Abstract
The biomagnification of Hg and Se was studied using nitrogen stable isotope analysis during four seasons in a coastal lagoon of the eastern central Gulf of California. This lagoon receives agricultural, municipal, and shrimp aquaculture effluents. The species were categorized into organism groups and presented a significant accumulation of Hg and Se with respect to the sources, while the concentration of both elements in sediment and suspended particulate matter (SPM) was low. Our data confirms the positive transfers (biomagnification factors >1) of Hg and Se in the entire studied food web, and it was structured in five trophic levels across all seasons. Additionally, there were no linear correlations between the molar Se:Hg ratios and the trophic levels of the organism groups. However, the Se:Hg ratios among organism groups were >1, which indicates that there is an excess of Se and that it is not a limiting factor for the detoxification of Hg.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) contamination is a worldwide problem given it is a persistent and highly toxic contaminant that occurs ubiquitously in the environment. Its bioaccumulation in marine food webs is several times higher compared to baseline levels, and the greatest concern is related to the transformation of reactive Hg into organic species, which are highly toxic even at low concentrations (Fitzgerald and Lamborg 2005; Dehn et al. 2006). The accumulation of Hg species depends on abiotic (e.g., pH, salinity, redox, and chemical speciation) and biotic factors (e.g., diet, age, sex, the physiological role of each element, and trophic position) (Lavoie et al. 2013). Conversely, selenium (Se) is an essential element for all organisms given it is a cofactor of enzymes (e.g., glutathione peroxidase or thioredoxin reductase); its deficiency results in the increased risk of the development of many chronic degenerative diseases, and the action as a protective agent against the toxicity of Hg was proposed by the formation of insoluble Hg–Se complexes (Sørmo et al. 2011; Kehrig et al. 2013). A molecular excess of Se ratio to methyl-mercury (CH3Hg) prevents the inhibition of selenoenzyme activities, thereby alleviating Hg–exposure risks (Ralston et al. 2019). However, high levels of Se can be toxic for organisms, and their interactions with Hg may also have synergistic effects depending on the sensitivity of the organ or organism which is determined by the threshold concentrations of both elements (Khan and Wang 2009; Dang and Wang 2011).
Although Hg biomagnification has been usually documented (e.g., Dehn et al. 2006; Jara-Marini et al. 2012; Kehrig et al. 2013; Lavoie et al. 2013), evidence for Se biomagnification is inconsistent with cases of biomagnification (e.g., Kehrig et al. 2013; Økelsrud et al. 2016) and no biomagnification (e.g., Ouédraogo et al. 2015). According to Reinfelder et al. (1998), the principal factors that drive the Hg and Se transference in food webs are the assimilation efficiency, the ingestion rate, the physiological loss, and the dilution by growth; however, Stewart et al. (2004) proposed that the dominant factor controlling this transference may vary under specific conditions.
The variation in the ratio of stable isotopes of nitrogen (δ15N=15N/14N) has been used in ecology to determine the relative trophic position of species as a function of its time-integrated dietary history (Minagawa and Wada 1984). Due to the relatively constant enrichment rate of the heavier isotope (15N) across trophic levels (2–4‰), nitrogen isotopic signatures provide valuable information on the trophic position of an organism in a food web (Post 2002). Additionally, the biomagnification of contaminants in food webs may be determined by the correlation between the trophic position of organisms and their contaminant concentrations (Post 2002; Power et al. 2002; Dehn et al. 2006; Jara-Marini et al. 2009, 2012; Økelsrud et al. 2016).
Marine ecosystems associated with the Gulf of California are ecologically and socioeconomically important in Mexico due to their elevated biodiversity, productivity, and commercial fisheries (Páez-Osuna et al. 2017). Specifically, the El Tobari (ET) lagoon is an ecosystem with the highest priority for conservation as a result of its rich biodiversity, high rates of biological productivity, and endemism of invertebrates, fish, and bird species (Aguilar et al. 2008). However, this lagoon is ecologically impacted by discharges of municipal, agriculture, and shrimp farm effluents that contain a myriad of pollutants, and some of the ecological (Martínez-Durazo et al. 2019) and metal accumulation (Jara-Marini et al. 2020) impacts have been previously reported. Approximately 230,000 ha of irrigated agriculture surface in the Yaqui Valley (Gortáres-Moroyoqui et al. 2011; McCullough and Matson 2016) extensively uses a great variety of agrochemicals and constitutes significant metal inputs for the ET lagoon. Furthermore, inputs of Hg and Se from intensive shrimp farming may be significant through the use of artificial feed, fertilizers, and other chemical additives, including acidity correctors and algaecides (Lyle-Fritch et al. 2006). Similar conditions occur in other coastal ecosystems in the ecoregion of the Gulf of California. In this study, we examined the biomagnification of Hg and Se in the food web of the ET lagoon during four typical seasons (summer and autumn 2011 and winter and spring 2012). Our study objectives were to (a) characterize the trophic position of food web species through stable nitrogen isotopic ratios; (b) assess the Hg and Se transfer and biomagnification through the ecosystem food web; and (c) determine the Se:Hg molar ratios in the organisms of the food web.
Materials and methods
Study area
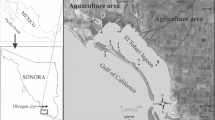
The ET lagoon is located in northwestern Sonora, Mexico, along the eastern central coast of the Gulf of California (Fig. 1). The characteristics and prevailing climatic conditions of the ET lagoon have been described in previous studies (Jara-Marini et al. 2013a, b). This ecosystem plays a crucial ecological role in the support of a variety of endemic and migratory organisms, and for this reason, it was included among the Conservation of Coastal and Oceanic Priority Zones of Mexico (Aguilar et al. 2008). However, the ET lagoon also receives the regular effluent discharges from (a) agriculture (10 irrigation districts ~230,000 ha); (b) municipal untreated and partially treated sewage from Obregon City and other surrounding towns (~375,800 habitants); and )c) aquaculture (1190 ha of shrimp ponds) (Gortáres-Moroyoqui et al. 2011; INEGI 2015; McCullough and Matson 2016).
Sampling and processing
Four sample collections were conducted during the summer (August 2011), autumn (October 2011), winter (February 2012), and spring (May 2012) at seven highly biodiverse sites inside the ET lagoon and in one reference site outside of the lagoon (Fig. 1). In each site, samples of subsurface water (<1 m depth, 1 L Nalgene bottles), surface sediment (0–2.5 cm from the top of the sedimentary column), and numerous organisms were collected following standardized sampling protocols. Details of the sampling and processing of the samples were previously reported in Martínez-Durazo et al. (2019) and Jara-Marini et al. (2020).
The surface water samples were filtered through a precleaned and precombusted (500°C, 4 h) glass fiber filter (GF/F), and the total suspended particulate matter (SPM) contents were gravimetrically determined. The organic carbon (Loring and Rantala 1992), carbonates (Rauret et al. 1988), and the Hg and Se contents in bioavailable fractions (chemical sequential extraction scheme of Huerta-Diaz and Morse 1990) were determined in surface sediments. The composited samples of representative species of different organism groups in the ET lagoon food web were identified according to Fischer et al. (1995a, b, c) and Sibley (2000) and processed for isotopic and metal analysis according to Martínez-Durazo et al. (2019) and Jara-Marini et al. (2020). The organism groups and the characteristics of the abiotic samples are listed in Table 1.
Hg and Se analyses
Lyophilized tissues of the biological samples (0.30 ± 0.003 g on dry weight basis) were digested with 5 mL of concentrated HNO3 and heated in three steps (100 °C for 5 min, then 120 °C for 5 min, and finally 140 °C for 10 min) using a microwave system (MARS-X). The bioavailable extracts from the sediments and the biological digested tissues were analyzed for Hg and Se using cold vapor and hydride generation (Model VGA 110), respectively, coupled to an atomic absorption spectrophotometer (Varian, Model SpectrAA-240-FS). Certified reference material (marine sediment PACS-2 and dogfish muscle DORM-2 from the National Research Council Canada) and blanks were used for metal quality control purposes. The resulting agreement with certified values for both elements was higher than 10% in sediment fractions and in the biological tissues. The limits of detection for the Hg and Se analyses were estimated as 0.21 and 0.33 ng g−1 for the reactive fraction, 0.28 and 0.35 ng g−1 for the pyrite fraction, and 0.32 and 0.38 ng g−1 for the biological analysis, respectively.
Stable isotope analyses
Subsamples of dried SPM, sediment, and biological tissues were processed for the removal of carbonates, after which they were dried and packed into tin cups for N isotope analysis (see details in Martínez-Durazo et al. 2019). The 15N analyses were performed in the Environmental Isotope Laboratory of the Department of Geosciences (University of Arizona, Tucson, AZ) using a Finnigan Delta Plus XL continuous-flow gas-ratio Mass Spectrometer Carlo Erba NA 2100 ANCA-NT 20-20 Stable Isotope Analyzer (precision was ≤0.2‰ in n=100, as estimated by the international standards IAEA-N-1 and IAEA-N-2 from the International Atomic Energy Agency). The isotopic composition (δ15N) was expressed as the relative difference between the isotopic ratios in the sample and conventional standards (atmospheric N2):
where R= 15N /14N.
Data analyses
Since the δ15N and Hg and Se values were non-normally distributed (Shapiro-Wilks and Bartlett tests, p<0.05), Kruskal-Wallis and Student-Newman-Keuls tests (Glantz 2002) were used to compare the isotopic and metal concentrations among seasonal groups. The estimation of the most important trophic relations (carbon and nitrogen sources) was reported previously by Del Rio-Salas et al. (2019) using a stable isotope Bayesian SIAR mixing model (Parnell et al. 2010).
The trophic level (TL) was estimated assuming a 15N trophic enrichment factor of 2.54‰ (Vanderklift and Ponsard 2003), according to the following formula (Hobson and Welch 1992; Post 2002):
where δ15Nsecondary consumer is the δ15N composition of the secondary or higher consumer, and δ15Nprimary consumer is the δ15N composition of the baseline consumer (the composition used was zooplankton).
The biomagnification factor (BMF) corrected for differences in trophic position (based on δ15N) was calculated according to the procedure of Dehn et al. (2006) for the selected predator-prey scenarios as:
where MPredator and MPrey are the concentrations of each metal (μg g−1 DW) in the predator and prey species, and δ15NPredator/δ15NPrey is the N composition (‰) of the predator and prey pair.
The Hg and Se concentrations in the groups of organisms were logarithm-transformed (log-transformed) to normalize the distribution. A simple linear regression model using TL values as the independent variable and element concentrations in organisms as the dependent variables were then performed to establish the trophic relationships between both of these variables. The trophic biomagnification factor (TBMF) for the two studied elements was estimated from the antilogarithm of the slopes of these linear correlations. Seasonal differences of TBMF values for Hg and Se were evidenced using a t-student test (α=0.05). A TBMF>1 indicates the entire biomagnification of a contaminant in the food web (Mackay et al. 2016). All of the statistical analyses were performed using the NCSS Statistical Software (NCSS 2007).
Results
Hg and Se concentrations in sources and organisms
The concentrations of Hg in the bioavailable (reactive plus pyrite) fraction of sediment and in the SPM on the August (0.10 to 0.11 μg g−1) and February (0.09 to 0.11 μg g−1) samples evidenced nonsignificant differences; however, their levels increased significantly (p<0.05) in the May (0.27 to 0.29 μg g−1) and October (0.26 to 0.30 μg g−1) samples (Table 2). Significant accumulations of Hg with respect to sediments and SPM were determined in macroalgae species, which varied from 0.84 μg g−1 for Spyridia filamentosa in May to 1.57 μg g−1 for Ulva lactuca in August (Table 2). The two mangrove species showed similar (p>0.05) Hg concentrations ranging from 0.82 μg g−1 in October to 1.15 μg g−1 in February, while phytoplankton presented the highest accumulation of this element among primary producers and varied from 1.14 μg g−1 in May to 1.69 μg g−1 in August.
The primary consumers showed increases in the levels of Hg with respect to primary producers that were most significant in August (from 1.43 μg g−1 for Mugil cephalus juvenile fish to 3.52 μg g−1 for Callinectes arcuatus juvenile crab) and February (from 1.66 μg g−1 for M. cephalus juvenile fish to 4.28 μg g−1 for Haemulopsis leuciscus juvenile fish). Similarly, the accumulation of Hg in secondary and tertiary consumers was significant (p<0.05) with respect to primary consumers, and this was most evident in August (varying from 2.44 μg g−1 in adults of M. cephalus to 5.16 μg g−1 in adults of C. arcuatus) and February (varying from 4.02 μg g−1 in adults of M. cephalus adult to 5.18 μg g−1 in adult fishes of Lutjanus argentivensis). The highest Hg levels were determined in the quaternary consumers (birds) during May, and they ranged from 3.09 μg g−1 in Platalea ajaja to 6.44 μg g−1 in Fregata magnificens. Among this group of species, F. magnificens presented the highest concentrations of Hg during all seasons ranging from 4.19 μg g−1 in October to 6.44 μg g−1 in May.
In addition, Table 2 presents the Se concentrations in sources and organism groups. The concentrations of Se in the sediment (0.07 to 0.19 μg g−1) and SPM (0.09 to 0.12 μg g−1) varied slightly during the four sampling seasons. The levels of Se in primary producers with respect to sediment and SPM increased significantly (p<0.05), with seasonal variations and ranges from 1.24 to 2.69 μg g−1 in August, from 1.80 to 3.31 μg g−1 in February, from 2.34 to 3.22 μg g−1 in May, and from 2.12 to 2.92 μg g−1 in October. This group exhibited seasonal variation in their Se accumulation, with the most significant (p<0.05) high levels for macroalgae species (from 3.02 to 3.31 μg g−1) in February and in the mangrove Avicennia germinans (3.22 μg g−1) in May. The Se accumulation in primary consumers with respect to primary producers was significant (p<0.05) during all seasons, ranging from 2.29 μg g−1 for M. cephalus juveniles during October to 21.2 μg g−1 for the barnacle Fistulobalanus dentivarians in August. Some species of this group showed seasonal significant variations in Se content, with the lowest values in juveniles of M. cephalus (2.71 and 2.29 μg g−1 in August and May, respectively), C. arcuatus (2.74 μg g−1 in October), and L. argentivensis (2.71 μg g−1 in May). The secondary and tertiary consumers presented significantly (p<0.05) higher levels of Se than the primary consumers, ranging from 4.81 μg g−1 in adults of C. arcuatus in October to 12.9 μg g−1 in adults of L. argentivensis. Both groups also presented seasonal significant (p<0.05) variations in some species, with the highest values of 9.33 μg g−1 for M. cephalus adults in May, 9.17 μg g−1 for C. arcuatus adults in August, and 12.9 μg g−1 for L. argentivensis adults in October. The Se accumulation in the quaternary consumers was significantly (p<0.05) higher than in other groups, with seasonal variations in the birds Rallus longirostris (from 14.6 μg g−1 in October to 28.3 μg g−1 in August) and F. magnificens (from 19.1 μg g−1 in May to 34.8 μg g−1 in February).
No spatial significant (p>0.05) variations were determined regarding Hg and Se concentrations in sediments nor in organisms. The homogeneous distribution of these elements in the sediments may be related to hydrodynamic conditions of the ET lagoon, given site three is close to the northern mouth and the currents spread contaminants in the ecosystems according to tidal regime. Concerning organisms, the slight spatial variation could be related to the effects of marine currents on metal distribution and to the mobility of species through the ecosystem.
Trophic transfer of Hg and Se and molar Se:Hg ratios
The log-transformed Hg concentrations versus the trophic levels of the ET lagoon food web presented significant (p<0.05) linear correlations (R values from 0.64 to 0.83; Fig. 2), evidencing increases of Hg levels from primary producers to tertiary consumers. Similarly, significant positive (p<0.05) correlations between log-transformed Se concentrations versus the trophic levels of the ET lagoon were also observed (R values from 0.47 to 0.55). Thus, the increase in Se content through the studied food web was evident.
The TBMF is the slope of the correlation between logarithm-contaminant concentrations versus TL. A TBMF>1 indicates the entire biomagnification of a contaminant in the food web (Mackay et al. 2016). The ranges of TBMF that were determined in the ET lagoon varied from 1.38 to 1.62 (no seasonal differences, p>0.05) for Hg and from 1.45 to 1.70 (no seasonal differences, p>0.05) for Se (Table 2), which is indicative of the biomagnification of both elements. Table 2 also shows the BMF for the different prey-predator relations in the studied food web, with ranges from 0.52 to 14.2 for Hg and from 0.52 to 36.4 for Se. There are increases in the BMF through the trophic relationships of the ET lagoon relations, with ranges of 0.52–0.67 to 0.84–1.81 for Hg and of 0.43–0.81 to 3.24–6.66 for Se for primary consumers and from 1.15–2.03 to 1.33–3.88 for Hg and of 3.50–3.96 to 3.91–7.00 for Se for tertiary consumers. Values of BMF>1 are also indicative of a positive transfer of contaminants between the different organism groups (Dehn et al. 2006).
The linear correlations between the molar Se:Hg ratios and the TL of the organism groups of the ET lagoon did not present a defined pattern and were only significant (p<0.05) for the summer (August 2011) season (R2=0.30; Fig 2). The tendency in three of the four seasons was to decrease according to the TL increments in the ET lagoon food web. The ranges of molar Se:Hg ratios in the entire food web were 0.36–2.91 for summer (August 2011), 0.42–2.37 for winter (February 2012), 0.20–1.69 for spring (May 2012), and 0.35–1.63 for autumn (October 2012).
Discussion
Hg and Se sources in El Tobari lagoon
In the present study, the concentrations of Hg and Se found in the sediment were similar to those reported in mangrove or coastal ecosystems around the world; however, they were significantly lower than those reported in urbanized harbors (Haris et al. 2017). These authors also reported levels of Hg lower than those found in this study in an anthropogenically impacted mangrove ecosystem.
The measurements of bioavailable Hg concentrations in sediments were compared to those previously reported in the ET lagoon (0.22–1.38 μg g−1; Jara-Marini et al. 2013a). These were similar to the recent samples collected during the summer and winter seasons but were significantly lower than those collected during the spring and autumn seasons. Jara-Marini et al. (2013b) also reported enrichment factors for Hg ranging from 2.8 to 12.9, which is classified as strong enrichment and is indicative of the anthropogenic sources of this element (Birch 2017). The seasonal variations of Hg and Se concentrations in the sediment and SPM in the ET lagoon evidenced seasonal and yearly variations probably associated to the anthropogenic inputs of both elements. Specifically, values of bioavailable Hg in the sediments that were collected in the spring and autumn seasons (0.27 and 0.29 μg g−1, respectively) did not exceeded the threshold effect level (TEL= 130 μg g−1) (Birch 2018); this would not represent adverse biological effects. However, the transference of metals and metalloids from sediments may occur even if the levels are considered low (Birch 2017, 2018).
As previously reported, the ET lagoon is an ecosystem that is affected by agriculture activities from the Yaqui Valley, which is one of the most extensive agricultural areas in Mexico and discharges 10 agricultural drains into this ecosystem (Jara-Marini et al. 2013a, b, 2020; Martínez-Durazo et al. 2019). Mercurial fungicides (e.g., methyl-mercury, methoxy-ethyl mercury, and phenylmercury acetate) were used as a seed preservative between 1950 and 1970 (Gupta and Aggarwal 2007). Although these fungicides were prohibited in the early 1970s, their extensive application resulted in Hg accumulation in soils and posterior mobilization to adjacent coastal ecosystems (Birch 2018) and thus to the potential for transference to aquatic food webs (Calle et al. 2018; Fang et al. 2019).
Diverse studies have reported the application of Se mineral fertilizers with a load from 5 to 20 g ha−1 to increase the element bioavailability levels in soils (Curtin et al. 2008; Jiang et al. 2015; Garousi 2017). An annual discharge between 1150 and 4600 kg Se year−1 from the 230,000 ha of the Yaqui Valley during the 1950–1970 period has been estimated. The irrigation in each crop cycle provokes the Se lixiviation and may be an important source of the element to adjacent ecosystems. Santos et al. (2015) reported that agriculturally drained water contains 140–1400 mg Se L−1, which could explain the accumulation and transfer of this element in the organisms of the ET lagoon by its continued exportation through the drains. However, the presence of a natural source of Se cannot be ignored. There is no precise quantitative information regarding the flows in Yaqui Valley drains for the estimation of this Se load into the ET lagoon.
The ET lagoon possesses an extensive surface of shrimp aquaculture in the north zone (1190 ha) with effluents that are another potential Hg source to this ecosystem. The inputs of this element from intensive shrimp farming may be significant through the use of artificial feed, fertilizers, and other chemical additives, including acidity correctors and algaecides (Lyle-Fritch et al. 2006). Lacerda et al. (2011) estimated Hg emission factors of ~83.5 mg ha−1 in each culture cycle of a coastal ecosystem in Brazil. Assuming that the same conditions operate in the ET lagoon, the shrimp area ponds represent a load of 99.4 g Hg yr−1. Conversely, assuming the calculated Hg emission factors for urban wastewaters (200 mg ha−1 yr−1) that were reported by Lacerda et al. (2011), the inputs of Hg from Obregon city and other towns located in the basin of the ecosystem (surface of 331,200 ha) represent a load of 66.2 kg Hg yr−1. However, a correct and more realistic estimation is required considering that wastewaters are partially treated, and part of these effluents are not entirely discharged to the ET lagoon.
There is no available information for the calculation of the shrimp farm load of Se from shrimp aquaculture. However, by assuming a Se content of 1.22 μg Se g−1 in the shrimp feed (Wang et al., 2019), a feed conversion ratio (wet food added/wet weight of shrimp harvested) of 1.4 (Lyle-Fritch et al. 2006) and considering that the Se content in the shrimp farming is 2.23 μg g−1 (Silva et al. 2016), it is possible to deduce that the 1190 ha of shrimp ponds in the adjacent shrimp farms receives a Se contribution through the food of 2433 g per cycle. The Se recovery through the harvested shrimp is estimated to be 637 g; therefore, we estimated that 1.8 kg of Se is released per cycle, of which a proportion is discharged to the ET lagoon via shrimp effluents.
Trophic transfer of Hg and Se through the food web
Many studies have reported the biomagnification of Hg and/or Se in food webs in freshwater (Arcagni et al. 2013; Økelsrud et al. 2016), coastal (Kehrig et al. 2013; Seixas et al. 2014), pelagic (Escobar-Sánchez et al. 2011; Ordiano-Flores et al. 2012; Lavoie et al. 2013), and artic (Dehn et al. 2006; Lavoie et al. 2013) ecosystems. The elevated biomagnification potentials of both elements are caused by their high assimilation efficiencies and ingestion rates and low efflux rates (Reinfelder et al. 1998; Wang 2002), and these variables depend on the biotic factors (e.g., growth rate, species diversity, and length of the food web) and physico-chemical conditions of the ecosystems (e.g., pH, salinity, dissolved organic matter, redox potential) (Wang 2002). Lavoie et al. (2013) found that polar and temperate regions have higher Hg trophic magnification slopes than tropical ecosystems, which is explained by the low growth and excretion rates at cold temperatures and the low species diversity (less complex food chains). Since diet dominates Se uptake, trophic relations might be the limiting factor for the biomagnification of this element. Stewart et al. (2004) reported that clams and crustaceans cause the efficient transfer of Se to upper trophic levels by their low excretion rates. Similarly, the predominant species of phytoplankton may limit the positive transfer of Se to predators given the dinoflagellate group has high assimilation efficiencies and ingestion rates and/or low efflux rates, as compared to chlorophytes. Nonetheless, the precise mechanisms are still uncertain (Stewart et al. 2010). Our data indicates that bivalves could be significant sources of Se to higher trophic positions. In addition, the formation of an equimolar Hg–Se complex binding to selenoprotein P may lead to a positive correlation between Hg and Se (Sasakura and Suzuki 1998), which would explain the Hg and Se biomagnification found in the ET lagoon.
The antagonistic effect of Se against Hg toxicity is widely recognized (Ralston et al. 2019). The Hg–Se antagonism is based on the formation of certain Hg–Se compounds that limit the mobility, bioavailability, and affinity to the target sites. Most of the organism groups presented molar Se:Hg ratios >1 during the four seasons, which is indicative of an excess of Se in relation to their essentiality, but this is not a limiting factor for the detoxification of Hg (Sørmo et al. 2011). The effects of Se against Hg toxicity include the redistribution of Hg in the tissues, the competition for binding sites, and the formation of un-reactive Hg–Se complexes (Stewart et al. 2010). It was proposed that Se acts against Hg2+ and methyl–Hg, reducing their toxicity and possible transfer through the food web by the formation of insoluble mercury selenides (Se–Hg complexes) that interrupt the activity of Se-dependent functions (e.g., selenoenzymes) (Sørmo et al. 2011), since they may be less solubilized from the prey and could be absorbed by the predator digestive tract. Wang et al. (2004) reported that the dietary assimilation of Hg2+ and methyl–Hg by the diatom Thalassiosira pseudonana and the green mussel Perna viridis was not significantly affected by the different Se6+ and Se4+ concentrations. In contrast, seleno-L-methionine significantly inhibited the uptake of methyl–Hg and enhanced the uptake of Hg2+ by the diatoms and the mussels at a relatively low concentration, possibly by the complexation of Hg2+.
Conclusions
The biomagnification of Hg and Se studied during four seasons in a subtropical coastal lagoon (ET) of the eastern central Gulf of California included the characterization of the trophic position of food web species through stable nitrogen isotopic ratios. For this, a total of thirty-three species belonging to five organism groups were examined. The BMF ranges for Hg and Se in the organisms were 0.52–1.81 and 0.90–6.66 for primary consumers (zooplankton); 0.70–1.45 and 0.52–1.34 for secondary consumers (crustaceans); 1.09–14.2 and 0.76–36.4 for tertiary consumers (mainly fishes); and 1.15–3.88 and 3.16–7.00 for quaternary consumers (birds), respectively. The estimated TBMF for the trophic web was 1.38–1.62 and 1.45–1.70 for Hg and Se, respectively. These data confirm the biomagnification of Hg and Se (BMF and TBMF>1) during the four seasons examined. Additionally, it evidences that primary consumers are the main sources of Hg and Se to higher trophic levels. Conversely, although the linear correlations between the molar Se:Hg ratios and the TL of the organism groups of the ET lagoon did not present a defined pattern, the Se:Hg ratios among organism groups were >1, which suggests an excess of Se, and that it is not a limiting factor for the detoxification of Hg.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguilar V, Kolb M, Hernández D, Urquiza T, Koleff P (2008) Prioridades de conservación de la biodiversidad marina de México. CONABIO Biodivers 79:1–15
Arcagni M, Campbell L, Arribére MA, Marvin-DiPasquale M, Rizzo A, Ribeiro Guevara S (2013) Differential mercury transfer in the aquatic food web of a double basined lake. Sci Total Environ 454–455:170–180. https://doi.org/10.1016/j.scitotenv.2013.03.008
Birch GF (2017) Determination of sediment metal background concentrations and enrichment in marine environments – A critical review. Sci Total Environ 580:813–831. https://doi.org/10.1016/j.scitotenv.2016.12.028
Birch GF (2018) A review of chemical-based sediment quality assessment methodologies for the marine environment. Mar Pollut Bull 133:218–232. https://doi.org/10.1016/j.marpolbul.2018.05.039
Calle P, Monserrate L, Medina F, Calle Delgado M, Tirapé A, Montiel M, Ruiz Barzola O, Alvarado Cadena O, Dominguez GA, Alava JJ (2018) Mercury assessment, macrobenthos diversity and environmental quality conditions in the Salado Estuary (Gulf of Guayaquil, Ecuador) impacted by anthropogenic influences. Mar Pollut Bull 136:365–373. https://doi.org/10.1016/j.marpolbul.2018.09.018
Curtin D, Hanson R, Van Der Weerden TJ (2008) Effect of selenium fertiliser formulation and rate of application on selenium concentrations in irrigated and dryland wheat (Triticum aestivum). New Zeal J Crop Hort 36:1–7. https://doi.org/10.1080/01140670809510216
Dang F, Wang W-X (2011) Antagonistic interaction of mercury and selenium in a marine fish is dependent on their chemical species. Environ Sci Technol 45:3116–3122. https://doi.org/10.1021/es103705a
Dehn LA, Follmann EH, Thomas DL, Sheffield GG, Rosa C, Duffy LK, O'Hara TM (2006) Trophic relationships in an Arctic food web and implications for trace metal transfer. Sci Total Environ 362:103–123. https://doi.org/10.1016/j.scitotenv.2005.11.012
Del Rio-Salas M, Martínez-Durazo A, Castro-Longoria R, Jara-Marini ME (2019) Temporal variabilities of δ13C and δ15N in an ecosystem from the Central-East Gulf of California: estimating trophic relationships. Aquat Ecosyst Health Manag 22(1):40–52. https://doi.org/10.1080/14634988.2018.1547581
Escobar-Sánchez O, Galván-Magaña F, Rosíles-Martínez R (2011) Biomagnification of mercury and selenium in blue shark Prionace glauca from the Pacific Ocean of Mexico. Biol Trace Elem Res 144:550–559. https://doi.org/10.1007/s12011-011-9040-y
Fang T, Lu W, Cui K, Li J, Yang K, Zhao X, Liang Y, Li H (2019) Distribution, bioaccumulation and trophic transfer of trace metals in the food web of Chaohu Lake, Anhui, China. Chemosphere 218:1122–1130. https://doi.org/10.1016/j.chemosphere.2018.10.107
Fischer W, Krupp W, Schneider W, Sommer C, Carpenter KE, Niem VH (1995a) Plantas e invertebrados. Guía FAO para la identificación de especies para los fines de la pesca. vol. I. Organización de las Naciones Unidas para la Agricultura y la Alimentación. FAO, Roma, pp 1–646
Fischer W, Krupp W, Schneider W, Sommer C, Carpenter KE, Niem VH (1995b) Vertebrados. Guía FAO para la identificación de especies para los fines de la pesca. vol. II. Organización de las Naciones Unidas para la Agricultura y la Alimentación, Roma, pp 646–1200
Fischer W, Krupp W, Schneider W, Sommer C, Carpenter KE, Niem VH (1995c) Vertebrados. Guía FAO para la identificación de especies para los fines de la pesca. vol. III. Organización de las Naciones Unidas para la Agricultura y la Alimentación, Roma, pp 1201–1813
Fitzgerald WF, Lamborg CH (2005) Geochemistry of mercury in environment. In: Lollar S, Holland HD, Turekian KK (eds) Environmental Geochemistry. Treatise of Geochemistry. Elsevier-Pergamon, Oxford, pp 108–148
Garousi F (2017) Toxicity of selenium, application of selenium in fertilizers, selenium treatment of seeds, and selenium in edible parts of plants. Acta Univ Sapientiae Aliment 10:61–74. https://doi.org/10.1515/ausal-2017-0004
Glantz SA (2002) Alternatives to analysis of variance and the t-test based in ranks. In: Primer of biostatistics. McGraw-Hill, USA, pp 351–385
Gortáres-Moroyoqui P, Castro-Espinoza L, Naranjo JE, Karpiscak MM, Freitas RJ, Gerba CP (2011) Microbiological water quality in a large irrigation system: El Valle del Yaqui, Sonora México. J Environ Sci Health Part A 46:1708–1712. https://doi.org/10.1080/10934529.2011.623968
Gupta PK, Aggarwal M (2007) Toxicity of fungicides. In: Gupta RC (ed) Veterinary Toxicology. Elsevier, New York, pp 587–601
Haris H, Aris AZ, Mokhtar MB (2017) Mercury and methylmercury distribution in the intertidal surface sediment of a heavily anthrophogenically impacted saltwater mangrove-sediment interplay zone. Chemosphere 166:323–333. https://doi.org/10.1016/j.chemosphere.2016.09.045
Hobson KA, Welch HE (1992) Determination of trophic relationship within a high arctic marine food web using δ13C and δ15N analysis. Mar Ecol Prog Ser 84:9–18
Huerta-Diaz MA, Morse JW (1990) A quantitative method for determination of trace metal concentrations in sedimentary pyrite. Mar Chem 29:119–144. https://doi.org/10.1016/0304-4203(90)90009-2
INEGI (2015) Instituto Nacional de Estadística y Geografía (México). Anuario estadístico y geográfico por entidad federativa 2015. Ciudad de México. 723 p
Jara-Marini ME, Molina-García A, Martínez-Durazo Á, Páez-Osuna F (2020) Trace metal trophic transference and biomagnification in a semiarid coastal lagoon impacted by agriculture and shrimp aquaculture. Environ Sci Pollut Res 27:5323–5336. https://doi.org/10.1007/s11356-019-06788-2
Jara-Marini ME, Soto-Jiménez M, Páez-Osuna F (2009) Trophic relationships and transference of cadmium, copper, lead and zinc in a subtropical coastal lagoon food web from SE Gulf of California. Chemosphere. 77:1366–1373. https://doi.org/10.1016/j.chemosphere.2009.09.025
Jara-Marini ME, Soto-Jiménez M, Páez-Osuna F (2012) Mercury transfer in a subtropical coastal lagoon food web (SE Gulf of California) under two contrasting climatic conditions. Environ Toxicol 27:526–536. https://doi.org/10.1002/tox.20670
Jara-Marini ME, Tapia-Alcaraz JN, Dumer-Gutiérrez JA, García-Rico L, García-Hernández J, Páez-Osuna F (2013a) Comparative bioaccumulation of trace metals using six filter feeder organisms in a coastal lagoon ecosystem (of the central-east Gulf of California). Environ Monit Assess 185:1071–1085. https://doi.org/10.1007/s10661-012-2615-z
Jara-Marini ME, Tapia-Alcaraz JN, Dumer-Gutiérrez JA, García-Rico L, García-Hernández J, Páez-Osuna F (2013b) Distribution and accumulation of Cd, Cu, Hg, Pb and Zn in the surface sediments of El Tobari Lagoon, central-East Gulf of California: an ecosystem associated with agriculture and aquaculture activities. J Environ Sci Health Part A 48:1842–1851. https://doi.org/10.1080/10934529.2013.823341
Jiang Y, Zeng ZH, Bu Y, Ren CZ, Li JZ, Han JJ, Tao C, Zhang K, Wang XX, Lu GX, Li YJ, Hu YG (2015) Effects of selenium fertilizer on grain yield, Se uptake and distribution in common buckwheat (Fagopyrum esculentum Moench). Plant Soil Environ 61:371–377. https://doi.org/10.17221/284/2015-PSE
Kehrig HA, Seixas TG, Malma O, Di Beneditto APM, Rezende CE (2013) Mercury and selenium biomagnification in a Brazilian coastal food web using nitrogen stable isotope analysis: a case study in an area under the influence of the Paraiba do Sul River plume. Mar Pollut Bull 75:283–290. https://doi.org/10.1016/j.marpolbul.2013.06.046
Khan MAK, Wang FY (2009) Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environ Toxicol Chem 28:1567–1577
Lacerda LD, Soares TM, Costa BGB, Godoy MDP (2011) Mercury emission factors from intensive shrimp aquaculture and their relative importance to the Jaguaribe River Estuary, NE Brazil. Bull Environ Contam Toxicol 87(6):657–661. https://doi.org/10.1007/s00128-011-0399-4
Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, Campbell LM (2013) Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environ Sci Technol 47:13385–13394. https://doi.org/10.1021/es403103t
Loring DH, Rantala RTT (1992) Manual for the geochemical analyses of marine sediments and suspended particulate matter. Earth Sci Rev 32:235–283
Lyle-Fritch ML, Romero-Beltrán E, Páez-Osuna F (2006) A survey on use of chemical and biological products in shrimp farming from Sinaloa (NW Mexico). Aquac Eng 35:135–146. https://doi.org/10.1016/0012-8252(92)90001-A
Mackay D, Celsie AKD, Arnot JA, Powell DE (2016) Processes influencing chemical biomagnification and trophic magnification factors in aquatic ecosystems: implications for chemical hazard and risk assessment. Chemosphere 154:99–108. https://doi.org/10.1016/j.chemosphere.2016.03.048
Martínez-Durazo A, García-Hernández J, Páez-Osuna F, Soto-Jiménez MF, Jara-Marini ME (2019) The influence of anthropogenic organic matter and nutrient inputs on the food web structure in a coastal lagoon receiving agriculture and shrimp farming effluents. Sci Total Environ 664:635–646. https://doi.org/10.1016/j.scitotenv.2019.01.343
McCullough EB, Matson PA (2016) Evolution of the knowledge system for agricultural development in the Yaqui Valley, Sonora, Mexico. Proc Natl Acad Sci 113:4609–4614. https://doi.org/10.1073/pnas.1011602108
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between 15N and animal age. Geochim Cosmochim Acta 48:1135–1140. https://doi.org/10.1016/0016-7037(84)90204-7
NCSS (2007) Number Cruncher Statistical Software. Statistical System for Windows, Utah
Økelsrud A, Lydersen E, Fjeld E (2016) Biomagnification of mercury and selenium in two lakes in southern Norway. Sci Total Environ 566–567:596–607. https://doi.org/10.1016/j.scitotenv.2016.05.109
Ordiano-Flores A, Rosíles-Martínez R, Galván-Magaña F (2012) Biomagnification of mercury and its antagonistic interaction with selenium in yellowfin tuna Thunnus albacares in the trophic web of Baja California Sur, Mexico. Ecotoxicol Environ Saf 86:182–187. https://doi.org/10.1016/j.ecoenv.2012.09.014
Ouédraogo O, Chételat J, Amyot M (2015) Bioaccumulation and trophic transfer of mercury and selenium in African Sub-Tropical fluvial reservoirs food webs (Burkina Faso). PLoS One 10(4):e0123048. https://doi.org/10.1371/journal.pone.0123048
Páez-Osuna F, Álvarez-Borrego S, Ruiz-Fernández AC, García-Hernández J, Jara-Marini ME, Bergés-Tiznado ME, Piñón-Gimate A, Alonso-Rodríguez R, Soto-Jiménez MF, Frías-Espericueta MG, Ruelas-Inzunza JR, Green-Ruiz CR, Osuna-Martínez CC, Sanchez-Cabeza JA (2017) Environmental status of the Gulf of California: a pollution review. Earth Sci Rev 166:181–205. https://doi.org/10.1016/j.earscirev.2017.01.014
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5(3):e9672. https://doi.org/10.1371/journal.pone.0009672
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods and assumptions. Ecology 83:703–718. https://doi.org/10.2307/3071875
Power M, Klein GM, Guigue KRRA, Kwan MKH (2002) Mercury accumulation in the fish community of a sub-Arctic lake in relation to trophic position and carbon sources. J Appl Ecol 39:819–830. https://doi.org/10.1046/j.1365-2664.2002.00758.x
Ralston NVC, Kaneko JJ, Raymond LJ (2019) Selenium health benefit values provide a reliable index of seafood benefits vs. risks. J Trace Elem Med Biol 55:50–57. https://doi.org/10.1016/j.jtemb.2019.05.009
Rauret G, Rubio R, López-Sánchez JF, Cassasas E (1988) Determination and speciation of cooper of a Mediterranean river (River Tenes, Catalonia, Spain). Water Res 22(4):449–455. https://doi.org/10.1016/0043-1354(88)90039-5
Reinfelder JR, Fisher NS, Luoma SN, Nichols JW, Wang WX (1998) Trace element trophic transfer in aquatic organisms: a critique of the kinetic model approach. Sci Total Environ 219:117–135. https://doi.org/10.1016/S0048-9697(98)00225-3
Santos S, Ungureanu G, Boaventura R, Botelho C (2015) Selenium contaminated waters: an overview of analytical methods, treatment options and recent advances in sorption methods. Sci Total Environ 521–522:246–260. https://doi.org/10.1016/j.scitotenv.2015.03.107
Sasakura C, Suzuki KT (1998) Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J Inorg Biochem 71:159–162. https://doi.org/10.1016/S0162-0134(98)10048-X
Seixas TG, Moreira I, Siciliano S, Malm O, Kehrig HA (2014) Mercury and selenium in tropical marine plankton and their trophic successors. Chemosphere 111:32–39. https://doi.org/10.1016/j.chemosphere.2014.03.003
Sibley DA (2000) The Sibley Guide to Birds. National Audubon Society. Alfred A. Knopf Inc., United States
Silva E, Viana ZCV, Onofre CRE, Korn MGA, Santos VLCS (2016) Distribution of trace elements in tissues of shrimp species Litopenaeus vannamei (Boone, 1931) from Bahia, Brazil. Braz J Biol 76(1):194–204. https://doi.org/10.1590/1519-6984.17114
Sørmo EG, Ciesielski TM, Overjordet LB, Lierhagen S, Eggen GS, Berg T, Jenssen BM (2011) Selenium moderates mercury toxicity in free-ranging freshwater fish. Environ Sci Technol 45:6561–6566. https://doi.org/10.1021/es200478b
Stewart AR, Grosell M, Buchwalter D, Fisher N, Luoma S, Mathews T, Orr P, Wang W-X (2010) Bioaccumulation and trophic transfer of selenium. In: Chapman PM, Adams WJ, Brooks ML, Delos CG, Luoma SN, Maher WA, Ohlendorf HM, Presser TS, Shaw DP (eds) Ecological Assessment of Selenium in the Aquatic Environment. Taylor & Francis CRC Press. Pensacola, Florida, pp 93–139
Stewart AR, Luoma SN, Schlekat CE, Doblin MA, Hieb KA (2004) Food web pathway determines how selenium affects aquatic ecosystems: a San Francisco Bay case study. Environ Sci Technol 38:4519–4526. https://doi.org/10.1021/es0499647
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182. https://doi.org/10.1007/s00442-003-1270-z
Wang WX (2002) Interactions of trace metals and different marine food chains. Mar Ecol Prog Ser 243:295–309. https://doi.org/10.3354/meps243295
Wang W-X, Wong RSK, Wang J, Yen Y-F (2004) Influences of different selenium species on the uptake and assimilation of Hg(II) and methylmercury by diatoms and green mussels. Aquat Toxicol 68:39–50. https://doi.org/10.1016/j.aquatox.2004.02.003
Wang L, Zhang L, Ma X, Wang LZ, Xing GZ, Yang L, Yu T, Lu L, Liao XD, Li SF, Luo XG (2019) A survey on distribution of selenium contents in feedstuffs for livestock and poultry in China. Sci Agric Sin 52(11):2011–2020
Acknowledgements
The authors would like to thank G. Leyva-García, D. Aguilera-Márquez, A. Martínez-Durazo, and M. del Río-Salas for their technical support in sample collection and preparation. We also thank Dr. Chris Eastoe and his work group of the Environmental Isotope Laboratory of the Department of Geosciences of the University of Arizona at Tucson, AZ, USA, for the use of their facilities for the stable isotope analyses.
Funding
This study was funded by the Consejo Nacional de Ciencia y Tecnología de México (grant number CONACYT CB-2008-C01-103522).
Author information
Authors and Affiliations
Contributions
AMG sampled, processed, and analyzed sediment and biota samples. JGH designed the sampling and sampled, processed, and analyzed avifauna samples. MFSJ interpreted stable isotopes data. FPO interpreted Hg and Se data. MEJM designed the sampling, processed and analyzed sediment and biota samples, performed the dataset, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors. Consent to participate is “Not applicable.”
Consent to participate or publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: V. V.S.S. Sarma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Molina-García, A., García-Hernández, J., Soto-Jiménez, M.F. et al. Mercury and selenium biomagnification in a coastal food web from the Gulf of California influenced by agriculture and shrimp aquaculture. Environ Sci Pollut Res 28, 56175–56187 (2021). https://doi.org/10.1007/s11356-021-14524-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14524-y