Abstract
In this research, we intended to appraise the hydrogeochemistry and human health risks of groundwater (GW) in southwestern Bangladesh, applying hydrogeochemical techniques, GW quality index (GWQI), several pollution indices, and mathematical health risk models. The trace elements (TEs) and ionic composition of GW samples were analyzed by atomic absorption spectroscopy and ion chromatography (AAS-IC) technique. The evaporate dissolution, silicate weathering, and ionic exchange processes control the hydrogeochemistry in GW. The GWQI revealed that 34% of samples were poor to very poor quality for drinking purposes, whereas irrigation water quality indices suggested moderate suitability of GW. The mean hazard quotient (HQ) and hazard index (HI) exceeded the tolerable level for adults and children, making substantial chronic health impacts on humans. The estimated carcinogenic risk of As and Pb surpassed the upper level of 1 × 10–4 for both aged populations. Overall, the results indicate that the local inhabitants have detrimental health risks; hence, effective regulation and proper measures should be concentrated for continuous monitoring, assessment, and remediation of As, Mn, Pb, and Hg in the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bangladesh utilizes approximately 4% of world GW resources which met up its 98% national water requirement (Hanasaki et al. 2018; Shamsudduha et al. 2018). Protection of GW quality from diverse geogenic (naturally occurring arsenic, fluoride, salinity, iron, and manganese) and anthropogenic inputs such as agriculture, industry or human settlements are key issues in Bangladesh (Rahman et al. 2017a; Islam et al. 2021). An earlier study revealed that approximately 97% population of Bangladesh uses GW as drinking water, and nearly 70–86% of water is used for irrigation purposes (Kabir et al. 2021). GW scarcity is severe in the southern coastal belt of Bangladesh compared to the northern part, because of the elevated level of As, Fe, and salinity problems. Furthermore, the southern part has 710 km coastline which is vulnerable to different natural disasters, i.e., cyclone, storm surges, saltwater intrusion, and sea-level rise. As a result, the soil, surface water, and even GW quality of this province has been intensifying through trace elements (TEs) pollution and increased salinity levels (Anny et al. 2017; Kabir et al. 2017; Kabir et al. 2018; Salam et al. 2019; Kayes et al. 2019; Kabir et al. 2020; Islam et al. 2019, 2020a; Hasan et al. 2021). Water quality relies on physicochemical and biological features (Hassan et al. 2016); different natural and anthropogenic factors control these properties (Kumar et al. 2021). Chemical characteristics of GW vary with different hydrogeochemical processes and those mechanisms are well established by many researchers (Bhuiyan et al. 2016; Islam et al. 2017a, 2017b, 2017c; Ahmed et al. 2020; Rahman et al. 2021).
Thus, the origin of the chemical composition can be determined through the knowledge of hydrogeochemistry of GW (Thilagavathi et al. 2012), and this knowledge is also required to understand GW resources management (Islam et al. 2018). However, TEs are toxic depending on their level of concentration, persistent, and carcinogenic (Islam et al. 2017c), consumption of TEs-contaminated GW causes different problems in human health (Smith et al. 2000; Mridul et al. 2020; Kabir et al. 2021a; Tasneem et al. 2021; Ahmed et al. 2021), hence require efforts to assess the human health risk (HHR) and subsequent management applying different mathematical and traditional health risk models (Kabir et al. 2020). Therefore, integration of water quality appraisal and monitoring for drinking and agricultural purposes along with HHR assessment can protect life and the environment (Islam et al. 2020b).
Many researchers have employed water quality index (WQI) for evaluating the water quality of any region where the index value depends on the input parameters in GW (Singh et al. 2018; Shahid et al. 2014) but WQI values alone cannot exhibit any evidence of the sources of GW pollution. Thus, for a better evaluation of drinking water quality, different pollution evaluation indices have been used with the WQI analysis (Shahid et al. 2014). Different types of pollution appraisal indices such as heavy metal evaluation, heavy metal pollution, and degree of contamination indices are usually adopted to assess the level of TEs contamination in water (Edet and Offiong 2002; Rikta et al. 2016; Sultana et al. 2016). To appraise the irrigation water quality, several indices have frequently been used, such as sodium adsorption ratio (SAR), magnesium adsorption ratio (MAR), residual sodium bicarbonate (RSBC), or soluble sodium percentage (SSP) (Ashraf and Afzal 2011).
Spatial patterns of irrigation water quality indices can determine the possible sources of contamination and identify the polluted area. Few studies have addressed on the health effects of TEs contamination for drinking use; however, a systematic investigation on the usage of GW for irrigation is still less explored. The GW contamination in the southern part of Bangladesh is increasing gradually by both geogenic and anthropogenic sources as reported by several scattered research (Islam et al. 2020c; Hasan et al. 2019).
We hypothesize that Upazilla wise characterization of GW hydrogeochemistry, quality, and potential health risks to the local community of southwestern Bangladesh could ensure sustainable GW management. Moreover, so far, no prior research has intended to appraise the hydrogeochemistry, quality, and probable health risk to humans of GW over the study basin. Such analysis can give a comprehensive idea of the GW quality status in the designated area. This study scientifically appraises water hydrogeochemistry and quality in the Jashore Sadar Upazila (JSU). Thus, it is imperative to appraise the GW quality and explore the key hydrogeochemical factors influencing hydrochemistry which ultimately affect GW suitability for drinking as well as agricultural usage. The major objectives of this research are (1) to characterize the hydrogeochemistry of the study area, (2) to detect the key factors influencing the hydrogeochemical process of GW, (3) to evaluate the GW and irrigation water suitability and their spatial variation in the study area, and (4) to appraise the potential HHR posed by TEs in water to humans via oral ingestion. The outcomes of this research will be helpful for sustainable GW management and protecting GW resources in the study area.
Materials and methods
Study site description
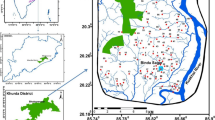
JSU under Jashore district is positioned in the south-western section of Bangladesh. It is a coastal region and geographically, the area encompasses between latitude 23°06′ to 23°20′ N and longitude 89°04′ and 89°22′ E (Fig. 1). According to the physiographic subdivision, the study area falls in the old Ganges River floodplain (Brammer 2012). The approximate space of the upazila is 435.22 km2 with a total population of 742.898 (BBS 2011). The study area falls into a tropical monsoon climatic region with a mean annual rainfall of 2000–2400 mm, increasing from southwest to northeast (Islam et al. 2019). The JSU shows seasonal rainfall variation with 70–80% of rainfall happening in the monsoon rainy season (June–September), often led to heavy flood. Generally, GW is the prime source of drinking water for local respondents of the study area. The aquifer system of the area is mostly unconfined to leaky confined type and GW can be found within a few meters of the surface. Lithologically, the upper shallow aquifers are comprised of fine to very fine sands and the deeper aquifers are consisting of medium to coarse sand (Ahmed et al. 2004; Rahman et al. 2020). The alluvial deposit is developed in the southern Bengal basin and floodplain, and there is a distribution of thin water pocket, fine sand bearing particles bounded by coarse sand layers. This region embodies by a smooth hydraulic gradient, higher runoff, and good water variation (Islam et al. 2016a, b).
Hydrogeological settings of the study area
The investigated area falls within the western part of Faridpur Trough of Bengal Foredeep (Alam 1990) and is located on a natural levee of the Rupsha and Bhairab Rivers and characterized by Ganges tidal floodplains with low relief, criss-crossed by rivers and water channels, and surrounded by tidal marshes and swamps. The surface lithology of the area is of deltaic deposits which are composed of tidal deltaic deposits, deltaic silt deposits, and mangrove swamp deposits (Alam 1990). The aquifers in and around the study area are generally multilayered varying from unconfined to leaky-confined in the shallow alluvial deposits and confined in the deeper alluvial deposits (Uddin and Lundberg 1998). The aquifer systems of the study area can be classified into two major classes: the shallow aquifers ranging from depth ~ 10 to 150 m and deep aquifers generally > 180 m depth. The water of this aquifer is generally brackish or saline with few isolated fresh water pockets (DPHE 2006).
Analytical procedures
Thirty samples were collected on a purposive random basis from the tube wells which cover each union of the JSU (Fig. 1) at depths ranging from 120 to 220 m during June–July in 2019. The Geographical positions of the well were identified by a GPS receiver (Kansas, USA). The depths of the well were obtained from the owners of the well and the previous record of the LGED (Local Government Engineer Department) (LGED 2014). Before sampling, each well was purged for 10 min for obtaining steady-state chemical conditions and samples were filtered to eradicate insoluble materials using 0.45 μm filter membranes. The experimental layout of this research is presented in Fig. 2. Portable digital equipment (Model: SensionTM1, HACH 146 International, USA) was employed in situ to estimate pH, electrical conductivity (EC), dissolved oxygen (DO), and temperature of all the studied samples. Appropriate standards were applied for calibrating field meters. From each site, two sets of samples were taken in prewashed high-density polypropylene (HDPP) bottles. At first, 1% HNO3 was used for cleaning the sample bottle and subsequently washed with distilled water.
The concentrations of main cations (Li+, Na+, K+, Ca2+, NH4+, and Mg2+) and anions (F−, Cl−, NO2−, Br−, NO3−, and SO42−, PO43−) were determined by ion chromatography (Model: Ion Chromatograph, Dionex DX-3000, USA) using IonPac CS17 and IonPac AS11-HC analytical columns respectively. Six Cation-II standard and Seven Anion-II standard were applied for calibrating the instrument. Alkalinity (HCO3−) was determined by titration method using 0.02 N H2SO4 (Kabir et al. 2021). The trace elements (Fe, Cd, Pb, Cr, Zn, Mn, Hg, Co, Ni, and Cu) in groundwater samples were estimated by flame atomic absorption spectrophotometer (Model No. AA240, Varian, Australia) using acetylene and air gas mixture tools. Arsenic (As) was estimated by hydride generation technique. The concentration of As was calculated by the hydride generation method. Duplicate analysis on selected samples was used for assuring the precision of the analyses, and the average results for all analyses represented the data. Furthermore, the precision of the datasets was confirmed by the charge balance error (CBE) of major ions occupying within ± 5% in all tested samples of the study area.
Pollution level indices of GW
Groundwater quality index (GWQI) acts an important role for setup the water quality and security for purposes of drinking uses (Vasanthavigar et al. 2010; Bodrud-Doza et al. 2016; Islam et al. 2017d). Estimation of GWQI has been performed by applying the Eq. (1).
According to Vasanthavigar et al. (2010), variables, weight factors, and limit values were regarded for calculating the GWQI which are summarized in the supplementary Table S1.
Heavy metal pollution index (HPI) is computed by the following Eqs. (2) and (3) (Bhuiyan et al. 2016)
Heavy metal evaluation index (HEI) was computed by the methods described by Prasad and Jaiprakas (1999) with the following Eq. (4):
The degree of contamination (Cd) value is estimated by the Eq. (5) (Backman et al. (1997):
Where, \( \mathrm{Cfi}=\frac{CAi}{CNi}-1 \)
Irrigation water quality indices
The irrigation quality index is calculated by the following equations:
Irrigation water evaluation indices were quantified using their respective equations (Eqs. 3–9).
Soluble sodium percentages,
The residual sodium carbonate,
Doneen (1964) defined PI as:
Magnesium absorption ration (MAR) also is known as magnesium hazard,
Finally, Kelley’s ratio (KR) is calculated by (Kelly 1940):
Ionic concentrations were measured in meq/L. All of the calculated chemical parameters were contrasted with national and international standards to evaluate the irrigation water suitability.
Human health risk evaluation
Non-carcinogenic risk
The value of six TEs was adopted to appraise the health risks through oral contact ways for the people of JSU, in Jashore District of Bangladesh. In this study, traditional USEPA (2004) models applied to measure the NHR and the lifetime carcinogenic risk (CR). The slope factor (SF) and reference dose (RfD) was regarded as the ultimate toxicity factor for both risk categories (Lim et al. 2008). Here, CDI and NCRfD represent the averages of daily intake (mg L−1) and non-carcinogenic risk (NCR) reference dose (μg kg−1 day−1), correspondingly. Therefore, chronic daily intake (CDI) from ingestion (CDIoral) of GW were estimated by followings Eqs. (1) and (2) suggested by the USEPA (US EPA 2004).
Here, CW indicates the average content of the TEs in GW (μg L−1), IR represents the daily ingestion rate (L day−1), ED is the exposure duration (year), EF denotes the exposure frequency (day/year), BW is the mean body weight (kg), AT denotes the mean time of exposure (days), SA denotes the exposed skin area (cm2), ET is the exposure time (h/day), and Kp is the dermal permeability coefficient in water (cm/h). All the enlisted variables were used from the previous literature (USEPA 2004; Wu et al. 2009; Karim 2011a, b).
Hazard quotient (HQ) value of associated TEs were calculated by the following Eq. (16)
Where HQ = hazard quotient (unitless) and RfD = reference dose (μg/kg/day). The HQ < 1 indicated that there is no obvious NHR on residents, and the HQ > 1 considered as an unacceptable adverse NHR for individual elements from drinking water (USEPA 2004; Gao et al. 2019). Addition of HQ values of each metal provides the overall NHR, which denoted as a hazard index (HI) value (USEPA 1989).
The Eq. (17) of HI is given below:
The HI > 1 indicates the NHR and HI < 1 represents no health risk in the designated area (Yang et al. 2012a, b).
Carcinogenic risk
The CR was computed as a lifetime cancer risk through the multiplication of daily intake (CDIoral) with the cancer slope factor (SForal), using the Eqs. (18) and (19) (USEPA 1989)
where SF is the slope factor of pollutants (mg/kg/day). Equation (19) was applied when the computed value was greater than 0.01. In this study, we computed the CR for arsenic and lead. The CSF values of As and Pb were 0.0015 and 0.0085 μg/kg/day for oral exposure, correspondingly (Yang et al. 2012a, b). A tolerable level was considered at 1 × 10−6 and 1 × 10−4 value (USEPA 2004; Yang et al. 2012a, b).
Data analysis and statistics
The spatial variability of the major chemical constituents was performed by the inverse distance weighted (IDW) interpolation model with the ArcGIS (Version 10.2) environment. The SPSS software (Version 25.00) was employed to perform the statistical analysis of the analyzed datasets. Kolmogorov–Smirnov (K-S) method was adopted to detect the normality of the analyzed physicochemical parameters, while the Levene test was applied to check the consistency of the GW datasets. Piper diagram (Piper 1944) for identifying the major hydro-chemical facies in the GW system was performed by Rockworks (Version 16) software. The suitability of GW within the study area for irrigation purpose was appraised using irrigation water quality (IWQI), sodium adsorption ratio, magnesium absorption ratio, permeability index, total hardness, sodium %, Kelley’s ratio, residual sodium carbonate, and soluble sodium % (Islam et al. 2017a; Xiao et al. 2019). The detailed procedures of the computations and equations employed for the GW quality assessment and health risk appraisal can be found elsewhere in earlier literature (Liu et al. 2021; Xiao et al. 2019; Islam et al. 2020c) and supplementary materials.
Results and discussion
Hydrogeochemical properties of GW
The statistical outcomes of physicochemical variables in the tested water samples are outlined in Table 1. The average concentrations of cations including Na+, Ca2+, Mg2+, K+, NH4+, and Li2+ in the tested samples are 68.31, 67.74, 24.40.31, 1.38, 0.60, and 0.001 mg/L, respectively and the mean concentrations of anions such as HCO3−, F−, Cl−, NO2−, NO3−, SO42, and PO43− in GW are 405, 0.30, 43.55, 5.04, 10.62, 0.90, and 0.01 mg/L, respectively. As shown in Fig. 3a, Na+ is the most prevalent cation, followed by Ca2+, Mg2+, K+, NH4+, and Li2+, whereas HCO3− is the predominant anion, followed by Cl−, NO2−, NO3−, SO42, F−, and PO43−. The elevated content of Na+ may be due to the ionic alteration between Ca2+, Na+, and the weathering of halite and silicate minerals (Islam et al. 2017a) while high contents of HCO3− may be derived from the dissolution of silicate minerals (Saha et al. 2020). Meanwhile, the mean values of Na+, K+, Ca2+, Mg2+, F−, Cl−, NO2−, SO42, and PO43− were observed at acceptable limits. Groundwater K+ is derived from K-feldspar weathering, cation altercation, anthropogenic fertilizer contamination, and wastewater effluent (Griffioen 2001). The observed low content of K+ in GW can be due to the less weathering of K-bearing minerals, but this also indicates that not much K of fertilizer infiltrates into the GW.
Dissolution of carbonate, silicate, and sulfate minerals is the likely sources of GW Ca2+ and Mg2+ (Fisher and Mullican Iii 1997). Solubility of halite, Cl−-bearing silicate minerals, and gypsum are found as responsible for Cl−, SO42− in GW (Fisher and Mullican Iii 1997). The average value of pH was 7.94, which indicates weakly alkaline GW which falls within the tolerable limits of Bangladesh and international laws. The electrical conductivity (EC) values ranged from 576 to 1353 μS cm−1. The total dissolve solid (TDS) values ranged from 374 to 860 mg/L. The mean values of EC and TDS fall within the tolerable levels set by Bangladesh drinking water quality (1997) and WHO (2017) standards (Table 2). The low salinity of GW is the reason for the weathering of silicate minerals, and halite. The elevated NO3− contents were found in the study region, suggesting that the nitrate contamination exists which surpassed the Bangladesh water quality standard (DoE 1997). NO3− is presently most concerning pollutants affecting the water quality worldwide, with the major sources of NO3− pollution contributed to man-made inputs including animal and human wastes (Zhang et al. 2020). The agricultural by-products and fertilizers may be the main source of the high NO3− content in the studied GW aquifer (Islam et al. 2017b). Based on the concentration, the trace elements (TEs) can be demarcated into three categories: (1) metals of high abundance including Fe and Mn; (2) metals of moderate abundance such as Zn and Pb; and (3) metals of low abundance including Hg, Cr, Cd, Co, Ni, Cu, and As. The mean concentration of As and Hg was 15.97 and 0.06 μg/L, respectively. The average concentrations of Mn, Hg, and Pb have surpassed the allowable thresholds of drinking water regulation/law which is set by BIS (2012), DoE (1997), and WHO (2011). The remaining TEs fall within the permissible limits set by Bangladesh and international laws. As seen in Fig. 3b, the concentrations of most TEs varies significantly in the tested elements, especially Mn, Fe, Ni, and Zn, whereas the rest of TEs concentrations were comparatively constant.
The hydro-chemical facies of the tested samples is shown through the piper trilinear plot (Piper 1953). As seen from Fig. 4, the types of GW are mostly Ca2+–Mg2+–Cl−–SO42−, Na+–K+–Cl−–SO42−, and Na+–K+–HCO3−; however, the predominant water type is of Ca–Mg–HCO32− which may control the water quality of the studied area. According to Bodrud-doza et al. (2019), GW types in Kusiyara River and Surma River are of Ca–Mg–SO4–HCO3− and Ca–Mg–HCO3−. Meanwhile, Na–Ca–Mg–HCO3− type GW was found in Pleistocene aquifer well at Gowainghat, indicating slight Na+ content for Ca2+ and Mg2+ contents.
Factors influencing the hydrogeochemical processes of GW
The ratio between the major ions in water can be employed to further confirm the key drivers influencing the GW chemistry of an aquifer (Kumar et al. 2021; Liu et al. 2021). As seen from Fig. 5 a and b that lithogenic influences had been represented by the bi-plots of (Ca2+/Na+) vs. (HCO3−/Na+) and (Ca2+/Na+) vs. (Mg2+/Na+). These plots imply that weathering of silicate rocks and evaporate dissolution were the key factor influencing the hydrogeochemical properties of GW (Islam et al. 2017b). Similar results were reported by Saha et al. (2020) while characterizing the GW hydrogeochemistry in Faridpur district of central Bangladesh. However, dissolution of carbonate is almost absent in the GW system. Furthermore, bi-plots of (Ca2++Mg2+) vs. HCO3− and (Na++K+) vs. HCO3– also agree with the outcomes and similar findings is drawn in Fig. 5 c and d. Besides, Na+ in water of the JSU may have derived from the silicate weathering or the ionic change mechanisms. Bodrud-Doza et al. (2019) also observed the analogous result in Dhaka city of Bangladesh where silicate weathering and evaporation dissolution took part as the major processes that control the GW solute content in capital. On the other hand, all of the samples possibly represent silicate weathering which demonstrates a molar (Na + K)/Cl ratio greater than 1 (Mukherjee et al. 2008). The Na+/Cl− plot is adopted to evaluate the either salinity available or interaction of rock-water (Sivasubramanian et al. 2013). Generally, most of the analyzed GW samples positioned near to the trend line 1:1 indicating the evaporite dissolution is the preliminary source of Na+ and Cl− in GW (Islam et al. 2018) (Fig. 5e). Water samples having Cl/Σanions ratio < 0.8 and Na/(Na + Cl) ratio > 0.5 suggest no influence of sea water was observed in the water system (Table S3). Ca2+ in GW aquifer may be extracted from the plagioclase feldspar dissolution (Al-Ruwaih and Qabazard 2005), which was further supported by strong moderate-significant relationship with Mg2+. Moreover, Na+ normalized Ca2+ vs. Mg2+ plot (Fig. 5f) implies that Mg2+ might be originated from weathering of silicate with moderate significant relationship. This result can be elucidated as the elevated content of calcium related to cation exchange in the aquifer (Fig. 5g). Cl−/total anions ratio and HCO3−/total anions ratio suggested the predominance of bicarbonate over the alkaline metal ions. Interaction of carbonate ions and water molecules releases HCO3− and OH− in water and enhancing the alkalinity or pH of the GW aquifer (Saha et al. 2020). The ratios of Ca2+/Mg2+ could be employed to analyze the impact of the weathering of both carbonate and silicate parent rocks on GW hydrogeochemical properties (Giri and Singh 2015) (Fig. 5h). Furthermore, ~ 95% of water samples fall with 1:1 line thereby suggesting that majority of GW samples were impacted by weathering of silicate rocks. Carbonate-silicate dominance withering process of minerals dissolution in the GW of Bangladesh has been reported by many research scholars (Bhuiyan et al. 2016; Ahmed et al. 2019; Bodrud-Doza et al. 2016; Bodrud-Doza et al. 2019; Saha et al. 2020).
Bivariate plot of a Ca2+/Na+ vs. HCO3−/Na+ and b Ca2+/Na+ vs. Mg2+ /Na+ to identify the minerals weathering of groundwater in the study area. Bivariate plots of c Ca2++Mg2+ vs. HCO3− and d Na++K+ vs. HCO3− identify the silicate weathering of groundwater in in the study area. Bivariate plots showing the relationships between e Na+ and Cl−, f Cl− and Mg2+/Ca2+, g Na+-normalized Ca2+ and Mg2+, and h Ca2+ and Mg2+
Pollution level appraisal of GW
To measure appropriateness of the groundwater quality for drinking water purposes, international (WHO 2011) and Bangladesh standard (1997) values were used in this work (Table 2). The GWQI values demonstrated that 3.33, 30, and 66.66% of the GW samples fall into the very poor, poor, and good classes, respectively. Generally, the GWQI appraisal indicated that most analyzed samples came from the study area were fit for drinking purpose. Figure 6a represents the spatial pattern of the GWQI values. The water quality of the study basin can be widely demarcated into three quality categories, e.g., good, poor, and very poor. Good quality water was irregularly distributed and poor water quality occurred in the southwestern and small portion of eastern regions of the study site. Although the results of GW quality appraisal showed good quality water in most sampling sites of the study area, however poor GW quality appeared southwestern site. This outcome can be contributed by the heavy rainfall during the monsoon period and enhanced water recharge may results dilutions impacts on water quality (Shammi et al. 2017). Nevertheless, the elevated rainfall can also trigger the contaminants including NO3−, Hg, Mn, and Pb, which may result to fluctuation of GW quality (Rahman et al. 2017b). Surprisingly, significant amount of Hg (0.06 μg/L) was recorded in the studied GW samples which also exceeded both local and international laws (Table 1) and presumed to be linked with igneous activities and anthropogenic sources like emissions from coal-fired power plants which falls to the ground in rain or snow, contaminating waterways (Kabir et al. 2021).
The HPI, HEI, and Cd were used to show the pollution status of GW in the study basin (Table 2). The variation of HPI was found 32.56 to 134.76 with a mean value of 73.93. The critical limit for HPI was 100, demonstrating that 10% of the samples surpassed the critical level (Table S4). This study showed that 26.66% of the samples revealed a high level of heavy metal pollution, which is regarded as unsuitable for drinking and domestic use. On the contrary, 53.33 and 20% of samples indicate medium and low levels of heavy metal pollution, respectively (Table 2). Spatial distribution of HPI showed that some sporadic points such as Noapara, Ichali, Kachua, Arabpur, and Chanchra unions were highly polluted compared to other parts of the Jassore Sadar upazila (Fig. 6b). The high contents of As, Fe, Mn, Pb, Cr, and Cd are the key reasons for the GW pollution of the study area. A similar pattern of HPI was recorded by Bodrud-Doza et al. (2016) while evaluating the GW quality in central Bangladesh.
The HEI shows 10% of the samples surpassed the critical limit (Table S3). The HEI criteria for GW samples also revealed that only 6.66% samples score a high level of contamination, while 66.66 and 36.66% of samples are characterized as medium and low levels of pollution respectively (Table 2). Spatial map of HEI revealed two hot spots in Noapara and Chanchra unions that surpassed the critical value, suggesting a high heavy metal contamination (Fig. 6c).
The Cd was adopted for examining the status of trace element contamination in the water system (Bhuiyan et al. 2010). The Cd values imply that approximately 70% of GW samples crossed the critical limit based on Edet and Offiong (2002). Thus, Cd values recommended those sample locations were highly polluted in the JSU and 10% of samples represented a low degree of pollution according to the findings of Cd, whereas 26.67% of the GW samples considered as the medium degree of pollution and 63.33% of samples fall within the high degree of pollution. The spatial distribution of Cd values suggested that most sampling sites were heavily contaminated. Overall findings depict that Noapara, Canchra, Arabpur, Diara, and Kachua unions are polluted with heavy metal (Fig. 6d). The elevated concentration of Hg, Pb, Fe, and Mn can be attributed to the high contamination of trace elements in JSU (Shammi et al. 2017).
Potential human health risks appraisal
Toxic TEs such as Pb, As, Cr, and Cd in GW enter human health mostly via the ingestion of water and could have a significant impact on the human body (Rasool et al. 2016; Rasool et al. 2017; Ahmed et al. 2019). Long-term ingestion of polluted GW with a high Pb and As contents could cause skin cancer (Gao et al. 2019; Islam et al. 2019). Human health risks from the TEs via oral ingestion of GW for children and adults were represented in Table S5 and S6. Non-carcinogenic (NCR) health effects of different TEs were determined by calculating the hazard quotient (HQ). HQ > 1 suggested the probability of detrimental health impact (Leung and Jiao 2006; Giri and Singh 2015). In general, HQ values for Fe, Cd, Zn, Cr, and Cu were < 1 for both aged groups, suggesting individual presence of these TEs could pose only a minor health hazard in the residents of the study area (Giri and Singh 2015). Wu et al. (2009) and Karim (2011a, b) also stated that HQs of TEs in drinking water were lower than the unity, which suggests that these TEs could pose a minimum hazard to residents. The HQ values for Hg ranged from 10.11 to 30.11 with a mean value of 22.11 for children. On the other hand, the HQ for Hg for adult population ranged from 2.34 to 34.1 with an average value of 10.33. For both age populations, the acute human health risk is considered to be extremely high; similar patterns of acute HHR was noted by Islam et al. (2019) and Kabir et al. (2021).
The computed HI values ranged from 20.64 to 53.34 for children with a mean value of 31.98 thereby surpassed the tolerable limit (HI = 1). For the adults, the HQ values ranged from 9.71 to 25.14 with an average value of 15.07. Only Pb and As concentrations in GW were found to be harmful to both age groups having HQ values > 1. The risk is superior to 1 for arsenic which may be associated with natural activities including parent rock-water exchange and also manmade inputs like excessive utilization of agrochemicals (Karim 2011a, b; Habib et al. 2020). The computed HQ values for each TEs are presented in Fig. 7. For both age groups, the maximum risk was observed in sampling point GW-28, but the minimum risk was observed in sampling site GW-3 due to intake of the groundwater and these sites either have scarce geological enrichment or heavily anthropogenic effect. Analogous studies were performed by many research scholars all over the globe, although the outcomes are quite diverse which rely on the characteristics of GW quality and consumption behavior (Yang et al. 2012; Wongsasuluk et al. 2014; Giri and Singh 2015; Bhutiani et al. 2016; Nkpaa et al. 2018; Barzegar et al. 2019). Children have 2-times higher NCR due to elevated absorption of As and Pb via drinking water than adults in the JSU. The main cause for the elevated NCR for children may be due to the lower body weight and less developed enzyme metabolism compared to adults (Kabir et al. 2020). In fact, the majority of the studied GW samples posed a high risk to children’s health which was substantially higher than that for adult’s health and elevated NCR for both aged populations was caused by Mn, Pb, and As contents. The analogous findings have also been stated by earlier literature (Tiwari and Maio 2017; Sharma et al. 2019; Habib et al. 2020; Liu et al. 2021; Zhang et al. 2020; Bodrud-Doza et al. 2020).
The carcinogenic risk (CR) of As for children varied from 0.54 × 10−3 to 4.98 × 10−3 with an average value of 1.60 × 10−3 while for adults it ranged from 2.55 × 10−4 to 23.48 × 10−4 with a mean value of 7.53 × 10−4 (Table S7). Similarly, CR of Pb for children ranged from 2.02 × 10−2 to 4.8 × 10−2 with an average value of 3.1 × 10−2 while for adults it varied from 9.9 × 10−3 to 2.2 × 10−2 with a mean value of 1.4 × 10−2 (Table S8).
The CR for both aged groups are much greater than the tolerable limit of 1 × 10−6, implying that inhabitants have significant cancer risk by Pb and As. However, interesting CR also found to be higher in children compared to adults. In general, the key influential factors for elevated cancer risk suffered by children than adults are the higher absorption rate and larger exposure duration (Zhang et al. 2020). Analogous findings were also reported in central Bangladesh (Saha et al. 2020), northwestern Bangladesh (Islam et al. 2017b), and also in the GW of northeastern region (Ahmed et al. 2019). The health risk appraisal suggested proper GW treatment to reduce the contents of As and Pb for drinking or other agricultural uses. Furthermore, sustainable management and pre-treatment of lead-bearing battery factory and anthropogenic arsenic mobilization process in GW system should be monitored to protect GW pollution generated by man-made inputs (Islam et al. 2020a).
Irrigation water quality appraisal
The chemical analyses of water in the study basin were assessed to check aptness for agricultural purpose and drinking water quality was compared with World Health Organization (WHO) (1997, 2017) standard. GW of JSU is also the major source of water for irrigation use. High contents of salinity in irrigation water could influence soil physical structure, lessen water permeability, enhance soil compaction, and easily impact crop growth (Rahman et al. 2017a; Nematollahi et al. 2015). The SAR, PI, MAR, Na%, TH, KR, RSC, and SSP are the vital indices to determine the suitability of water for irrigation purpose (Liu et al. 2021).
Salt water is a good conductor of electricity, thus EC is a measurement of electrical conductance in water (Islam et al. 2018). In this study, the EC values varied from 576 to 1353 with an average value of 868, representing that GW samples are within excellent to the permissible limit for irrigation purposes (Table 2). However, according to the classification of Wilcox (1955), ~ 20% of water samples demonstrate excellent quality, and 80% of samples were within the permissible limit, and none of the sampling sites were found to unsuitable for irrigation. A high concentration of EC was reported in the northern portion of JSU (Fig. 8a). TH (total hardness) values varied from 37.12 to 389.45 mg/L with an average value of 287.02 mg/L. Nearly 96.67% of the samples were considered as hard, and only one GW sample (3.33%) was considered as a soft category. The elevated value of TH was identified in the southern portion of the JSU (Fig. 8b). Hence, the measured water samples are demarcated as moderately hard to very hard water (Sawyer and McCarty 1967). The IWQI values varied from 26 to 40 within the average value of 31.3, which indicates medium suitability of water for irrigation uses. According to the suitability level, 80% of the sampling sites were moderately appropriate, and the rest of 20% were regarded as suitable (Table 2 and Table S9 & S2).
Crop yield and soil fertility worsen under irrigation water comprising some chemical element originated from either the geogenic or from human activities (Jalali 2009). The accomplishment of irrigation projects mostly relies on the supply and control of salts and alkalis in the soil (Haritash and Kaushik 2008). The use of irrigation water aids to supply salts to the crop root zone (Jalali 2011a, 2011b). Parameters such as SAR, SSP, RSBC, and PI are the main determinants for irrigation water aptness (Raju 2007). High IWQI values were detected in the southeast and south-western parts of the JSU (Fig. 8c). Na+ is absorbed on clay minerals as a superfluous for Ca2+ and Mg2+ and thereby making soil compact and impermeable (Singh et al. 2008). Based on the Wilcox (1955) classification, about 53.33% of water samples were in the permissible range, 26.66% were good, and the remaining 20% were in the excellent quality range. The high score of SSP was observed in Canchra, Kashimpur, Noapara, Haibatpur, and Ichali union of JSU (Fig. 8d).
The results of our study exhibited that SAR value varied from 2.30 to 48.42 with an average value of 14.29. Weathering of rocks, anthropogenic sources, leaching from topsoil, and minor influences on climate can cause salinity hazard in GW (Prasanna et al. 2011; Islam et al. 2018). The use of the classification model based on the USSL diagram (USSL 1954) exhibited that almost all GW samples were distributed in the C2S1 (48%) and C3S1 (52%) regions, thus indicated that the water of the study area is fit for irrigation uses (Fig. 9). The use of the Wilcox diagram (Wilcox 1955) also indicates that majority GW samples fall in the excellent to good classes (48%) or good to permissible classes (50%), suggesting that water is usually good quality for agricultural crop activities. Overall, the SAR values of the JSU supported its suitability as a source of water for irrigation use. However, Canchra and Ichali unions of the study area showed the highest score of SAR value (Fig. 8e).
Groundwater classification for irrigation according to US Salinity Laboratory’s (USSL) diagram (Richards 1954)
KR is the vital parameter for IWQI assessment. KR > 1 reflects excess sodium and KR < 1 signifies sodium deficit (Kelly 1940). Waters with KR < 1 are fit for irrigation while those with a greater ratio are considered unsuitable (Sundaray et al. 2009a, b). Kelly’s ratio varied from 0.12 to 2.42, with a mean value of 0.79. According to this category, almost 21 water samples (70%) JSU were suitable for irrigation purposes, and the rest of the samples (30%) were not suitable for irrigation (Table 2). These KR values imply a higher Na+ % which can be obtained from weathering of feldspars from the lithologic origin (Vasanthavigar et al. 2012). A high score of KR value was found in Chanchra, Diara, Noapara, and Ichhali union of JSU (Fig. 8f).
Magnesium absorption ratio (MAR) reveals an association between the concentration of Mg+2 and Ca+2 in water (Ragunath 1987). The high levels of exchangeable Mg2+ affects the quality of soil through infiltration problems and resulting in poor agricultural outputs (Islam et al. 2017a; Ayers and Westcott 1985). Hence, MAR is considered an important factor for assessing irrigation water suitability, and the value exceeding 50 indicates deterioration of the soil quality (Kaçmaz and Nakoman 2009; Islam et al. 2017d). Magnesium adsorption ratio (MAR) values varied from 14.51 to 100, with an average value of 31.27 (Table 2) in the present study. However, 93.33% of the samples fall in MAR < 50, indicating water quality is excellent for irrigation, although the higher value of MAR was observed in Noapara, Kashimpur, Haibatpur, Lebutala, and Ichhali union of JSU (Fig. 8g).
The ratio of carbonate and bicarbonate to calcium and magnesium influences the aptness of groundwater for irrigation use. Excess sodium HCO3 and carbonate can enhance the dissolution of organic matter in the soil, leaving a black stain on the surface upon drying (Kumar et al. 2007). Residual sodium carbonate (RSC) values of all the water samples in the study area fall within an unsuitable category which means potentially harmful water for irrigation purposes. The highest RSC value was observed in the northern and central parts, whereas the lowest value was noted in the southern part of JSU (Fig. 8h). Overall, GW in JSU is moderately suitable for agricultural activities. The long-term GW application for the usage of irrigation purpose may result in slight sodium and moderate salinity hazards, the associated loss of soil characteristics will affect crop production. The present study recommends improvement of the irrigation network in the sampling sites, enhancement of surface water irrigation facilities, and agricultural rotation system can be introduced in the study area in order to minimize the adverse impacts of water irrigation.
Conclusions
In this research, we intended to characterize the hydrogeochemistry, quality of water, potential health risks posed to humans, as well as the factors governing these issues in JSU, southwestern Bangladesh using hydrogeochemical analysis, GW pollution indices, and health risk models. GW in the study area is poorly alkaline and falls in the hard brackish water types; Na+ and HCO3− are the predominant chemicals in the GW of JSU. The piper diagram revealed that the predominant GW type is Ca–Mg–HCO32− and the major factors influencing the hydrogeochemistry of GW were found to be silicate rock weathering, evaporate dissolution, ion exchange, and anthropogenic inputs. The outcomes of GWQI reported that GW quality for drinking use is poor due to the high contents of Pb, Mn, Hg, and NO3− in most of the studied samples. The results of the health risk appraisal for both age groups depicted that GW of the JSU posed an undesirable health risk to the local community. Children were two times higher susceptible to health risk than adults regarding carcinogenic and non-carcinogenic risks. The results from the irrigation water quality indices indicated that GW is moderately fit for irrigation activities. Based on the key findings of the present study, the following recommendations can be helpful for the Upazila wise sustainable GW management in Bangladesh:
-
A comprehensive GW quality monitoring and management tool to regulate the water quality based on the local geochemical characteristics is must.
-
Special focus should be given on As, Hg, Fe, and Pb as they could result significant health burden to the local community.
-
Agrochemicals especially TEs containing pesticides and insecticides use should be banned; proper monitoring of agrochemicals usage should be ensured by the local government.
-
Inauguration of a GW quality and health risk appraisal framework for the local community for sustainable GW management and health hazards reduction.
Data availability
Data are available upon request on the corresponding author.
References
Ahmed KM, Bhattacharya P, Hasan MA, Akhter SH, Alam SMM, Bhuyian MAH, Imam MB, Khan AA, Sracek O (2004) Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overview. Appl Geochem 19(2):181–200
Ahmed N, Bodrud-Doza M, Islam ARMT, Hossain S, Moniruzzaman M, Dev N, Bhuiyan MAQ (2019) Appraising spatial variations of As, Fe, Mn and NO3 contaminations associated health risks of drinking water from Surma basin Bangladesh. Chemosphere 218:726–740. https://doi.org/10.1016/j.chemosphere.2018.11.104
Ahmed S, Kayes I, Shahriar SA, Kabir M, Salam MA, Mukul S (2020) Soil salinity and nutrients pattern along a distance gradient in coastal region. Global J Environ Sci Manage 6:59–72
Ahmed F, Fakhruddin ANM, Fardous Z, Chowdhury MAZ, Kabir MM (2021) Accumulation and translocation of chromium (Cr) and lead (Pb) in chilli plants (Capsicum annuum L.) grown on artificially contaminated soil. Nat Environ Pollut Technol 20:63–70
Al-Ruwaih FM, Qabazard HA (2005) Aquifer characteristics and water quality of Miocene–Pleistocene sediments Kuwait. Bull Eng Geol Environ 64:175–191
Anny F, Kabir M, Bodrud-Doza M (2017) Assessment of surface water pollution in urban and industrial areas of Savar Upazila, Bangladesh. Pollut 3:243–259
Ashraf M, Afzal M, Ahmad R, Ali S (2011) Growth and yield components of wheat genotypes as influenced by potassium and farm yard manure on a saline sodic soil. Soil Environ 30:115–121
Ayers RS, Westcott DW (1985) Water quality for agriculture (No 29): Food and agriculture organization of the United Nations, Rome, Italy
Backman B, Bodis D, Lahermo P, Rapant S, Tarvainen T (1997) Application of a groundwater contamination index in Finlandand Slovakia. Environ Geol 36:55–64
Barzegar R, Moghaddam AA, Soltani S, Fijani E, Tziritis E, Kazemian N (2019) Heavy metal(loid)s in the groundwater of Shabestar Area (NW Iran): source identification and health risk assessment. Expo Health 11:251–265
BBS (2011) Bangladesh bureau of statistics and information division, ministry of the people republic of Bangladesh
Bhuiyan MAH, Islam MA, Dampare SB, Parvez L, Suzuki S (2010) Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. J Hazard Mater 179:1065–1077. https://doi.org/10.1016/jjhazmat201003114
Bhuiyan MAH, Bodrud-Doza M, Islam ARMT, Rakib MA, Rahman MS, Ramanathan AL (2016) Assessment of groundwater quality of Lakshimpur district of Bangladesh using water quality indices, geostatistical methods, and multivariate analysis. Environ Earth Sci 75:1020. https://doi.org/10.1007/s12665-016-5823-y
Bhutiani R, Kulkarni DB, Khanna DR, Gautam A (2016) Water quality, pollution source apportionment and health risk assessment of heavy metals in groundwater of an industrial area in North India. Expo Health 8:3–18
BIS (2012) Bureau of Indian standards drinking water specifications, BIS. 10500:2012 New Delhi, India
Bodrud-Doza M, Islam ARMT, Ahmed F, Das S, Saha N, Rahman MS (2016) Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Sci 33(1):19–40. https://doi.org/10.1016/j.wsj.2016.05.001
Bodrud-Doza M, Islam SMDU, Hasan MT, Alam F, Haque MM, Rakib MA, Md Asad MA, Rahman MA (2019) Groundwater pollution by trace metals and human health risk assessment in central west part of Bangladesh. Groundw Sustain Dev 9:100219. https://doi.org/10.1016/j.gsd.2019.100219
Bodrud-Doza M, Islam SMD, Rume T, Quraishi SB, Safiur Rahman M, Bhuiyan MAH (2020) Groundwater quality and human health risk assessment for safe and sustainable water supply of Dhaka City dwellers in Bangladesh. Groundw Sustain Dev 10:100374
DoE (1997) Department of environment, the environment conservation rules Government of the People’s Republic of Bangladesh, Dhaka
Doneen LD (1964) Notes on water quality in agriculture, water science and engineering University of California, Davis
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a study case fromAkpabuyo-Odukpani area Lower Cross River Basin (southeastern Nigeria). GeoJournal 5:295–304
Fisher RS, Mullican Iii WF (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the northern Chihuahuan Desert, Trans-Pecos, Texas, USA. Hydrogeol J 5:4–16
Giri S, Singh AK (2015) Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin India. Environ Monit Assess 187:63. https://doi.org/10.1007/s10661-015-4265-4
Griffioen J (2001) Potassium adsorption ratios as an indicator for the fate of agricultural potassium in groundwater. J Hydrol 254:244–254
Habib MA, Islam ARMT, Bodrud-Doza et al (2020) Simultaneous appraisals of pathway and probable health risk associated with trace metals contamination in groundwater from Barapukuria coal basin Bangladesh. Chemosphere 242:125183. https://doi.org/10.1016/j.chemosphere.2019.125183
Hanasaki N, Yoshikawa S, Pokhrel Y, Kanae S (2018) A global hydrological simulation to specify the sources of water used by humans. Hydrol Earth Syst Sci 22:789–817
Haritash AK, Kaushik CP (2008) Suitability assessment of groundwater for drinking, irrigation andindustrial use in some North Indian villages. Environ Monit Assess 145:397–408
Hasan MK, Shahriar A, Jim KU (2019) Water pollution in Bangladesh and its impact on public health. Heliyon. 5:e02145. https://doi.org/10.1016/j.heliyon.2019.e02145
Hasan MF, Nur-E-Alam M, Salam MA, Rahman MH, Paul SC, Rak AE, Ambade B, Islam ARMT (2021) Health risk and water quality assessment of surface water in an urban river of Bangladesh. Sustainability. https://doi.org/10.3390/su1158077
Hassan M, Islam SMD, Ahmed F, MATMT R (2016) Quality analysis of drinking water provided for the readymade garment workers in Dhaka, Bangladesh. Pollution 2(3):289–298. https://doi.org/10.5194/hess-22-789-2018
Islam SMD, Bhuiyan MAH, Rume T, Mohinuzzaman M (2016a) Assessing heavy metal contamination in the bottom sediments of Shitalakhya River Bangladesh; using pollution evaluation indices and geo-spatial analysis. Pollution 2(3):299–312. https://doi.org/10.7508/pj201603005
Islam SMDU, Bhuiyan MAH, Rume T, Azam G (2016b) Hydrogeochemical investigation of groundwater in shallow coastal aquifer of Khulna District, Bangladesh. Appl Water Sci 7:4219–4236. https://doi.org/10.1007/s13201-017-0533-5
Islam ARMT, Shen S, Bodrud-Doza M, Rahman SM (2017a) Assessing irrigation water quality in Faridpur district of Bangladesh using several indices and statistical approaches. Arab J Geosci 10:418. https://doi.org/10.1007/s12517-017-3199-2
Islam ARMT, Ahmed N, Bodrud-Doza M, Chu R (2017b) Characterizing groundwater quality ranks for drinking purposes in Sylhet district, Bangladesh, using entropy method, spatial autocorrelation index, and geostatistics. Environ Sci Pollut Res 24(34):26350–26374. https://doi.org/10.1007/s11356-017-0254-1
Islam ARMT, Shen S, Bodrud-Doza M (2017c) Assessment of arsenic health risk and source apportionment of groundwater pollutants using multivariate statistical techniques in Chapai-Nawabganj district, Bangladesh. J Geol Soc India 90(2):239–248. https://doi.org/10.1007/s12594-017-0705-9
Islam ARMT, Shen S, Bodrud-Doza M et al (2017d) Assessment of trace elements of groundwater and their spatial distribution in Rangpur district, Bangladesh. Arab J Geosci 10(4):95. https://doi.org/10.1007/s12517-017-2886-3
Islam ARMT, Shen S, Haque MA, Bodrud-Doza M, Maw KW, Habib MA (2018) Assessing groundwater quality and its sustainability in Joypurhat district of Bangladesh using GIS and multivariate statistical approaches, Environment. Dev Sustain 20(5):1935–1959. https://doi.org/10.1007/s10668-017-9971-3
Islam ARMT, Bodrud-doza M, Rahman MS, Amin SB, Chu R, Mamun HA (2019) Sources of trace elements identification in drinking water of Rangpur district Bangladesh and their potential health risk following multivariate techniques and Monte-Carlo simulation. Groundw Sustain Dev 9:100275. https://doi.org/10.1016/j.gsd.2019.100275
Islam ARMT, Siddiqua MT, Zahid A, Tasnim SS, Rahman MM (2020a) Drinking appraisal of coastal groundwater in Bangladesh: an approach of multi-hazards towards water security and health safety. Chemosphere 254:126933. https://doi.org/10.1016/jchemosphere.2020.126933
Islam ARMT, Mamun AA, Rahman MM, Zahid A (2020b) Simultaneous comparison of modified-integrated water quality and entropy weighted indices: implication for safe drinking water in the coastal region of Bangladesh. Ecol Indic 113:106229. https://doi.org/10.1016/j.ecolind.2020.106229
Islam ARMT, Islam HM et al (2020c) Co-distribution, possible origins, status and potential health risk of trace elements in surface water sources from six major river basins Bangladesh. Chemosphere 249:126180. https://doi.org/10.1016/j.chemosphere.2020.126180
Islam ARMT, Kabir MM, Faruk S, al Jahin J, Bodrud-Doza M, Didar-ul-Alam M, Bahadur NM, Mohinuzzaman M, Fatema KJ, Safiur Rahman M, Choudhury TR (2021) Sustainable groundwater quality in southeast coastal Bangladesh: co-dispersions, sources, and probabilistic health risk assessment. Environ Dev Sustain. https://doi.org/10.1007/s10668-021-01447-4
Jalali M (2009) Phosphorous concentration, solubility and species in the groundwater in a semi-arid basin, southern Malayer, western Iran. Environ Geol 57:1011–1020
Jalali M (2011a) Nitrate pollution of groundwater in Toyserkan, western Iran. Environ Earth Sci 62:907–913
Jalali M (2011b) Hydrogeochemistry of groundwater and its suitability for drinking and agricultural use in Nahavand, Western Iran. Nat Resour Res 20:65–73
Kabir MM, Fakhruddin ANM, Chowdhury MAZ, Fardous Z, Islam R (2017) Characterization of tannery effluents of Hazaribagh area, Dhaka, Bangladesh. Pollut 3:395–406
Kabir MM, Fakhruddin ANM, Chowdhury MAZ, Pramanik MK, Fardous Z (2018) Isolation and characterization of chromium (VI)-reducing bacteria from tannery effluents and solid wastes. World J Microbiol Biotechnol 34:126
Kabir MM, Sultana F, Rahman MM, Uddin MK (2020) Chromium (VI) removal efficacy from aqueous solution by modified tea wastes-polyvinyl alcohol (TW-PVA) composite adsorbent. Desalination Water Treat 174:311–323
Kabir MM, Akter S, Ahmed FT, Mohinuzzaman M, Didar-ul-Alam M, Mostofa KMG, Islam ARMT, Niloy NM (2021) Salinity-induced fluorescence dissolved organic matter influence co contamination, quality and risk to human health of tube well water in southeast coastal Bangladesh. Chemosphere 275:130053. https://doi.org/10.1016/j.chemosphere.2021.130053
Kabir MM, Mouna SSP, Akter S, Khandaker S, Didar-ul-Alam M, Bahadur NM, Mohinuzzaman M, Islam MA, Shenashen MA (2021a) Tea waste based natural adsorbent for toxic pollutant removal from waste samples. J Mol Liq 322:115012
Kaçmaz H, Nakoman ME (2009) Hydrochemical characteristics of shallow groundwater in aquifer containing uranyl phosphate minerals, in the Köprübaşı (Manisa) area. Turkey Environ Earth Sci 59:449–457. https://doi.org/10.1007/s12665-009-0043-3
Karim Z (2011a) Risk assessment of dissolved trace metals in drinking water of Karachi Pakistan. Bull Environ Contam Toxicol 86:676–678
Karim Z (2011b) Risk assessment of dissolved trace metals in drinking water of Karachi Pakistan. Bull Environ Contam Toxicol 86:676–678
Kayes I, Shahriar SA, Hasan K, Akhter M, Kabir MM, Salam MA (2019) The relationships between meteorological parameters and air pollutants in an urban environment. Global J Environ Sci Manage 5:265–278
Kelly WP (1940) Permissible composition and concentration of irrigated waters In: Proceedings of the ASCF, 607
Kumar M, Kumari K, Ramanathan AL, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ Geol 53:553–574
Kumar S, Islam ARMT, Islam HMT, Hasanuzzaman M, Ongoma V, Khan R, Mallick J (2021) Water resources pollution associated with risks of heavy metals from Vatukoula Goldmine region, Fiji. J Environ Manag 293:112868. https://doi.org/10.1016/j.jenvman.2021.112868
Lim HS, Lee JS, Chon HT, Sager M (2008) Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J Geochem Explor 96:223–230
Liu J, Peng Y, Li C, Gao Z, Chen S (2021) An investigation into the hydrochemistry, quality and risk to human health of groundwater in the central region of Shandong Province, North China. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.125416
Local government engineering department (LGED) (2014) National strategy for water supply and sanitation. Government of the People's Republic of Bangladesh, Ministry of Local Government, Rural Development and Cooperatives Local Government Division 1-18
Mridul MMI, Huda ME, Khan M, Roy SK, Akter S, Kabir MM, Mouna SSP (2020) Groundwater quality and vulnerability assessment in Savar Dhaka Bangladesh. J Biol Environ Sci 17:1–9
Mukherjee A, Bhattacharya P, Savage K, Foster A, Bundschuh J (2008) Distribution of geogenic arsenic in hydrologic systems: controls and challenges. J Contam Hydrol 99:1–7
Nkpaa KW, Amadi BA, Wegwu MO (2018) Hazardous metals levels in groundwater from Gokana, Rivers State Nigeria: non-cancer and cancer health risk assessment. Hum Ecol Risk Assess 24:214–224
Prasad B, Jaiprakas KC (1999) Evaluation of heavy metals in ground water near mining area and development of heavy metal pollution index. J Environ Sci Health A 34(1):91–102
Prasanna MV, Chidambaram S, Gireesh TV, Jabir Ali TV (2011) A study on hydrochemical characteristics of surface and subsurface water in and around Perumal Lake Cuddalore District, Tamil Nadu, South India. Environ Earth Sci 64:1419–1431
Ragunath HM (1987) Groundwater Wiley Eastern, New Delhi 563
Rahman MM, Islam MA, Bodrud-Doza M, Muhib MI, Zahid A, Shammi M, Tareq SM, Kurasaki M (2017a) Spatio-temporal assessment of groundwater quality and human health risk: a case study in Gopalganj Bangladesh. Expo Health 10:167–188. https://doi.org/10.1007/s12403-017-0253-y
Rahman MS, Saha N, Islam ARMT et al (2017b) Evaluation of water quality for sustainable agriculture in Bangladesh. Water Air Soil Pollut 228:385. https://doi.org/10.1007/s11270-017-3543-x
Rahman MM, Bodrud-Doza M, Siddique T, Zahid A, Islam ARMT (2020) Spatiotemporal distribution of fluoride in drinking water and associated probabilistic human health risk appraisal in the coastal region Bangladesh. Sci Total Environ 724:138316. https://doi.org/10.1016/j.scitotenv.2020.138316
Rahman MR, Islam ARMT, Shammi M (2021) Emerging trends of water quality monitoring and applications of multivariate tools. Water Eng Model Mathematic Tools 14:271–283. https://doi.org/10.1016/B978-0-12-820644-7.00020-7
Raju NJ (2007) Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhara Pradesh, South India. Environ Geol 52:1067–1074
Rasool A, Xiao T, Farooqi A, Shafeeque M, Masood S, Ali S, Fahad S, Nasim W (2016) Arsenic and heavy metal contaminations in the tube well water of Punjab, Pakistan and risk assessment: a case study. Ecol Eng 95:90–100. https://doi.org/10.1016/j.ecoleng.2016.06.034
Rasool A, Xiao T, Farooqi A, Shafeeque M, Liu Y, Kamran MA, Katsoyiannis IA, Eqani SAMA (2017) Quality of tube well water intended for irrigation and human consumption with special emphasis on arsenic contamination at the area of Punjab, Pakistan. Environ Geochem Health 39:847–863
Richards LA (1954) Diagnosis and improvement of saline and alkali soils, vol 60 US Department of Agricultural Handbook, Washington DC 160
Rikta SY, Rahaman MS, Mehjabin JJ, Uddin MK, Kabir MM, Tareq SM, Ehsan D (2016) Evaluation of water quality parameters and humic substance status of Bangshi Dhaleshwari and Padma Rivers in Bangladesh. Int J Environ Sci 6:1129–1139
Saha N, Bodrud-doza M, Islam ARMT et al (2020) Hydrogeochemical evolution of shallow and deeper aquifers in central Bangladesh: arsenic mobilization process and health risk implications from the potable use of groundwater. Environ Earth Sci 79(20):477. https://doi.org/10.1007/s12665-020-09228-4
Salam MA, Kabir MM, Yee LF, Khan MS (2019) Water quality assessment of Perak River, Malaysia. Pollution 5:637–648
Sawyer CN, McCarty PL (1967) Chemistry for Sanitary Engineers McGraw-Hill New York
Shahid SU, Iqbal J, Hasnain J (2014) Groundwater quality assessment and its correlation with gastroenteritis using GIS: a case study of Rawal Town, Rawalpindi Pakistan. Environ Monit Assess 186:7525–7537. https://doi.org/10.1007/s10661-014-3945-9
Shammi M, Rahman MM, Islam MA, Bodrud-Doza M, Zahid A, Akter Y, Quaiyum S, Kurasaki M (2017) Spatio-temporal assessment and trend analysis of surface water salinity in the coastal region of Bangladesh. Environ Sci Pollut Res 24:14273–14290. https://doi.org/10.1007/s11356-017-8976-7
Shamsudduha M, Zahid A, Burgess WG (2018) Security of deep groundwater against arsenic contamination in the Bengal aquifer system: a numerical modeling study in southeast Bangladesh. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-40018-40275-z
Singh AK, Mondal GC, Kumaar S, Sinngh TB, Sinha A (2008) Majorion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin. India Environ Geol 54:745–758
Singh AK, Sathya M, Verma S, Jayakumar S (2018) Health risk assessment of heavy metals in crop grains grown on open soils of Kanwar wetland India Euro-Mediterr. J Environ Integr 3:29. https://doi.org/10.1007/s41207-018-0073-x
Smith A, Lingas E, Rahman M (2000) Contamination of drinking water by arsenic in Bangladesh: a public health emergency. Bulletin of the World Health Organisation 83:177–186. https://doi.org/10.1590/S0042-96862000000900005
Sultana F, Uddin MK, Kabir MM, Sultana N, Sikder MT, Rahman SM (2016) Removal efficiency of chromium (VI) ions from aqueous solution by adsorption of rice husk and saw dust ash as a homogeneous composite. Int J Environ Sci 6:1129–1139
Sundaray SK, Nayak BB, Bhatta D (2009a) Environmental studies on river water quality with reference to suitability for agricultural purposes. Mahanadi river estuarine system, India—a case study. Environ Monit Assess 155:227–243
Sundaray SK, Nayak BB, Bhatta D (2009b) Environmental studies on river water quality with reference to suitability for agricultural purposes: Mahanadi river estuarine system, India-a case study. Environ Monit Assest 155:227–243. https://doi.org/10.1007/s10661-008-0431-2
Tasneem A, Sarker P, Akter S, Mouna SSP, Rahaman MS, Mohinuzzaman M, Uddin MK, Kabir MM (2021) Textile wastewater treatment by combination of chemical and phytoremediation processes. Pollution 7:43–54
Thilagavathi R, Chidambaram S, Prasanna MV, Thivya C, Singaraja C (2012) A study on groundwater geochemistry and water quality in layered aquifers system of Pondicherry region, southeast India. Appl Water Sci 2:253–269. https://doi.org/10.1007/s13201-012-0045-2
US EPA (2004) Risk assessment guidance for superfund volume I: human health evaluation manual (part E)
USEPA (1989) Risk assessment guidance for superfund volume I human health evaluation manual (Part A)
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi RR, Chidambaram S, Anandhan P, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin Tamilnadu, India. Environ Monit Assess 171:595–609
Vasanthavigar M, Srinivasamoorthy K, Rajiv Ganthi R, Vijayaraghavan K, Sarma LS (2012) Characterisation and quality assessment of groundwater with a special emphasis on irrigation utility: thirumanimuttar sub-basin, Tamil Nadu, India. Arab J Geosci 5:245–258
WHO (2011) WHO guidelines for drinking-water quality, 4th ed World Health Organization, Geneva
WHO (2017) Guidelines for drinking-water quality. World Health Organization, Geneva
Wilcox LV (1955) Classification and use of irrigation waters Agric circ 969 USA, Washington DC 19
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province Thailand. Environ Geochem Health 3:169–182
World Health Organization (WHO) (1997) Guidelines for drinking water quality. Geneva 1:53–73
World Health Organization (WHO) (2017) Guidelines for drinking water quality: first addendum to the fourth edition. Licence: CC BY-NC-SA 3.0 IGO, Geneva. http://apps.who.int/iris/bitstream/10665/254636/1/9789241550017-eng.pdf?ua=1. Access 16 April 2021
Wu B, Zhao DY, Jia HY, Zhang Y, Zhang XX, Cheng SP (2009) Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing Section China. Bull Environ Contam Toxicol 82:405–409
Yang M, Fei Y, Ju YMZ, Li H (2012a) Health risk assessment of groundwater pollution—a case study of typical city in north China. Plain J Earth Sci 23:335–348
Yang M, Fei Y, Ju YMZ, Li H (2012b) Health risk assessment of groundwater pollution—a case study of typical city in north. China Plain J Earth Sci 23(3):335–348
Zhang Y, Xiong Y, Chao Y, Fan R, Rena F, Xu B, Liu Z (2020) Hydrogeochemistry and quality assessment of groundwater in Jinghui canal irrigation district of China. Human and Ecological Risk Assessment: an International Journal 26:2349–2366. https://doi.org/10.1080/10807039.2020.1774737
Acknowledgements
Authors are grateful to the anonymous reviewers for their constructive suggestions and recommendations to improve the quality of this manuscript significantly. Authors greatly acknowledged the Department of Environmental Science and Disaster Management, Noakhali Science and Technology University for completion of this research project. Authors gratefully acknowledged the technical support and preliminary draft checking of this paper by Mahfuzur Rahman, Lecturer, Department of Oceanography, Noakhali Science & Technology University. Authors also thankful to Bangladesh Atomic Energy Commission and Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh for other sorts of experimental support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
M.M.K. and N.H designed, planned, conceptualized, and drafted the original manuscript, and S.A., M.H., and M.M. were involved in statistical analysis and interpretation; K.J.F., L.N.H., and T.R.C. contributed to instrumental setup, data analysis, and validation; A.R.M.T.I and M.D.A contributed to editing the manuscript, literature review, and proofreading; M.M.K, A.R.M. T.I., and M.H.S. were involved in software, mapping, and proofreading during the manuscript drafting stage.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Xianliang Yi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 60 kb)
Rights and permissions
About this article
Cite this article
Kabir, .M., Hossain, N., Islam, A.R.M.T. et al. Characterization of groundwater hydrogeochemistry, quality, and associated health hazards to the residents of southwestern Bangladesh. Environ Sci Pollut Res 28, 68745–68761 (2021). https://doi.org/10.1007/s11356-021-15152-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15152-2