Abstract
Antimicrobial additives in personal care products (PCPs) such as triclosan (TCS) and triclocarban (TCC) are of environmental concern due to their potential toxicity in non-target aquatic organisms. In this study, the histological, genotoxic (micronucleus assay), and embryotoxic effects of sublethal and environmentally relevant concentrations of TCS and TCC were evaluated in Clarias gariepinus (the African sharptooth catfish) over a period of 28 days. The 96 hLC50 values of TCS and TCC against fingerlings of C. gariepinus were 16.04 mg/L and 41.57 mg/L respectively. The 24 hLC50 and 26 hEC50 (non-hatching) values for C. gariepinus embryos were 16.48 mg/L and 11.08 mg/L for TCS and 46.08 mg/L and 41.93 mg/L for TCC respectively. TCS was ×3 to ×4 more toxic to C. gariepinus fingerlings and embryos than TCC. Gill histological alterations ranged from mild to severe lamellar necrosis in the exposed fishes with Gill Alteration Index (GAI) of 1.60 on day 14 and 3.20 on day 28. There were significant dose-dependent increases (p < 0.05) in micronuclei and binucleated cells in the erythrocytes of exposed fishes compared to control. Embryotoxic effects assessed from 0 to 72 h post fertilization showed significant decreases (p < 0.05) in hatching success and number of heartbeats per minute, and significant increase (p < 0.05) in percentage abnormalities in the exposed embryos compared to control. The study demonstrates the need for regulatory measures and monitoring of the use of TCS and TCC in PCPs in order to mitigate potential adverse effects to non-target aquatic organisms. This will support the United Nations Sustainable Development Goal 14 on sustaining life below water.
Graphical abstract

Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antimicrobial additives in personal care products (PCPs) are environmental threats because of their potential harmful ecological impacts in aquatic ecosystems (Dann and Hontela 2011). The use of soaps, shampoos, toothpastes, or other PCPs generates discharges of chemicals into the environment with consequent ecotoxicological effects such as endocrine disruption, fertility reduction, and developmental abnormalities (Bedoux et al. 2012). Chemical substances used in the formulation of PCPs are often reported in aquatic ecosystems (Brausch and Rand 2011). Although present at minute concentrations, bioaccumulation, flow through the food chain and reaction with other chemicals might potentiate their risk to aquatic organisms (Oliveira et al. 2009). Triclosan (TCS) and triclocarban (TCC) are two prevalent antimicrobial agents observed in many PCPs. Thus, they are reported severally in wastewater, surface waters, sediments, and aquatic organisms (Chen et al. 2014).
TCC is mostly added to antimicrobial soaps while the application of TCS is broader such as its use in antibacterial mouthwash and toothpaste, and household items such as textiles, furniture, plastic cutting boards, and sports equipment (Bester 2003). Typically, antimicrobial soap bars constitute 2% by weight of TCC; lower concentrations of TCS in this and other personal care and household products are primarily 0.1–0.3% by weight (Sabaliunas et al., 2003). Considerable amounts of triclosan and triclocarban are mostly released into the aquatic environment through wastewater (Halden and Paull 2005). Since wastewater treatment plants (WWTPs) are unable to eliminate TCS and TCC, these compounds remain highly stable for a long period of time leading to the release of high concentrations of TCS and TCC into water bodies (Vijitha et al. 2017). In sewage treatment plants, TCS and TCC are partially adsorbed onto biosolids (31% and 76% respectively) and partially biodegraded (65% and 21% respectively) before discharge via effluents (4% and 3% respectively) (Heidler and Halden 2008). Upon application of biosolids on land and effluent discharge into streams, these chemicals undergo further partitioning in soil, sediment, water, and other environmental media. Therefore, they are observed frequently in agricultural soils (Chen et al. 2011), surface water (Zhao et al. 2010), and sediments (Miller et al. 2008), although they are not deemed to be recalcitrant organic pollutants (Sabaliunas et al., 2003). The half-life of triclosan in aqueous media ranges between less than 1 h and 10 days in freshwater bodies (Helbing et al. 2011). The half-lives of triclosan and triclocarban in aerobic soil are 18 days and 108 days respectively (Ying et al. 2007). TCS has been identified in aquatic life ranging from algae, fishes, to dolphins as well as in human urine and blood (Orvos et al. 2002; Adolfsson-Erici et al. 2002).
Triclocarban can act as endocrine disruptors at high concentrations by increasing the activity of testosterone and other steroids (Chen et al. 2008). A 96 hLC50 value of 0.34 mg/L has been reported in adult Danio rerio exposed to TCS while D. rerio larvae exposed to 0.5 mg/L TCS showed spine malformations, pericardial edema at 96 h, and a major delay in hatching at 48 h and 72 h (Oliveira et al. 2009). Concentration-dependent increases in shortened primary lamella, loss of secondary lamella, hyperplasia of gill arches, and cytoplasmic vacuolization in the hepatocytes have been observed in Anabas testudineus exposed to TCS (Priyatha and Chitra 2018). Similarly, in Tetrehymena thermophila, increasing concentrations (1, 100, 500 μg/L) of both TCS and TCC for 1 h caused concentration-dependent DNA damage with TCC causing more severe DNA damage than TCS (Gao et al. 2015). Fish are good bioindicators for assessing aquatic health, especially for monitoring the effect of aquatic contaminants (Priyatha and Chitra 2018). The African sharptooth catfish (Clarias gariepinus, Burchell 1822) is an ecologically and commercially important freshwater fish that is found in the wild and one of the most cultured fish species in several African countries especially Nigeria (Esenowo and Ugwumba 2010). There is a dearth of studies using environmentally relevant concentrations of TCS and TCC and their effects on early life stages of fish species. The aim of this study was to evaluate the acute toxicity, histological, genotoxic, and embryotoxic effects of sublethal and environmentally relevant concentrations (Table 1) of triclosan and triclocarban on C. gariepinus. The results are envisaged to provide evidence-based data for ecological risk assessment and standards setting. This will support global efforts on risk assessment of emerging pollutants in order to provide targeted interventions to sustain life below water (UN SDG 14).
Materials and methods
Chemicals
TCS (5-chloro-2-(2,4-dichlorophenoxy)phenol) of 97% purity (Dev Impex, Bileshwarpura, India) and TCC (N-(4-chlorophenyl)-N-(3,4-dichlorophenyl) urea) of 98% purity (Kancor pharmaceutical company, India) were purchased from a commercial store in Lagos, Nigeria, and were stored at room temperature (25 °C). Triclosan and triclocarban were dissolved in absolute ethanol (analytical grade; 99.8% purity; Fisher Scientific, UK). Stock solutions of TCS and TCC were 1 g/20 mL or 50 mg/mL. Triclosan (Irgasan-DP300) is a white to off-white crystalline powder with a molecular weight of 289.54 g/mol which has a slight, faintly aromatic odor. It is readily dissolved in organic solvents. Triclocarban is a fine powder with a slight odor with a molecular weight of 315.58 g/mol which is soluble in fats and many organic solvents (Greenwood and Earnshaw 2006).
Test organisms: collection and acclimatization
Fingerlings (weight 6–9 g; length 4.5–5.5 cm) and juveniles (weight 17–22 g and length 11.5–13 cm of Clarias gariepinus (Chordata, Osteichthyes, Siluriformes, Clariidae) were obtained from a fish farm in Lagos, Nigeria. The fishes were acclimatized to laboratory conditions prior to the experiment in aerated, dechlorinated, static water (40-L capacity). A photoperiod of light and dark (12 h:12 h) was maintained throughout the study. During acclimatization, the fish were fed twice daily with a commercial fish feed (Coppens) and the water in the holding tanks was renewed daily (this was done by replenishing 50% of the water in the stock tank to avoid stress to the fish) to avoid accumulation of waste metabolites and decayed food materials (Otitoloju 2006). Feeding was stopped 24 h prior to commencement of the bioassays.
Procedure for spawning Clarias gariepinus embryos
Two male breeders of C. gariepinus (weight 1.00 ± 0.5 kg; length 49.7–49.9 cm) were procured from the Aquaculture section of the Department of Marine Sciences, University of Lagos, while one female breeder of C. gariepinus (weight 1.00 ± 0.5 kg; length 48–50 cm) was procured from a commercial fish farm in Surulere, Lagos, Nigeria. Embryos were spawned as described in Sogbanmu et al. (2018) and Mumuni and Sogbanmu (2018). The female breeder was injected with OVAPRIM hormone (salmon gonadotropin-releasing hormone analogue and domperidone injection manufactured by Syndel Laboratories Ltd, Canada) at 0.5 mL/kg of fish. After 10 h of latency period, the female was stripped to induce the release of eggs collected in a plastic bowl by applying slight pressure (by hand) on the female’s abdomen. The two male C. gariepinus breeders were euthanized (American Veterinary Medical Association (AVMA), 2013), dissected with a new razor blade, and the testes were removed carefully. These were slightly dissected to collect the milt used for fertilizing the eggs. Fertilization was facilitated by adding saline water to the milt and egg mixture and the bowl was gently swirled to ensure adequate mixing of the milt with the eggs at 27 ± 2 °C within 3 min. Using a dissecting microscope, fertilized eggs were detected and verified (Ceti Star–13 ED Stereomicroscope, Medline Scientific, UK). Fertilization was considered to have occurred when the egg yolk was transparent greenish-orange and cell division was clearly visible in the blastodisc (Oellermann, 1995). Fertilized eggs were then transferred into petri dishes.
Range finding tests and physicochemical analysis of test media
The physicochemical parameters of the dechlorinated tap water utilized for the experiments were evaluated according to APHA guidelines (American Public Health Association (APHA), 1998). Water temperature in the test media ranged from 26 ± 2 °C and pH was maintained between 7.4 and 7.6, which were monitored throughout the study using the standardized procedures. Preliminary tests were conducted to provide guidance on a range of concentration for TCS and TCC to use in the bioassay, that is, before the definitive tests. The range of concentrations of TCS and TCC used in this study was 4, 8, 16, and 32 mg/L for TCS and 20, 30, 40, and 60 mg/L for TCC. Four fishes were placed in each test tank and were maintained in each of the test and control groups in duplicates. Tanks are covered with monofilament nets to prevent the fishes from leaping out of test solutions. Mortality was recorded daily throughout the study.
Acute toxicity studies with Clarias gariepinus fingerlings and embryos exposed to triclosan and triclocarban
This was conducted with fingerlings of C. gariepinus and embryos (age, 2 min post-fertilization) in 5-L glass tanks and petri dishes (diameter, 90 mm) respectively. Based on the preliminary tests conducted, various concentrations were determined for the test compounds. A working solution was prepared from a stock solution of 1 g/L of the test chemicals. Varying concentrations were measured out from the stock solution with syringes. For the definitive tests, four fingerlings of similar sizes in duplicates and fifty fertilized embryos in triplicate were exposed to varying concentrations of 5, 10, 15, 20, and 25 mg/L of TCS; 35, 40, 45, 50, and 55 mg/L of TCC and untreated control (water alone). A static non-renewable bioassay protocol was employed in which test medium was not renewed throughout the duration of the experiment (96 h for fingerlings, 24 h for embryos) (American Public Health Association (APHA), 1998; Organization for Economic Cooperation and Development, 2013). Mortality was assessed every 24 h over a period of 96 h for fingerlings (Organization for Economic Cooperation and Development, 1992) and every 6 h over a period of 24 h for embryos, with the aid of a dissecting microscope. The percentage of embryos that did not hatch (non-hatching) after 26 h was also assessed (Mumuni and Sogbanmu 2018) to establish the 26 hEC50 (effective concentration that will elicit a response (non-hatching) in 50% of the exposed embryos). A fish was considered dead when it did not respond to mechanical contact stimulation or when there was no body or opercular movement when prodded with a glass rod (Otitoloju 2006).
Sublethal and environmentally relevant studies with Clarias gariepinus juveniles and embryos exposed to triclosan and triclocarban
The sublethal concentration (1/10th or 10% of 96 hLC50) was derived from the results of the acute toxicity studies with C. gariepinus fingerlings. The environmentally relevant concentration (ERC) was derived from an extensive literature search on the levels of TCS and TCC detected in the aquatic environment and the highest levels of the test chemicals detected were 26.80 μg/L (~ 0.030 mg/L) for TCS (McAvoy et al. 2002) and 2.84 μg/L (~ 0.003 mg/L) for TCC (Lehutso et al. 2017). These concentrations were adopted for this study as the ERC. Tenfold (×10) of the ERC was used due to the possibility of TCS and TCC increasing over time in the environment. Tenfold (×10) of the ERC was thus adopted for TCS and TCC as 0.30 mg/L and 0.03 mg/L respectively. A semi-static bioassay test protocol was implemented in which the test media were replaced with a fresh medium of the same concentration and untreated control once every 72 h (Mumuni and Sogbanmu, 2018). Six juveniles of similar sizes and fifty fertilized embryos in triplicate were exposed to sublethal concentrations of triclosan and triclocarban.
Histological studies
C. gariepinus juveniles were exposed to sublethal concentrations of TCS and TCC in triplicate for 28 days. At 14 and 28 days post-exposure, three fishes from each treatment group and control were subjected to histological studies. The fishes were euthanized and the tissues/organs (gills and liver) were extracted. The organs were fixed in 10% buffered formalin, transferred into an automated tissue processor, embedded in paraffin wax, and sectioned with a rotary microtome at 5 μm. Sections were stained with hematoxylin and eosin (Adeboyejo et al. 2013). All sections were examined and photographed by a professional pathologist using an optical microscope (Olympus BX 51, Tokyo Japan) at × 100 objective (Alimba et al. 2015).
The histopathological changes were evaluated according to Ekpendu et al. (2020), which involves the calculation of the Histopathological Alteration Index (HAI) for each fish using the formula: HAI = 1 ΣI + ΣII + ΣIII
Because I, II, and III correspond to the number of stages of change, the mean Gill Alteration Index (GAI)/Hepatic Alteration Index (HAI) was categorized on a 5-point scale: 0 = normal tissue; 2.0 = mild damage to the tissue, 4.0 = moderate damage to the tissue, 6.0 = partially severe damage to the tissue, 8.0 = severe and irreparable damage to the tissue. These stages of change and scoring system or scale are given in Table 2.
Genotoxicity studies (micronucleus assay)
C. gariepinus juveniles were exposed to sublethal concentrations of TCS and TCC in triplicates for 28 days. At 14 and 28 days post-exposure, blood samples were collected from fishes exposed to the test chemicals and the control groups for micronucleus assay. The peripheral blood was collected from the caudal vein of the fishes with a 1-mL syringe (Alimba et al. 2015). A smear was prepared on a grease-free slide. The smeared slides were air-dried for 60 min at 25 °C, fixed for 30 min in absolute ethanol, stained with 5% Giemsa stain, and counter-stained with May-Grunwald stain. Prepared slides for micronuclei (MN) and binucleated (BN) cells were scored at × 100 objective. Cells with small nuclei (micronuclei) within the cytoplasm and near the main nucleus were classified as MN (Singh et al. 2005). Cells of approximately the same size with two nuclei were scored as BN (Costa and Costa 2007). The slides were staged and analyzed for 1000 cells/individual with micronuclei and binucleated cells (Supplementary material, SM 1).
Embryotoxicity studies
C. gariepinus embryos were exposed to sublethal concentrations of TCS and TCC. Fifty fertilized embryos were exposed to test media in triplicate including control from 0 to 72 hpf. Hatching success, developmental abnormalities, and number of heartbeats per minute (NHBpM) were assessed at 26 hpf according to Mumuni and Sogbanmu (2018). Hatching success was measured as the percentage of embryos that hatched (fully emerge from the chorion) at 26 hpf. Developmental abnormalities were calculated as the percentage of embryos observed under the dissecting microscope with one or more developmental abnormalities such as C-shaped curvature, lordosis, stunted growth, tail tip curvature, and pericardial hemorrhage as described by Osman et al. (2007) and Sogbanmu et al. (2016). The NHBpM in the fish embryos was evaluated as the number of heartbeats recorded as viewed with the aid of a dissecting microscope for 30 s. This number was extrapolated to cover the NHBpM (60 s).
Statistical analysis
The relative acute toxicity data for fingerlings and embryos was analyzed by probit using SPSS 20.0 version. One-way analysis of variance (ANOVA), Fisher’s LSD (least significance difference) test, and multiple comparison analysis were utilized to evaluate the significant differences between treatment means and control at p < 0.05. Results are presented as mean ± standard error. Figures were prepared using Microsoft Office Excel 2010 version.
Results
Relative acute toxicity of triclosan and triclocarban to Clarias gariepinus fingerlings and embryos
The results showed that C. gariepinus fingerlings (96 hLC50—16.04 mg/L for TCS and 41.57 mg/L for TCC) were more susceptible to the test chemicals compared to the embryos (24 hLC50—16.48 mg/L for TCS and 46.08 mg/L for TCC) (Table 3). The median concentration (26 hEC50—11.08 mg/L for TCS and 41.93 mg/L for TCC) for embryos that did not hatch revealed that the endpoint (non-hatching) was a precursor for lethality to the embryos (Table 3). Furthermore, triclosan was found to be approximately three times more toxic than triclocarban to embryos and fingerlings of C. gariepinus based on the median lethal concentration. On the basis of the median effective concentration for non-hatching of C. gariepinus embryos, TCS was four times more toxic than TCC (Table 3).
Histopathological effects in Clarias gariepinus juveniles exposed to sublethal and environmentally relevant concentrations of triclosan and triclocarban
At day 14, microscopic observations of gill tissues of the control group (Fig. 1a) showed the presence of primary and secondary lamellae, with no areas of necrosis or inflammation, and revealed apparently normal gills, whereas the treatment groups (Fig. 1b–e) showed some histopathological lesions which include shortening/blunting of secondary lamellae but preservation of the primary lamellae indicating mild lamellar necrosis at day 14 (Fig. 1). At day 28, gill tissues of the control group (Fig. 1f) showed the presence of primary and secondary lamellae with no areas of necrosis or inflammation and revealed apparently normal gills, whereas treatment groups (Fig. 1g, i) showed shortening/blunting of secondary lamellae but preservation of the primary lamellae with areas of edema indicating severe lamellar necrosis and (Fig. 1h, j) showed shortening/blunting of secondary lamellae but preservation of the primary lamellae indicating mild lamellar necrosis at day 28 (Fig. 1).
Photomicrographs of longitudinal sections through the gills of C. gariepinus juveniles at days 14 and 28 exposed to sublethal concentrations of triclosan (TCS), triclocarban (TCC), and controls. (A) Control at day 14 showing normal appearing gills with secondary lamellae (SL) and primary lamellae (PL) (mag. × 100). (B) TCS (1.60 mg/L) and (C) TCS (0.30 mg/L) at day 14 showing shortening/blunting of secondary lamellae (SL) but preservation of the primary lamellae (PL) indicating mild lamellar necrosis MN (mag. × 100). (D) TCC (4.16 mg/L) and (E) TCC (0.03 mg/L) at day 14 showing shortening/blunting of secondary lamellae (SL) but preservation of the primary lamellae (PL) indicating mild lamellar necrosis MN (mag. × 100). (F) Control at day 28 showing normal appearing gills with secondary lamellae (SL) and primary lamellae (PL) (mag. × 100). (G) TCS (1.60 mg/L) at day 28 showing shortening/blunting of secondary lamellae (SL) but preservation of the primary lamellae (PL) with areas of edema indicating severe lamellar necrosis SN (mag. × 100). (H) TCS 0.30 mg/L at day 28 showing mild lamellar necrosis MN (mag. × 100). (I) TCC (4.16 mg/L) at day 28 showing shortening/blunting of secondary lamellae SL but preservation of the primary lamellae PL with areas of edema indicating severe lamellar necrosis SN (mag. × 100). (J) TCC (0.03 mg/L) at day 28 showing mild lamellar necrosis MN (mag. × 100)
Gill Alteration Index of Clarias gariepinus exposed to the sublethal and environmentally relevant concentrations of triclosan and triclocarban
Five key alterations were evaluated: shortening of secondary lamella (SSGL), degeneration of primary lamella (DPL), oedema (OD), mild necrosis (MN), and severe necrosis (SN). There was an increase in the gill alteration index of Clarias gariepinus exposed to the sublethal and environmental relevant concentrations of triclosan and triclocarban from 1.60 on day 14 to 3.20 on day 28 (Table 4).
Histologic section through the liver of control (Fig. 2a) and treatment groups (Fig. 2b–e) revealed apparently normal hepatocytes arranged in parallel radiating plates. No abnormalities were seen indicating normal liver at day 14 (Fig. 2). At day 28, the liver of the control (Fig. 2f) and treatment groups (Fig. 2g–j) showed general structure, central vein CV, blood sinusoids BS, portal vein PV and the basophilic portion with nucleus and the acidophilic cytoplasm of the acinar cells indicating normal liver at day 28 (Fig. 2).
Photomicrographs of longitudinal sections through the liver of C. gariepinus juveniles at days 14 and 28 exposed to sublethal concentrations of triclosan (TCS), triclocarban (TCC), and controls. (A) Control at day 14 showing normal hepatocytes arranged in parallel radiating plates indicating normal liver (mag. × 100). Plate of hepatocytes (PH). (B) TCS (1.60 mg/L) at day 14 showing normal hepatocytes arranged in parallel radiating plates indicating normal liver (mag. × 100). (C) TCS (0.30 mg/L) at day 14 showing normal hepatocytes arranged in parallel radiating plates indicating normal liver (mag. × 100). (D) TCC (4.16 mg/L) at day 14 showing normal hepatocytes arranged in parallel radiating plates indicating normal liver (mag. × 100). (E) TCC (0.03 mg/L) at day 14 showing normal hepatocytes arranged in parallel radiating plates indicating normal liver (mag. × 100). (F) Control at day 28 showing general structure, blood sinusoids (BS), central vein (CV), portal vein (PV), and the basophilic portion with nucleus and the acidophilic cytoplasm of the acinar cells indicating normal liver (mag. × 100). (G) TCS (1.60 mg/L) at day 28 showing general structure, blood sinusoids (BS), central vein (CV), portal vein (PV), and the basophilic portion with nucleus and the acidophilic cytoplasm of the acinar cells indicating normal liver (mag. × 100). (H) TCS (0.30 mg/L) at day 28 showing general structure, blood sinusoids (BS), central vein (CV), portal vein (PV), and the basophilic portion with nucleus and the acidophilic cytoplasm of the acinar cells indicating nor mal liver (mag. × 100). (I) TCC (4.16 mg/L) at day 28 showing general structure, blood sinusoids (BS), central vein (CV), portal vein (PV), and the basophilic portion with nucleus and the acidophilic cytoplasm of the acinar cells indicating normal liver (mag. × 100). (J) TCC (0.03 mg/L) at day 28 showing general structure, blood sinusoids (BS), central vein (CV), portal vein (PV), and the basophilic portion with nucleus and the acidophilic cytoplasm of the acinar cells indicating normal liver (mag. × 100)
Hepatic Alteration Index of Clarias gariepinus exposed to the sublethal and environmentally relevant concentrations of triclosan and triclocarban
Five key alterations were evaluated: hypertrophy (SSGL), degeneration of primary lamella (DPL), edema (OD), mild necrosis (MN), and severe necrosis (SN). There was no lesion or any alteration in the liver of the exposed Clarias gariepinus and control. Hence, the Hepatic Alteration Index was 0 on day 14 and day 28 (Table 5).
Genotoxic effects in Clarias gariepinus juveniles exposed to sublethal and environmentally relevant concentrations of triclosan and triclocarban
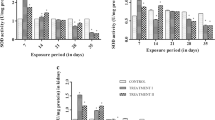
The frequency of micronuclei (an index of genotoxicity) increased statistically from days 14 to 28 in the exposed fish compared to control (Fig. 3). There was a significant dose-dependent increase (p < 0.05) in the micronuclei in the erythrocytes of exposed fishes; micronuclei were higher in fish exposed at the highest concentration of 1.60 mg/L (day 14: 1.44 ± 0.53‰; day 28: 2.67 ± 0.87‰) compared to the lowest concentration of 0.30 mg/L (day 14: 0.56 ± 0.53‰; day 28: 1.22 ± 0.44‰) of triclosan. Also, micronuclei were higher in fish exposed at the highest concentration of 4.16 mg/L (day 14: 1.44 ± 0.53‰; day 28: 2.33 ± 0.71‰) compared to the lowest concentration of 0.03 mg/L (day 14: 0.44 ± 0.53‰; day 28: 1.33 ± 0.50‰) of triclocarban. Micronuclei in the erythrocytes of the control fish were 0.22 ± 0.44‰ and 0.33 ± 0.50‰ on days 14 and 28 respectively.
There was a significant dose-dependent increase (p < 0.05) in the number of binucleated cells in the erythrocytes of exposed fishes (Fig. 3). Binucleated cells were higher in fish exposed at the highest concentration of 1.60 mg/L (day 14: 1.78 ± 0.83‰; day 28: 3.44 ± 0.88‰) compared to the lowest concentration of 0.30 mg/L (day 14: 0.78 ± 0.44‰; day 28: 1.67 ± 0.71‰) of triclosan. Also, binucleated cells were higher in fish exposed at the highest concentration of 4.16 mg/L (day 14: 1.56 ± 0.53‰; day 28: 3.78 ± 0.67‰) compared to the lowest concentration of 0.03 mg/L (day 14: 1.11 ± 0.60‰; day 28: 1.78 ± 0.67‰) of triclocarban. Binucleated cells in the erythrocytes of the control fish were 0.11 ± 0.33‰ and 0.22 ± 0.44‰ on days 14 and 28 respectively.
Embryotoxicity and developmental toxicity studies with Clarias gariepinus embryos
There were statistically significant (p < 0.05) differences in the hatching success of exposed embryos and control (Fig. 4). Percentage hatching success was highest for embryos in the control (84.66 ± 1.15%) followed by embryos in the lowest treatment of 0.30 mg/L (78.66 ± 2.30%) and the highest treatment of 1.60 mg/L (68.66 ± 8.08%) for TCS (Fig. 4). For TCC, percentage hatching success was highest for embryos in the control (84.00 ± 2.00%) followed by embryos in the lowest treatment of 0.03 mg/L (78.00 ± 2.00%) and the highest treatment of 4.16 mg/L (70.00 ± 2.00%) (Fig. 4).
The number of heartbeats per minute (NHBpM) in the exposed embryos was significantly lower (p < 0.05) compared to the control while developmental abnormalities were statistically higher (p < 0.05) compared to the control in the exposed embryos. The NHBpM was highest in control (131.20 ± 2.49) followed by the 0.30 mg/L treatment (124.53 ± 0.61) and 1.60 mg/L treatment was (123.60 ± 1.44) for TCS (Fig. 5). For TCC, the NHBpM was highest in the control (132.40 ± 1.74) followed by the 0.03 mg/L treatment (126.40 ± 0.40) and 4.16 mg/L treatment (125.20 ± 0.69) for TCC (Fig. 5).
Developmental abnormalities observed were C-shaped embryo, lordosis, stunted growth, and tail tip curvature at 24 hpf (Plate 1). Pericardial hemorrhage and lordosis (dorsoventral curvature) were observed at 48 hpf (Plate 2) and 72 hpf (Plate 3). These abnormalities were highest in the 1.60 mg/L treatment (46.07 ± 8.49% at 24 hpf and 27.44 ± 4.49% at 48 and 72 hpf), followed by the 0.30 mg/L treatment (3.45 ± 3.97% at 24 hpf and 2.56 ± 2.56% at 48 and 72 hpf) for TCS (Fig. 6). Abnormalities were highest in the 4.16 mg/L treatment (43.80 ± 5.94% at 24 hpf and 27.61 ± 5.94% at 48 and 72 hpf), followed by the 0.03 mg/L treatment (4.27 ± 2.96% at 24 hpf and 3.41 ± 1.47% at 48 and 72 hpf) for TCC (Fig. 6). Embryos in the control group had no morphological abnormalities (0.00%).
Representative images of developmental abnormalities observed at 24 hpf following exposure of Clarias gariepinus to triclosan and triclocarban. (A) Normal embryo (control); (B) C-SC, C-shaped curvature (0.30 mg/L and 1.60 mg/L TCS, 4.16 mg/L TCC); (C) TTC, tail tip curvature (1.60 mg/L TCS, 4.16 mg/L and 0.03 mg/L TCC); (D) L, lordosis (0.30 mg/L and 1.60 mg/L TCS, 4.16 mg/L and 0.03 mg/L TCC); (E) SG, stunted growth (1.60 mg/L TCS, 4.16 mg/L TCC)
Representative images of developmental abnormalities observed at 48 hpf following exposure of Clarias gariepinus to triclosan and triclocarban. (A) Normal embryo (control); (B) L, lordosis (0.30 mg/L and 1.60 mg/L TCS, 0.03 mg/L and 4.16 mg/L TCC); (C) PH, pericardial hemorrhage (0.30 mg/L and 1.60 mg/L TCS, 0.03 mg/L and 4.16 mg/L TCC)
Representative images of developmental abnormalities observed at 72 hpf following exposure of Clarias gariepinus to triclosan and triclocarban. (A) Normal embryo (control); (B) L, lordosis, and PH, pericardial hemorrhage (0.30 mg/L and 1.60 mg/L TCS, 0.03 mg/L and 4.16 mg/L TCC); (C) PH, pericardial hemorrhage (0.30 mg/L and 1.60 mg/L TCS, 0.03 mg/L and 4.16 mg/L TCC)
Discussion
Data from acute toxicity studies based on mortality are of biological and ecological importance as they can be used to determine application factors for derivation of water quality (Kumar et al. 2016).
In this study, the mortality of fingerlings of C. gariepinus was dose-dependent and agrees with the results of Priyatha and Chitra (2018) and Oliveira et al. (2009) who reported an increase in the mortality of mature freshwater teleost fish, Anabas testudineus and adult Danio rerio, respectively, with increasing concentration of triclosan, while Vijitha et al. (2017) reported a decrease in the survival of Oreochromis niloticus with increasing concentration of triclosan. In previous studies, the median lethal concentrations of triclosan in various fish species were 4.4 mg/L in rainbow trout, Oncorhynchus mykiss (Adolfsson-Erici et al. 2002); 2.81 mg/L in Nile tilapia, O. niloticus (Vijitha et al. 2017); 0.34 mg/L in zebrafish, Danio rerio (Oliveira et al., 2009); 0.26 mg/L in fathead minnow, Pimephales promelas and 0.37 mg/L in sunfish, Lepomis macrochirus (Orvos et al. 2002). Conversely, the 96 hL50 values of TCS and TCC (16.04 mg/L and 41.57 mg/L) respectively to C. gariepinus in this study are much higher (less toxic) than the values reported in previous studies. The differences in acute toxicity of triclosan in various fish species may be due to numerous factors such as variation in species, strain and gender, size of test animal, purity of triclosan used, sensitivity and resistance of the species, mode of entry, adaptation of the animal, detoxifying mechanisms, among others (Priyatha and Chitra 2018). Following exposure of C. gariepinus to triclosan and triclocarban, drastic changes in behavior were observed such as air gulping, mucous deposition, erratic swimming, and surfacing especially at the highest concentration when compared to control groups. Similar behavioral responses have been reported in the native fish, Anabas testudineus, exposed to triclosan (Priyatha and Chitra 2018), D. rerio exposed to TCS at 0.5 mg/L and 0.4 mg/L (Oliveira et al. 2009). The increase in surfacing and gulping of air from surface water after triclosan exposure could be an attempt of the animal to escape from the toxicant and to avoid breathing in the contaminated water (Priyatha and Chitra 2018).
The tolerance of the C. gariepinus embryos compared to the fingerlings could be attributed to the presence of the egg chorion which acts as a barrier for embryo protection preventing the test compounds from penetrating the embryos (Oliveira et al. 2009). It is understood that the embryonic stages of fish are more resistant than those immediately after hatching (eleutheroembryo) and continue through the juvenile stages (Leonard et al. 2005). The relatively high mortality rate of embryos reported in this study suggests TCS and TCC are integrated into the cell, which is associated with their high lipophilicity (Hallare et al. 2006). The evaluation on non-lethal end points in acute toxicity studies provides an indication of sublethal effects of exposure to toxic substances within a short time which usually precedes mortality (Mumuni and Sogbanmu 2018). In this study, the 26 hEC50 value (11.07 mg/L, TCS; 41.93 mg/L, TCC) for non-hatching of exposed C. gariepinus embryos is indicative that non-hatching at lower concentrations would invariably precede mortality of the embryos at higher concentrations of the test chemicals. In other words, if the concentration of the test compound in the environment is increased beyond the EC50 level, non-hatching would precede death eventually.
Histopathological examination is known as the most sensitive endpoint for detecting organ toxicity during exposure to toxicants (Lanning et al. 2002). Also, it has the capability to provide vital information on the acute and chronic effects of toxicants on targeted organs that may not be identified by functional biomarkers (Amacher et al. 2006). Histological lesions on tissues therefore serve as warning symptoms for the health of organisms (Alimba et al. 2015). Gill and liver are considered the most targeted organs of toxicity (Priyatha and Chitra 2018). In the present study, the vulnerability of tissue damage was concentration-dependent, which reflected the direct effect of triclosan and triclocarban. Shortening/blunting of primary lamellae significantly reduces the gill surface and thus decreases the contact between the pollutant and gill epithelium (Kaur and Dua 2015). Therefore, triclosan and triclocarban exposure affects osmoregulation and gill respiration which may be correlated to the behavioral changes as observed by engulfing air from the water surface. These changes are consistent with the findings of Priyatha and Chitra (2018) who reported uplifting and hyperplasia of gill epithelium, lamellar disorganization such as shortened primary lamella, fusion or loss of secondary lamella, and hyperplasia of gill arches in Anabas testudineus exposed to triclosan. The liver is associated with detoxification and biotransformation of toxicants and also one of the organs most susceptible to toxicant exposure (Priyatha and Chitra 2018). Histologic section of liver tissue revealed apparently normal hepatocytes arranged in parallel radiating plates; no abnormalities were observed. The lack of alteration in the liver though observed in the gills is possibly because gills are the first point of contact with xenobiotics and hence usually have more pronounced effects observed in them compared to the liver.
Fishes live in close contact with their environment and are thus highly susceptible to physical and chemical changes in aquatic environments that can be expressed in their blood (Wilson and Taylor 1993). In fishes, the micronucleus test is a useful in vivo technique for genotoxicity testing to predict the toxic effect of exposed chemicals (Vijitha et al. 2017). The induction of micronuclei in peripheral erythrocytes of C. gariepinus juveniles exposed to sublethal concentrations of the test chemicals is consistent with the results of Vijitha et al. (2017), Emery (2012), and Binelli et al. (2009) who reported that triclosan increased micronuclei frequency in Oreochromis niloticus, tadpoles of the American bullfrog, Lithobates catesbeianus, and the freshwater bivalve Zebra mussel (Dreissena polymorpha) respectively. Genetic damage in fish cells could play a major role in decreasing the fitness of fish populations with short- and long-term consequences on their survival (Alimba et al. 2015). Significant increase in binucleated cells observed in C. gariepinus implies that TCS and TCC are cytotoxic. This may be indicative of their capability to block cytokinesis of a normal dividing cell during M phase of the cell cycle and may result in genetic imbalance and carcinogenesis (Rodilla 1993).
The developmental abnormalities and embryo mortality observed in this study were dose-dependent. Similar results were observed in Danio rerio embryos exposed to triclosan concentrations of 0.1–0.9 mg/L (Oliveira et al. 2009), Oryzias latipes exposed to triclosan concentrations of 5–80 μg/L (Rodilla 1993), and Oncorhynchus mykiss exposed to triclosan concentrations of 78–2500 μg/L (Orvos et al. 2002). There is a paucity of studies that report developmental abnormalities and embryotoxicity of triclocarban during embryogenesis in C. gariepinus. The significant decrease in hatching rate observed in this study agrees with the findings of Ishibashi et al. (2004) in which the hatchability of fertilized eggs/embryos of O. latipes exposed to 0.31 mg/L of TCS was significantly decreased relative to the control groups. Multiple toxic mechanisms may explain hatching failure or delay such as the activation of an irregular activity of the enzyme, chorionase, and/or the inability of the emerging larvae to break the shell of the egg (Hallare et al. 2006). Embryos with developmental abnormalities namely C-shaped curvature, tail tip curvature, and stunted growth died after 24 hpf in both TCS and TCC treatments. Spine deformations such as C-shaped curvature and lordosis may be ascribed to the reduction or deregulation of ions such as calcium and phosphorus or the reduction of myosin and myotonia, both of which are essential for normal development (Cheng et al. 2000).
In conclusion, this study showed that exposure of various life stages of C. gariepinus to sublethal concentrations of triclosan and triclocarban led to mortality, and mild to severe histological, genotoxic, embryotoxic, and developmental effects. Toxic effects of triclosan and triclocarban on C. gariepinus and mortality rate increased in a dose-dependent manner. The dose-dependent induction of micronuclei and binucleated cells examined in this study suggests that an increase in TCS and TCC concentrations will induce more genotoxicity. The sublethal effects of TCS and TCC therefore show that there may be potential effects to aquatic organisms through continuous exposure over longer periods. Widespread use of triclosan and triclocarban as antimicrobial agents contributes to their detection in aquatic ecosystems and consequent effects on aquatic life even when exposed for short durations. In addition, increased production and use of TCS and TCC would lead to higher environmental concentrations of both compounds. Therefore, regulatory measures should be taken by stakeholders such as manufacturers, consumers, and regulatory agencies to reduce the use of TCS and TCC in personal care products in order to sustain life below water (UN SDG 14). Monitoring of these chemicals in the receiving waters is of great importance in designing appropriate interventions and management strategies from diffuse and point sources.
References
Adeboyejo OA, Fagbenro OA, Adeparusi EO, Clarke EO (2013) Acute toxicity of industrial effluents from Agbara environs of Ologe lagoon on early life stages of African catfish Clarias gariepinus. Am J Res Com 1(3):50–60
Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J (2002) Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 46:1485–1489
Alimba CG, Saliu JK, Ubani-Rex OA (2015) Cytogenotoxicity and histopathological assessment of Lekki Lagoon and Ogun River in Synodontis clarias (Linnaeus, 1758). Toxicol Environ Chem 97(2):221–234
Amacher DE, Schomaker SJ, Boldt SE, Mirsky M (2006) The relationship among microsomal enzyme induction, liver weight and histological change in cynomolagus monkey toxicology studies. Food Chem Toxicol 44:528–537
American Veterinary Medical Association (AVMA) (2013)AVMA Guidelines for the euthanasia of animals: 2013 edn 38 (AVMA, Schaumburg, IL, 2013). https://www.avma.org/KB/Policies/Documents/euthanasia.pdf
American Public Health Association (APHA) (1998) Standard methods for the examination of water and wastewater, 16th edn. American Public Health Association, New York 215pp
Bedoux G, Roig B, Thomas O, Dupont V, Le Bot B (2012) Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ Sci Pollut Res 19:1044–1065
Bester K (2003) Triclosan in a sewage treatment process – balances and monitoring data. Water Res 37:3891–3896
Binelli A, Cogni D, Parolini M, Riva C, Provini A (2009) In vivo experiments for the evaluation of genotoxic and cytotoxic effects of triclosan in Zebra mussel hemocytes. Aquat Toxicol 91:238–244
Brausch JM, Rand GM (2011) A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82(11):15–32
Chen F, Ying GG, Kong LX, Wang L, Zhao JL, Zhou LJ, Zhang LJ (2011) Distribution and accumulation of endocrine disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei, China. Environ Pollut 159(6):1490–1498
Chen J, Ahn KC, Gee NA, Ahmed MI, Duleba AJ, Zhao L, Gee SJ, Hammock BD, Lasley BL (2008) Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrinology 149(3):1173–1179
Chen ZF, Ying GG, Liu YS (2014) Triclosan as a surrogate for household biocides: an investigation into biocides in aquatic environments of a highly urbanized region. Water Res 58:269–279
Cheng SH, Wai AWK, So CH, Wu RS (2000) Cellular and molecular basis of cadmium-induced deformities in zebrafish embryos. Environ Toxicol Chem 19:3024–3031
Costa PM, Costa MH (2007) Genotoxicity assessment in fish peripheral blood: a method for more efficient analysis of micronuclei. J Fish Biol 71:148–151
Dann AB, Hontela A (2011) Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol 31:285–311
Ekpendu E, Saliu J, Otitoloju A, Ukwa U (2020) Hematological variations, histopathology and reversibility of liver function enzymes in post juvenile Clarias gariepinus exposed singly to five botanical piscicides. Egypt J Aquatic Biol Fish 24(3):169–180
Emery D (2012) Assessing the genotoxicity of triclosan in tadpoles of the American Bullfrog, Lithobates catesbeianus. A thesis submitted in partial fulfillment of the requirements for the degree of Master of Biology at Virginia Commonwealth University. 58 pp
Esenowo IK, Ugwumba OA (2010) Growth response of catfish (Clarias gariepinus) exposed to water soluble fraction of detergent and diesel oil. Environ Res J 4(4):298–301
Gao AB, Tao Yuan A, Cheng C, Bai A, Zhou A, Wang A, Zhang H (2015) Effects of triclosan and triclocarban on the growth inhibition, cell viability, genotoxicity and multixenobiotic resistance responses of Tetrahymena thermophila. Chemosphere 139:434–440
Greenwood N, Earnshaw A (2006) Chemistry of the elements, 2nd edn. Butterworth-Heinemann, Oxford ISBN 978-0-7506-3365-9
Halden RU, Paull DH (2005) Co-occurrence of triclocarban and triclosan in U.S. water resources. J. Environ. Sci. Technol. 39(6):1420–1426
Hallare A, Nagel K, Kohler HR, Triebskorn R (2006) Comparative embryotoxicity and proteotoxicity of three carrier solvents to zebrafish (Danio rerio) embryos. Ecotoxicol Environ Saf 63:378–388
Helbing CC, Propper CR, Veldhoen N (2011) Triclosan affects the thyroid axis of amphibians. Toxicol Sci 123(2):601–602
Heidler J, Halden RU (2008) Meta-analysis of mass balances examining chemical fate during wastewater treatment. Environ Sci Technol 42(17):6324–6332
Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, Takao Y, Arizono K (2004) Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol 67:167–179
Kaur R, Dua A (2015) 96 h-LC50, behavioural alterations and histopathological effects due to wastewater toxicity in a freshwater fish Channa punctatus. Environ Sci Pollut Res 22(5):100–110
Kumar N, Ambasankar K, Krishnani KK, Gupta SK, Bhushan S, Minhas PS (2016) Acute toxicity biochemical and histopathological responses of endosulfan in Chanos chanos. Ecotoxicol Environ Saf 131:79–88
Lanning LL, Creasy DM, Chapin RE, Mann PC, Barlow NJ, Regan KS, Goodman DG (2002) Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol Pathol 30:507–520
Lehutso RF, Daso AP, Okonkwo JO (2017) Occurrence and environmental levels of triclosan and triclocarban in selected wastewater treatment plants in Gauteng Province, South Africa. Emerging Contam 3:107–114
Leonard M, Vanpoucke M, Petit-Poulsen V, Porcher JM (2005) Evaluation of the fish embryo test as a potential alternative to the standard acute fish toxicity test OECD 203. In: International Symposium on Toxicology Assessment, Skathios, Greece
McAvoy DC, Schatowitz B, Jacob M, Hauk A, Eckhoff WS (2002) Measurement of triclosan in wastewater treatment systems. Environ Toxicol Chem 21:1323–1329
Miller TR, Heidler J, Chillrud SN, Delaquil A, Ritchie JC, Mihalic JN, Bopp R, Halden RU (2008) Fate of triclosan and evidence for reductive dechlorination of triclocarban in estuarine sediments. Environ Sci Technol 42(12):4570–4576
Mumuni AA, Sogbanmu TO (2018) Embryotoxic, developmental and genotoxic evaluations of a endosulfan and deltamethrin mixture on the African sharptooth catfish (Clarias gariepinus). West Afr J App Ecol 26(1):1–10
Oellermann LK (1995) A comparison of the aquaculture potential of Clarias gariepinus (Burchell, 1822) and its hybrid with Heterobranchus longiflis valenciennes, 1840 in Southern Africa. PhD Thesis, Rhodes University, South Africa, 152 pp
Oliveira R, Domingues I, Grisolia K, Soares VM (2009) Effects of triclosan on zebrafish early-life stages and adults. Environ Sci Pollut Res 16:679–688
Organization for Economic Cooperation and Development (2013) OECD Guidelines for the testing of chemicals Section 2: effects on biotic systems test no. 236. Fish embryo acute toxicity (FET) test, Paris, France
Organization for Economic Cooperation and Development (1992) OECD guideline for testing of chemicals, fish acute toxicity test. 21 pages
Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstein A, Cunningham V (2002) Aquatic toxicity of triclosan. Environ Toxicol Chem 21:1338–1349
Osman A, Wuertz S, Mekkawy I, Exner HJ, Kirschbaum F (2007) Lead induced malformations in embryos of African catfish, Clarias gariepinus (Burchell, 1822). Environ Toxicol 22(4):375–389
Otitoloju AA (2006) Joint action toxicity of spent lubrication oil and laundry detergent against. Poecilia reticulata (Teleostei: Poeciliidae). Afr J Aquat Sci 31(1):125–129
Priyatha V, Chitra C (2018) Acute toxicity of triclosan on the native freshwater fish, Anabas testudineus (Bloch, 1792): behavioral alterations and histopathological lesions. Intl J Life Sci 6(1):166–172
Rodilla V (1993) Origin and evolution of binucleated cells and binucleated cell with micronuclei in cisplatin-treated cho cultures. Mutat Res 300:281–291
Sabaliunas D, Webb SF, Hauk A, Jacob M, Eckhoff WS (2003) Environmental fate of triclosan in the River Aire Basin, UK. Water Res 37(13):3145–3154
Singh PJ, Pandey S, Sharma S (2005) Micronucleus assay for evaluation of in vivo genotoxicity in fishes: training on genotoxic assays in fishes. TOGAIF-2005 edited by Kapoor D, Nagpure NS Lucknow: National Bureau of Fish. Genet Res 1:1–68
Sogbanmu TO, Osibona AO, Oguntade AO, Otitoloju AA (2018) Biomarkers of toxicity in Clarias gariepinus exposed to sublethal concentrations of polycyclic aromatic hydrocarbons. Afr J Aquat Sci 43(3):281–292
Sogbanmu TO, Nagy E, Phillips DH, Arlt VM, Otitoloju AA, Bury NR (2016) Lagos lagoon sediment organic extracts and polycyclic aromatic hydrocarbons induce embryotoxic, teratogenic and genotoxic effects in Danio rerio (zebrafish) embryos. Environ Sci Pollut Res Int 23:14489–14501
Vijitha CK, Asifa KP, Chitra KC (2017) Assessment of genotoxic and haematological consequence of triclosan in the fish, Oreochromis niloticus (Linnaeus, 1758) International J. Appl Theatr Res 3(2):101–109
Wilson RW, Taylor EW (1993) Differential responses to copper in rainbow trout (Oncorhynchus mykiss) acclimated to sea water and brackish water. J Comp Physiol B 163:239–246
Ying GG, Yu XY, Kookana RS (2007) Triclosan in wastewaters and biosolids from Australian wastewater treatment plants. Environ Int 33(2):199–205
Zhao JL, Ying GG, Liu YS, Chen F, Yang JF, Wang L (2010) Occurrence and risks of triclosan and triclocarban in the Pearl River system, South China: from source to the receiving environment. J Hazard Mater 179(1-3):215–222
Acknowledgements
The authors would like to acknowledge Mr Monday of the Department of Marine Sciences, Faculty of Science, University of Lagos, for his technical support in the handling and spawning of African sharptooth catfish embryos.
Availability of data and materials
Data and materials are available on request.
Author information
Authors and Affiliations
Contributions
ROJ: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, writing - original draft, writing - review and editing. TOS: conceptualization, methodology, project administration, resources, supervision, writing - review and editing.
Corresponding author
Ethics declarations
Ethical approval
This research was in accordance with the principles in the AVMA Guidelines for the euthanasia of animals (AVMA 2013) and the principles in the Declaration of Helsinki on the humane treatment of animals used in research (https ://www.wma.net/en/30pub lications/10pol icies /a18/).
Consent to participate
Not Applicable
Consent to publish
Both co-authors consent to the publication of the manuscript.
Conflict of interest
The authors have no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 211 kb)
Rights and permissions
About this article
Cite this article
Jimoh, R.O., Sogbanmu, T.O. Sublethal and environmentally relevant concentrations of triclosan and triclocarban induce histological, genotoxic, and embryotoxic effects in Clarias gariepinus (Burchell, 1822). Environ Sci Pollut Res 28, 31071–31083 (2021). https://doi.org/10.1007/s11356-021-12820-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12820-1