Abstract
Sustainable groundwater quality has become a major concern for the agro-based country like Bangladesh. Integrated approaches of various irrigation water quality indices and geostatistical modeling were applied to evaluate the suitability and for spatial mapping of groundwater quality of Faridpur District in central Bangladesh. The irrigation water quality index (IWQI) revealed that majority of the samples were suitable for irrigation. Similar outcomes were recorded from other indices including Na%, sodium adsorption ratio (SAR), residual sodium bicarbonate (RSBC), total hardness (TH), Kelley’s ratio (KR), and magnesium adsorption ratio (MAR). Classifications based on Wilcox diagram and permeability index (PI) plot indicated a similar conclusion wherein almost all the samples were safe for agricultural uses without posing considerable effect on the soil fertility and overall crop yield. Principal component analysis (PCA) grouped the major cations and anions into three principal components including dissolution of calcite minerals, leaching of silicate sediments, and ion exchange process. Spatial mapping of IWQI identified that groundwater in the northern side of Faridpur region were more suitable for irrigational uses relative to central and southern side, possibly due to gradients of domestic discharges and agricultural activates from north to south side. These findings would provide useful information to water distributors, managers, and decision makers for taking adaptive measures in irrigation water quality management systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Irrigation plays an important role to increase yield of agricultural production and to maintain a continuous supply of increased food demands for the expanding world population. Sustainable yield of agricultural products largely relies on proper management of water quality used for irrigation. About 70% of total irrigated area in Bangladesh depends on groundwater sources (Kinniburgh and Smedley 2001). Nonetheless, contamination of groundwater by human activates (e.g., agricultural development, rapid urbanization, and anthropogenic pollution) and/or inherent aquifer material composition is a matter of serious concern (Rahman and Islam 2009; Rahman et al. 2014a, b; Saha et al. 2016; Liu et al. 2017). Thus, a continuous monitoring of regional groundwater geochemistry to identify the degree of contamination from various drivers is highly needed to ensure a sustained crop-yield capacity of the agricultural lands. A conceptual model for the contamination of groundwater by human activates is shown in Fig. 1. However, it is worthy to note that recharge of groundwater occurs through lateral seepage of river and canal water, infiltration of rain, flood, and pond water, and also through the return flow of irrigated water from the agricultural fields (Bhattacharya 2010).
Several index techniques are generally used in the literature to assess water quality. Horton (1965) initially proposed an integrated index of water quality. Since then, many water quality indices have been reported by several authors (e.g., Ayers 1977; Liou et al. 2004; Simsek and Gunduz 2007; Boyacioglu 2007; Song and Kim 2009; Hussain et al. 2012; Romanelli et al. 2012; Bhuiyan et al. 2016). Debels et al. (2005) have used a modified water quality index, which is composed of physicochemical parameters for evaluating the quality status of a river in central Chile. Simsek and Gunduz (2007) have developed a GIS (Global information system)-based irrigation water quality index (IWQI) by combining well-known hydrochemical parameters (electrical conductivity—EC, sodium adsorption ratio—SAR, residual sodium bicarbonate—RSBC and others) to assess the irrigation water quality of the Simav Plain in Turkey. Subsequently, Romanelli et al. (2012) have introduced IWQI by integrating hydrochemical, hydrogeological, and topographic parameters (EC, SAR, RSC, slops, hydraulic gradient, aquifer thickness) to assess groundwater suitability for irrigation in Wet Pampa Plain, Argentina. Recently, Bozdag (2015) has used an analytic hierarchy process (AHP) integrated with GIS to infer water quality for irrigation in central Anatolia, Turkey.
Hydrochemical analysis of groundwater can identify the degree of contamination and allow us to understand the suitability of water for agricultural, drinking, and other uses. GIS-based spatial mapping of IWQ parameters and indices can play a significant role in understanding the possible contamination sources and identifying the extent of contaminated area (Dixon 2005). Moreover, geostatistical techniques can be used to predict the status of water quality parameters at unmeasured locations. Among various geostatistical interpolation techniques (e.g., kriging, inverse distance weighting), the ordinary kriging (OK) that consider the spatial correlation between the sample points is commonly accepted and widely used interpolation method in hydrology and soil science for spatial mapping (e.g., Isaaks and Srivastava 1989; Goovaerts 1997; Webster and Oliver 2001; Kumari et al. 2013; Ağca et al. 2014.
Although, many studies were conducted in different regions of Bangladesh to assess the suitability of groundwater for irrigation (Shammi et al. 2016; Bhuiyan et al. 2014; Rahman et al. 2014a, b), a few comprehensive assessments have been found in the literature that incorporated major hydrochemical parameters including salinity hazard, permeability hazard, specific ion toxicity, and miscellaneous effect. Besides, GIS-integrated IWQ assessment has not done yet in a wide range of area in central Bangladesh. Mapping of IWQ parameters is a vital tool for monitoring spatial variance of water quality.
Since groundwater is intensively used for irrigation in Faridpur District (central part of Bangladesh), the present study is designed to evaluate its irrigational suitability utilizing an integrated approach including hydrogeochemistry, multiple water quality indices, geostatistical modeling, GIS-based mapping of IWQ parameters, and IWQI.
2 Materials and Methods
2.1 Study Area
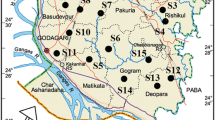
The present study area, Faridpur upazila/sub-district is a small administrative unit in Faridpur district of central Bangladesh. Geographically, Faridpur is positioned at 22.50–23.55° N latitude and 89.15–90.40° E longitude. Total area of this district is ~ 2072.72 km2 and is bounded by the Padma River to the north and east (Fig. 2). Physiographical, the soil is highly fertile as this region lies in the floodplain of the Padma River (also called the Lower Ganges plain). Surface geology of Faridpur consists of alluvial sands in the northern part and deltaic sands and silts in the south. The alluvial sands is part of the active floodplain of the Padma River and oversteps the deltaic sand and slit deposits, which crop out further south (Kinniburgh and Smedley 2001). Bangladesh is characterized by tropical monsoon climate with ~ 85% of annual rainfall from May to September monsoon. This coincides with peak inflow of the major rivers and annual flooding. Less than 5% of the mean annual rainfall occurs dry season between November and March (Shahid 2010). Agricultural activates during dry period with almost zero effective rainfall depend entirely on groundwater irrigation. Thus, understanding the regional groundwater hydrochemistry is critical (Bodrud-Doza et al. 2016). The economy of this region is predominantly agri-based and groundwater is a main source of irrigation water supply. However, household garbage and wastewater from small-scale industries (e.g., sugar and jute mills and plastic industries) are major players for groundwater contamination in this area.
2.2 Sample Collection and Analytical Procedure
A total of 180 samples (3 samples per sampling spot) was collected from 60 sampling points (Fig. 2). A portable global positioning system (GPS) meter (Explorist 200, Magellan) was used for geographical location of each sampling point (1 to 60). Groundwater samples were collected randomly from the pumping wells with variable depths from 14 to 204 m. Each well was pumped until steady pH and electrical conductivity were obtained. All water samples were collected in prewashed high-density polypropylene (HDPP) bottles following the standard method (APHA-AWWA-WEF 2005). The sample bottles were kept in a cooler box and shifted to our laboratory. All water samples were labeled and then kept in freeze until chemical analysis.
Electrical conductivity (EC), pH, and total dissolved solids (TDS) were recorded on field using a portable Accumet electrode and Accumet Excel, XL50 (dual channel pH/ion/conductivity) meter (Fisher Scientific, Singapore). The pH meter was standardized daily using a three-point calibration with pH 4 (SB101–500), pH 7 (SB107–500), and pH 10 (SB115–500) buffer solutions (Fisher Scientific, USA) before taking a reading in samples. Conductivity meter was calibrated with reference solution just prior to measuring EC. Concentrations of major anions (Chloride—Cl−, Bicarbonate—HCO3 −, and Sulfate—SO4 2−) in water samples were measured by an ion chromatograph (761 Compact IC, Metrohm). Cation concentrations (i.e., Sodium—Na+, Potassium—K+, Calcium—Ca2+, Magnesium—Mg2+, and Boron—B) were measured using inductively coupled plasma mass spectrometry (Thermo Scientific X-Series2 ICP-MS), which was linearly calibrated from 10 to 100 μg/L with custom multi-element standards (SPEX CertiPrep, Inc., NJ, USA) before running the real samples. All solutions were prepared using double distilled deionized water. The accuracy and precision of analyzes were tested through running duplicate analyzes on selected samples. Samples were diluted several times if needed, and the relative standard deviations of measured major ions and elements were within ± 3%.
2.3 Irrigation Water Quality Assessment
2.3.1 Irrigation Water Quality Index
The IWQI is based on linear relation of four groups of IWQ parameters that are linked to form a unique index value to assess the usability of irrigation water in the study area. Based on the guidelines presented by Ayers and Westcot (1985), four groups of IWQ parameters such as salinity hazard, permeability hazard, specific ion toxicity, and miscellaneous effect were selected (supplementary information (SI); Table SI 1) for interpreting our data. Detailed standard procedures for estimating IWQI were adapted from Simsek and Gunduz (2007). All parameters were given a weight coefficient from the highest (4) to the lowest (1) points in the technique (Table SI 2 and SI 3). Salinity hazard is the highest priority factor, while the miscellaneous effects to sensitive crops are the least important factor in IWQ assessment. Another two parameters, such as permeability hazard and specific ion toxicity are rated in decreasing order according to importance for water quality. The IWQI is calculated by the following equation (Eq. 1).
where i is an incremental index and G represents the contribution of each one of the four hazard categories that are important to assess the quality of an irrigation water resource. The values of G can be calculated using the following equation (Eq. 2).
where k is an incremental index, n is the total number of parameter available for the analysis, w is the weight value of the groups, and r is the rating value of each parameter (Table SI 2 and SI 3).
2.3.2 Irrigation Water Quality Parameters
The irrigation quality parameters were calculated using the following equations (Eqs. 3 to 9),
Total Hardness (TH)
The total hardness (TH) in parts per million (Todd 1980; Ragunath 1987) is calculated by the following equation (Eq. 3).
Percentage of Sodium (Na%)
The Na+ in irrigation water is usually denoted as Na% and can be determined using Eq. 4 (Todd 1980).
Sodium Adsorption Ratio
According to US Salinity Laboratory (Richards 1954), SAR is expressed as Eq. 5. The higher the SAR values in the water, the greater the risk of Na+, which leads to the development of a crop-unfriendly alkaline soil.
Permeability Index
Based on soil permeability, which is influenced by the presence of cations and anion in irrigation water, Doneen (1964) and WHO (2004) developed a criterion to test the suitability of groundwater for irrigational uses. The authors defined the permeability index (PI) as follows:
Residual Sodium Bicarbonate
The RSBC is expressed as Eq. 7 (Gupta 1983)
Kelley’s Ratio
The Kelley’s ratio (KR) (Kelley 1963) is obtained as described by Eq. 8.
Magnesium Adsorption Ratio
The magnesium adsorption ratio (MAR) (Raghunath 1990), also known as magnesium hazard (MH), which is calculated by Eq. 9.
2.4 Geostatistical Modeling and Statistical Analysis
Ordinary kriging (OK) and semivariogram models were used for spatial distribution of the groundwater parameters (Masoud 2014; Tapoglou et al. 2014). Out of various kriging techniques, the OK method is used in the present study of its simplicity and prediction accuracy in comparison to other Kriging methods (Gorai and Kumar 2013). Different semivariogram models (e.g., linear, exponential and spherical) were tested and the best-fitted theoretical semivariogram models for all groundwater parameters were prepared based on selecting the trial and error parameters. Predictive performances of the fitted models were checked based on cross validation tests (Gorai and Kumar 2013). The mean error (ME), mean square error (MSE), root mean error (RMSE), average standard error (ASR), and root mean square standardized error (RMSSE) values were assessed to establish the fitted models performances. After completing the cross validation process, kriging maps were produced for a graphical representation of distribution of the groundwater quality parameters. These kriged maps were generated using the ArcGIS 10.2. All other statistical analyses were performed using IBM-SPSS 19 for windows (IBM-SPSS Inc., Chicago, IL, USA).
3 Results and Discussion
3.1 Characterization of Groundwater
General characteristics of physicochemical parameters of groundwater for the study area are summarized in Table 1, along with the IWQ standard values recommended by Department of Environment (DoE) of Bangladesh, University of California Committee of Consultants (UCCC), and Food and Agriculture Organization (FAO) (UCCC 1974; DoE 1997; FAO 1985). pH varied from 6.38 to 10.02 with an average value of 7.53 ± 0.77, indicating slightly acidic (6.38) to strongly alkaline (10.02) groundwater. Elevated pH levels beyond the permissible limit (pH 8.33) indicate carbonate content in the bed rocks. The EC values varied from 344 to 1760 μs/cm with a mean value of 788.87 ± 242.83 μs/cm, which was quite lower than the Bangladesh standard of 2250 μs/cm (DoE 1997). The TDS ranged from 276.70 to 1298.40 mg/L with a mean value of 748.61 ± 198.28 mg/L. Based on the TDS classification, all the groundwater samples were belonged to freshwater category (TDS < 1000 mg/L) (Freeze and Cherry 1979). Concentrations of Na+, K+, Ca2+, and Mg2+ ranged from 5.10 to 175, 2.60 to 8.60, 34.80 to 190, and 8.92 to 59.50 mg/L with the mean values of 35.34 ± 37.99, 5.00 ± 1.36, 103.75 ± 36.23, and 32.91 ± 10.38 mg/L, respectively. The concentration of dissolved anions such as Cl−, HCO3 −, and SO4 2− varied from 1.80 to 195, 0.02 to 0.40, 200 to 848, and 1.10 to 12.30 mg/L with the mean concentrations of 23.95 ± 36.64, 0.12 ± 0.06, 542.36 ± 130.71, and 5.26 ± 2.40 mg/L, respectively (Table 1). The mean value of B was 0.04 ± 0.03 mg/L and it ranged from 0.01 to 0.10 mg/L. The chronological order of major cations were Ca2+ > Na+ > Mg2+ > K+, and major anions were HCO3 − > Cl− > SO4 2−, respectively. Higher standard deviation of both EC and TDS indicated heterogeneity in groundwater hydrochemistry. Moreover, higher concentration of HCO3 − and Ca2+ ions suggested dissolution of carbonate mineral in groundwater.
A trilinear diagram (also known as Piper diagram) developed by Piper is a very useful and popular method for primary characterization of water quality (Piper 1944). The diamond shaped graph is used to represent the composition of water with respect to both cations and anions. This trilinear diagram suitably discloses the similarity and differences among groundwater samples. Figure 3 displays that Ca2+, Mg2+, and HCO3 − play a vital role for defining ground water type. The Ca–Mg–HCO3 type groundwater indicated dominance of alkaline earth metal ions (Ca2+, Mg2+) and weak acid (HCO3 −) rather than alkali metal ions (Na+ and K+) and strong acid (Cl− and SO4 2−). Ca2+ and Mg2+ might have been contributed through dissolution of carbonate content in bedrocks (Zhou et al. 2012). The results are consistent with the findings for groundwater resources in the northwestern part of Takurgaon District, Bangladesh (Bhuiyan et al. 2014). However, it has been suggested that most of the major ions present in groundwater originated from natural sources in the study area.
3.2 Evaluation of Groundwater Quality for Irrigation
The IWQI values were determined following the Eq. (1) to evaluate the quality of the studied water for irrigation, which is graphically shown in Fig. 4. Based on IWQI, groundwater was classified into three classes, i.e., low, medium, and high suitability water (Simsek and Gunduz 2007) (Table 2). This study revealed that ~ 70% groundwater was suitable for irrigation purpose. Reversely, the computed IWQI < 22 was observed in 18 locations (S3-S4, S9, S11-S13, S15-S16, S20, S35, S38, S44-S48, S50, and S58), demonstrating that 30% groundwater in this region was classified as low suitability for irrigation (Table 2). Thus, the groundwater extracted from those locations should be used with caution.
EC and Na+ are two important factors for classifying IWQ. The presence of higher amount of salt in irrigation water results in osmotic pressure in soil solution (Thorne and Peterson 1954). In addition, salt affects the structure, texture, and permeability of soil and restrict the growth of plants making the soil hard (Trivedy and Geol 1984). The values of EC in the groundwater samples indicated that most of the sampling areas fall within the category of good (48.33%) to fair (51.67%) for irrigation purposes (Table 2). The Na% ranged from 2.13 to 55.34%, with a mean value of 14.97% (Fig. 4a). According to the classification of groundwater based on Na% (Table 2), 76.67% samples were belonged to the category of “excellent”, while the rest of them were occurred in “good (16.67%)” to “permissible (6.67%)” categories. Classification of analyzed groundwater based on EC and Na% following Wilcox (1955) (Fig. 5) illustrated that 51.67% of water samples fall in the group of “excellent to good”, while 48.33% of samples belonged to the group of “good to permissible” for irrigation. Hakim et al. (2009) and Rahman et al. (2014a, b) also found similar groundwater quality around northern and northwestern part of Bangladesh, respectively.
The SAR is generally considered as an effective groundwater evaluation index for irrigation as it is a measure of sodium (or alkali) hazard to crops (Todd 1980). High SAR values indicate Ca2+ or Mg2+ substitution for Na+ in soil through cation exchange process. This exchange process reduces the soil permeability and restrict the circulation of air and water through soil system. The calculated SAR values varied from 0.11 to 3.94 with a mean value of 0.79 (Fig. 4b). According to US salinity diagram (Fig. 6), wherein SAR is used as an index of sodium/alkalinity hazard (S) and EC is used as an index of salinity hazard (C), 48.33% samples fall in S1-C2 field (i.e., low alkalinity and medium salinity hazards water that can be used to all types of soil without the risk of moveable Na+) and 51.67% samples occurred in S1-C3 field (i.e., low alkalinity and high salinity hazards). The elevated salinity could be attributed to bedrock formation, saline soil, agricultural activities, and close proximity to Padma River system that carries domestic sewage and animal waste.
The PI values are plotted against total ionic content on Doneen’s chart (Doneen 1964), where three water types are used to explain the agricultural water quality (Fig. 7). Class I water type (> 75% of maximum permeability) with low PI values is the best water quality for irrigation, while class II water type (25–75% of maximum permeability) is considered as acceptable for irrigation. On the contrary, class III water type (< 25% of maximum permeability) is unacceptable for irrigational uses. The PI values of the groundwater samples ranged from 27.47 to 83.43% with an average of 47.79%. (Fig. 4a). Results showed that 99.99% of the samples fall under class I, indicating that Faridpur groundwater is suitable for irrigation.
The water containing RSBC < 5, 5–10, and > 10 mg/L is considered as safe, marginal, and unsatisfactory categories, respectively (Gupta and Gupta 1987). The RSBC values varied from 0.82 to 8.96 mg/L with an average of 3.70 mg/L (Fig. 4a), indicating that the water was suitable for irrigation purpose. About 80% samples were in safe class (Table 2), while the rest belonged to marginal class.
This study revealed the TH values from 157.67 to 685.06 mg/L with a mean value of 394.51 mg/L. About 81.67% samples were belonged to a very hard water category (Fig. 4c). On the other hand, KR exhibited a balance between Na+, Ca2+, and Mg2+ ions in groundwater samples. KR ratio > 1 indicates a surplus level of Na+ in water samples. Kelley (1963) suggested that the ratio for irrigation water should not exceed 1. About 98.33% samples indicated excellent irrigation water quality considering KR value (Table 2).
The presence of higher concentration of Mg2+ in groundwater results in alkaline irrigation soil, which in turn decreases the crop yielding capacity of soil. MAR values in groundwater greater than 50% are considered harmful for irrigation (Gupta and Gupta 1987; Raghunath 1990). The examined groundwater samples showed MAR values between 21.4 and 61.4% with a mean value of 35.32% (Fig. 4a). About 88.33% of studied samples showed MAR values < 50%, signifying no risk for soil and crop yield (Table 2). This study showed that Mg2+ and Ca2+ ratios varied from 0.16 to 0.95 with a mean value of 0.35. Similarly, Na+ and Ca2+ ratios ranged from 0.04 to 2.52 with an average value of 0.42 (Fig. 4b). In both cases, ratios were < 3, suggesting no threat of soil infiltration in using the studied groundwater (Table 2). However, the values of various irrigation water quality indices for each sampling points (n = 60) are listed in Table SI 4.
3.3 Principal Component Analysis
In order to understand the geochemical processes and the sources of major anions and cations in the groundwater, hydrochemical data were subjected to principal component analysis (PCA) that allows to group them based on their inherited properties. PCA was performed with an orthogonal Kaiser’s Varimax rotation to make the factors more interpretable without altering the underlying mathematical structure (Mertler and Vannatta 2005). Three principal components (PCs), with eigenvalues > 1 were extracted that represented 87.41% of the total variance (Table 3). The first principal component explaining 31.64% of the total variance is positively loaded with EC, TDS, Ca2+, and HCO3 −. This factor defines the natural origin of the ions through dissolution of calcite by carbonic acid. Among the analyzed cations and anions, Ca2+ and HCO3 − contributed the most and grouped together in first PC. Both Ca2+ and HCO3 − are very strongly correlated with EC [r 2 (Ca-EC) = 0.51 and r 2 (HCO3-EC) = 0.77)] as well as TDS [r 2 (Ca-TDS) = 0.58 and r 2 (HCO3-TDS) = 0.96] at 95% confidence level. The second PC explains 28.69% of the total variance and is positively loaded with EC, Na+, and Cl−. This factor explains the weathering of silicate sediments. Finally, the third PC explaining 27.57% of the total variance was positively loaded with K+ and Mg2+, whereas negatively with pH. This factor may represent the ion exchange of K+ and Mg2+ for Ca2+ in clays and this exchange is facilitated at less pH, i.e., acidic condition.
3.4 Semivariogram Modeling
The nugget/sill (N/S) ratio indicates the spatial dependence of IWQ parameters. Three classifications are used to explain the models: (i) strong spatial dependence (N/S ratio < 25%); (ii) moderate spatial dependence (N/S ratio is25–75%); and (iii) weak spatial dependence (N/S ratio > 75%) (Shi et al. 2007). However, the experimental semivariogram (binned sign) around the omnidirectional semivariogram model (blue line) and the average of semivariogram models (plus sign) are shown in Fig. 8. Subsequently, the exponential semivariogram model was identified as the best-fitted models for EC and SAR, while the circular semivariogram model fitted best for TH (Table 4). The Gaussian semivariogram model was best-fitted for IWQI. The major ranges varied from 3.09 to 7.88 km, where the highest range was measured for SAR (7.39 km) and the lowest for EC (3.09 km). The variation of ranges may be related to topographic and geometric factors of groundwater. Small-scale factors such as precipitation, runoff, and fertilizer application may also affect the variation of groundwater quality parameters. According to ordinary kriging results, IWQI and EC values showed a weak spatial dependence (See Table 4 and also Fig. 8a, b), while SAR and TH represented a moderate spatial dependence (See Table 4 and also Fig. 8c, d). Thus, most of the groundwater quality parameters exhibited less nugget effects in the semivariogram shapes.
3.5 Spatial Distribution Maps
Spatial distribution of IWQI, EC, SAR, and TH is shown in Fig. 9. Based on IWQI map, ~ 72% of groundwater in Faridpur is suitable for irrigational uses (Fig. 9a). The IWQI map demonstrated a decreasing trend from the northeast to southeast direction in the study area. The medium IWQI values were found near Padma River, which was located to the north and northeast parts of central Bangladesh. The low scores of IWQI value (i.e., low suitability) were observed in the southern and central parts of the study area, probably due to the influence of domestic sewage discharges, effective leaching of ions, over-exploitation of groundwater, and agricultural impact (Sahu and Sikdar 2008).
On the other hand, EC demonstrated an increasing trend from the southwest to the northeast direction in the study area (Fig. 9b). The higher values of EC were found in the northern portion. Major Padma River system might have influenced the point sources of EC in groundwater. The spatial map of SAR exhibited higher values in the central and southern part, but opposite in northern part of the study area (Fig. 9c). On the contrary, the spatial map of TH values revealed a complex spatial variability and pattern in different directions (Fig. 9d). It shows a decreasing trend from the Southwest toward the center part and an increasing trend from center toward the northeast part of the study area. Moderate spatial dependence has found in the TH values based on semivariogram studies. The higher TH values in the study area may be linked to excess use of lime on land and its transition through hydraulic connection of groundwater with river water. Weathering of limestone, sedimentary rock, and Ca-bearing minerals may also cause higher value of TH. This finding is in good agreement with the result of Bahar and Reza (2010), who conducted the groundwater quality study in southeastern Bangladesh.
4 Conclusion
Several water quality indices and geostatistical modeling were utilized to explore the suitability of groundwater for irrigational uses and for spatial mapping of water quality in Faridpur District (central Bangladesh). The order of abundance of cations in studied groundwater samples were Ca2+ > Na+ > Mg2+ > K+, and major anions were HCO3 − > Cl− > SO4 2−. Based on IWQI, it was observed that ~ 70% of the samples belonged to high suitability water type, whereas 30% exhibited low suitability type for irrigational uses. Results of EC, Na%, and SAR inferred that majority of the groundwater samples belonged to “excellent to good” classes for irrigation uses. Most of all the groundwater samples were classified as safe for irrigation based on RSBC, TH, KR, and MAR. Wilcox diagram-aided classification of water suggested that groundwater in Faridpur region were permissible for agricultural uses. Similar result was inferred by the total ionic content vs. PI plot, wherein all the samples were belonged to class I water type (i.e., > 75% of maximum permeability). The results of PCA among the major anions, cations, pH, EC, and TDS identified three PC explaining 87.41% of the total variance. Dissolution of calcite minerals, leaching of silicate sediments, and ion exchange mechanism were identified as probable causes for clustering the data into three PC. The spatial analysis of IWQI identified high suitability groundwater in the northern side, while the low suitability water in the central and southern parts of Faridpur region. This may be attributed to the gradients of domestic sewage discharges, and agricultural activates from northern to south side of Faridpur district. Since the IWQI map showed the spatial distribution of groundwater quality in the Faridpur region, decision makers can easily find most appropriate locations for drilling wells in order to extract suitable irrigation water to maintain existing soil productivity with the benefit of high crop yield.
References
Ağca, N., Karanlık, S., & Ödemiş, B. (2014). Assessment of ammonium, nitrate, phosphate, and heavy metal pollution in groundwater from Amik plain, southern Turkey. Environmental Monitoring and Assessment, 186, 5921–5934.
APHA-AWWA-WEF. (2005). Standard methods for the examination of water and wastewater (20th ed.). Washington DC: APHA, AWWA and WEF.
Ayers, R. S. (1977). Quality of water for irrigation. Journal of Irrigation and Drainage Division ASCE, 103(IR2), 135–154.
Ayers, R.S., & Westcot, D.W. (1985). Water quality for agriculture, FAO Irrigation and Drainage Paper 29, Rev. I, UN Food and Agriculture Organization, Rome.
Bahar, M. M., & Reza, M. S. (2010). Hydrochemical characteristics and quality assessment of shallow groundwater in a coastal area of Southwest Bangladesh. Environmental Earth Sciences, 61, 1065–1073.
Bhattacharya, A. K. (2010). Artificial ground water recharge with a special reference to India. International Journal of Research and Reviews in Applied Sciences, 4, 214–221.
Bhuiyan, M. A. H., Ganyaglo, S., & Suzuki, S. (2014). Reconnaissance on the suitability of the available water resources for irrigation in Thakurgaon District of northwestern Bangladesh. Applied Water Science, 5, 229–239.
Bhuiyan, M. A. H., Bodrud-Doza, M., Islam, A. R. M. T., Rakib, M. A., Rahman, M. S., & Ramanathan, A. L. (2016). Assessment of groundwater quality of Lakshimpur district of Bangladesh using water quality indices, geostatistical methods, and multivariate analysis. Environmental Earth Sciences, 75, 1020.
Bodrud-Doza, M., Islam, A. R. M. T., Ahmed, F., Das, S., Saha, N., & Rahman, M. S. (2016). Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Science Journal, 30, 19–40.
Boyacioglu, H. (2007). Development of a water quality index based on a European classification scheme. Water SA, 33, 101–106.
Bozdag, A. (2015). Combining AHP with GIS for assessment of irrigation water quality in Cumra irrigation district (Konya), Central Anatolia, Turkey. Environmental Earth Sciences, 73, 8217–8236.
Debels, P., Figueroa, R., Urrutia, R., Barra, R., & Niell, X. (2005). Evaluation of water quality in the Chillan River (Central Chile) using physicochemical parameters and a modified water quality index. Environmental Monitoring and Assessment, 110, 301–322.
Dixon, B. (2005). Groundwater vulnerability mapping: a GIS and fuzzy rule based integrated tool. Applied Geography, 25, 327–347.
DoE. (1997). The environment conservation rules. Dhaka: Government of the People’s Republic of Bangladesh.
Doneen, L. D. (1964). Notes on water quality in agriculture, water science and engineering. Davis: University of California.
FAO (1985). Water quality for agriculture. Food and Agriculture Organization. http://www.fao.org/docrep/003/T0234E/T0234E00.htm. Accessed 26 June 2016.
Freeze, R. A., & Cherry, J. A. (1979). Groundwater (p. 56). New Jersey: Prentice-Hall.
Goovaerts, P. (1997). Geostatistics for natural resource evaluation. New York: Oxford University Press.
Gorai, A. K., & Kumar, S. (2013). Spatial distribution analysis of groundwater quality index using GIS: a case study of Ranchi municipal corporation (RMC) area. Geoinfor Geostat: An Overview, 1, 2. https://doi.org/10.4172/2327-4581.1000105.
Gupta, S. K. (1983). Variations of water table in Yamuna drainage basin of Haryana-implications and management strategies. In Seminar on Strategies for Irrigation Water Management, Patna Google Scholar.
Gupta, S. K., & Gupta, I. C. (1987). Management of saline soils and waters. New Delhi: Oxford and IBH Publishing Company.
Hakim, M. A., Juraimi, A. S., Begum, M., Hasanuzzaman, M., Uddin, M. K., & Islam, M. M. (2009). Suitability evaluation of groundwater for irrigation, drinking and industrial purposes. American Journal of Environmental Science, 5, 413–419.
Horton, R. K. (1965). An index number system for rating water quality. Journal of Water Pollution Control Federation, 37, 300–306.
Hussain, H. M., Joshi, H., Singhal, D. C., Kumar, S., & Rao, M. S. (2012). Development of an index of aquifer water quality within GIS environment. Iran Journal of Earth Sciences, 4, 44–50.
Isaaks, E. H., & Srivastava, R. M. (1989). An introduction to applied geostatistics (p. 561). New York: Oxford University Press.
Kelley, W. P. (1963). Use of saline irrigation water. Soil Science, 95, 355–391.
Kinniburgh, D.G., & Smedley, P.L. (2001). Arsenic contamination of groundwater in Bangladesh. BGS Technical Report WC/00/19, Vol. 2.
Kumari, S., Singh, A. K., Verma, A. K., & Yaduvanshi, N. P. S. (2013). Assessment and spatial distribution of groundwater quality in industrial areas of Ghaziabad, India. Environmental Monitoring and Assessment, 186, 501–514.
Liou, S., Lo, S., & Wang, S. (2004). A generalized water quality index for Taiwan. Environmental Monitoring and Assessment, 96, 35–52.
Liu, S., Tao, A., Dai, C., Tan, B., Shen, H., Zhong, G., Lou, S., Chalov, S., & Chalov, R. (2017). Experimental study of tidal effects on coastal groundwater and pollutant migration. Water, Air, & Soil Pollution, 228, 163.
Masoud, A. A. (2014). Groundwater quality assessment of the shallow aquifers west of the Nile Delta (Egypt) using multivariate statistical and geostatistical techniques. Journal of African Earth Sciences, 95, 123–137.
Mertler, C. A., & Vannatta R.A. (2005). Advanced and multivariate statistical methods: practical application and interpretation (3rd ed), Glendale, CA. pp. 120.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analyses. Transactions American Geophysical Union, 25, 914–923.
Raghunath, H. M. (1990). Ground water. Wiley eastern limited (2nd ed.p. 563). New Delhi.
Ragunath, H. M. (1987). Groundwater (p. 563). New Delhi: Wiley Eastern.
Rahman, M. S., & Islam, M. R. (2009). Effects of pH on isotherms modeling for Cu(II) ions adsorption using maple wood sawdust. Chemical Engineering Journal, 149, 273–280.
Rahman, M.A.T.M.T., Saadat, A.H.M., Islam, M.S., Al-Mansur, M.A., & Ahmed, S. (2014a). Groundwater characterization and selection of suitable water type for irrigation in the western region of Bangladesh. Applied Water Science, 7, 233–243.
Rahman, M. S., Saha, N., & Molla, A. H. (2014b). Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environmental Earth Sciences, 71, 2293–2308.
Ravikumar, P., Somashekar, R. K., & Angami, M. (2011). Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State, India. Environmental Monitoring and Assessment, 173(1), 459–487.
Richards, L.A. (1954). Diagnosis and improvement of saline and alkaline soils, US Salinity Laboratory, US Department of Agriculture Hand Book, pp 60.
Romanelli, A., Lima, M. L., Londoño, O. M. Q., Martínez, D. E., & Massone, H. E. (2012). A Gis-based assessment of groundwater suitability for irrigation purposes in flat areas of the wet pampa plain, Argentina. Environmental Management, 50, 490–503.
Saha, N., Rahman, M. S., Ahmed, M. B., Zhou, J. L., Ngo, H. H., & Guo, W. (2016). Industrial metal pollution in water and probabilistic assessment of human health risk. Journal of Environmental Management, 185, 70–78.
Sahu, P., & Sikdar, P. K. (2008). Hydrochemical framework of the aquifer in and around East Kolkata wetlands, West Bengal. Environmental Geology, 55, 823–835.
Shahid, S. (2010). Recent trends in the climate of Bangladesh. Climate Research, 42, 185–193.
Shammi, M., Rahman, R., Rahman, M. M., Moniruzzaman, M., Bodrud-Doza, M., Karmakar, B., & Uddin, M. K. (2016). Assessment of salinity hazard in existing water resources for irrigation and potentiality of conjunctive uses: a case report from Gopalganj District, Bangladesh. Sustainable Water Resources Management, 2, 369–378.
Shi, J., Wang, H., Xu, J., Wu, J., Liu, X., Zhu, H., & Yu, C. (2007). Spatial distribution of heavy metals in soils: a case study of Changxing, China. Environmental Geology, 52, 1–10.
Simsek, C., & Gunduz, O. (2007). IWQ index: a GIS-integrated technique to assess irrigation water quality. Environmental Monitoring and Assessment, 128, 277–300.
Song, T., & Kim, K. (2009). Development of a water quality loading index based on water quality modeling. Journal of Environmental Management, 90, 1534–1543.
Tapoglou, E., Karatzas, G. P., Trichakis, J. C., & Varouchakis, E. A. (2014). A spatio-temporal hybrid neural network-kriging model for groundwaterlevel simulation. Journal of Hydrology, 519, 3193–3203.
Thorne, D. W., & Peterson, H. B. (1954). Irrigated soils. London: Constable and Company.
Todd, D. K. (1980). Groundwater hydrology (2nd ed.). New York: Wiley.
Trivedy, R. K., & Geol, P. K. (1984). Chemical and biological methods for water pollution studies. Karad: Environ Publications.
UCCC. (1974). Guidelines for interpretations of water quality for irrigation. California: University of California Committee of Consultants.
Webster, R., & Oliver, M. A. (2001). Geostatistics for environmental scientists (p. 330). New York: Wiley.
WHO. (2004). Guidelines for drinking-water quality (3rd ed.). Geneva: WHO.
Wilcox, L. V. (1955). Classification and use of irrigation water. Washington DC: US Department of Agriculture.
Zhou, T., Wu, J., & Peng, S. (2012). Assessing the effects of landscape pattern on river water quality at multiple scales: a case study of the Dongjiang River watershed, China. Ecological Indicators, 23, 166–175.
Acknowledgements
The authors would like to acknowledge the Department of Environmental Sciences, Jahangirnagar University, Bangladesh and Chemistry Division, Atomic Energy Center, Dhaka Bangladesh for providing some technical supports.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 40.3 kb)
Rights and permissions
About this article
Cite this article
Safiur Rahman, M., Saha, N., Islam, A.R.M.T. et al. Evaluation of Water Quality for Sustainable Agriculture in Bangladesh. Water Air Soil Pollut 228, 385 (2017). https://doi.org/10.1007/s11270-017-3543-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3543-x