was no change in the SOD, CAT, and GSH in the 0.5% group, there was a significant reduction in TBARS content, indicating a direct scavenging of free radicals by curcumin.
In the long term also, there was an increase in protein concentration. But the antioxidant enzyme activity varied without affecting lipid peroxidation. The nonenzymatic antioxidant, glutathione (GSH) increased in both the treated groups. This may mean that curcumin inclusion increased cellular GSH levels likely reflecting the antioxidant and therefore, GSH sparing properties of curcumin (Rinaldi et al. 2002) as a long-term effect. Since liver is the chief organ concerned with detoxification process, availability of increased amount of GSH could improve this function. In the native gel, SOD activity increased in the 0.5% group may be due to the time-dependent effect of curcumin. CAT activity unaltered in the densitogram, may be due to the direct scavenging of H2O2 by curcumin. Studies have shown that curcumin prevented oxidative damage during indomethacin-induced gastric lesion, not only by blocking inactivation of gastric peroxides but also by direct scavenging of H2O2 and the hydroxyl radical (Halliwell and Gutteridge 1990; Bandyopadhyay et al. 1999). Another mechanism by which curcumin protects oxidative stress in endothelial cells is by induction of heme oxygenase-1 (Motterlini et al. 2000).
In conclusion, the present study confirms the protective effect of curcumin in a sub-mammalian vertebrate group that was investigated in vitro recently in our laboratory. A time- and dose-dependent effect of curcumin on fish lipid peroxidation is confirmed, as has been reported by Ambegaokar et al. (2003), Swarnakar et al. (2005), Ahuja et al. (2006) and Manju et al. (2008a, b). Curcumin may directly scavenge free radicals, stimulate antioxidant enzymes pathway, and increases the antioxidant contents in the cells. It uses different pathways to bring about its effect. More than eighty molecular targets have been reported for curcumin so far. Even though antioxidant enzymes activity inhibited, there was no increase in the TBARS content in the liver assuring its role as a promising nontoxic feed additive in the aquaculture field. This inhibition may be a part of maintaining the normal physiology of the curcumin-treated groups. Histological data also support the protective effect. Curcumin also facilitated growth through an increase in protein concentration, which is characteristic of true growth in fish to suggest that curcumin in very low doses can be used in the aquaculture feed in order to increase the quality and quantity of fish.
References
Ahuja KD, Kunde DA, Ball MJ, Geraghty DP (2006) Effects of capsaicin, dihydrocapsaicin, and curcumin on copper-induced oxidation of human serum lipids. J Agric Food Chem 54:6436–6439
Allen PC, Danforth HD, Augustine PC (1998) Dietary modulation of avian coccidiosis. Int J Parasitol 28:1131–1140
AL-Sultan SI (2003) The effect of Curcuma longa (turmeric) on overall performance of broiler chickens. Int J Poult Sci 5:351–353
Ambegaokar SS, Wu L, Alamshahi K, Lau J, Jazayeri L, Chan S, Khanna P, Hsieh E, Timiras PS (2003) Curcumin inhibits dose-dependently and time dependently neuroglial cell proliferation and growth. Neuro Endocrinol Lett 24:469–473
Antunes LMG, Dias FL, Takahashi CS (1999) Potentiation by turmeric and curcumin of radiation induced chromosome aberration in Chinese hamster ovary cells. Teratogen Carcin Mut 19:9–18
Apisariyakul A, Vanittanakom N, Buddhasukh D (1995) Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae). J Ethnopharmacol 49(3):163–169
Araujo MC, Dias FL, Kronka SN, Takahashi CS (1999a) Effect of turmeric and its active principle, curcumin on bleomycin induced chromosome aberrations in Chinese hamster ovary cells. Genet Mol Biol 22:407–413
Araujo MC, Dias FL, Takahashi CS (1999b) Potentiation of turmeric and curcumin of γ-radiation induced chromosome aberrations in Chinese hamster ovary cells. Teratogenesis Carcinog Mutagen 19:9–18
Bandyopadhyay U, Das D, Banerjee RK (1999) Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci 77:658–666
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–278
Benke GM, Cheevar KC (1974) Comparative toxicity, anti-cholinesterase action and metabolism of methyl parathion and parathion in sun fish and mice. Toxicol Appl Pharmacol 28:97–109
Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J fish diseases 22:25–34
Bhavanishankar TN, Shanta NV, Ramesh HP, Murthy Indira AS, Sreenivasmurthy V (1980) Toxicity studies on turmeric (Curcuma longa): Acute toxicity studies in rats, guinea pigs and monkeys. Ind J Exp Biol 18:73–75
Bille N, Larsen JC, Hansen EV, Wurtzen G (1985) Subchronic oral toxicity of turmeric oleoresin in pigs. Food Chem Toxicol 23:967–973
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
Clark G (ed) (1981) Staining procedures used by the Biological Stains Commission In: Staining procedures, 4th edn. Williams and Wilkins, Baltimore, pp 471–472
D’Souza HP, Prabhu HR (2006) In vitro inhibition of lipid peroxidation in fish by turmeric (Curcuma longa). Indian J Clin Biochem 21(2):138–141
David M, Richard JS (1983) Glutathione reductase. Method Enzymol Anal 3:258–265
Deshpande UR, Gadre SG, Raste AS, Pillai D, Bhide SV, Samuel AM (1998) Protective effect of turmeric (Curcuma longa L.) extract on carbon tetrachloride induced liver damage in rats. Ind Exp Biol 36:573–577
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Elizabeth K, Rao MNA (1990) Oxygen radical scavenging activity of curcumin. Int J Pharm 58:237–240
Eshrat H, Ali Hussain M (2002) Hypoglycemic, hypolipidemic and antioxidant properties of combination of curcumin from Curcuma longa, linn, and partially purified product from Abroma augusta, linn. in streptozotocin induced diabetes. Indian J Clin Biochem 17(2):33–43
Gabriel O (1971) Analytical disc gel electrophoresis. Methods Enzymol 22:565–578
Halliwell B, Gutteridge JMC (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186:59–85
Hardy R (1980) Fish feed formulation. Lectures presented at the FAO/UNDP Training course in fish feed technology, held at the College of Fisheries. University of Washington, Seattle, pp 233–240
Johnson C (2004) Influence of certain plant extract on growth and metabolism of teleosts (Anabas testudineus and Labeo rohita) and a mammal (Rattus norvegicus). Ph.D thesis, University of Kerala, Thiruvananthapuram, Kerala, India
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Kamalakkannan N, Rajagopalan R, Suresh PV, Viswanathan P, Rajasekharan KN, Menon VP (2005) Comparative Effects of curcumin and an analogue of curcumin in carbon tetrachloride-induced hepatotoxicity in rats. Basic Clin Pharmacol Toxicol 97(1):15–21
Kandarkar SV, Sharda SS, Ingle AD, Deshpande SS, Maru GB (1998) Subchronic oral hepatotoxicity of turmeric in mice—histopathological and ultrastructural studies. Ind J Exp Biol 36:675–679
Kato K, Ito H, Kamei K, Iwamoto I (1998) Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo. Cell Stress Chap 3:152–160
Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW et al (2000) Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr 130:467S–471S
Kelly MR, Xu J, Alexander KE, Loo G (2001) Disparate effects of similar phenolic phytochemicals as inhibitors of oxidative damage to cellular DNA. Mut Res 485:309–318
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Livingstone DR (2001) Contaminant stimulated reactive oxygen production and oxidative damage in aquatic organisms. Mar Pollut Bull 42:656–666
Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev Med Vet 154:427–430
Maehly A, Chance B (1954) The assay of catalases and peroxidases. Methods Biochem Anal 1:357–424
Manju M, Sherin TG, Rajeesha KN, Sreejith P, Rajasekharan KN, Oommen OV (2008a) Curcumin and its derivatives prevent hepatocyte lipid peroxidation in Anabas testudineus. J Fish Biol 73:1701–1713
Manju M, sherin TG, Rajasekharan KN, Oommen OV (2008b) Curcumin analogue inhibits lipid peroxidation in a freshwater teleost, Anabas testudineus (Bloch)—an in vitro and in vivo study. Fish Physiol Biochem. doi:10.1007/s10695-008-9266-6
Mishra HP, Fridovich I (1977) Superoxide dismutase and peroxidase; a positive activity staining applicable to poly acrylamide gel electrophorograms. Arch Biochem Bio phys 183:511–515
Motterlini R, Foresti R, Bassi R, Green CJ (2000) Curcumin, an antioxidant and anti-inflammatory agent induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med 28:1303–1312
Nichans WG, Samuelsson B (1968) Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130
Park EJ, Jeon CH, Ko G, Kim J, Sohn DH (2000) Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J Pharm Pharmacol 52:437–440
Pulla Reddy AC, Lokesh BR (1992) Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol Cell Biochem 111:117–124
Ramý′rez-Tortosa MC, Mesa MD, Aguilera MC, Quiles JL, Baro′ L, Ramirez-Tortosa CL et al (1999) Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis 147:371–378
Rasmussen HB, Christensen SB, Kvist LP, Karazmi A (2000) A simple and efficient separation of the curcumins, the antiprotozoal constituents of Curcuma longa. Planta Med 66:396–398
Rinaldi AL, Morse MA, Fields HW, Rothas DA, Pei P, Rodrigo KA, Renner RJ, Mallery SR (2002) Curcumin activates the aryl hydrocarbon receptor yet significantly inhibits (−)-Benzo(a)pyrene-7R-trans-7,8-dihydrodiol bioactivation in oral squamous cell carcinoma cells and oral mucosa. Cancer Res 62:5451–5456
Ruby JA, Kuttan G, Dinesh Babu KV, Rajasekharan KN, Kuttan R (1995) Anti-tumour and free radical scavenging activity of synthetic curcuminoids. Int J Pharm 131:1–7
Ruby JA, Kuttan G, Dinesh Babu KV, Rajasekharan KN, Kuttan R (1998) Anti-inflammatory activity of natural and synthetic curcuminoids. Pharm Pharmacol Commun 4:103–106
Soudamini KK, Kuttan R (1989) Inhibition of chemical carcinogenesis by curcumin. J Ethnopharmacol 27:227–233
Sun Y, Elwell JH, Oberley LW (1988) A simultaneous visualization of the antioxidant enzymes glutathione peroxidase and catalase on polyacrylamide gels. Free Radic Res Commun 5:67–75
Swarnakar S, Ganguly K, Kundu P, Banerjee A, Maity P, Sharma AV (2005) Curcumin regulates expression and activity of matrix metalloproteinase 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J Biol Chem 280:9409–9415
Urbina-Cano P, Morales BL, Herrera MA, Rivera JR, Magna ML, Sanroman R, Rivera A (2006) DNA damage in mouse lymphocytes exposed to curcumin and copper. J Appl Genet 47:377–382
Wahlstrom B, Blennow G (1978) A study on the fate of curcumin in the rat. Acta Pharmcol Toxicol 43:86–92
Weston DP (1996) Ecological effects of the use of chemicals in aquaculture. In: Use of chemicals in aquaculture in Asia. Arthur JR, Lavilla-Pitogo CR, Subasinghe RP (eds) Proceedings of the Meeting on the Use of chemicals in aquaculture in Asia 20–22 May 1996. Tigbauan, Iloilo, Philippines
Acknowledgments
The authors are grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi for providing the Senior Research Fellowship (M.M) and University Grants Commission–Special Assistance Programme (UGC-SAP) for the infrastructural facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manju, M., Akbarsha, M.A. & Oommen, O.V. In vivo protective effect of dietary curcumin in fish Anabas testudineus (Bloch). Fish Physiol Biochem 38, 309–318 (2012). https://doi.org/10.1007/s10695-011-9508-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-011-9508-x