Abstract
Air pollution is a major cause of specific deaths worldwide. This review article aimed to investigate the results of cohort studies for air pollution connected with the all-cause, cardio-respiratory, and lung cancer mortality risk by performing a meta-analysis. Relevant cohort studies were searched in electronic databases (PubMed/Medline, Web of Science, and Scopus). We used a random effect model to estimate the pooled relative risks (RRs) and their 95% CIs (confidence intervals) of mortality. The risk of bias for each included study was also assessed by Office of Health Assessment and Translation (OHAT) checklists. We applied statistical tests for heterogeneity and sensitivity analyses. The registration code of this study in PROSPERO was CRD42023422945. A total of 88 cohort studies were eligible and included in the final analysis. The pooled relative risk (RR) per 10 μg/m3 increase of fine particulate matter (PM2.5) was 1.080 (95% CI 1.068–1.092) for all-cause mortality, 1.058 (95% CI 1.055–1.062) for cardiovascular mortality, 1.066 (95%CI 1.034–1.097) for respiratory mortality and 1.118 (95% CI 1.076–1.159) for lung cancer mortality. We observed positive increased associations between exposure to PM2.5, PM10, black carbon (BC), and nitrogen dioxide (NO2) with all-cause, cardiovascular and respiratory diseases, and lung cancer mortality, but the associations were not significant for nitrogen oxides (NOx), sulfur dioxide (SO2) and ozone (O3). The risk of mortality for males and the elderly was higher compared to females and younger age. The pooled effect estimates derived from cohort studies provide substantial evidence of adverse air pollution associations with all-cause, cardiovascular, respiratory, and lung cancer mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution is a well-known risk factor for mortality and burden of disease [1]. Recent estimates from the Global Burden of Disease (GBD) study estimated that ambient air pollution caused 4.2 (95% confidence interval 3.7–4.8) million excess deaths (7.6% of total global mortality) for the year 2015 [2]. World Health Organization (WHO) in 2019, estimated that 99% of the global population lived in areas where air quality levels exceeded the air quality guideline values [3]. The major ambient air pollutants include particulate matter (PM), nitrogen dioxide (NO2), nitrogen oxide (NO), sulfur dioxide (SO2), carbon monoxide (CO), ozone (O3), and volatile organic compounds (VOCs) [4, 5]. PM is classified according to particle size. Fine particulate matter (PM2.5) is defined as particles with a diameter equal to or less than 2.5 μm and coarse particulate matter (PM10) is defined as particles with a diameter of 10 μm or less [6].
Long-term exposure to air pollution induces many health problems such as respiratory problems, cardiovascular disease, neurologic disorders, stroke, and cancer [6,7,8]. Air pollution is also recognized as the fourth largest risk factor for premature and lung cancer death [6,7,8,9]. Among air pollutants, PM2.5 and PM10 have been strongly connected to mortality and morbidity [10,11,12]. PM2.5 exposure in the long term increases the relative risk of all-cause mortality by 8% and cardiovascular events by as much as 10% [13,14,15]. The presence of NO2 and ground-level O3 have been reported as important contributors to mortality or morbidity due to respiratory and cardiovascular diseases [5, 16, 17]. NO2 is positively linked to increased mortality from cancer [18]. Evidence also documented that higher levels of O3 are connected with a higher risk of cognitive disorders, preterm birth, and reproductive health [19, 20].
Several studies are available that investigated the adverse health effects of air pollution [21], but the findings are inconsistent because air pollution is a complex mixture of pollutants from various sources. Some recent meta-analysis and cohort studies found an increased risk of mortality accompanied by air pollution [6, 22,23,24,25,26,27]. For instance, a cohort study by Peng et al. (2017) indicated that exposure to PM2.5 was significantly connected with mortality from all-cause (HR 1.30, 95% CI 1.19–1.42), respiratory diseases (HR 1.19, 95% CI 1.02–1.38), lung cancer (HR 1.72, 95% CI 1.36–2.19) and other cancers (HR 1.76, 95% CI 1.33–2.32) [23]. Kim et al. (2018) in a meta-analysis of cohort studies indicate that exposure to PM2.5, PM10, and NO2 were associated with increased mortality from all cancers [28]. Conversely, Tseng et al. (2015) in a cohort study found that exposure to PM2.5 was not significantly connected to all-cause (HR 0.92, 95% CI 0.72–1.17) and cardiovascular (HR 0.80, 95% CI 0.43–1.50) mortality [29].
Most existing meta-analysis focus on short-term effects of air pollution, especially for cardio-respiratory mortality [11, 30, 31], and the pooled associations between long-term exposure to air pollution with cardio-respiratory and lung cancer health is poorly understood. Further, some recent reports providing additional evidence of the associations from areas with low levels of air pollution, which found stronger associations between ambient air pollution, and all-cause, cardio-respiratory, and lung cancer mortality. Similarly, considering the gaseous air pollutants, the estimated associations between these pollutants (e.g. SO2, NO, NO2, and O3) with specific causes of mortality in long-term are still unclear. Thus, we decided to carry out a comprehensive literature review of cohort studies and perform a meta-analysis on the long-term association between particulate and gaseous air pollutants with all-cause and specific-cause of mortality. This review also systematically summarizes the effects of air pollution and mortality based on latest published evidence. The main goal of this systematic review and meta-analysis was to summarize the findings of cohort studies linked to air pollution with all-cause, cardiovascular, respiratory, and lung cancer mortality.
Method
Search of studies and selection
The current meta-analysis complies with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) methods (Table S1). The protocol of this study was registered on PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=422945 with registration code = CRD42023422945). Electronic databases including Google Scholar, PubMed, Scopus, and Web of Science were independently searched by two researchers (B.K. and S.S.) to obtain the eligible studies up to 30 May 2022. The search for relevant literature was conducted with no restriction for regarding language, or publication date using appropriate keywords in the title and abstract, as well as Medical Subject Headings (MeSH). The search strategy is detailed in Appendix A (Supplementary Table). The reference lists of relevant studies were investigated for additional papers. We applied a combination of the following keywords: “air pollution”, “air pollutant*”, “particulate*”, “particle*”, "PM10", "PM2.5", “nitrogen oxide*”, “nitrogen dioxide”, “NO2”, “sulfur dioxide”, “SO2”, “black carbon”, “BC”, “ozone”, “O3”, “carbon monoxide”, “CO” linked with “mortality”, “all-cause mortality”, “cardiovascular mortality”, “respiratory mortality”, “lung cancer mortality”. The search was narrowed to prospective and retrospective cohort studies.
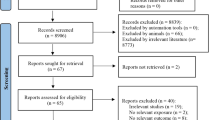
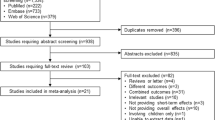
The title and abstract of the studies were screened and the full text of the selected studies was investigated for further assessment. The following inclusion criteria were applied for the extraction of information: (1) cohort studies explored the risk of mortality for all-cause, cardiovascular, respiratory, and lung cancer (2) studies investigated the exposure concentrations to PM10, PM2.5, BC, NO2, NO, SO2 and O3 (3) studies provided information about relative risk (RR), hazard ratio (HR), odds ratio (OR) and beta slope of regression models with 95% CIs (Confidence Intervals) which linked with air pollutants. All included studies clearly described the outcomes according to the International Classification of Diseases (ICD) codes including ICD-10 A00-R99 or ICD-9 001–779 to describe all-causes mortality, ICD-10 I10–I70 or ICD-9 400–440 to explain cardiovascular mortality, ICD-10 J00–J99 or ICD-9 460–519 to explain respiratory mortality and ICD-9 C34 to define lung cancer mortality. Review studies, letters to the editor, news articles, poster and conference abstracts were excluded. The detailed stepwise literature selection is presented in Fig. 1.
Collection of data
Two investigators (B.K and S.S) separately extracted information including study location, study design, publication years, sample size, period of follow-up, method of exposure measurement, the concentration of air pollutants and their corresponding standard deviations, risk estimates of outcomes (RR, OR, HR) and their associated 95% CIs, mortality diagnosis by ICD codes, death rate, main findings and adjustment covariates (Table 1). We also reviewed the extracted data by the authors for quality control and assurance.
Risk of bias assessment
The risk of bias and internal validity of studies was assessed by the Office of Health Assessment and Translation (OHAT) method as suggested by the National Institutes of Environmental Health Sciences-National Toxicology program [32]. Six domains including selection bias, confounders variable, exposure measurement bias, assessment of outcome, selective reporting bias, and missing data bias were evaluated. The risk of bias is categorized per domain as “low”, “probably low”, “probably high”, “high” and “not applicable” (Table 3 and see additional Table S2-S89). Furthermore, the Newcastle–Ottawa Quality Assessment Scale (NOQAS) method was also applied to evaluate the methodological quality of the selected studies. The methodological quality for each study is based on estimated scores categorized as “ ≥ 7, high”, “4–6, intermediate” and “ ≤ 3, low” (see Table S90- S91).
Data synthesis
Risk estimates obtained from included studies were expressed as RR, OR, HR, or beta (β) coefficients of regression. Estimates of OR, and HR were converted to RR by the following formulas:
where r is the rate of death among the reference group. If r was not proved in the studies, the Human Mortality Database was used to obtain death rates based on gender, and age, and the year of study [28, 33]. The percent change of mortality is computed from the following equation (Percent change (%) = (RR-1) × 100%) [6, 33]. To obtain the RR of mortality from the beta (β) coefficients, we exponentiated the regression coefficient. Additionally, 95% Cl was estimated using an exponentiated regression coefficient with their standard error [β ± (1.96*SE)] [6, 34].
When exposure measurements were reported as ppb or ppm, these findings converted to μg/m3 as follows: NO2, 1 ppb = 1.88 μg/m3; O3, 1 ppb = 1.96 μg/m3; SO2, 1 ppb = 2.66 μg/m3; CO, 1 ppb = 1.15 μg/m3 [35]. If studies did not report the RR based on a unit of 10 μg/m3 increment in each pollutant, the estimates of RR across studies were standardized using the following formula [34]:
Meta-analysis
Statistical analyses and forest plots were created by STATA12 and R version 3.6.1. The pooled effect (RR) of studies was computed using the random-effect and fixed-effect model based on the Mantel–Haenszel procedure. The presence of statistical heterogeneity between the estimated effect of studies was evaluated by I2 and Cochran’s Q-test (Significance level < 0.1). The I2 value equals 25% indicating a “low” degree of heterogeneity, 50% moderate, and I2 exceeds 75% suggesting a “high” degree of heterogeneity [36]. To assess the possible sources of heterogeneity, subgroup analyses were performed for sex, mean of age, and study location (Asia, Europe, Canada, and the United States). The presence of publication bias was evaluated by a funnel plot of the log RR against the standard error (SE). Funnel plot asymmetry was also tested by Egger's test with a significance level < 0.10. We applied the trim-and-fill method for detecting and adjusting the publication bias in our meta-analysis [6, 36]. Sensitivity analyses were achieved to investigate the robustness of our main analyses by analyzing the impact of excluding each study on the consistency of the results.
Results and discussion
Studies included
After searching databases, 3692 records were recognized. Eighteen publications were also added from the reference lists. Removing the duplicates and unrelated articles after the screening of abstracts, 127 studies were eligible to assess the title and full text. Finally, 88 articles were included in the meta-analysis and fulfilled the quality assessment criteria (Fig. 1) [14, 23,24,25,26,27, 29, 37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116]. Eighty-one studies involved an adult population with both genders, and five studies included only adult females [44, 67, 80, 86, 110] and two studies included only adult males [64, 75]. Among the included studies, 66 studies investigated all-cause mortality, 64 studies evaluated cardiovascular mortality, 37 studies assessed respiratory mortality, and 31 studies considered lung cancer mortality as an outcome. The included studies were carried out in Europe (31 studies), the United States (27 studies), Canada (8 studies), and Asia (22 studies) (Fig. 2A and B). The total number of participants in these studies was 270877792 individuals (Table 1).
The concentrations of pollutants and methods of exposure assessment are diverse between the studies. The overall mean concentration of PM2.5 derived from studies was 19.47 ± 4.26 µg/m3, with a mean concentration of 39.81 ± 0.78 µg/m3 in Asia, 14.92 ± 2.20 µg/m3 in Europe, 19.69 ± 3.33 µg/m3 in the USA, and 10.7 ± 2.08 µg/m3 in Canada, (Fig. 3, See more in Table S3). Pooled average concentrations of PM10, NO2, SO2, and O3 derived from studies were 45.3 ± 17.68, 23.54 ± 8.92, 16.1 ± 4.65 and 60.67 ± 14.96 μg/m3, respectively (Table 2). We found higher levels of air pollution in Asian developing countries (Fig. 3).
Methodological quality and risk of bias
The risk of bias rating based on investigated studies is presented in Table 3 (also see more detailed of risk of bias assessment in supplementary Table S4-S91). All individual studies had a high quality. The findings showed that the risk of bias for exposure measurement, assessment of outcome, and selective reporting bias differed among the studies. Selection bias for one study was rated as “probably high” risk [42] while other studies were “low” risk. For exposure measurement bias, 7 studies were rated as “probably high” risk because the sampling technique of these studies was not clearly described [27, 29, 42, 54,55,56,57]. Outcome assessment bias for 11 studies rated as “probably high” risk [41, 42, 51, 53, 57,58,59, 81,82,83,84]. The selective reporting bias of 10 studies rated as “probably high” risk [29, 41, 42, 48, 53,54,55, 57,58,59]. Results of the risk of bias assessment are displayed in Table 3, and summary tables justify for the judgments given for each bias domain presented in supplementary Table S4-S91.
Air pollutants and mortality
This is a meta-analysis investigating the relationship between exposure to air pollutants with the risk of mortality for all-cause, cardiovascular, respiratory, and lung cancer. We used data derived from 88 cohort studies carried out in 20 countries involving more than 270 million subjects. Using a random-effect model, exposure to PM2.5, PM10, NO2, and SO2 was strongly linked with mortality, whereas O3 was not significantly related. The pooled estimates of all-cause mortality with air pollutants are presented in Table 4. Positive and significant associations were observed between the percent changes of all-cause mortality with a 10 μg/m3 increment in PM2.5 (RR 1.08, 95% CI 1.07–1.09), PM10 (RR 1.10, 95% CI 1.06–1.14), and BC (RR 1.04, 95% CI 1.02–1.07) (Fig. 4a-c). The estimates of heterogeneity between studies were found to be high for PM2.5, PM10, and BC (Table 4, see Forest plot in Supplementary Fig. S1-7). Elevated risk of all-cause mortality significantly linked with a 10 μg/m3 increment in NO2 (RR 1.04, 95% CI 1.03–1.06), NOx (RR 1.02, 95% CI 1.01–1.04) and SO2 (RR 1.03, 95% CI 1.00–1.06) (Fig. 4a-c), but the association was not significant for O3 (RR 0.98, 95% CI 0.97–1.01) (Table 4, see Forest plot in Supplementary Fig. S1-7). Heterogeneities between the studies were found to be high for NOX, NO2, SO2, and O3.
The highest elevated risk of cardiovascular mortality was significantly linked with a 10 μg/m3 increment in PM10 (RR 1.15, 95% CI 1.08–1.22), the second highest with NO2 (RR 1.06, 95% CI 1.04–1.08), followed by SO2 (RR 1.06, 95% CI 1.01–1.11), PM2.5 (RR 1.06, 95% CI 1.05–1.06), NOx (RR 1.03, 95% CI 1.01–1.06), and BC (RR 1.03, 95% CI 1.01–1.05) (Fig. 4a-e). A non-significant association was observed between the risk of cardiovascular mortality with exposure to O3 (RR 0.99, 95% CI 0.95–1.03) (Table 4, see Forest plot in Supplementary Fig. S8-14). The relationships between the risk of cardiovascular mortality with exposure to PM10, PM2.5, BC, NO2, NOx, SO2, and O3 were investigated by 46, 17, 13, 24, 5, 9, and 9 studies, with “high” levels of heterogeneities (I2 > 75%) (Table 5).
Significant positive relations were observed between the risk of respiratory mortality with a 10 μg/m3 increment in PM2.5 (RR 1.066, 95% CI 1.034–1.097), PM10 (RR 1.196, 95% CI 1.114–1.279), BC (RR 1.048, 95% CI 1.025–1.07) and NO2 (RR 1.061, 95% CI 1.033–1.089), except for NOx (RR 1.026, 95%CI 0.998–1.055), SO2 (RR 1.041, 95% CI 0.964–1.118) and O3 (RR 0.971, 95% CI 0.944–0.998) (Table 4, Supplementary Fig. S15-21).
We observed significant relations between the risk of lung cancer mortality with a 10 μg/m3 increment in PM2.5 (RR 1.118, 95% CI 1.076–1.159, I2 77.40%, τ2 0.0042), PM10 (RR 1.127, 95%CI 1.029–1.224), BC (RR 1.048, 95% CI 1.025–1.07), NO2 (RR 1.067, 95%CI 1.039–1.095), NOx (RR 1.057, 95%CI, 1.005–1.11), and SO2 (RR 1.087, 95% CI 1.011–1.163). Applying the forest plot, the risk of lung cancer mortality was negatively related to exposure to O3 (RR 0.921, 95% CI 0.865–0.978) (Table 4, Supplementary Fig. S22-28).
Exposure to PM2.5 per 10 µg/m3 increment is associated with an elevated risk of mortality for all-cause (8%), cardiovascular (6%), respiratory (7%), and lung cancer (11.8%). Less or more the same associations have been reported between exposure to PM2.5 and all-cause mortality by two other studies [117, 118]. Our finding was higher than that (3.9%) obtained by Hart et al. [119], but considerably lower than those findings (53% and 17%) reported by studies conducted in China and the United States [37, 120]. The association between exposure to PM2.5 and cardiovascular mortality in the current study was comparable to that finding previously reported by Pope Lii et al. [90], but higher than the result obtained by a cohort study conducted in the United States [121]. The same but not significantly elevated risk was reported by Beelen et al. [69]. Consistent with our findings, several studies found an elevated relationship between exposure to PM2.5 with mortality due to respiratory disease and lung cancer [69, 80, 122, 123]. Air pollution related to traffic is likely to contribute as a source of PM2.5, which possibly leads to elevated deaths due to lung cancer [124].
The association between exposure to PM10 and all-cause mortality in the present study (10%) was considerably lower than the result reported by a previous study of meta-analysis (18%) [28], but the association was substantially higher than the estimated percent changes of 5% and 3.9% obtained two studies [36, 119]. The onset of mortality associated with exposure to PM2.5 and PM10 could be explained through numerous underlying mechanisms such as oxidative stress and systemic inflammation, which leads to direct neurotoxicity, hormonal dysregulation, promotion of cell turnover, epigenetic changes in the genome, suppression of DNA repair, DNA methylation, and consequently prompt carcinogenesis [125,126,127,128,129,130]. PM2.5 has also improved the production of inflammatory cytokines (interleukin (IL) -6 and IL-8) due to the mitochondrial generation of hydroxyl radical (•OH) as a reactive oxygen species (ROS) [126, 131,132,133,134,135].
The estimated percent changes of all-cause mortality per 10 μg/m3 increment in exposure to NO2 (4.5%), and SO2 (3.5%) in the present study were less or more similar to those findings obtained by meta-analyses studies [36, 136, 137]. In the present investigation, the significant relations between exposure to NO2 with all-cause (4.5%), cardiovascular (6.3%), and respiratory (6.1%) mortality were markedly higher than those findings reported by an earlier study of meta-analysis including all-cause (1.58%), cardiovascular (1.72%) and respiratory (2.05%) mortality [30]. Findings of a recent pooled analysis of 67 studies showed elevated associations between exposure to SO2 with respiratory (1.0067) and all-cause (1.0059) mortality, but the findings were lower than our results [31]. Our finding for all-cause mortality was substantially lower than the previous results obtained for NO2 (8.2% and 14%) and SO2 6.9% [79]. NO2 and SO2 can exacerbate the effects of oxidative stress and promote the progression of respiratory disease and lung cancers [138, 139].
No significant associations in the present study were found between exposure to O3 with all-cause (RR 0.99) and respiratory (RR 0.97). Similar results were reported by a study of meta-analysis for all-cause (RR 0.97) and respiratory (RR 0.99) mortality [136]. The same findings were also reported by a previous study [140].
Subgroup analyses
Figure 4A–E presents the pooled effects of air pollutants on cause-specific mortality with stratification for sex (male and female) and age (< 0–64 and ≥ 65). The RR of all-cause mortality related to PM2.5 exposure was highest in males (RR per 10 µg/m3 = 1.075, 95% CI = 1.030–1.09) compared to the female (1.039, 95% CI: 1.02–1.058) and individuals with age ≥ 65 years (RR per 10 µg/m3 = 1.046, 95% CI = 1.005–1.075), compared to the individuals with age < 64 years (1.028, 95% CI = 0.965–1.085) (Fig. 4). The RR of all-cause mortality due to PM10, NO2 and SO2 for male and individual age ≥ 65 years were also highest compared to the female and individuals with age < 64 years.
In sex-stratified analysis detected a significant association between exposure to PM2.5, PM10, BC NO2, and SO2, and mortality in males compared with females, but no significant association was found for O3. This finding could be related to diverse physiological functions in men and women. Moreover, individual characteristics (e.g., smoking, physical activity, alcohol consumption, work- exposures, etc.) are an important risk factor in air pollution-related mortality [122, 141]. Similarly, the risk of mortality from coronary heart disease, cardiorespiratory disease, and myocardial infarction, which is attributed to air pollution was higher in males and the elderly [142, 143].
Individual over 65 years old was more susceptible to ambient PM2.5, NO2, and SO2 exposure, while the younger (< 65 years old) age were more susceptible to BC and O3. Previous studies supported these findings [141, 142, 144, 145]. The elderly are typically more exposed to outdoor air pollution compared to the younger age [141, 146]. The physiological structures and body functions diminish with age, which might enhance the risks of air pollution-related mortality among the elderly [147].
Additional analyses
According to Egger’s test, we found significant publication bias for O3 (P-value 0.03), SO2 (P-value 0.005), and NOx (P-value 0.012) with all-cause mortality, but not for PM2.5 (P-value 0.52), PM10 (P-value 0.33) and BC (P-value 0.44). The sub-stratified analysis of the association between all-cause mortality with PM2.5 by location illustrated that the RR was higher in studies conducted in Canada (RR 1.17, 95% CI 1.15–1.20, I2 68.0%, p-value 0.008) compared with Asia (RR 1.04, 95% CI 1.01–1.08, I2 98.1%, p-value 0.000) and United states (1.08, 95% CI 1.06–1.09, I2 91.8%, p-value 0.000) (Supplementary Fig. S29-S35).
The trim and fill method result shows that about 7 records are essential to creating a complete asymmetry in the Funnel plot (P-value < 0.001) of All-Cause with PM2.5 (Table 5, Supplementary Fig. S36-S59). The graphical funnel plots appeared to be slightly asymmetrical for exposure to PM10, BC, NOx, O3, and SO2, suggesting the presence of publication bias for studies (p-value ≤ 0.05). Sensitivity analyses showed that the results were stable for the combination of pollutants with all-cause cardiovascular, and respiratory mortality, and the pooled RR did not alter when any individual record was excluded, indicating the robustness of the results (Table 5).
The current study has some limitations. First, we included only cohort studies, and other types of study designs such as case–control, cross-sectional, time-series, or case-crossover designs were not evaluated. Second, most of the included studies were performed in developed countries especially in urban areas of Europe and North America, while there was no study available for African countries. Third, the exposure measurements were conducted in outdoor environments, and indoor air pollution (e.g. home, school, office) was not considered. Fourth, we were also unable to perform meta-regression analyses to identify the sources of heterogeneity for some air pollutants due to low sample size.
Conclusion
The most considered air pollutants were associated with an increased risk of mortality due to cardiovascular and respiratory diseases, but the effects of PM10 and PM2.5 were stronger. Exposure to PM10 and PM2.5 is the predominant factor for mortality risk, contributing to RR 1.104 and 1.08 in all-cause mortality and RR 1.149 and 1.058 in cardiovascular mortality. The highest respiratory and lung cancer mortality was associated with exposure to PM10 (1.196 and 1.127), followed by PM2.5 (RR 1.066 and 1.118). The male and elderly adults seemed to be more susceptible to exposure to particle air pollution compared to the female and younger age groups. Thus, policymakers need to pay more attention to establishing new regulations and intervention strategies to enhance air quality. This subsequently leads to a diminishing of morbidity and mortality. Further population-based studies in this field are required to enhance the understanding of the adverse health effects of air pollution among vulnerable subgroups.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wang K, Wang W, Lei L, Lan Y, Liu Q, Ren L, Wu S. Association between short-term exposure to ambient air pollution and biomarkers of coagulation: A systematic review and meta-analysis. Environ Res. 2022;215:114210.
Vohra K, Vodonos A, Schwartz J, Marais EA, Sulprizio MP, Mickley LJ. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: Results from GEOS-Chem. Environ Res. 2021;195: 110754.
Salud, O.M.d.l., Weltgesundheitsorganisation, Organization, W.H. and Environment, E.C.f. WHO global air quality guidelines: particulate matter (PM2. 5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. World Health Organization; 2021.
Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Prüss-Ustün A, Lahiff M, Rehfuess EA, Mishra V, Smith KR. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environ Health Perspect. 2013;121:784–90.
Karimi B, Shokrinezhad B. Air pollution and the number of daily deaths due to respiratory causes in Tehran. Atmos Environ. 2021;246: 118161.
Karimi B, Shokrinezhad B. Air pollution and mortality among infant and children under five years: a systematic review and meta-analysis. Atmos Pollut Res. 2020;11:61–70.
Edwards L, Wilkinson P, Rutter G, Milojevic A. Health effects in people relocating between environments of differing ambient air pollution concentrations: A literature review. Environ Pollut. 2022;292: 118314.
Karimi B, Moradzadeh R, Samadi S. Air pollution and COVID-19 mortality and hospitalization: An ecological study in Iran. Atmos Pollut Res. 2022;13(7):101463.
Bai X, Chen H, Oliver BG. The health effects of traffic-related air pollution: A review focused the health effects of going green. Chemosphere. 2022;289: 133082.
Anenberg SC, Henze DK, Lacey F, Irfan A, Kinney P, Kleiman G, Pillarisetti A. Air pollution-related health and climate benefits of clean cookstove programs in Mozambique. Environ Res Lett. 2017;12: 025006.
Orellano P, Reynoso J, Quaranta N, Bardach A, Ciapponi A. Short-term exposure to particulate matter (PM10 and PM2. 5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ Int. 2020;142:105876.
Karimi B, Shokrinezhad B. Spatial variation of ambient PM2. 5 and PM10 in the industrial city of Arak, Iran: A land-use regression. Atmos Pollut Res. 2021;12:101235.
Pope CA III, Coleman N, Pond ZA, Burnett RT. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ Res. 2020;183: 108924.
Pope CA III, Lefler JS, Ezzati M, Higbee JD, Marshall JD, Kim S-Y, Bechle M, Gilliat KS, Vernon SE, Robinson AL. Mortality risk and fine particulate air pollution in a large, representative cohort of US adults. Environ Health Perspect. 2019;127: 077007.
Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2054–70.
Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The Lancet. 2017;389:1907–18.
Shin S, Bai L, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Lavigne E, Weichenthal S, Copes R, Martin RV. Air pollution as a risk factor for incident chronic obstructive pulmonary disease and asthma. A 15-year population-based cohort study. Am J Respir Crit Care Med. 2021;203:1138–48.
Turner MC, Krewski D, Diver WR, Pope CA III, Burnett RT, Jerrett M, Marshall JD, Gapstur SM. Ambient air pollution and cancer mortality in the cancer prevention study II. Environ Health Perspect. 2017;125: 087013.
Merhi Z, Garg B, Moseley-LaRue R, Moseley AR, Smith AH, Zhang J. Ozone therapy: a potential therapeutic adjunct for improving female reproductive health. Med Gas Res. 2019;9:101.
Rappazzo KM, Nichols JL, Rice RB, Luben TJ. Ozone exposure during early pregnancy and preterm birth: A systematic review and meta-analysis. Environ Res. 2021;198: 111317.
Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. 2020;8:1–13.
Zhao L, Liang H-R, Chen F-Y, Chen Z, Guan W-J, Li J-H. Association between air pollution and cardiovascular mortality in China: a systematic review and meta-analysis. Oncotarget. 2017;8:66438.
Peng Z, Liu C, Xu B, Kan H, Wang W. Long-term exposure to ambient air pollution and mortality in a Chinese tuberculosis cohort. Sci Total Environ. 2017;580:1483–8.
Hales S, Atkinson J, Metcalfe J, Kuschel G, Woodward A. Long term exposure to air pollution, mortality and morbidity in New Zealand: cohort study. Sci Total Environ. 2021;801: 149660.
Bauwelinck M, Chen J, de Hoogh K, Katsouyanni K, Rodopoulou S, Samoli E, Andersen ZJ, Atkinson R, Casas L, Deboosere P. Variability in the association between long-term exposure to ambient air pollution and mortality by exposure assessment method and covariate adjustment: a census-based country-wide cohort study. Sci Total Environ. 2022;804: 150091.
So R, Andersen ZJ, Chen J, Stafoggia M, de Hoogh K, Katsouyanni K, Vienneau D, Rodopoulou S, Samoli E, Lim Y-H. Long-term exposure to air pollution and mortality in a Danish nationwide administrative cohort study: Beyond mortality from cardiopulmonary disease and lung cancer. Environ Int. 2022;164: 107241.
Krewski D, Burnett R, Jerrett M, Pope CA, Rainham D, Calle E, Thurston G, Thun M. Mortality and long-term exposure to ambient air pollution: ongoing analyses based on the American Cancer Society cohort. J Toxicol Environ Health A. 2005;68:1093–109.
Kim H-B, Shim J-Y, Park B, Lee Y-J. Long-term exposure to air pollutants and cancer mortality: a meta-analysis of cohort studies. Int J Environ Res Public Health. 2018;15:2608.
Tseng E, Ho W-C, Lin M-H, Cheng T-J, Chen P-C, Lin H-H. Chronic exposure to particulate matter and risk of cardiovascular mortality: cohort study from Taiwan. BMC Public Health. 2015;15:1–9.
Wang M, Li H, Huang S, Qian Y, Steenland K, Xie Y, Papatheodorou S, Shi L. Short-term exposure to nitrogen dioxide and mortality: a systematic review and meta-analysis. Environ Res. 2021;202: 111766.
Orellano P, Reynoso J, Quaranta N. Short-term exposure to sulphur dioxide (SO2) and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ Int. 2021;150: 106434.
OHAT. Handbook for conducting a literature-based health assessment using OHAT approach for systematic review and evidence integration. Office of Health Assessment and Translation, Division of National Toxicology Program, National Institute of Environmental Health Sciences; 2015.
Shor E, Roelfs D, Vang ZM. The “Hispanic mortality paradox” revisited: Meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants’ mortality. Soc Sci Med. 2017;186:20–33.
Borroni E, Pesatori AC, Bollati V, Buoli M, Carugno M. Air pollution exposure and depression: A comprehensive updated systematic review and meta-analysis. Environ Pollut. 2022;292: 118245.
Hu C-Y, Gao X, Fang Y, Jiang W, Huang K, Hua X-G, Yang X-J, Chen H-B, Jiang Z-X, Zhang X-J. Human epidemiological evidence about the association between air pollution exposure and gestational diabetes mellitus: Systematic review and meta-analysis. Environ Res. 2020;180: 108843.
Karimi B, Samadi S. Mortality and hospitalizations due to cardiovascular and respiratory diseases associated with air pollution in Iran: A systematic review and meta-analysis. Atmos Environ. 2019;198:438–47.
Jerrett M, Finkelstein MM, Brook JR, Arain MA, Kanaroglou P, Stieb DM, Gilbert NL, Verma D, Finkelstein N, Chapman KR. A cohort study of traffic-related air pollution and mortality, Canada. Environ Health Perspect. 2009;117:772–7.
Li T, Zhang Y, Wang J, Xu D, Yin Z, Chen H, Lv Y, Luo J, Zeng Y, Liu Y. All-cause mortality risk associated with long-term exposure to ambient PM2· 5 in China: a cohort study. Lancet Public Health. 2018;3:e470-7.
Kim I-S, Yang P-S, Lee J, Yu HT, Kim T-H, Uhm J-S, Kim J-Y, Pak H-N, Lee M-H, Joung B. Longterm fine particulate matter exposure and cardiovascular mortality in the general population: a nationwide cohort study. J Cardiol. 2020;75:549–58.
Yang Y, Tang R, Qiu H, Lai P-C, Wong P, Thach T-Q, Allen R, Brauer M, Tian L, Barratt B. Long term exposure to air pollution and mortality in an elderly cohort in Hong Kong. Environ Int. 2018;117:99–106.
Zhang Y, Li Z, Wei J, Zhan Y, Liu L, Yang Z, Zhang Y, Liu R, Ma Z. Long-term exposure to ambient NO2 and adult mortality: a nationwide cohort study in China. J Adv Res. 2022a.
Villeneuve PJ, Goldberg MS, Krewski D, Burnett RT, Chen Y. Fine particulate air pollution and all-cause mortality within the Harvard six-cities study: variations in risk by period of exposure. Ann Epidemiol. 2002;12:568–76.
Zhang Z, Wang J, Kwong JC, Burnett RT, van Donkelaar A, Hystad P, Martin RV, Bai L, McLaughlin J, Chen H. Long-term exposure to air pollution and mortality in a prospective cohort: the Ontario health study. Environ Int. 2021;154.
So R, Jørgensen JT, Lim Y-H, Mehta AJ, Amini H, Mortensen LH, Westendorp R, Ketzel M, Hertel O, Brandt J. Long-term exposure to low levels of air pollution and mortality adjusting for road traffic noise: a danish nurse cohort study. Environment International. 2020;143.
Klompmaker JO, Hoek G, Bloemsma LD, Marra M, Wijga AH, van den Brink C, Brunekreef B, Lebret E, Gehring U, Janssen NA. Surrounding green, air pollution, traffic noise exposure and non-accidental and cause-specific mortality. Environ Int. 2020;134.
Hanigan IC, Rolfe MI, Knibbs LD, Salimi F, Cowie CT, Heyworth J, Marks GB, Guo Y, Cope M, Bauman A. All-cause mortality and long-term exposure to low level air pollution in the ‘45 and up study’cohort, Sydney, Australia, 2006–2015. Environ Int. 2019;126:762–70.
Crouse DL, Pinault L, Balram A, Brauer M, Burnett RT, Martin RV, Van Donkelaar A, Villeneuve PJ, Weichenthal S. Complex relationships between greenness, air pollution, and mortality in a population-based Canadian cohort. Environ Int. 2019;128:292–300.
Dehbi H-M, Blangiardo M, Gulliver J, Fecht D, De Hoogh K, Al-Kanaani Z, Tillin T, Hardy R, Chaturvedi N, Hansell AL. Air pollution and cardiovascular mortality with over 25 years follow-up: a combined analysis of two British cohorts. Environ Int. 2017;99:275–81.
Xi Y, Richardson DB, Kshirsagar AV, Wade TJ, Flythe JE, Whitsel EA, Peterson GC, Wyatt LH, Rappold AG. Effects of short-term ambient PM2. 5 exposure on cardiovascular disease incidence and mortality among US hemodialysis patients: a retrospective cohort study. Environ Health. 2022;21:1–12.
Li Z-H, Wang X-M, Chen Y-J, Zhang P-D, Shen D, Zhang X-R, Zhong W-F, Chen P-L, Huang Q, Song W. Association between long-term concomitant exposure to various ambient air pollutants and all-cause and cause-specific mortality: Data from a nationwide prospective cohort study.
Weichenthal S, Pinault LL, Burnett RT. Impact of oxidant gases on the relationship between outdoor fine particulate air pollution and nonaccidental, cardiovascular, and respiratory mortality. Sci Rep. 2017;7:1–10.
Jalali S, Karbakhsh M, Momeni M, Taheri M, Amini S, Mansourian M, Sarrafzadegan N. Long-term exposure to PM2. 5 and cardiovascular disease incidence and mortality in an eastern Mediterranean country: findings based on a 15-year cohort study. Environ Health. 2021;20:1–16.
Christidis T, Erickson AC, Pappin AJ, Crouse DL, Pinault LL, Weichenthal SA, Brook JR, van Donkelaar A, Hystad P, Martin RV. Low concentrations of fine particle air pollution and mortality in the Canadian Community Health Survey cohort. Environmental Health. 2019;18:1–16.
Dimakopoulou K, Samoli E, Beelen R, Stafoggia M, Andersen ZJ, Hoffmann B, Fischer P, Nieuwenhuijsen M, Vineis P, Xun W. Air pollution and nonmalignant respiratory mortality in 16 cohorts within the ESCAPE project. Am J Respir Crit Care Med. 2014;189:684–96.
Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187:721–7.
Carey IM, Atkinson RW, Kent AJ, Van Staa T, Cook DG, Anderson HR. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med. 2013;187:1226–33.
Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F, Schwartz JD. Air pollution and mortality in the Medicare population. N Engl J Med. 2017;376:2513–22.
Lefler JS, Higbee JD, Burnett RT, Ezzati M, Coleman NC, Mann DD, Marshall JD, Bechle M, Wang Y, Robinson AL. Air pollution and mortality in a large, representative US cohort: multiple-pollutant analyses, and spatial and temporal decompositions. Environ Health. 2019;18:1–11.
Sanyal S, Rochereau T, Maesano CN, Com-Ruelle L, Annesi-Maesano I. Long-term effect of outdoor air pollution on mortality and morbidity: a 12-year follow-up study for metropolitan France. Int J Environ Res Public Health. 2018;15:2487.
Kim O-J, Kim S-Y, Kim H. Association between long-term exposure to particulate matter air pollution and mortality in a south Korean National Cohort: comparison across different exposure assessment approaches. Int J Environ Res Public Health. 2017;14:1103.
Zhang P, Dong G, Sun B, Zhang L, Chen X, Ma N, Yu F, Guo H, Huang H, Lee YL. Long-term exposure to ambient air pollution and mortality due to cardiovascular disease and cerebrovascular disease in Shenyang. China PloS One. 2011;6.
Enstrom JE. Fine particulate air pollution and total mortality among elderly Californians, 1973–2002. Inhal Toxicol. 2005;17:803–16.
Héritier H, Vienneau D, Foraster M, Eze IC, Schaffner E, de Hoogh K, Thiesse L, Rudzik F, Habermacher M, Köpfli M. A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: a nationwide cohort study in Switzerland. Eur Heart J. 2019;40:598–603.
Yin P, Brauer M, Cohen A, Burnett RT, Liu J, Liu Y, Liang R, Wang W, Qi J, Wang L. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese men. Environ Health Perspect. 2017;125:117002.
Fischer PH, Marra M, Ameling CB, Hoek G, Beelen R, de Hoogh K, Breugelmans O, Kruize H, Janssen NA, Houthuijs D. Air pollution and mortality in seven million adults: the Dutch environmental longitudinal study (DUELS). Environ Health Perspect. 2015;123:697–704.
Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, Forastiere F. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121:324–31.
Ostro B, Lipsett M, Reynolds P, Goldberg D, Hertz A, Garcia C, Henderson KD, Bernstein L. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California teachers study. Environ Health Perspect. 2010;118:363–9.
Zeger SL, Dominici F, McDermott A, Samet JM. Mortality in the Medicare population and chronic exposure to fine particulate air pollution in urban centers (2000–2005). Environ Health Perspect. 2008;116:1614–9.
Beelen R, Hoek G, van Den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, Jerrett M, Hughes E, Armstrong B, Brunekreef B. Long-term effects of traffic-related AIR pollution on mortality in a Dutch cohort (NLCS-AIR study). Environ Health Perspect. 2008;116:196–202.
Bentayeb M, Wagner V, Stempfelet M, Zins M, Goldberg M, Pascal M, Larrieu S, Beaudeau P, Cassadou S, Eilstein D. Association between long-term exposure to air pollution and mortality in France: a 25-year follow-up study. Environ Int. 2015;85:5–14.
Cesaroni G, Porta D, Badaloni C, Stafoggia M, Eeftens M, Meliefste K, Forastiere F. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health. 2012;11:1–10.
Strak M, Weinmayr G, Rodopoulou S, Chen J, De Hoogh K, Andersen ZJ, Atkinson R, Bauwelinck M, Bekkevold T, Bellander T. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: pooled analysis. bmj. 2021:374.
Abbey DE, Nishino N, McDonnell WF, Burchette RJ, Knutsen SF, Lawrence Beeson W, Yang JX. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159:373–82.
Cao J, Yang C, Li J, Chen R, Chen B, Gu D, Kan H. Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J Hazard Mater. 2011;186:1594–600.
Zhou M, Liu Y, Wang L, Kuang X, Xu X, Kan H. Particulate air pollution and mortality in a cohort of Chinese men. Environ Pollut. 2014;186:1–6.
Pope CA, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW. Particulate air pollution as a predictor of mortality in a prospective study of US adults. Am J Respir Crit Care Med. 1995;151:669–74.
Raaschou-Nielsen O, Andersen ZJ, Jensen SS, Ketzel M, Sørensen M, Hansen J, Loft S, Tjønneland A, Overvad K. Traffic air pollution and mortality from cardiovascular disease and all causes: a Danish cohort study. Environ Health. 2012;11:1–12.
Hales S, Blakely T, Woodward A. Air pollution and mortality in New Zealand: cohort study. J Epidemiol Community Health. 2012;66:468–73.
Filleul L, Rondeau V, Vandentorren S, Le Moual N, Cantagrel A, Annesi-Maesano I, Charpin D, Declercq C, Neukirch F, Paris C. Twenty five year mortality and air pollution: results from the French PAARC survey. Occup Environ Med. 2005;62:453–60.
Heinrich J, Thiering E, Rzehak P, Krämer U, Hochadel M, Rauchfuss KM, Gehring U, Wichmann H-E. Longterm exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup Environ Med. 2013;70:179–86.
Jerrett M, Burnett RT, Ma R, Pope CA III, Krewski D, Newbold KB, Thurston G, Shi Y, Finkelstein N, Calle EE. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005:727–36.
Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet. 2002;360:1203–9.
Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer P, Nieuwenhuijsen M. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383:785–95.
Lipfert F, Wyzga R, Baty J, Miller J. Traffic density as a surrogate measure of environmental exposures in studies of air pollution health effects: long-term mortality in a cohort of US veterans. Atmos Environ. 2006;40:154–69.
Coleman CJ, Yeager RA, Pond ZA, Riggs DW, Bhatnagar A, Pope CA III. Mortality risk associated with greenness, air pollution, and physical activity in a representative US cohort. Sci Total Environ. 2022;824:153848.
Gehring U, Heinrich J, Krämer U, Grote V, Hochadel M, Sugiri D, Kraft M, Rauchfuss K, Eberwein HG, Wichmann H-E. Long-term exposure to ambient air pollution and cardiopulmonary mortality in women. Epidemiology. 2006:545–51.
Eftim SE, Samet JM, Janes H, McDermott A, Dominici F. Fine particulate matter and mortality: a comparison of the six cities and American Cancer Society cohorts with a medicare cohort. Epidemiology. 2008:209–16.
Hansell A, Ghosh RE, Blangiardo M, Perkins C, Vienneau D, Goffe K, Briggs D, Gulliver J. Historic air pollution exposure and long-term mortality risks in England and Wales: prospective longitudinal cohort study. Thorax. 2016;71:330–8.
Bauleo L, Bucci S, Antonucci C, Sozzi R, Davoli M, Forastiere F, Ancona C. Long-term exposure to air pollutants from multiple sources and mortality in an industrial area: a cohort study. Occup Environ Med. 2019;76:48–57.
Pope Iii CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama. 2002;287:1132–41.
Stafoggia M, Oftedal B, Chen J, Rodopoulou S, Renzi M, Atkinson RW, Bauwelinck M, Klompmaker JO, Mehta A, Vienneau D. Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: results from seven large European cohorts within the ELAPSE project. Lancet Planet Health. 2022;6:e9–18.
Yazdi MD, Wang Y, Di Q, Requia WJ, Wei Y, Shi L, Sabath MB, Dominici F, Coull B, Evans JS. Longterm effect of exposure to lower concentrations of air pollution on mortality among US Medicare participants and vulnerable subgroups: a doubly-robust approach. Lancet Planet Health. 2021;5:e689-97.
Pope CA III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–7.
Liang R, Chen R, Yin P, van Donkelaar A, Martin RV, Burnett R, Cohen AJ, Brauer M, Liu C, Wang W. Associations of long-term exposure to fine particulate matter and its constituents with cardiovascular mortality: a prospective cohort study in China. Environ Int. 2022;162:107156.
Hystad P, Larkin A, Rangarajan S, AlHabib KF, Avezum Á, Calik KBT, Chifamba J, Dans A, Diaz R, Du Plessis JL. Associations of outdoor fine particulate air pollution and cardiovascular disease in 157 436 individuals from 21 highincome, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet Planet Health. 2020;4:e235-45.
Niu Y, Zhou Y, Chen R, Yin P, Meng X, Wang W, Liu C, Ji JS, Qiu Y, Kan H. Long-term exposure to ozone and cardiovascular mortality in China: a nationwide cohort study. Lancet Planet Health. 2022;6:e496-503.
Pope CA III, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015;116:108–15.
Hayes RB, Lim C, Zhang Y, Cromar K, Shao Y, Reynolds HR, Silverman DT, Jones RR, Park Y, Jerrett M. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int J Epidemiol. 2020;49:25–35.
Chen, X., Wang, X., Huang, J-j., Zhang, L-w., Song, F-j., Mao, H-j., Chen, K-x., Chen, J., Liu, Y-m. Jiang, G-h. Nonmalignant respiratory mortality and long-term exposure to PM10 and SO2: a 12-year cohort study in northern China. Environ Pollut (Barking, Essex : 1987). 2017;231:761–7.
Dong G-H, Zhang P, Sun B, Zhang L, Chen X, Ma N, Yu F, Guo H, Huang H, Lee YL. Long-term exposure to ambient air pollution and respiratory disease mortality in Shenyang, China: a 12-year population-based retrospective cohort study. Respiration. 2012;84:360–8.
Hvidtfeldt UA, Geels C, Sørensen M, Ketzel M, Khan J, Tjønneland A, Christensen JH, Brandt J, RaaschouNielsen O. Long-term residential exposure to PM2.5 constituents and mortality in a Danish cohort. Environ Int. 2019a;133:105268.
Wang Y, Xiao S, Zhang Y, Chang H, Martin RV, Van Donkelaar A, Gaskins A, Liu Y, Liu P, Shi LL. Exposure to PM2.5 major components and mortality in the southeastern United States. Environ Int. 2022;158:106969.
Kazemiparkouhi F, Honda T, Eum K-D, Wang B, Manjourides J, Suh HH. The impact of long-term PM2. 5 constituents and their sources on specific causes of death in a US Medicare cohort. Environ Int. 2022;159:106988.
Beelen R, Hoek G, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer PH. Natural-cause mortality and long-term exposure to particle components: an analysis of 19 European cohorts within the multi-center ESCAPE project. Environ Health Perspect. 2015;123:525–33.
Sommar JN, Hvidtfeldt UA, Geels C, Frohn LM, Brandt J, Christensen JH, Raaschou-Nielsen O, Forsberg B. Long-term residential exposure to particulate matter and its components, nitrogen dioxide and ozone—a northern Sweden cohort study on mortality. Int J Environ Res Public Health. 2021;18:8476.
Chen Y, Chen R, Chen Y, Dong X, Zhu J, Liu C, van Donkelaar A, Martin RV, Li H, Kan H. The prospective effects of long-term exposure to ambient PM2. 5 and constituents on mortality in rural East China. Chemosphere. 2021a;280:130740.
Wang M, Beelen R, Stafoggia M, Raaschou-Nielsen O, Andersen ZJ, Hoffmann B, Fischer P, Houthuijs D, Nieuwenhuijsen M, Weinmayr G. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: results from the ESCAPE and TRANSPHORM projects. Environ Int. 2014;66:97–106.
Liu L, Zhang Y, Yang Z, Luo S, Zhang Y. Long-term exposure to fine particulate constituents and cardiovascular diseases in Chinese adults. J Hazard Mater. 2021;416:126051.
Pond ZA, Saha PK, Coleman CJ, Presto AA, Robinson AL, Pope CA III. Mortality risk and long-term exposure to ultrafine particles and primary fine particle components in a national US cohort. Environ Int. 2022;167:107439.
Ostro B, Hu J, Goldberg D, Reynolds P, Hertz A, Bernstein L, Kleeman MJ. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California teachers study cohort. Environ Health Perspect. 2015;123:549–56.
Badaloni C, Cesaroni G, Cerza F, Davoli M, Brunekreef B, Forastiere F. Effects of long-term exposure to particulate matter and metal components on mortality in the Rome longitudinal study. Environ Int. 2017;109:146–54.
Liu L, Luo S, Zhang Y, Yang Z, Zhou P, Mo S, Zhang Y. Longitudinal impacts of PM2. 5 constituents on adult mortality in China. Environ Sci Technol. 2022.
Pinault LL, Weichenthal S, Crouse DL, Brauer M, Erickson A, van Donkelaar A, Martin RV, Hystad P, Chen H, Finès P. Associations between fine particulate matter and mortality in the 2001 Canadian census health and environment cohort. Environ Res. 2017;159:406–15.
Crouse DL, Peters PA, van Donkelaar A, Goldberg MS, Villeneuve PJ, Brion O, Khan S, Atari DO, Jerrett M, Pope CA III. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: a Canadian national-level cohort study. Environ Health Perspect. 2012;120:708–14.
Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard six cities study from 1974 to 2009. Environ Health Perspect. 2012;120:965–70.
Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P, Nieuwenhuijsen MJ, Brunekreef B. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European study of cohorts for air pollution effects (ESCAPE). Lancet Oncol. 2013;14:813–22.
Hvidtfeldt UA, Sørensen M, Geels C, Ketzel M, Khan J, Tjønneland A, Overvad K, Brandt J, RaaschouNielsen O. Long-term residential exposure to PM2. 5, PM10, black carbon, NO2, and ozone and mortality in a Danish cohort. Environ Int. 2019b;123:265–72.
Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Brook JR. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario. Canada Environ Health Perspect. 2013;121:804–10.
Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. Long-term ambient multipollutant exposures and mortality. American journal of respiratory and critical care medicine. 2011;183:73–8.
Sui X, Zhang J, Zhang Q, Sun S, Lei R, Zhang C, Cheng H, Ding L, Ding R, Xiao C. The short-term effect of PM2. 5/O3 on daily mortality from 2013 to 2018 in Hefei, China. Environ Geochem Health. 2021;43:153–69.
Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–58.
Zhang Y, Li Z, Wei J, Zhan Y, Liu L, Yang Z, Zhang Y, Liu R, Ma Z. Long-term exposure to ambient NO2 and adult mortality: a nationwide cohort study in China. J Adv Res. 2022;41:13–22.
Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, Coelho MS, Saldiva PH, Lavigne E, Matus P. Ambient particulate air pollution and daily mortality in 652 cities. New Engl J Med. 2019;381:705–15.
Yang X, Zhang T, Zhang X, Chu C, Sang S. Global burden of lung cancer attributable to ambient fine particulate matter pollution in 204 countries and territories, 1990–2019. Environ Res. 2022;204:112023.
Alexeeff SE, Liao NS, Liu X, Van Den Eeden SK, Sidney S. Long‐term PM2. 5 exposure and risks of ischemic heart disease and stroke events: review and meta-analysis. J Am Heart Assoc. 2021;10:e016890.
Xing Y-F, Xu Y-H, Shi M-H, Lian Y-X. The impact of PM2. 5 on the human respiratory system. J Thorac Dis. 2016;8:E69.
Fiordelisi A, Piscitelli P, Trimarco B, Coscioni E, Iaccarino G, Sorriento D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail Rev. 2017;22:337–47.
Magalhaes S, Baumgartner J, Weichenthal S. Impacts of exposure to black carbon, elemental carbon, and ultrafine particles from indoor and outdoor sources on blood pressure in adults: A review of epidemiological evidence. Environmental research. 2018;161:345–53.
Magalhaes S, Baumgartner J, Weichenthal S. Impacts of exposure to black carbon, elemental carbon, and ultrafine particles from indoor and outdoor sources on blood pressure in adults: a review of epidemiological evidence. Environ Res. 2018;161:345–53.
Karimi, B. and Samadi, S. Mortality associated with fine particulate and its components: A systematic review and meta-analysis. Atmospheric Pollution Research, 2023, 14, 101648.
Veranth JM, Moss TA, Chow JC, Labban R, Nichols WK, Walton JC, Watson JG, Yost GS. Correlation of in vitro cytokine responses with the chemical composition of soil-derived particulate matter. Environ Health Perspect. 2006;114:341–9.
Chen J, Rodopoulou S, de Hoogh K, Strak M, Andersen ZJ, Atkinson R, Bauwelinck M, Bellander T, Brandt J, Cesaroni G. Long-term exposure to fine particle elemental components and natural and cause-specific mortality—a pooled analysis of eight European cohorts within the ELAPSE project. Environ Health Perspect. 2021;129:04700.
Straif, K., Cohen, A. and Samet, J. IARC scientific publication no. 161: air pollution and cancer. International Agency for Research on Cancer, Lyon. 2013. Available (28 April 2024) from ULR: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Air-Pollution-And-Cancer-2013
Hvidtfeldt UA, Chen J, Andersen ZJ, Atkinson R, Bauwelinck M, Bellander T, Brandt J, Brunekreef B, Cesaroni G, Concin H. Long-term exposure to fine particle elemental components and lung cancer incidence in the ELAPSE pooled cohort. Environ Res. 2021;193:110568.
Pouri N, Karimi B, Kolivand A, Mirhoseini SH. Ambient dust pollution with all-cause, cardiovascular and respiratory mortality: a systematic review and meta-analysis. Sci Total Environ. 2023;168945.
Huangfu P, Atkinson R. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: a systematic review and meta-analysis. Environ Int. 2020;144:105998.
Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manag Assoc. 2002;52:470–84.
Kruk J, Aboul-Enein Y, H. Reactive oxygen and nitrogen species in carcinogenesis: implications of oxidative stress on the progression and development of several cancer types. Mini Rev Med Chem. 2017;17:904–19.
Zhu, M., Du, J., Liu, A.D., Holmberg, L., Tang, C. and Jin, H. Effect of endogenous sulfur dioxide in regulating cardiovascular oxidative stress. 2014.
Atkinson RW, Butland BK, Dimitroulopoulou C, Heal MR, Stedman JR, Carslaw N, Jarvis D, Heaviside C, Vardoulakis S, Walton H. Long-term exposure to ambient ozone and mortality: a quantitative systematic review and metaanalysis of evidence from cohort studies. BMJ Open. 2016;6:e009493.
Guo Y, Zeng H, Zheng R, Li S, Barnett AG, Zhang S, Zou X, Huxley R, Chen W, Williams G. The association between lung cancer incidence and ambient air pollution in China: a spatiotemporal analysis. Environ Res. 2016;144:60–5.
Tsai D-H, Guessous I, Riediker M, Paccaud F, Gaspoz J-M, Theler J-M, Waeber G, Vollenweider P, Bochud M. Short-term effects of particulate matters on pulse pressure in two general population studies. J Hypertens. 2015;33:1144–52.
Hartiala J, Breton CV, Tang WW, Lurmann F, Hazen SL, Gilliland FD, Allayee H. Ambient air pollution is associated with the severity of coronary atherosclerosis and incident myocardial infarction in patients undergoing elective cardiac evaluation. J Am Heart Assoc. 2016;5:e003947.
Zeka A, Sullivan JR, Vokonas PS, Sparrow D, Schwartz J. Inflammatory markers and particulate air pollution: characterizing the pathway to disease. Int J Epidemiol. 2006;35:1347–54.
Chen R, Kan H, Chen B, Huang W, Bai Z, Song G, Pan G. Association of particulate air pollution with daily mortality: the China air pollution and health effects study. Am J Epidemiol. 2012;175:1173–81.
Tong L, Li K, Zhou Q. The association between air pollutants and morbidity for diabetes and liver diseases modified by sexes, ages, and seasons in Tianjin. China Environ Sci Pollut Res. 2015;22:1215–9.
Collins K. Effects of cold on old people. Br J Hosp Med. 1987;38(506–8):510–2. 514.
Acknowledgements
This research was financially supported by Arak University of medical sciences, Arak, Iran. The authors would like to thank the staff of the Department of Environment Health, Arak University of medical sciences, Iran, for their collaboration in this research.
Funding
This study was financially supported by the Arak University of medical sciences, Arak, Iran.
Author information
Authors and Affiliations
Contributions
B.K. and S.S. conceived of the presented idea, designed the study, participated in the data collection and wrote the main manuscript. B.K. performed the statistical analysis and assisted in the data collection and approved the final version. All authors discussed the results, reviewed the paper and contributed to the final manuscript. Corresponding Author: Behrooz Karimi. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This paper is the result of a research project supported by the Arak University of medical sciences, Arak, Iran.
Consent to participate
Not applicable (The manuscript does not report on or involve the use of any animal or human data or tissue).
Consent to publication
Arak University of medical sciences has allowed to publish the information obtained from this research. This work was supported by the Arak University of medical sciences, Arak, Iran.
Competing interests
The authors declare that they have no known competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karimi, B., Samadi, S. Long-term exposure to air pollution on cardio-respiratory, and lung cancer mortality: a systematic review and meta-analysis. J Environ Health Sci Engineer 22, 75–95 (2024). https://doi.org/10.1007/s40201-024-00900-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-024-00900-6