Abstract
The causes of leukemia remain largely unknown; our aims were to examine the association between the exposure to outdoor air pollution and leukemia risk and to explore the effect of this exposure during different periods of pregnancy and early life. We searched for all case–control and cohort studies published before February 20, 2021, which measured the risk of leukemia in relation to exposure to the air pollutants: particulate matter, benzene, nitrogen dioxide (NO2), and nitrogen oxides (NOx). We then carried out a meta-analysis and calculated the summary relative risks (RRs) of leukemia by using a random-effects model. The potential dose–response relationship was further explored. The results showed that the highest exposure to benzene (RR: 1.20, 95%CI: 1.06–1.35) and NO2 (RR: 1.04, 95%CI; 1.02–1.08) were positively correlated with leukemia risk when compared to the lowest exposure categories for each air pollutant. During pregnancy, exposure to benzene in the third trimester, as well as exposure to NO2 in the second trimester and entire pregnancy, could also increase the risk of leukemia. In the dose–response analysis, benzene exposure and NO2 exposure were linearly associated with the risk of leukemia. Other air pollutants did not have a statistical correlation with leukemia risk. There was a certain degree of publication bias in studies on benzene. Overall, our results support a link between outdoor air pollution and leukemia risk, particularly due to benzene and NO2. Prospero Registration Number: PROSPERO CRD42020207025.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Leukemia is a common cancer not only among adults (Rosenberg et al. 2012) but also among children and adolescents. In the recent years, the incidence of leukemia and the number of patients seeking medical advice showed an upward trend (Barrington-Trimis et al. 2017; Isaevska et al. 2017). Although the causes of leukemia remain largely unknown, some studies have suggested that exposure to outdoor air pollution is one of the risk factors for leukemia (Konstantinoudis et al. 2020; Metayer et al. 2016; Schraufnagel et al. 2019). Additionally, according to WHO’s report, air pollution is a major environmental health problem affecting low-, middle-, and high-income countries. Recently published studies have inconsistent results; a case–control study suggested that their results did not support the relationship between outdoor air pollution and leukemia (Peckham-Gregory et al. 2019). The effects of different exposure periods on leukemia are different. Two meta-analyses showed that the risk estimates were generally higher for exposures that happened during the postnatal period compared to the prenatal period (Filippini et al. 2019; Filippini et al. 2015). However, a cohort study showed different results which suggested that postnatal exposure was not associated with leukemia, whereas prenatal exposure could lead to leukemia (Lavigne et al. 2017). The prenatal period was further divided into three exposure windows, such as the first trimester, second trimester, and third trimester. One study showed that only exposure to air pollution in the second trimester could increase the risk of leukemia (Ghosh et al. 2013), and another study suggested that only exposure to air pollution in the first trimester was associated with the risk of leukemia (Lavigne et al. 2017). In addition, a study showed that exposure to air pollutants could initiate leukemia in utero by causing chromosomal rearrangements and mutations that are on the causal pathway to these malignancies in fetal hematopoietic stem cells (Carlos-Wallace et al. 2016). Given that a number of the most recent studies assessed more time windows of exposure, an updated review and meta-analysis are warranted.
Methods
Search strategy
We performed a systematic search in the PubMed and Web of Science databases from inception to February 20, 2021. Keywords included air pollution, traffic-related pollution, particulate matter, benzene, nitrogen dioxide, nitrogen oxides, outdoor air pollution, air pollutants, outdoor air pollutants, leukemia, childhood leukemia, adult leukemia, acute lymphoblastic leukemia, acute myeloid leukemia, chronic leukemia, chronic myeloid leukemia, and chronic lymphoblastic leukemia. Full details on the related database search strings are reported in Supplementary Table S1. We also searched the reference lists of eligible studies and checked which other articles cited the eligible studies. The protocol for this meta-analysis can be found in the International Prospective Register of Systematic Reviews (PROSPERO), the registration number is CRD42020207025.
Study selection
Inclusion criteria were (a) epidemiologic case–control or cohort studies, (b) any type of assessment of exposure to outdoor air pollutants, and (c) reporting of risk estimates or ability to compute them from the reported data. Exclusion criteria included (a) ecologic study design, (b) exposure assessment limited to only occupational activities, (c) exposure assessment limited to only factory emissions, and (e) editorials, letters, commentaries, and reports.
Data extraction and quality assessment
Two authors independently extracted the data. Any disagreement was resolved through discussion until a consensus was reached or by consulting a third author. We extracted information on author, year of publication, country, study period, sample size, study design, age, air pollutant, type of leukemia, exposure window, matching variables, and statistical methods. We categorized these exposure periods into 7 time frames: first trimester, second trimester, third trimester, entire pregnancy, child's first year, residence at birth, and residence at diagnosis. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of each included study. Each study was judged based on three broad perspectives: the selection of the study groups; the comparability of the groups; and the ascertainment of the outcome of interest for case–control or cohort studies, respectively. A higher score indicates that the study is of a higher quality GA Wells et al. (2019).
Exposure assessment methods
In our meta-analysis, the ranges of exposure levels for most studies were roughly comparable, although cut-points for the highest level of exposure identification differed across the various studies to some extent. We extracted relative risks (RRs) for each category of air pollutant exposure from all studies, by abstracting the odds ratios (ORs) from 15 studies (Hvidtfeldt et al. 2020; Puett et al. 2020; Taj et al. 2020; Janitz et al. 2017; Symanski et al. 2016; Raaschou-Nielsen et al. 2016; Janitz et al. 2016; Houot et al. 2015; Heck et al. 2014; Heck et al. 2013; Ghosh et al. 2013; Badaloni et al. 2013; Vinceti et al. 2012; Amigou et al. 2011; Weng et al. 2008) and hazard ratios (HRs) or rate ratios (RRs) from 5 studies (Raaschou-Nielsen et al. 2018; Lavigne et al. 2017; Crosignani et al. 2004; Raaschou-Nielsen et al. 2001; Feychting et al. 1998), as also used in previous review (Filippini et al. 2019). For the dose–response analysis, except for the above information, the median or mean exposure level was also abstracted. When the mean (or median) of an exposure strata was not directly reported, we calculated the midpoint of reported upper and lower boundaries. If the category of exposure had an open interval, we entered a value that was 20% higher or lower than the closest cut point (Filippini et al. 2019).
Statistical analysis
We used a random-effects model to calculate the summary RR and 95% confidence intervals (CIs) to provide an overall estimate of the strength of the association between air pollution and the leukemia outcome during different exposure periods, comparing the highest and lowest exposure categories for each metric of interest. Furthermore, we conducted stratified analyses by leukemia subtype (ALL and AML).
For dose–response analysis, we performed a two-stage random-effects dose–response meta-analysis (Orsini et al. 2012). In the first stage, a restricted cubic spline model with three knots at the 25th, 50th, and 75th centiles of the exposure distribution was estimated using generalized least square regression. Then the study-specific estimates were combined using the restricted maximum likelihood method in a multivariate random-effects meta-analysis (Sun et al. 2018; Orsini et al. 2012). The P value for nonlinearity was assessed by testing the null hypothesis that the coefficient of the second spline equals to zero (Zhang et al. 2020). Initially, a nonlinear model was applied if model verification indicated significance (P < 0.05), otherwise, a linear model was adopted (Lu et al. 2016).
We used the Q test and I2 statistic to assess the heterogeneity. Furthermore, we used Egger’s test to assess publication bias. If there had the publication bias, we would use the trim and fill method to test whether the publication bias affected the results (Duval and Tweedie 2000). All data analyses were conducted using the STATA/SE software, version 15, and P values were two-sided. P<0.05 indicated a statistically significant result.
Results
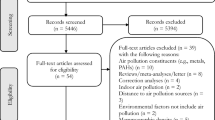
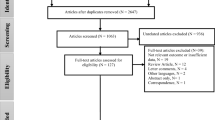
We identified 2742 studies from the PubMed and Web of Science databases. Three hundred and thirty-eight studies were duplicates, leaving 2404 studies for title and abstract screening. After screening, forty-six studies needed to be assessed for their eligibility. Twenty-seven studies were excluded with reasons, and the remaining nineteen articles. After screening the reference lists of eligible studies and checked the articles cited the eligible studies, 1 study was included. Finally, 20 studies were included in our meta-analysis. Figure 1 shows the flowchart of study selection.
Table 1 shows the characteristics of the included studies, 19 studies were case–control studies, and 1 study was a cohort study. The study period ranged from 1968 to 2014. Seventeen studies included children aged under 20 years at diagnosis. Air pollutants included in these studies were the following: nitrogen dioxide (NO2) (n=12), benzene (n=8), fine particulate matter (PM2.5) (n=6), inhalable particles (PM10) (n=2), and nitrogen oxides (NOX) (n=2). Twelve studies were conducted in Europe, six studies in the USA, one in Canada, and one in Taiwan, China. Twelve studies used conditional logistic regression, six studies used unconditional logistic regression, one study used the mixed-effects logistic regression model, and the cohort study used the Cox proportional hazards models. The total score of the NOS scale was 9. For included studies, the median value of the NOS scale was 8, which meant that these studies were of good quality and had a low risk of bias. The detail of NOS scale scores of included studies were reported in Supplementary Table S2.
Figure 2a shows that exposure to benzene was a risk factor for leukemia, (RR: 1.20, 95%CI: 1.06–1.35). Additionally, in subgroup analysis, when the exposure period was taken into account, exposure in the 3rd trimester and exposure associated with the residence at birth was correlated with leukemia risk. Figure 3a demonstrates the exposure period subgroup meta-analysis for leukemia, which indicates that there was a positive correlation between NO2 and leukemia risk (RR: 1.04, 95%CI; 1.02–1.08), especially in the 2nd trimester and entire pregnancy. There was no significant correlation between the PM2.5, NOX, and leukemia risk (Figs. 4a and 5). For PM10, there were insufficient results to conduct a meta-analysis (n=2). Heterogeneity between studies on benzene, NO2, NOX, and PM2.5 were 44.3%, 25.7%, 43.2%, and 30.9%, respectively. Both studies on the relationship between PM10 and leukemia showed that there was no significant correlation between exposure to PM10 and leukemia risk, either at the birth address or at the diagnosis address (Badaloni et al. 2013; Vinceti et al. 2012).
In the stratified analyses results of disease subtypes, we found that summary RR estimates differ markedly for benzene and PM2.5 exposures. For benzene, when we limited the studies enrolling only the ALL subtype, we estimated a summary RR of 1.07 (95% CI: 0.97–1.17) with I2=18.5% (Fig. 2b) while for the AML subtype, a summary RR of 1.61 (95% CI: 1.30–2.01) with I2=0.0% (Fig. 2c) was observed. No such change in RR according to leukemia subtype was found for NO2 and PM2.5.
In the dose–response meta-analysis, for benzene (Raaschou-Nielsen et al. 2018; Janitz et al. 2017; Vinceti et al. 2012; Crosignani et al. 2004), there was a linear increase in estimated risk (P for nonlinearity=0.654). The RR of leukemia was 1.02 (95%CI: 0.99–1.05) for every 1 μg/m3 increment in benzene exposure (Fig. 6). A linear relationship was also found between NO2 exposure (Janitz et al. 2016; Amigou et al., 2011; Weng et al. 2008; Feychting et al. 1998) and the risk of leukemia (P for nonlinearity=0.598). The RR of leukemia was 1.19 (95%CI: 1.07–1.32) for per 10 μg/m3 increment in NO2 exposure (Fig. 7).
Publication bias was found in studies which estimated the risk of exposure to benzene by Egger’s test (P=0.001, Supplementary Table S3). We then used the trim and fill method to test the effect of publication bias on the results (Supplementary Fig. S1). The results of the trim and fill analysis indicated that the summary effects of benzene showed a change to RR=1.11 (95%CI: 0.98–1.26). Publication bias was not observed in studies on NO2, NOX, PM2.5, and PM10 (Supplementary Table S3).
Discussion
Our meta-analysis indicated that outdoor air pollution could increase the risk of leukemia, especially with exposure to benzene and NO2. When the exposure time window was divided into 7 periods, we found that exposure at different periods might cause different outcomes. An exposure to benzene in the 3rd trimester of pregnancy, residence at birth exposure, as well as exposure to NO2 in the 2nd trimester and entire pregnancy, might increase the risk of leukemia. The results from our subgroup studies suggested benzene exposure has different effects on the two subtypes of leukemia which could increase the risk of AML, not ALL. The dose–response analyses showed that there was a linear correlation between benzene exposure and leukemia risk. And NO2 exposure was also linearly associated with risk of leukemia. We found that increase of NO2 exposure contributed to elevated leukemia risk significantly.
In the meta-analyses, Egger’s test and the trim and fill method showed that studies which focused on benzene had a publication bias. The occurrence and potential influence of publication bias is very difficult to assess (Filippini et al. 2015). The heterogeneity between studies and the number of studies could influence the asymmetry of the funnel plot (Sterne et al. 2011). Exposure time windows, leukemia types (ALL or AML), and cutoffs for exposure categories are used in different studies to some extent are the causes of heterogeneity among included studies. The results of the trim and fill analysis indicated that the summary effects of benzene showed a slightly changed. The main goal of the trim and fill analysis should be seen as providing methods for sensitivity analyses, and does seem to give good indications of which meta-analyses do not suffer from publication bias and which need to be evaluated much more carefully (Duval and Tweedie 2000). In our meta-analysis, the single RR estimates (1.20/1.11) generated by pooling all studies independently of the metrics adopted, the timing of the exposure assessment and the disease subtype (ALL or AML), a choice that considerably decreases the ability to detect and assess associations that can be seen (and likely exist) only in stratified analyses.
Although there was an inconsistency in conclusions on the correlation between air pollution and leukemia (Schüz and Erdmann 2016), the following mentioned studies, meta-analyses (Carlos-Wallace et al. 2016; Gong et al. 2019) and reviews (Pourvakhshoori et al. 2020; Schraufnagel et al. 2019) tended to support that outdoor air pollution was the risk factor for leukemia. These studies divided the exposure window into postnatal and prenatal (Boothe et al. 2014) or residence at diagnosis and residence at birth (Filippini et al. 2015), which showed that the risk of postnatal exposure was higher than that of prenatal exposure. Our meta-analysis divided the exposure window into seven periods which obtained similar results. Meanwhile, although some exposure period subgroups included fewer studies, we also found that the risk was different at different periods of pregnancy which was not mentioned in previous meta-analyses.
Laboratory studies comparing benzene exposed children after refinery emitted and unexposed children confirmed that exposure to outdoor air pollution could decrease hemoglobin levels (D'Andrea and Reddy 2016), and a review also discussed that the children lived near petrochemical estate region more likely to suffer from bone marrow injury resulting in hematologic toxicity leading to changes in blood cell formation than unexposed children (D'Andrea and Reddy 2018). The studies in mice showed that air pollution could increase the expression levels of inflammatory factors, IL-2, IL-10, IL-17A, and TNFα mRNA in leukemia cells which indicated that air pollution might lead to the occurrence of leukemia by affecting cytokine expression (Chen et al. 2018; Jin et al. 2016). One article mentioned that air pollution had toxic effects through inducing DNA-single strand breaks and DNA modifications to cause cancer (Gong et al. 2019). A review based on human studies concluded that air pollution caused leukemia via proinflammatory cytokine production and impaired immunosurveillance (Guo et al. 2020). During pregnancy, air pollution can cross the placenta (Dowty et al. 1976), leading to the increase of reactive oxygen species (ROS) which might play an important role in the development of childhood leukemia through disruption of hematopoietic cell signaling pathways (Badham and Winn 2010). And the mice experiment confirmed that the air pollution exposure in utero could also increase the frequency of micronuclei and DNA recombination events that lead to childhood leukemia (Lau et al. 2009).
There are some limitations in our meta-analysis that need to be declared. First, some included studies were old; second, some air pollutants and subgroups included fewer studies which might have affected the credibility of our results; and last, we could not entirely rule out the occurrence of publication bias. Nevertheless, as far as we know, this is the first meta-analysis to assess air pollution and leukemia by dividing the exposure window into detailed periods.
Overall, outdoor air pollution could increase the risk of leukemia, especially exposure to benzene and NO2. Exposure at different periods and different leukemia subtypes had different effects. It is therefore necessary to formulate policies that aim at reducing air pollution exposure and protect people from leukemia. Furthermore, there existed a linear relationship between benzene and NO2 exposure and leukemia risk. More epidemiological studies are needed to confirm our findings.
References
Amigou A, Sermage-Faure C, Orsi L, Leverger G, Baruchel A, Bertrand Y, Nelken B, Robert A, Michel G, Margueritte G, Perel Y, Mechinaud F, Bordigoni P, Hémon D, Clavel J (2011) Road traffic and childhood leukemia: the ESCALE study (SFCE). Environ Health Perspect 119:566–572. https://doi.org/10.1289/ehp.1002429
Badaloni C, Ranucci A, Cesaroni G, Zanini G, Vienneau D, al-Aidrous F, de Hoogh K, Magnani C, Forastiere F, on behalf of the SETIL Study Group (2013) Air pollution and childhood leukaemia: a nationwide case-control study in Italy. Occup Environ Med 70:876–883. https://doi.org/10.1136/oemed-2013-101604
Badham HJ, Winn LM (2010) In utero exposure to benzene disrupts fetal hematopoietic progenitor cell growth via reactive oxygen species. Toxicol Sci 113:207–215. https://doi.org/10.1093/toxsci/kfp242
Barrington-Trimis JL, Cockburn M, Metayer C, Gauderman WJ, Wiemels J, McKean-Cowdin R (2017) Trends in childhood leukemia incidence over two decades from 1992 to 2013. Int J Cancer 140:1000–1008. https://doi.org/10.1002/ijc.30487
Boothe VL, Boehmer TK, Wendel AM, Yip FY (2014) Residential traffic exposure and childhood leukemia: a systematic review and meta-analysis. Am J Prev Med 46:413–422. https://doi.org/10.1016/j.amepre.2013.11.004
Carlos-Wallace FM, Zhang L, Smith MT, Rader G, Steinmaus C (2016) Parental, in utero, and early-life exposure to benzene and the risk of childhood leukemia: a meta-analysis. Am J Epidemiol 183:1–14. https://doi.org/10.1093/aje/kwv120
Chen T, Zhang J, Zeng H, Zhang Y, Zhang Y, Zhou X, Zhao D, Feng Y, Zhou H (2018) The impact of inflammation and cytokine expression of PM2.5 in AML. Oncol Lett 16:2732–2740. https://doi.org/10.3892/ol.2018.8965
Crosignani P, Tittarelli A, Borgini A, Codazzi T, Rovelli A, Porro E, Contiero P, Bianchi N, Tagliabue G, Fissi R, Rossitto F, Berrino F (2004) Childhood leukemia and road traffic: a population-based case-control study. Int J Cancer 108:596–599. https://doi.org/10.1002/ijc.11597
D'Andrea MA, Reddy GK (2016) Adverse health effects of benzene exposure among children following a flaring incident at the British petroleum refinery in Texas City. Clin Pediatr (Phila) 55:219–227. https://doi.org/10.1177/0009922815594358
D'Andrea MA, Reddy GK (2018) Health risks associated with benzene exposure in children: a systematic review. Glob Pediatr Health 5:2333794x18789275. https://doi.org/10.1177/2333794x18789275
Dowty BJ, Laseter JL, Storer J (1976) The transplacental migration and accumulation in blood of volatile organic constituents. Pediatr Res 10:696–701. https://doi.org/10.1203/00006450-197607000-00013
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463. https://doi.org/10.1111/j.0006-341x.2000.00455.x
Feychting M, Svensson D, Ahlbom A (1998) Exposure to motor vehicle exhaust and childhood cancer. Scand J Work Environ Health 24:8–11. https://doi.org/10.5271/sjweh.272
Filippini T, Hatch EE, Rothman KJ, Heck JE, Park AS, Crippa A, Orsini N, Vinceti M (2019) Association between outdoor air pollution and childhood leukemia: a systematic review and dose–response meta-analysis. Environ Health Perspect 127:46002. https://doi.org/10.1289/ehp4381
Filippini T, Heck JE, Malagoli C, Del Giovane C, Vinceti M (2015) A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 33:36–66. https://doi.org/10.1080/10590501.2015.1002999
GA Wells BS, DO’Connell, J Peterson, V Welch, M Losos, P Tugwell (2019) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 27th 2020
Ghosh JKC, Heck JE, Cockburn M, Su J, Jerrett M, Ritz B (2013) Prenatal exposure to traffic-related air pollution and risk of early childhood cancers. Am J Epidemiol 178:1233–1239. https://doi.org/10.1093/aje/kwt129
Gong ZH, Li J, Wang XY, Yu Y, Ren MM, Zhou J (2019) A meta-analysis of traffic-related air pollution and risk of childhood leukemia. J Pediatr Hematol Oncol 41:267–274. https://doi.org/10.1097/mph.0000000000001413
Guo H, Ahn S, Zhang L (2020) Benzene-associated immunosuppression and chronic inflammation in humans: a systematic review. Occup Environ Med 78:377–384. https://doi.org/10.1136/oemed-2020-106517
Heck JE, Park AS, Qiu JH, Cockburn M, Ritz B (2014) Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int J Hyg Environ Health 217:662–668. https://doi.org/10.1016/j.ijheh.2013.12.003
Heck JE, Wu J, Lombardi C, Qiu J, Meyers TJ, Wilhelm M, Cockburn M, Ritz B (2013) Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ Health Perspect 121:1385–1391. https://doi.org/10.1289/ehp.1306761
Houot J, Marquant F, Goujon S, Faure L, Honoré C, Roth MH, Hémon D, Clavel J (2015) Residential proximity to heavy-traffic roads, benzene exposure, and childhood leukemia—the GEOCAP study, 2002–2007. Am J Epidemiol 182:685–693. https://doi.org/10.1093/aje/kwv111
Hvidtfeldt UA, Erdmann F, Urhøj SK, Brandt J, Geels C, Ketzel M, Frohn LM, Christensen JH, Sørensen M, Raaschou-Nielsen O (2020) Air pollution exposure at the residence and risk of childhood cancers in Denmark: a nationwide register-based case-control study. EClinicalMedicine 28:100569. https://doi.org/10.1016/j.eclinm.2020.100569
Isaevska E, Manasievska M, Alessi D, Mosso ML, Magnani C, Sacerdote C, Pastore G, Fagioli F, Merletti F, Maule M (2017) Cancer incidence rates and trends among children and adolescents in Piedmont, 1967–2011. PLoS One 12:e0181805. https://doi.org/10.1371/journal.pone.0181805
Janitz AE, Campbell JE, Magzamen S, Pate A, Stoner JA, Peck JD (2016) Traffic-related air pollution and childhood acute leukemia in Oklahoma. Environ Res 148:102–111. https://doi.org/10.1016/j.envres.2016.03.036
Janitz AE, Campbell JE, Magzamen S, Pate A, Stoner JA, Peck JD (2017) Benzene and childhood acute leukemia in Oklahoma. Environ Res 158:167–173. https://doi.org/10.1016/j.envres.2017.06.015
Jin XT, Chen ML, Li RJ, An Q, Song L, Zhao Y, Xiao H, Cheng L, Li ZY (2016) Progression and inflammation of human myeloid leukemia induced by ambient PM2.5 exposure. Arch Toxicol 90:1929–1938. https://doi.org/10.1007/s00204-015-1610-x
Konstantinoudis G et al (2020) Bayesian spatial modelling of childhood cancer incidence in Switzerland using exact point data: a nationwide study during 1985-2015. Int J Health Geogr 19:13. https://doi.org/10.1186/s12942-020-00211-7
Lau A, Belanger CL, Winn LM (2009) In utero and acute exposure to benzene: investigation of DNA double-strand breaks and DNA recombination in mice. Mutat Res 676:74–82. https://doi.org/10.1016/j.mrgentox.2009.04.001
Lavigne E, Bélair MA, Do MT, Stieb DM, Hystad P, van Donkelaar A, Martin RV, Crouse DL, Crighton E, Chen H, Brook JR, Burnett RT, Weichenthal S, Villeneuve PJ, To T, Cakmak S, Johnson M, Yasseen AS III, Johnson KC, Ofner M, Xie L, Walker M (2017) Maternal exposure to ambient air pollution and risk of early childhood cancers: a population-based study in Ontario, Canada. Environ Int 100:139–147. https://doi.org/10.1016/j.envint.2017.01.004
Lu W, Chen H, Niu Y, Wu H, Xia D, Wu Y (2016) Dairy products intake and cancer mortality risk: a meta-analysis of 11 population-based cohort studies. Nutr J 15:91. https://doi.org/10.1186/s12937-016-0210-9
Metayer C, Dahl G, Wiemels J, Miller M (2016) Childhood leukemia: a preventable disease. Pediatrics 138:S45–s55. https://doi.org/10.1542/peds.2015-4268H
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175:66–73. https://doi.org/10.1093/aje/kwr265
Peckham-Gregory EC, Ton M, Rabin KR, Danysh HE, Scheurer ME, Lupo PJ (2019) Maternal residential proximity to major roadways and the risk of childhood acute leukemia: a population-based case–control study in Texas, 1995–2011. Int J Environ Res Public Health 16. https://doi.org/10.3390/ijerph16112029
Pourvakhshoori N, Khankeh HR, Stueck M, Farrokhi M (2020) The association between air pollution and cancers: controversial evidence of a systematic review. Environ Sci Pollut Res Int 27:38491–38500. https://doi.org/10.1007/s11356-020-10377-z
Puett RC, Poulsen AH, Taj T, Ketzel M, Geels C, Brandt J, Christensen JH, Sørensen M, Roswall N, Hvidtfeldt U, Raaschou-Nielsen O (2020) Relationship of leukaemias with long-term ambient air pollution exposures in the adult Danish population. Br J Cancer 123:1818–1824. https://doi.org/10.1038/s41416-020-01058-2
Raaschou-Nielsen O, Hertel O, Thomsen BL, Olsen JH (2001) Air pollution from traffic at the residence of children with cancer. Am J Epidemiol 153:433–443. https://doi.org/10.1093/aje/153.5.433
Raaschou-Nielsen O, Hvidtfeldt UA, Roswall N, Hertel O, Poulsen AH, Sorensen M (2018) Ambient benzene at the residence and risk for subtypes of childhood leukemia, lymphoma and CNS tumor. Int J Cancer 143:1367–1373. https://doi.org/10.1002/ijc.31421
Raaschou-Nielsen O, Ketzel M, Poulsen AH, Sorensen M (2016) Traffic-related air pollution and risk for leukaemia of an adult population. Int J Cancer 138:1111–1117. https://doi.org/10.1002/ijc.29867
Rosenberg PS, Wilson KL, Anderson WF (2012) Are incidence rates of adult leukemia in the United States significantly associated with birth cohort? Cancer Epidemiol Biomark Prev 21:2159–2166. https://doi.org/10.1158/1055-9965.Epi-12-0910
Schraufnagel DE, Balmes JR, Cowl CT, de Matteis S, Jung SH, Mortimer K, Perez-Padilla R, Rice MB, Riojas-Rodriguez H, Sood A, Thurston GD, To T, Vanker A, Wuebbles DJ (2019) Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies' Environmental Committee, part 2: air pollution and organ systems. Chest 155:417–426. https://doi.org/10.1016/j.chest.2018.10.041
Schüz J, Erdmann F (2016) Environmental exposure and risk of childhood leukemia: an overview. Arch Med Res 47:607–614. https://doi.org/10.1016/j.arcmed.2016.11.017
Sterne JA et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj 343:d4002. https://doi.org/10.1136/bmj.d4002
Sun K, Zhang Y, Tian Y, Jiang X (2018) Environmental tobacco smoke exposure and risk of habitual snoring in children: a meta-analysis. J Epidemiol Community Health 72:1064–1070. https://doi.org/10.1136/jech-2018-210820
Symanski E, Lewis PGT, Chen TY, Chan WY, Lai DJ, Ma XM (2016) Air toxics and early childhood acute lymphocytic leukemia in Texas, a population based case control study. Environ Health 15:11. https://doi.org/10.1186/s12940-016-0154-8
Taj T et al (2020) Exposure to PM(2.5) constituents and risk of adult leukemia in Denmark: a population-based case-control study. Environ Res:110418. https://doi.org/10.1016/j.envres.2020.110418
Vinceti M, Rothman KJ, Crespi CM, Sterni A, Cherubini A, Guerra L, Maffeis G, Ferretti E, Fabbi S, Teggi S, Consonni D, de Girolamo G, Meggiato A, Palazzi G, Paolucci P, Malagoli C (2012) Leukemia risk in children exposed to benzene and PM10 from vehicular traffic: a case–control study in an Italian population. Eur J Epidemiol 27:781–790. https://doi.org/10.1007/s10654-012-9727-1
Weng HH, Tsai SS, Chen CC, Chiu HF, Wu TN, Yang CY (2008) Childhood leukemia development and correlation with traffic air pollution in Taiwan using nitrogen dioxide as an air pollutant marker. J Toxicol Environ Health A 71:434–438. https://doi.org/10.1080/15287390701839042
Zhang D, Liu Y, Cheng C, Wang Y, Xue Y, Li W, Li X (2020) Dose-related effect of secondhand smoke on cardiovascular disease in nonsmokers: systematic review and meta-analysis. Int J Hyg Environ Health 228:113546. https://doi.org/10.1016/j.ijheh.2020.113546
Author information
Authors and Affiliations
Contributions
TW and FL designed this study. TW and RJ selected studies. TW, RJ, and FL extracted data and assessed the quality of included studies. TW, RN, RJ, ZZ, YS2, YS1, CN, LZ, and XR analyzed the data. TW draft the manuscript. FL revised and made the decision to submit for publication. All authors contributed to manuscript revision and read and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Wei, T., Jiao, R., Nakyeyune, R. et al. Exposure to outdoor air pollution at different periods and the risk of leukemia: a meta-analysis. Environ Sci Pollut Res 28, 35376–35391 (2021). https://doi.org/10.1007/s11356-021-14053-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14053-8