Abstract
In the present study, a hydroponic experiment was performed to evaluate the effect of exogenous silicon (Si) and methyl jasmonate (MeJA) on the mitigation of Cd toxicity in tomato seedlings. The results revealed that Cd-stressed plants exhibited growth inhibition, increased lipid peroxidation, and impaired photosynthetic pigment accumulation. However, Si and MeJA applied alone or in combination significantly ameliorated the above-mentioned adverse effects induced by Cd. Among all treatments, Cd+Si+MeJA treatment elevated the dry mass of roots, stems, and leaves by 317.39%, 110.85%, and 119.71%, respectively. The chlorophyll a, chlorophyll b, and carotenoid contents in Cd+Si+MeJA-treated group were dramatically elevated (p < 0.05). Meanwhile, the malondialdehyde content in roots and shoots were reduced by 32.24% and 69.94%, respectively. The Si and MeJA applied separately or in combination also resulted in a prominent decrease of Cd influxes in tomato roots; therefore, a reduction of Cd content in tomato tissues were detected, and the Cd concentration in tomato roots were decreased by 27.19%, 25.18%, and 17.51% in Cd+Si, Cd+MeJA and Cd+Si+MeJA-treated plants, respectively. Moreover, in Cd+Si+MeJA-treated group, the percentage of Cd in cell wall fraction was enhanced while that in organelle fraction was decreased as compared with Cd-stressed plants. Collectively, our findings indicated that Si and MeJA application provide a beneficial role in enhancing Cd tolerance and reducing Cd uptake in tomato plants.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a divalent metallic element in nature; it is not an essential metal element for the growth and development of living organisms; instead, it is hazardous and an excessive amount of Cd is known to be toxic to various plants, animals, and microorganisms (Guo et al. 2020; Noor et al. 2020; Muhammad et al. 2021). However, due to increased mining activities, excessive usage of fertilizers and pesticides, and wastewater irrigation, Cd contamination of soil has become a serious environmental problem all over the world (Wei et al. 2018; Muhammad et al. 2021). Cd in soil not only disrupts the function of the soil ecosystem but also disturbs the metabolic, physiological, and biochemical processes in plants, thus leads to growth inhibition, photosynthetic rate decline, oxidative injury, metabolic disturbance, nutrient deficiency, and yield and quality reduction (Andresen and Küpper 2013; Guo et al. 2020; Wei et al. 2021). Moreover, Cd-contaminated crops pose serious food safety problems and health threats worldwide. Excessive Cd uptake induces serious diseases (including cardiovascular disease, heart disease, cancer, etc.) in human since Cd is a mutagenic and carcinogenic element (Houston 2007; Shi et al. 2018). Therefore, it is urgent to increase Cd tolerance and decrease Cd content in food crops. Various exogenous substances have been applied to mitigate Cd toxicity, including phytohormones and mineral elements such as silicon (Si) and selenium (Se) (Lin et al. 2012; Liu et al. 2017; Kaya et al. 2018; Wei et al. 2018; Jia et al. 2020).
Methyl jasmonate (MeJA), a derivative of jasmonic acid (JA), is an important phytohormone in plants (Cohen and Flescher 2009; Zhao et al. 2019). It participates in diverse physiological and biochemical processes in plants, including seed germination, metabolic regulation, defence response, and cell communication (Fahad et al. 2014a; Singh and Shah 2014; Dar et al. 2015; Wasternack and Strnad 2017). Several indicated that MeJA plays a key role in enhancing tolerance against various biotic and abiotic stresses, such as drought, salinity, UV radiation, osmotic shock, pathogens, and high-temperature stress (Cohen and Flescher 2009; Gaige et al. 2010; Noriega et al. 2012; Fahad et al. 2014b; Fahad et al. 2016a, b). Moreover, increasing evidence indicated that MeJA is implicated in defence response against heavy metal stress. It enhanced antioxidant enzyme activities in Vaccinium corymbosum under Al stress (Ulloa-Inostroza et al. 2017). MeJA is also observed to elevate heavy metal tolerance and plant growth by regulating the production of secondary metabolites and the expression of heavy metal resistance-related genes (Chen et al. 2014; Farooq et al. 2016).
Silicon (Si) is a beneficial quasi-essential mineral nutrient for plants. It not only plays a role in the regulation of nutrient balance in plants but also has the capability to protect plants from various biotic and abiotic stresses (Farooq and Dietz 2015). Si could protect plants against heavy metal toxicity via decreasing heavy metal uptake, root-to-shoot transport, lipid peroxidation, increasing nutrient element uptake, antioxidant capacity, and maintaining the morphological and anatomical features of plants (Farooq and Dietz 2015; Rahman et al. 2017, Wu et al. 2016). Si has been observed to mitigate heavy metal toxicity in a number of plants species, including rice, wheat, pea, and maize (Sumira et al. 2018; Wu et al. 2019; Nwugo and Huerta 2010; Liu et al. 2020). The protective effects of Si are plant-specific and correlate with the concentrations of Si in plant tissue (Bhat et al. 2019).
Tomato (Solanum lycopersicum L.), one of the most important vegetable crops with high nutritive value, is widely cultivated all over the world. However, the excessive amount of Cd in soil impaired the yield and quality of this economically important crop. Few investigations have shown that MeJA and Si are involved in the amelioration of heavy metal stress; however, whether and how MeJA, Si, or their combination alleviate Cd stress in tomato plants are still unknown. Therefore, in the present study, a hydroponic experiment was carried out to reveal the effects and possible underlying mechanisms of MeJA, Si, and their combination in alleviating Cd phytotoxicity in tomato plants. This study may provide a feasible way of reducing Cd content and toxicity in tomato plants
Materials and methods
Hydroponic experiment
The hydroponic experiment was performed in a growth chamber at Shaanxi University of Science and Technology, Xi’an, China (34° 22′ 44′′ N, 108° 58′ 20′′ E). Tomato seeds were sequentially sterilized with 3% NaOCl solution and rinsed with ultrapure water; healthy seeds were selected for germination in the growth chamber with the day and night temperature set up at 26 °C and 20 °C, respectively. The three-leaf stage seedings were used to cultivate in hydroponic pots (1.0 L) filled with 1/4 hoagland’s nutrient solution. The photoperiod was 16 h light and 8 h dark. The 6-week-old seedlings with similar size were transferred to 1/2 hoagland’s solution for 3 days and then subjected to the treatments as follow: (1) CK: control plants without treatment; (2) Cd: 2 mg/L Cd; (3) Si: 0.5 mmol/L Si; (4) Cd+Si: 2 mg/L Cd+0.5 mmol/L Si; (5) MeJA: 2.5 μmol/L MeJA; (6) Cd+MeJA: 2 mg/L Cd+2.5 μmol/L MeJA; (7) Cd+Si+MeJA: 2 mg/L Cd+0.5 mmol/L Si +2.5 μmol/L MeJA. Cd and Si were directly added into the nutrient solution in the form of CdCl2 and Na2SiO3, respectively. MeJA was foliar sprayed (8 mL for each plant) on the tomato leaves 24 h prior to Si and Cd treatment. The nutrient solution was refreshed every 2 days. Seven days after Cd treatment, plant samples were collected and separated into roots, stems, and leaves; part of the samples were rapidly frozen and stored at – 80 °C for physiological parameter analysis, another portion of the samples were washed with Na2EDTA solution (5 mmol/L) to remove surface bound Cd and then dried and subjected for Cd content analysis. The whole experiment was repeated three times.
Determination of plant growth parameters
Fresh plant samples were employed to determine the root length and shoot height; samples were separated into roots, stems, leaves, and the fresh weight was recorded; afterward, these samples were dried in an oven till constant weight obtained and subjected for the measurement of dry weight. For each treatment, 12 plants were employed for the assessment of the above-mentioned growth parameters.
Cadmium tolerance index
Cadmium tolerance index was evaluated as described by Bali et al. (2019).
Cd tolerance index (%) = (Dry weight of treated plants/dry weight of untreated plants) ×100
Assessment of chlorophyll and carotenoid contents
The content of photosynthetic pigment was measured as described by Bali et al. (2019) with slight modification. Fresh leaf tissue (1.0 g) was homogenized with 4 mL ice-cold acetone (80%) for the extraction of pigments, including chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid (Car). The resulting homogenates were spinned in a centrifuge at 4 °C for 20 min with a rotating speed of 12,000 g. The obtained supernatant was used for measuring the absorbance at 663 nm, 646 nm, and 470 nm using a UV/V is spectrophotometer (EU-2200R, Onlab, China).
Malondialdehyde content analysis
The malondialdehyde (MDA) content was determined as described by Farooq et al. (2018).
Cadmium content analysis
Extraction and measurement of Cd concentration in tomato roots, stems, and leaves were carried out as described by Wei et al. (2018). Briefly, the dried samples were finely grounded, weighted, and transferred to digestion tubes and digested with HNO3; the digests were filtered through a 0.45 μm membrane and subsequently subjected to Cd content analysis using an atomic absorption spectroscopy (Analytik Jena AG, Germany).
Determination of net Cd2+ fluxes
The non-invasive microsensing system (NMT100-SIM-XY/YG; Younger USA, LLC, MA, USA) was employed for the determination of net Cd2+ fluxes according to the process described previously (Jia et al. 2020; Chen et al. 2020). Briefly, the commercial silanized glass micropipettes were filled with a solution containing 0.1 mM KCl and 10 mM Cd (NO3)2, then the tips of the micropipettes were filled with 45 μL commercially available ion-selective liquid exchanger to prepare the sensors. The sensors were calibrated prior to net Cd2+ fluxes assessment. The 15-day-old tomato seedlings were treated with the designated concentration of MeJA; 24 h later, the plants were fixed on a dish by resin piece and incubated in the test solution (0.1 mM KCl, 0.05 mM CdCl2, 0.3 mM MES, pH 5.8) in the presence or absence of Si for half an hour and then used for Cd fluxes measurement.
Subcellular distribution of Cd
The subcellular distribution of Cd was investigated by following the protocol of Li et al. (2019). In brief, root and shoot samples (1.0 g) were homogenized in 20 mL extraction buffer (50 mM Tris-HCl (pH 7.5), 250 mM sucrose, and 1 mM DTT) on ice. The homogenates were centrifuged at 300 g for 10 min; the deposition was designated as cell walls fraction, the supernatant was subjected to centrifugation at 15,000 g for 40 min, and the newly obtained supernatant and precipitate were identified as soluble fraction (cytoplasm) and organelle fraction, respectively. The cell wall fraction and organelle fraction were dried and subsequently digested with HNO3. The Cd content in each fraction was measured using an atomic absorption spectrometer (AAS, Analytik Jena AG, Germany).
Statistical analysis
Data were statistically analyzed using the SPSS 21.0 for Windows. One-way ANOVA and Turkey’s post hoc analysis were employed to evaluate the significant differences of tested parameters among treatments (p < 0.05).
Results
Effects of Si and MeJA on growth of Cd-stressed tomato plants
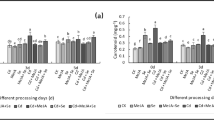
The growth parameters, including root and shoot length and fresh and dry mass of tomato seedlings were analyzed (Fig. 1). Cd exposure significantly repressed plant growth as evidenced by the reduction of all tested growth parameters (p < 0.05); among them, the root length and shoot length decreased by 19.29% and 6.87%, respectively (Fig. 1a, b). The fresh and dry weight of roots, stems, and leaves decreased by 61.41, 20.67%, 48.26%, and 28.13%, 37.38%, 33.55%, respectively (Fig. 1c, 1d). Si, MeJA, as well as their combination rescued the growth suppression caused by Cd. Compared with Cd-stressed plants, the root length of Cd+Si, Cd+MeJA, and Cd+Si+MeJA-treated plants increased by 23.37%, 54.41%, and 69.73%, respectively (Fig. 1a). Meanwhile, the fresh weight and dry weight of tomato plants in the above-mentioned groups were significantly enhanced (p < 0.05), especially in Cd+Si+MeJA-treated plants, the fresh and dry weight of roots, stems, and leaves were elevated by 291.77%, 67.06%, and 175.98%, 317.39%, 110.85%, and 119.71%, respectively (Fig. 1c, d), indicating that MeJA and Si play a synergistic effect in promoting the growth of tomato plants under Cd stress.
Effects of Si and MeJA on Cd tolerance index of tomato plants
A tolerance index of 100% in the control plants indicated the absence of Cd-induced adverse effects on tomato plants. In Cd-stressed tomato seedlings, the tolerance index was decreased to 50.55% (Fig. 2). Interestingly, the Cd tolerance index of Cd+Si, Cd+MeJA, and Cd+Si+MeJA-treated plants were significantly higher than that of the Cd-stressed plants (p < 0.05); especially in Cd+Si+MeJA-treated group, the Cd tolerance index reached 117.62%, indicating that Si and MeJA application significantly stimulated plant tolerance against Cd stress.
Effects of Si and MeJA on chlorophyll and carotenoid content of tomato plants
Cadmium exposure reduced the Chl a, Chl b, and Car content by 9.44%, 11.73%, and 40.32% as compared with the corresponding Cd-free plants (Fig. 3). Compared with Cd-stressed tomato seedlings, Cd+Si, Cd+MeJA, and Cd+Si+MeJA treatments elevated the Chl a, Chl b, and Car level by 15.98%, 19.17%, and 68.88%; 10.29%, 14.16%, and 55.15%; and 25.36%, 26.46%, and 77.85%, respectively. Indicating that Si, MeJA, and their combination could relieve the Cd-triggered damage to photosynthetic pigment in tomato plants.
Effects of Si and MeJA on MDA content of tomato plants
Malondialdehyde is a sensitive indicator of lipid peroxidation. The addition of Cd in the nutrient solution increased the MDA level in tomato shoots and roots by 64.50% and 10.7%, respectively (Fig. 4). Si and MeJA addition significantly mitigated the lipid peroxidation induced by Cd (p < 0.05), as evidenced by the lower MDA content detected in Cd+Si, Cd+MeJA, and Cd+Si+MeJA-treated plants; the MDA in shoots and roots were reduced by 37.61% and 11.83%, 66.19% and 29.19%, and 69.94% and 32.24%, respectively. Among all treatments, the combined application of Si and MeJA was more effective in alleviating lipid peroxidation.
Effects of Si and MeJA on Cd content and total Cd accumulation in tomato plants
Under all treatments, the highest Cd content was observed in tomato roots while that in stems and leaves were much lower (Fig. 5). Si application reduced the Cd level in roots, stems, and leaves by 27.19%, 37.40%, and 10.12%. Similarly, exogenous MeJA alone or in combination with Si also substantially reduced the Cd concentration in roots and leaves of tomato seedlings; the decreasing rate were 25.18% and 40.64%, 17.51% and 35.70%, respectively, indicating that Si and MeJA are implicated in the regulation of Cd content in tomato plants.
Effects of Si and MeJA on net Cd2+ fluxes of tomato roots
Cadmium treatment resulted in a vigorous Cd2+ inflow into tomato roots, with an average influx rate of 11.33 pmol cm−2 s−1 (Fig. 6a, b). Si and MeJA application significantly reduced the influx of Cd2+ (p < 0.05), and the average net Cd2+ influxes of Cd+Si, Cd+MeJA, and Cd+Si+MeJA-treated tomato plants were 3.73, 7.47, and 5.83 pmol cm−2 s−1, respectively. Compared with Cd-stressed plants, the average Cd2+ influx rate decreased by 67.08%, 34.07%, and 48.54%, respectively.
Effects of Si and MeJA on subcellular localization of Cd
The subcellular distribution of Cd in tomato roots and shoots were analyzed (Fig. 7). Generally, Cd was more abundant in cell wall fraction (34.92–67.66%) and soluble fraction (24.76–60.62%) than in organelle fraction (2.39–8.63%). In root tissues of Cd-stressed plants, the Cd compartmentalized in the cell wall fraction accounted for 34.92% of the total; Cd+Si, Cd+MeJA, and Cd+Si+MeJA treatment increased the cell wall-bound Cd to 38.98%, 41.02%, and 44.77%; meanwhile, the proportion of Cd in the organelle fraction was decreased from 5.04 to 3.26%, 2.41% and 2.39%, respectively (Fig. 7a). In tomato shoots, 48.44% of Cd was distributed in cell wall of Cd-stressed plants. Cd+MeJA and Cd+Si+MeJA treatments significantly enhanced the Cd proportion in cell wall fraction (p < 0.05), especially in Cd+Si+MeJA-treated tomato seedlings, the Cd in cell wall was increased to 67.66% (Fig. 7b).
Discussion
Exogenous Si and MeJA promoted the growth of tomato plants under Cd stress
Plants are sensitive to heavy metal stress, especially to those highly toxic ones, such as Cd. Cd induces growth inhibition in various plants via disturbing cell division, disrupting photosynthesis, inducing oxidative stress, interfering hormonal homeostasis, and nutrient uptake (Rizwan et al. 2018; Zhang et al. 2020; Huang et al. 2020; Dobrikova et al. 2021). In this study, Cd exposure significantly inhibited the growth of tomato seedlings, as evidenced by the reduced shoot length, root length, fresh weight, and dry weight (Fig. 1). However, the inhibitory effect was compensated by exogenous application of Si, MeJA, or their combination. Enhancement of plant growth by Si application has been demonstrated previously (Chen et al. 2019; Huang et al. 2020). Si is a beneficial element to plants; it could stimulate the uptake and utilization of other nutrient elements (Emamverdian et al. 2018). Besides, it might be involved in cell elongation and cell division (Korndörfer and Lepsch 2001), and could alleviate heavy metal toxicity and enhance heavy metal resistance (Wu et al. 2015; Huang et al. 2020), thus promoted the plant growth under stressed conditions. Previous studies indicated that MeJA could attenuate heavy metal-induced growth inhibition by changing the antioxidant enzyme activity (Yan et al. 2013), modulating the ascorbate-glutathione cycle (Farooq et al. 2018), elevating photosynthetic pigment content (Keramat et al. 2010), and depressing metal ion uptake (Singh and Shah 2014). Moreover, MeJA application may regulate the level of other internal hormones, such as ethylene and gibberellin, thus directly or indirectly enhanced the plant growth under Cd stress (Fahad et al. 2016c).
Exogenous Si and MeJA increased photosynthetic pigment accumulation under Cd stress
Suppression of plant growth by Cd is a direct consequence of alterations in photosynthetic rate and photosynthetic pigment content. In the current study, the concentration of Chl a, Chl b, and Car in tomato leaves was dramatically reduced upon Cd stress (Fig. 3). The reduced pigment content was attributed to the impaired chloroplast ultrastructure and chlorophyll metabolism in tomato plants (Piotrowska-Niczyporuk et al. 2012), suppressed level of enzymes that are involved in pigment biosynthesis (Keramat et al. 2010), perturbed Mg2+ and Fe2+ uptake that are crucial for chlorophyll synthesis (Khudsar and Mahmooduzzafar 2001), or the degradation of pigments by excessive ROS (Fahad et al. 2016d). Interestingly, compared with the Cd-stressed plants, the addition of Si, MeJA, and their combination enhanced the chlorophyll and carotenoids content. Previous studies indicated that appropriate concentration of MeJA exerts a positive role in terms of photosynthetic pigment accumulation under toxic metal stress (Chen et al. 2014; Rocha et al. 2013; Ahmed et al. 2015). Ueda and Saniewski (2006) suggested that MeJA could enhance the gene expression of some key enzymes involved in photosynthetic pigment synthesis. Si supply also exerts a beneficial effect in terms of photosynthetic pigment accumulation in various plants, including sorghum (Ahmed et al. 2014), tobacco (Lu et al. 2017), and cucumber (Feng et al. 2010). It changed the chloroplast structure and alleviated Cd stress to photosynthetic system in maize (Cunha and Nascimento 2009). Si could also stimulate the uptake of Mg2+ and Fe2+ and promote pigment synthesis (Greger et al. 2018). Moreover, exogenous Si induced a decline of MDA content in tomato shoots (Fig. 4), indicating that the peroxidation of chloroplast membrane was relieved; consequently, the degradation of pigments was prohibited.
Exogenous Si and MeJA alleviated lipid peroxidation induced by Cd
Exposure to heavy metals usually leads to oxidative stress in plants due to the overproduction of reactive oxygen species (ROS, including H2O2, ·OH, and O2−). These substances are toxic and highly reactive; they could oxidize biological macromolecules, such as proteins, carbohydrates, DNA, and lipids (Gill and Tuteja 2010). Both cellular and organelle membranes could be oxidated. Peroxidation of polyunsaturated fatty acids in membrane leads to the production of MDA, thus MDA usually acts as a marker of lipid peroxidation and lipid damage (Gawe et al. 2004). In the current study, Cd exposure elevated the MDA level in tomato seedlings (Fig. 4). MeJA, Si, as well as MeJA+Si application significantly reduced the MDA content in Cd-stressed plants, indicating that treatment with these substances alleviated the oxidative stress to cell membranes and intracellular membranes in tomato plants. Previous study indicated that Si application attenuated the oxidative stress in Cd-stressed Brassica napus L. via modulating AsA-GSH pathway (Hasanuzzaman et al. 2017). Similarly, Zhu et al. (2004) reported that Si-induced enhancement of Cd tolerance was associated with the increase of antioxidant defense response. Exogenous MeJA was reported to decrease the MDA content in Cd-stressed plants by enhancing the activities of APX, CAT, and SOD (Enteshari and Delavar 2011). MeJA also increased the accumulation of proline, glutathione, total phenols and flavonoids (Yan et al. 2015; Ulloa-Inostroza et al. 2017), thus enhanced the capability of plants to counteract ROS and reduced the production of MDA. Taken together, Si and MeJA-mediated Cd toxicity to lipid membrane was likely associated with the enhanced antioxidative capacity.
Exogenous Si and MeJA reduced Cd content in tomato plants
Cadmium in soil or nutrient solution could be readily uptake by plant roots and transfer to various plant tissues. In this study, the highest Cd concentration was detected in tomato roots while that in stems and leaves was much lower. Si, MeJA, and their combination significantly decreased the Cd concentration in roots and stems of tomato seedlings (Fig. 5). The reduced Cd2+ influx rate of tomato roots contributed to the reduced level of Cd in Cd+Si, Cd+MeJA, and Cd+Si+MeJA-treated tomato plants (Fig. 6). Toxic heavy metals compete with cations and get access into the root cell via the cation (Ca2+, Zn2+, Fe2+, Mn2+, Mg2+) transporters; it is likely that MeJA and Si application improved the physiological status of the tomato seedlings and enhanced the uptake of beneficial cations, thus depressed the Cd2+ influxes and Cd content in tomato plants (Sumira et al. 2018). Besides, Si and MeJA could modulate the expression of heavy metal transportation and detoxification-related genes, thus interfere with Cd uptake and translocation in plants (Ma et al. 2015; Greger et al. 2016; Lei et al. 2019). Moreover, Si deposited on the cell wall of plant roots and increased the strength and rigidity of cell walls, provided more metal biding site for Cd and reduced apoplastic by pass flow of metal ion, thus decreased Cd uptake (Ma and Yamaji 2006; Liu et al. 2013). Similarly, a study by Farooq et al. (2013) suggested that Cd and Si might form an unknown complex on root surface, decrease the porosity of cell wall and prevent Cd from entering into root cell.
Exogenous Si and MeJA modulated Cd subcellular localization in tomato plants
Deleterious effects of Cd in plants are usually associated with its subcellular distribution. Our findings indicated that the majority of Cd was compartmentalized in cell wall and cytoplasm in Cd-treated plants (Fig. 7). Sequestration of metal ions in less active parts (cell wall and cytosolic fraction) is an effective strategy employed by various plants to counteract Cd toxicity (Zhou et al. 2017; Li et al. 2019). Cell wall acts as a protective barrier against heavy metals. It is rich in functional groups (amino, carboxyl, hydroxyl, phosphoryl, and sulfhydryl groups) that could bind to metal ions and prevent these toxic ions from entering into the cytoplasm (Das and Guha 2007; Li et al. 2019). In addition, the sequestration of heavy metal ions in vacuole (which is located in cytoplasm) also contributes to the alleviation of metal toxicity in plants (Yang et al. 2018). Sulfur-rich peptides as well as organic acids are prevalent in vacuole; they could chelate and sequester metal ions; therefore, the amount of free metal ions in the cell will be reduced and the toxic effect to cellular organelles will be alleviated (Xu et al. 2018). Application of Si and MeJA individually enriched the cell wall-bound Cd while decreased that in organelle fraction, and a synergistic effect was detected in Cd+Si+MeJA treated plants (Fig. 7). A number of studies have demonstrated that Si could change the subcellular distribution of metal ions (Shi et al. 2010; Vaculík et al. 2012; Zhang et al. 2014). It binds to the negatively charged cell wall component, such as hemicellulose and cellulose, and inhibit the Cd uptake by forming a complex with Cd and get deposited on the cell wall (Ma et al. 2015). MeJA application increased the amount of cellulose in cell wall of Brachypodium distachyon (Napoleo et al. 2017), cellulose is a known macromolecular polysaccharide that could bind Cd and prevent its entry into the cell, thus enriched the amount of Cd in cell wall fractions (Jia et al. 2021). Fixation of metal ions by cell wall is regarded as an effective strategy to alleviate Cd toxicity.
Conclusions
In summary, exogenous application of Si and MeJA alone or in combination attenuates the damage caused by Cd in tomato seedlings. Their application stimulated plant growth and Cd tolerance index, increased photosynthetic pigment content, and decreased MDA level. Besides, their treatment reduced Cd content in various tomato organs and the Cd2+ influxes into tomato roots were prohibited. Moreover, the Cd distributed in cell wall fraction was increased while that in organelle fraction was decreased when Si and MeJA applied. Taken together, Si and MeJA provide a protective role against Cd stress in tomato seedlings; this study might provide a new strategy to counteract Cd toxicity in tomato plants. However, further experiments will be required to test the effects of MeJA, Si and their combination against different abiotic stresses in tomato and other crops under field conditions.
Data availability
The datasets used in the current study are available from the corresponding author on reasonable request.
References
Ahmed M, Hassan FU, Asif M (2014) Amelioration of drought in sorghum (Sorghum bicolor L.) by Silicon. Commun Soil Sci Plan 45(4):470–486. https://doi.org/10.1080/00103624.2013.863907
Ahmed F, Fanning K, Netzel M, Schenk PM (2015) Induced carotenoid accumulation in Dunaliellasalina and Tetraselmissuecica by plant hormones and UV-C radiation. Appl Microbiol Biotechnol 99(22):9407–9416. https://doi.org/10.1007/s00253-015-6792-x
Andresen E, Küpper H (2013) Cadmium toxicity in plants. Met Ions Life Sci 11:395–413. https://doi.org/10.1007/978-94-007-5179-8_13
Bali S, Jamwal VL, Kohli SK, Kaur P, Tejpal R, Bhalla V, Ohri P, Gandhi SG, Bhardwaj R, Al-Huqail AA, Siddiqui MH, Ali HM, Ahmad P (2019) Jasmonic acid application triggers detoxification of lead (Pb) toxicity in tomato through the modifications of secondary metabolites and gene expression. Chemosphere 235:734–748. https://doi.org/10.1016/j.chemosphere.2019.06.188
Bhat JA, Shivaraj SM, Singh P, Navadagi DB, Tripathi DK, Dash PK, Solanke AU, Sonah H, Deshmukh R (2019) Role of silicon in mitigation of heavy metal stresses in crop plants. Plants-Basel 8(3):71. https://doi.org/10.3390/plants8030071
Chen J, Yan ZZ, Li XZ (2014) Effect of methyl jasmonate on cadmium uptake and antioxidative capacity in Kandelia obovata seedlings under cadmium stress. Ecotox Environ Safe 104:349–356. https://doi.org/10.1016/j.ecoenv.2014.01.022
Chen DM, Chen DQ, Xue RR, Long J, Lin XH, Lin YB, Jia LH, Zeng RS, Song YY (2019) Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J Hazard Mater 367:447–455. https://doi.org/10.1016/j.jhazmat.2018.12.111
Chen SS, Jia HL, Wang XF, Shi C, Wang X, Ma PY, Wang J, Ren MJ, Li JS (2020) Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol Plant 13(5):732–744. https://doi.org/10.1016/j.molp.2020.01.004
Cohen S, Flescher E (2009) Methyl jasmonate: a plant stress hormone as an anti-cancer drug. Phytochemistry 70(13-14):1600–1609. https://doi.org/10.1016/j.phytochem.2009.06.007
Cunha KPVD, Nascimento CWAD (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Pollut 197(1-4):323–330. https://doi.org/10.1007/s11270-008-9814-9
Dar TA, Uddin M, Khan MMA, Hakeem KR, Jaleel H (2015) Jasmonates counter plant stress: A review. Environ Exp Bot 115:49–57. https://doi.org/10.1016/j.envexpbot.2015.02.010
Das SK, Guha AK (2007) Biosorption of chromium by termitomyces clypeatus. Colloid Surface B 60(1):46–54. https://doi.org/10.1016/j.colsurfb.2007.05.021
Dobrikova AG, Apostolova EL, Hanć A, Yotsova E, Borisova P, Sperdouli I, Adamakis IDS, Moustakas M (2021) Cadmium toxicity in Salvia sclarea L.: An integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotox Environ Safe 209(41):111851. https://doi.org/10.1016/j.ecoenv.2020.111851
Emamverdian A, Ding Y, Xie Y, Sangari S (2018) Silicon mechanisms to ameliorate heavy metal stress in plants. Biomed Res Int 2018(8492898):10. https://doi.org/10.1155/2018/8492898
Enteshari S, Delavar K (2011) Enhancing effect of Methyl jasmonate on antioxidative capacity of Bunium persicum under cadmium stress. Planta Med 77(12):1360–1360. https://doi.org/10.1055/s-0031-1282597
Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen Y, Wu C, Tabassum MA, Chun MX, Afzal M, Jan A, Jan MT, Huang J (2014a) Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut Res 22(7):4907–4921. https://doi.org/10.1007/s11356-014-3754-2
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Hassan S, Shan D, Khan F, Ullah N, Faiq M, Khan MR, Tareen AK, Khan A, Ullah A, Ullah N, Huang J (2014b) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75(2):391–404. https://doi.org/10.1007/s10725-014-0013-y
Fahad S, Hussain S, Saud S, Hassan S, Chauhan BS, Khan F, Ihsan MZ, Ullah A, Wu C, Bajwa AA, Alharby H, Amanullah NW, Shahzad B, Tanveer M, Huang JL (2016a) Responses of rapid viscoanalyzer profile and other rice grain qualities to exogenously applied plant growth regulators under high day and high night temperatures. PLoS One 11(7):e0159590. https://doi.org/10.1371/journal.pone.0159590
Fahad S, Hussain S, Saud S, Khan F, Hassan S, A J, Nasim W, Arif M, Wang F, Huang J (2016b) Exogenously applied plant growth regulators affect heat-stressed rice pollens. J Agron Crop Sci 202:139–150
Fahad S, Hussain S, Saud S, Hassan S, Ihsan Z, Shah AN, Wu C, Yousaf M, Nasim W, Alharby H, Alghabari F, Huang J (2016c) Exogenously applied plant growth regulators enhance the morphophysiological growth and yield of rice under high temperature. Front Plant Sci 7:1250. https://doi.org/10.3389/fpls.2016.01250
Fahad S, Hussain S, Saud S, Hassan S, Tanveer M, Ihsan MZ, Shah AN, Ullah A, Nasrullah KF, Ullah S, AlharbyH NW, Wu C, Huang J (2016d) A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol Biochem 103:191–198
Farooq MA, Dietz K-J (2015) Silicon as versatile player in plant and human biology: overlooked and poorly understood. Front Plant Sci 6:994. https://doi.org/10.3389/fpls.2015.00994
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotox Environ Safe 96:242–249. https://doi.org/10.1016/j.ecoenv.2013.07.006
Farooq MA, Gill RA, Islam F, Ali B, Liu H, Xu J, He S, Zhou W (2016) Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front Plant Sci 7:468. https://doi.org/10.3389/fpls.2016.00468
Farooq MA, Islam F, Yang C, Nawaz A, Athar HR, Gill RA, Ali B, Song W, Zhou W (2018) Methyl jasmonate alleviates arsenic-induced oxidative damage and modulates the ascorbate-glutathione cycle in oilseed rape roots. Plant Growth Regul 84:135–148. https://doi.org/10.1007/s10725-017-0327-7
Feng JP, Shi QH, Wang XF, Wei M, Yang FJ, Xu HN (2010) Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumissativus L. Sci Hortic-Amsterdam 123(4):521–530. https://doi.org/10.1016/j.scienta.2009.10.013
Gaige AR, Ayella A, Shuai B (2010) Methyl jasmonate and ethylene induce partial resistance in Medicago truncatula against the charcoal rot pathogen Macrophomina phaseolina. Physiol Mol Plant P 74(5-6):412–418. https://doi.org/10.1016/j.pmpp.2010.07.001
Gawe S, Wardas M, Niedworok E (2004) Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek 57(9-10):453–455
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Greger M, Ahmad HK, Landberg T, Pooja JM, Lindberg S (2016) Silicate reduces cadmium uptake into cells of wheat. Environ Pollut 211:90–97. https://doi.org/10.1016/j.envpol.2015.12.027
Greger M, Landberg T, Vaculík M (2018) Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 7(2):41. https://doi.org/10.3390/plants7020041
Guo JK, Muhammad H, Lv X, Wei T, Ren XH, Jia HL, Atif S, Hua L (2020) Prospects andapplications of plant growth promoting rhizobacteria tomitigate soil metal contamination: A review. Chemosphere 246:125823. https://doi.org/10.1016/j.chemosphere.2020.125823
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front Plant Sci 8:1061. https://doi.org/10.3389/fpls.2017.01061
Houston MC (2007) The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Altern Ther Health Med 13(2):S128–S133. https://doi.org/10.1016/j.jep.2006.09.034
Huang HL, Li M, Rizwan M, Dai ZH, Yuan Y, Hossain MM, Cao MH, Xiong SL, Tu SX (2020) Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J Hazard Mater 401:123393. https://doi.org/10.1016/j.jhazmat.2020.123393
Jia HL, Wang X, Shi C, Guo JK, Ma PY, Ren XH, Wei T, Liu HX, Li JS (2020) Hydrogen sulfide decreases Cd translocation from root to shoot through increasing Cd accumulation in cell wall and decreasing Cd2+ influx in Isatis indigotica. Plant Physiol Biochem 155:605–612. https://doi.org/10.1016/j.plaphy.2020.08.033
Jia HL, Wang XH, Wei T, Wang M, Liu X, Hua L, Ren XH, Guo JK, Li JS (2021) Exogenous salicylic acid regulates cell wall polysaccharides synthesis and pectin methylation to reduce Cd accumulation of tomato. Ecotoxicol Environ Saf 207:111550. https://doi.org/10.1016/j.ecoenv.2020.111550
Kaya C, Akram NA, Ashraf M (2018) Kinetin and indole acetic acid promote antioxidant defense system and reduce oxidative stress in maize (Zea mays L.) plants grown at boron toxicity. J Plant Growth Regul 37:1258–1266. https://doi.org/10.1007/s00344-018-9827-6
Keramat B, Kalantari KM, Arvin MJ (2010) Effects of methyl jasmonate treatment on alleviation of cadmium damages in soybean. J Plant Nutr 33(7):1016–1025. https://doi.org/10.1080/01904161003728685
Khudsar T, Mahmooduzzafar IM (2001) Cadmium-induced changes in leaf epidermes, photosynthetic rate and pigment concentrations in Cajanus cajan. Biol Plant 44(1):59–64. https://doi.org/10.1023/A:1017918320697
Korndörfer GH, Lepsch I (2001) Effect of silicon on plant growth and crop yield. Studies in Plant Science 8:133–147. https://doi.org/10.1016/S0928-3420(01)80011-2
Lei GJ, Sun L, Sun Y, Zhu XF, Li GX, Zheng SJ (2019) Jasmonic acid alleviates cadmium toxicity in arabidopsis via suppression of cadmium uptake and translocation. J Integr Plant Biol 62(02):60–69. https://doi.org/10.1111/jipb.12801
Li XD, Ma H, Li LL, Gao YF, Li YZ, Xu H (2019) Subcellular distribution, chemical forms and physiological responses involved in cadmium tolerance and detoxification in Agrocybe Aegerita. Ecotox Environ Safe 171:66–74. https://doi.org/10.1016/j.ecoenv.2018.12.063
Lin L, Zhou WH, Dai HX, Cao FB, Zhang GP, Wu FB (2012) Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J Hazard Mater 235-236(2):343–351. https://doi.org/10.1016/j.jhazmat.2012.08.012
Liu JG, Zhang HM, Zhang YX, Chai TY (2013) Silicon attenuates cadmium toxicity in Solanum nigrum L. by reducing cadmium uptake and oxidative stress. Plant Physiol Biochem 68:1–7. https://doi.org/10.1016/j.plaphy.2013.03.018
Liu CG, Lu WK, Ma QN, Ma CC (2017) Effect of silicon on the alleviation of boron toxicity in wheat growth, boron accumulation, photosynthesis activities, and oxidative responses. J Plant Nutr 40(17):2458–2467. https://doi.org/10.1080/01904167.2017.1380817
Liu XX, Yin LN, Deng XP, Gong D, Du S, Wang SW, Zhang ZY (2020) Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J Hazard Mater 395:122679. https://doi.org/10.1016/j.jhazmat.2020.122679
Lu YG, Ma J, Teng Y, He JY, Christie P, Zhu LJ, Ren J, Zhang MY, Deng SP (2017) Effects of silicon on the growth, physiology and cadmium translocation of tobacco (Nicotiana tabacum L.) in cadmium contaminated soil. Pedosphere 28(4):680–689. https://doi.org/10.1016/S1002-0160(17)60417-X
Ma J, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11(8):392–397. https://doi.org/10.1016/j.tplants.2006.06.007
Ma J, Cai HM, He CW, Zhang WJ, Wang LJ (2015) A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol 206(3):1063–1074. https://doi.org/10.1111/nph.13276
Muhammad H, Wei T, Cao G, Yu SH, Ren XH, Jia HL, Saleem A, Hua L, Guo JK, Li YT (2021) Study of soil microorganisms modified wheat straw and biochar forreducing cadmium leaching potential and bioavailability. Chemosphere 273:129644. https://doi.org/10.1016/j.chemosphere.2021.129644
Napoleo TA, Soares G, Vital CE, Bastos C, Castro R, Loureiro ME, Giordano A (2017) Methyl jasmonate and salicylic acid are able to modify cell wall but only salicylic acid alters biomass digestibility in the model grass Brachypodium distachyon. Plant Sci 263:46–54
Noor Z, Khan SA, Noor M (2020) Assessment of cadmium toxicity and its possible effects on goldfish (Carassius auratus), employing microscopy and biochemical techniques. Microsc Res Tech 83(12):1441–1449. https://doi.org/10.1002/jemt.23536
Noriega G, Cruz DS, Batlle AB, Tomaro M, Balestrasse K (2012) Heme oxygenase is involved in the protection exerted by jasmonic acid against cadmium stress in soybean roots. J Plant Growth Regul 31:79–89. https://doi.org/10.1007/s00344-011-9221-0
Nwugo CC, Huerta AJ (2010) Silicon-induced cadmium resistance in rice (Oryza sativa). J Plant Nutr Soil Sci 171(6):841–848. https://doi.org/10.1002/jpln.200800082
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Żyłkiewiczb B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65. https://doi.org/10.1016/j.plaphy.2011.11.009
Rahman MF, Ghosal A, Alam MF, Kabir AH (2017) Remediation of cadmium toxicity in field peas (Pisumsativum L.) through exogenous silicon. Ecotox Environ Safe 135:165–172. https://doi.org/10.1016/j.ecoenv.2016.09.019
Rizwan M, Ali S, Zia Ur Rehman M, Rinklebe J, Tsang DCW, Bashir A, Maqbool A, Tack FMG, Ok YS (2018) Cadmium phytoremediation potential of Brassica crop species: a review. Sci Total Environ 631-632:1175–1191. https://doi.org/10.1016/j.scitotenv.2018.03.104
Rocha ASD, Rocha EK, Alves LM, Moraes BAD, Castro TCD, Albarello N, Simões-Gurgel C (2013) Production and optimization through elicitation of carotenoid pigments in the in vitro cultures of Cleome rosea Vahl (Cleomaceae). J Plant Biochem Biotechnol 24(1):105–113. https://doi.org/10.1007/s13562-013-0241-7
Shi GR, Cai QS, Liu CF, Wu L (2010) Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regul 61(1):45–52. https://doi.org/10.1007/s10725-010-9447-z
Shi Z, Taylor AW, Riley M, Byles J, Liu JH, Noakes M (2018) S. Clin Nutr 37(1):276–284. https://doi.org/10.1016/j.clnu.2016.12.025
Singh I, Shah K (2014) Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 108:57–66. https://doi.org/10.1016/j.phytochem.2014.09.007
Sumira J, Nasser AM, Leonard W, Pravej A, Kadambot HS, Parvaiz A (2018) Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol 18(1):146. https://doi.org/10.1186/s12870-018-1359-5
Ueda J, Saniewski M (2006) Methyl jasmonate-induced stimulation of chlorophyll formation in the basal part of tulip bulbs kept under natural light conditions. J Fruit Ornam Plant Res 14:199–210
Ulloa-Inostroza EM, Alberdi M, Meriño-Gergichevich C, Reyes-Díaz M (2017) Low doses of exogenous methyl jasmonate applied simultaneously with toxic aluminum improve the antioxidant performance of Vaccinium corymbosum. Plant Soil 412(1-2):81–96. https://doi.org/10.1007/s11104-016-2985-z
Vaculík M, Landberg T, Greger M, Luxova M, Stolarikova M, Lux A (2012) Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Ann Bot 110(2):433–443. https://doi.org/10.1093/aob/mcs039
Wasternack C, Strnad M (2017) Jasmonates are signals in the biosynthesis of secondary metabolites-Pathways, transcription factors and applied aspects-A brief review. New Biotechnol 48:1–11. https://doi.org/10.1016/j.nbt.2017.09.007
Wei T, Lv X, Jia HL, Hua L, Xu HH, Zhou R, Zhao J, Ren XH, Guo JK (2018) Effects of salicylic acid, Fe (II) and plant growth-promoting bacteria on Cd accumulation and toxicity alleviation of Cd tolerant and sensitive tomato genotypes. J Environ Manag 214:164–171. https://doi.org/10.1016/j.jenvman.2018.02.100
Wei T, Liu X, Dong MF, Lv X, Hua L, Jia HL, Ren XH, Yu SH, Guo JK, Li YT (2021) Rhizosphere iron and manganese-oxidizing bacteria stimulate root iron plaque formation and regulate Cd uptake of rice plants (Oryza sativa L.). J Environ Manag 278:111533. https://doi.org/10.1016/j.jenvman.2020.111533
Wu JW, Guo J, Hu YH, Gong HJ (2015) Distinct physiological responses of tomato and cucumber plants in silicon-mediated alleviation of cadmium stress. Front Plant Sci 6:453. https://doi.org/10.3389/fpls.2015.00453
Wu Z, Wang F, Liu S, Du Y, Li F, Du R, Wen D, Zhao J (2016) Comparative responses to silicon and selenium in relation to cadmium uptake, compartmentationin roots, and xylem transport in flowering Chinese cabbage (Brassica campestris L.ssp. chinensis var. utilis) under cadmium stress. Environ Exp Bot 131:173–180. https://doi.org/10.1016/j.envexpbot.2016.07.012
Xu XX, Zhang SR, Xian J, Yang ZB, Cheng Z, Li T, Jia YX, Pu YL, Li Y (2018) Subcellular distribution, chemical forms and thiol synthesis involved in cadmium tolerance and detoxification in Siegesbeckia orientalis L. Int J Phytoremediat 20(10):973–980. https://doi.org/10.1080/15226514.2017.1365351
Yan ZZ, Chen J, Li XZ (2013) Methyl jasmonate as modulator of Cd toxicity in Capsicum frutescens var. fasciculatum seedlings. Ecotox Environ Safe 98:203–209. https://doi.org/10.1016/j.ecoenv.2013.08.019
Yan Z, Zhang W, Chen J, Li X (2015) Methyl jasmonate alleviates cadmium toxicity in Solanum nigrum by regulating metal uptake and antioxidative capacity. Biol Plant 59(2):373–381. https://doi.org/10.1007/s10535-015-0491-4
Yang LP, Zhu J, Wang P, Zeng J, Tan R, Yang YZ, Liu ZM (2018) Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotox Environ Safe 160:10–18. https://doi.org/10.1016/j.ecoenv.2018.05.026
Zhang Q, Yan CL, Liu JC, Lu HL, Duan HH, Du JN, Wang WY (2014) Silicon alleviation of cadmium toxicity in mangrove (Avicennia marina) in relation to cadmium compartmentation. J Plant Growth Regul 33(2):233–242. https://doi.org/10.1007/s00344-013-9366-0
Zhang TT, Hong MH, Wu MJ, Cheng BB, Ma ZL (2020) Oxidative stress responses to cadmium in the seedlings of a commercial seaweed Sargassum fusiforme. Acta Oceanol Sin 39(10):147–154. https://doi.org/10.1007/s13131-020-1630-0
Zhao Q, Sun Q, Dong PY, Ma CC, Sun HW, Liu CG (2019) Jasmonic acid alleviates boron toxicity in Puccinellia tenuiflora, a promising species for boron phytoremediation. Plant Soil 445:397–407. https://doi.org/10.1007/s11104-019-04326-0
Zhou JT, Wan HX, He JL, Lyu DG, Li HF (2017) Integration of cadmium accumulation, subcellular distribution, and physiological responses to understand cadmium tolerance in apple rootstocks. Front Plant Sci 8:966. https://doi.org/10.3389/fpls.2017.00966
Zhu ZJ, Wei GQ, Li J, Qian QQ, Yu JQ (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumissativus L.). Plant Sci 167(3):527–533. https://doi.org/10.1016/j.plantsci.2004.04.020
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (41807123) and Natural Science Foundation of Shaanxi Province (2020JQ-718).
Author information
Authors and Affiliations
Contributions
WT and GJK designed the experiment. LX and YN performed the experiments and analyzed the data. YN and HL reviewed the manuscript. SYN, LH, and RXH have provided technical assistance.
Corresponding authors
Ethics declarations
Ethics approval
No ethical approval was necessary for this study.
Consent to participate
All participants in this study consent to participation.
Consent for publication
All authors consent to this publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Exogenous Si and MeJA restored Cd-induced growth inhibition in tomato plants
• Exogenous Si and MeJA elevated chlorophyll and carotenoid contents in Cd-stressed tomato plants
• Exogenous Si and MeJA alleviated lipid peroxidation in Cd-stressed tomato plants
• Application of Si and MeJA reduced net Cd2+ fluxes into tomato roots and decreased Cd content in different tomato organs
• Exogenous Si and MeJA modulated subcellular localization of Cd in both roots and shoots of tomato plants
Rights and permissions
About this article
Cite this article
Wei, T., Li, X., Yashir, N. et al. Effect of exogenous silicon and methyl jasmonate on the alleviation of cadmium-induced phytotoxicity in tomato plants. Environ Sci Pollut Res 28, 51854–51864 (2021). https://doi.org/10.1007/s11356-021-14252-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14252-3