Abstract
Cartagena Bay is an estuarine system located in the Caribbean Sea (Colombia, South America), that receives fresh water from Canal del Dique, which is connected to the Magdalena River, the most important river of Colombia, with some of the most prominent Colombian cities located in its watershed, which has a high sediment yield. An analysis of persistent organic pollutants and heavy metals was carried out on marine sediments from Cartagena Bay. Cartagena Bay sediments deployed the occurrence of total levels of pesticides (thiocarbamates, bromacil, triazines, organochlorines, and organophosphorus), polybrominated diphenyl ethers (PBDEs), and polychlorinated biphenyls (PCBs), in sediments ranging from 0.83–33.67 ng/g dry-weight, 0.05–0.34 ng/g dry-weight, and 0.06–19.58 ng/g dry-weight, respectively. Their concentrations were lower than those reported in NOAA Screening Quick Reference Tables. DDTs and PCBs are banned organochlorine compounds, since, even at low levels, their presence in sediments represents a threat to aquatic organisms and, therefore, to human health through the trophic chain. Sediments showed high concentrations of strontium (50–959.6 mg/kg). All metals evaluated in the marine sediments were found in the S6 sampling point; this was near tannery and hydrocarbon industries (Pb 37.1 mg/kg, Cr 137.2 mg/kg, Cd 1.7 mg/kg, Cu 64.4 mg/kg, As 13.1 mg/kg, Sr 318.9 mg/kg); these results exceeded the accepted values of threshold effect levels (TEL) used as an indicator of their potential risk on marine life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cartagena Bay, located on the Caribbean coast of Colombia in South America, behaves as an estuarine system (Cogua et al. 2012). The distribution of sediments in this bay is highly influenced by Canal del Dique (Restrepo et al. 2013), a human-made canal connecting the bay to the Magdalena River, an important river, as it is the largest in Colombia, with a length of 1,540 km, and approximately 70% of the Colombian population (about 38 million people) is located in its watershed, which has the highest sediment yield of any other large river in South America, that is, 560 t km−2. From 1984 to 2010, Canal del Dique has discharged approximately 52 Mt of sediments into Cartagena Bay, and 55–250 m3 s−1 of freshwater (Restrepo et al. 2016). Additionally, Cartagena Bay’s coastal water quality is affected by maritime transportation, as well as industrial and household waste from the coastal city of Cartagena de Indias. After 2013, the city’s sewage system was directed to a drain far north of the city so as not to affect the bay (Tosic et al. 2019).
Sediments are known to become sumps for pollutants. Depending on environmental conditions, contaminants in sediments can be released into the water column and circulate in ecosystems (Tosic et al. 2019). As a result, sediment characterization in terms of metal and persistent organic pollutant (POP) content is used as a method to describe the environmental health status of the aquatic ecosystem. POPs such as polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and organochlorine pesticides (OCPs) have high octanol-water (Kow) partition coefficients; hence, they are highly adsorbed and accumulate in sediments (Souza et al. 2018). They are harmful to the marine environment due to their detrimental effects on wildlife and humans and were added to the list of substances that should be banned or limited in the Stockholm Convention on such POPs (Commendatore et al. 2018). OCPs are also toxic, bio-accumulative in fatty tissue, become biomagnified through the food web, and are prone to long-range transport via air, water, and migratory animals (Pereira et al. 2015a). These compounds are known to cause carcinogenesis, neurotoxicity, immunotoxicity, and endocrine and reproductive disorders in living organisms (Elnar et al. 2012; Kraugerud et al. 2012). Due to their physicochemical characteristics, they are found in the environment and their destination is still of concern despite the fact that their manufacture and use have been banned in many countries (Gonzalez-Mille et al. 2013).
Other families of pesticides such as organophosphorus, triazines, thiocarbamates, and chloroacetanilides were later introduced as alternatives to OCPs because these compounds are less persistent. Nonetheless, some molecules from these new families of pesticides have also been found toxic to biota (Phyu et al. 2006; Velisek et al. 2013) and their continuous release into the environment have resulted in air, water, soil, vegetable and fruit contamination (Ferré et al. 2018; Suárez et al. 2018; Vaclavik et al. 2018).
In Colombia, during the last 30 years, pesticide registration doubled from one hundred eighty-six molecules in 1974 up to four hundred active ingredients in 2003 (Cárdenas et al. 2010), and a recent inventory of PCBs estimated that about 9.771 and 12.803 tons are still in the country (Vaca Bohórquez et al. 2014). PBDEs were used worldwide as flame retardants in everyday products such as electronic equipment, building materials, furniture, and textiles (Besis and Samara 2012). Additionally, metal pollutants are released into the environment via anthropogenic and natural sources and, contrary to most pollutants, do not undergo microbial or chemical degradation. Metals are similarly biomagnified in the food chain (Burgos-Núñez et al. 2017; Marrugo-Negrete et al. 2017), and their adsorption into sediment constitutes one of the most important processes governing their mobility and bioavailability in aquatic environments (Pinzón-Bedoya et al. 2020
Studies have reported the presence of heavy metals and persistent organic pollutants from natural, industrial sources, and accidental spills, which have found their way into ecosystems at Cartagena Bay (Aguirre-Rubí et al. 2019; Tosic et al. 2019; Jaramillo-Colorado et al. 2015). Residues of various metals have been found in the bay’s surface sediments (Tosic et al. 2019; Jaramillo-Colorado et al. 2016; Alonso et al. 2000).
A multi-residue method for the analysis of semi-volatile organic pollutants in marine sediments from Cartagena Bay was employed. The QUECHERS extraction technique and gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS) were used for the determination of plaguicides, PCBs, and PBDEs. GC-MS/MS offers better selectivity and sensitivity than conventional quadrupole GC/MS (Payá et al. 2007; Zhang et al. 2015). Metals were analyzed by inductively coupled plasma mass spectrometry (ICP-MS), considered the best appropriate tool for determination of elements at ultratrace levels in all types of matrices (Fernandes et al. 2012).
The goals of this study were to evaluate concentrations of PCBs, PBDEs, OCPs, newer generations of pesticides, and metals from Cartagena Bay sediments, and compare the levels of these chemicals with the concentrations reported in other similar environments from other countries. This research would contribute to detect pollutant trends and improve the understanding of their sources, occurrence, risk, and destination.
Material and methods
Chemicals and materials
Analytical pesticide standards, of purity ≥ 95%, were obtained from Phenova (Golden, CO, USA) and Sigma Aldrich (St. Louis, MO, USA). Surrogate mix (2,4,5,6-tetrachloro-m-xylene and decachlorobiphenyl) was purchased from Phenova (Golden, CO, USA). Internal standards (ISTD), 13C12 PCB 52 and 13C12 PCB 138, were purchased from Wellington Laboratories Inc. (Ontario, Canada). PBDE standards were obtained from Accustandard (New Haven, CT, USA). The WHO-coplanar PCB mix was acquired from Cambridge Isotope Laboratories (Andover, MA). PSA (Primary Secondary Amine) and C18 sorbents were obtained from Agilent Technologies (Santa Clara, CA, USA). MgSO4, NaCl, acetonitrile LC/MS grade, isooctane for pesticide residue analysis, and hydrogen peroxide 30% were purchased from Fisher Scientific (Pittsburg, PA, USA). Metal-grade nitric acid and standard metal mixtures (82026-108 and 82026-114 BDH Aristar® Plus) were purchased from VWR International (Radnor, PA, USA). NANOpureTM water was used in all experiments from Barnstead International (Dubuque, IA, USA).
Study area and sample collection
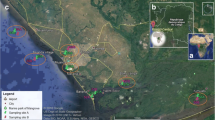
The Cartagena Bay has a surface area of 84 km2, approximately, with average and maximum water depths of 16 m and 26 m, respectively (Restrepo et al. 2013; Tosic et al. 2019). Ten sampling sites along the Cartagena Bay were selected to evaluate the spatial distribution of organic and inorganic pollutants in sediments. The sampling was carried out in 2015 at ten stations, during the rainy season.
Figure 1 shows the map of the study area with sampling locations, georeference, and land use around Cartagena Bay. Surficial sediments were collected using a Van Veen-type grab sampler; three sediment sub-samples were taken to complete about 400 g of a composite sample. The water depths of the sediment samples ranged from 6 to 15 m. The samples were immediately placed in dark amber bottles, refrigerated, and transported to the Agrochemical Research Group Laboratory at the University of Cartagena. Sediments were freeze-dried, ground, homogenized, and sieved (63-μm mesh). The samples were stored at – 40 °C for transportation to the University at Buffalo and until analysis was performed.
Sample extraction for organic pollutants
The Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERSTM) extraction method adapted from (Payá et al. 2007) was applied by using 4 g of freeze-dried sediment, which was weighed in 50-mL centrifuge tubes, and homogenized with 10 mL water and 10 mL acetonitrile. Then, 4 g of MgSO4 and 1 g of NaCl were added to promote phase separation. The mixture was agitated intensively in a vortex for 1 min and centrifuged at 3400 rpm for 8 min. An aliquot (4 mL) of the organic phase was cleaned up through dispersive solid-phase extraction (d-SPE) by adding 12.5 mg C18, 25 mg of PSA, and 150 mg of MgSO4 per mL extract. The mixture was shaken in a vortex for 1 min and centrifuged for 8 min at 3400 rpm. The supernatant was filtered (0.2-um PTFE), evaporated to dryness, and reconstituted with 1 mL of isooctane. An aliquot of the extract was spiked with 12.5 μL of 500 ng/mL spiking solution containing PCBs, PBDEs, and pesticides. This spiked sample was used as the single-point standard addition for quantification (Su et al. 2017).
GC/MS/MS analysis for organic pollutants
Organic compounds were analyzed using a Trace GC Ultra coupled to a TSQ Quantum XLS triple quadruple mass spectrometer (Thermo Scientific, West Palm Beach, FL) equipped with a programmable temperature vaporization injector (PTV). Separation was achieved on a DB-5HT column (30 m × 0.25 mm i.d × 0.10-μm film thickness; Agilent Technologies). The oven temperature was programmed to start at 60 °C (held for 1 min), ramped up to 160 °C at 15 °C/min (held for 2 min), then increased to 190 °C at 2.2 °C/min, ramped up to 290 °C at 50 °C/min (held for 3 min), and finally hiked up to 330 °C at 30 °C/min (held for 2 min). The carrier gas (helium, 99.99% purity) was set at 1 mL/min. Samples were injected (3 μL) using the PTV that was initially set at 89 °C and then increased at a rate of 7.5 °C /min for 0.2 min. The components were transferred to the column at a temperature of 330 °C for 1 min. A split flow of 125 mL/min and a split-less time of 1.4 min were used. The MS ionization energy was 70 eV, the transfer line temperature was 280 °C and the ion source was 200 °C. The mass spectrometer was operated under the selected reaction monitoring mode (SRM). Table 1 summarizes MS/MS transitions, retention times, collision energies, and recoveries for the targeted compounds.
Method validation
Sensitivity and linearity
Linearity was evaluated using a seven-point matrix-matched calibration curve that proved to be linear (r2 ≥ 0.999) for all the studied analytes over the studied scope (1.25 to 500 ng/mL). The sensitivity of the method was evaluated in terms of method quantification limit (MQL) and method detection limit MDL values stretch from 0.07 to 4.19 ng/g for pesticides and from 0.01 to 0.05 ng/g for PCBs and PBDEs, respectively (Table 1).
Some isomers such as demeton-s-methyl and demeton-o-methyl, beta-BHC, and delta-BHC were not completely separated by the column and therefore they were quantified as one.
Accuracy and precision
Standard addition was used for the quantification of targeted compounds to account for matrix effects associated with the analysis of such analytes in sediments. Organic components were confirmed based on their retention times, the presence of singled out quantifier and qualifier ions, and their expected ratios. Retention times had to be within ± 0.1 min of the expected time of the corresponding reference standard, and qualifier-to-quantifier ion ratios of the monitored m/z had to be within ±15% for positive confirmation.
Extraction recoveries for the organic pollutants were determined at two fortification levels, 10 ng/g and 100 ng/g. The samples used for determining such enhancements were subjected to the same extraction procedure as described above. Physical-chemical parameters of sediment used for validation are shown in Table S1. More than 96% of the pesticides under study presented Recuperation values between 70% and 120%. Similarly, PCBs and PBDEs such rates ranged from 70% to 99% (Table 1).
Some isomers such as demeton-s-methyl and demeton-o-methyl, beta-BHC, and delta-BHC were not completely separated by the column and therefore they were quantified as one.
Total organic carbon and metal analysis
Total organic carbon (TOC) was determined using the modified Wakley-Black titration method described by Gaudette et al. (1974). For trace determination of metals (Pb, Cr, Cd, As, Sr, Cd), freeze-dried sediments were acid-digested (HNO3 + H2O2) with a modified USEPA 3050B method (USEPA 1996), followed by metal detection using inductively coupled plasma mass spectrometry (ICP-MS). A detailed description of the metal analysis method is shown in the supplementary information.
Sample digestion for metal analysis
Freeze-dried sediment samples were digested in trace metal-grade nitric acid (Aristar Ultra) and hydrogen peroxide 30% (Fisher Scientific) following a method adapted from USEPA 3050B for 0.5 g of sample (USEPA 1996). Samples were extracted and analyzed by duplicated. Briefly, 0.5 g dry mass soil was covered with concentrated nitric acid and refluxed for 2 to 3 h in 50-mL digestion vessels, covered with reflux caps. Hydrogen peroxide was added before refluxing for an additional 1 to 3 h. Digestion was performed in a HotBlock® Pro system (Environmental Express). Samples were diluted to 50 mL with NANOpureTM water (Dubuque, IA). and filtered through 5-μm PTFE-faced polypropylene filters (Environmental Express, Charleston, SC, USA). A method blank solvent was analyzed with the samples.
Elemental metal analysis
Samples were analyzed by inductively coupled plasma mass spectrometry (ICP–MS) on a X-Series 2 instrument (SC2 DX autosampler) (Thermo Scientific). In Table S2, the operating conditions of the ICP-MS are described.
A list of the analyzed metals and the respective metal internal standard used are shown in Table S3. The isotopes used were chosen after evaluating the performance of the analysis when using the other abundant isotopes of each element. Internal standard solution of 10 ng/mL was introduced at a constant rate by the sample injection system to reduce errors due to instrument drift. Addition of 7% H2/He gas to the collision cell was utilized in conjunction with the pole bias and hexapole bias settings to remove interfering diatomic ions.
One-point standard addition was used for accurate quantification of the target metals to minimize matrix effects. Two 3-mL aliquots were taken from each digested sample. One aliquot received 7 mL of NANOpureTM water to represent a blank sample. The other aliquot received 1 mL of 200 ng/mL standard solution mixture to create a solution with a known concentration of 20 ng/mL, then 6 mL of NANOpureTM water was added to reach a total volume of 10 mL. The y-intercept and slope of the line connecting the 2 points were divided to calculate the actual sample concentration in ng/mL.
Method validation
For method validation, various sediment samples were pooled and homogenized. From this pool, sediments were spiked at two different levels (0.3 mg/kg and 3 mg/kg, respectively) and were digested to determine extraction recoveries of the metals (n = 3). Unspiked sediment samples were also digested and analyzed; the values from these were subtracted from the spiked samples to calculate % recoveries. The MQL and MDL were determined by pooling digested sediment and spiking them before injection at different levels. Linearity was evaluated using matrix-matched calibration over the range of 0.5 to 100 ng/mL. The MDL and MQL were defined as the minimum detectable amount of analyte with signal-to-noise (S/N) ratios of 3 and 10, respectively (Table 2). Metal extraction recovery and method performance rates are presented in Table 2. Recuperation levels overall range from 46 ± 10 % to 111 ± 7 %, while those of MDL go from 0.05 to 0.49 mg/kg.
Data processing
Spearman’s correlation analysis was performed using SPSS-25 Software to examine the relationships among metals, TOC, and pH. To differentiate the influence caused by natural or anthropogenic sources of metals, simple and integrated indices such as contamination factor (CF), geo-accumulation index (Igeo) and pollution load index (PLI) were calculated according to Kalender and Çiçek Uçar (2013). The Earth’s crust values were used (Lide 2008).
Principal component analysis was performed using Minitab 17.3.1 (Minitab, Inc. State College, PA). Data was pretreated using auto-scaling. A value of 0.00001 was assigned to variables exhibiting a non-detect (n.d.) to avoid loading bias. The analysis was performed using a covariance matrix to allow for full variable comparability and to account for differences in units and scales among variables. Significant principal components (PCs) were selected according to the Scree test (D’agostino and Russell 2005). Hierarchical Cluster Analysis was developed using the average linkage (between groups) method.
Results and discussion
In this study, organic pollutants were analyzed by gas chromatography-triple quadrupole mass spectrometry (GCMS/MS). With the arrival of tandem mass spectrometry detectors, sample preparation processes have been reduced and are more straightforward. Today, trends in organic pollutant residue analysis are aimed at decreasing solvent consumption, sample size, and minimum cleanup steps. One of the most used methods for the analysis of pesticide residues due to its versatility is the QuEChERS method, introduced by Annastasiades & Lehotay (Payá et al. 2007).
GC-MS/MS offers various advantages in selectivity and sensitivity at low concentrations in the most complex mixes, especially for identifying and quantifying organic contaminants in environmental samples. (Zhang et al. 2015; Fernandes et al. 2012). The utilization of tandem MS can effectively reduce co-extractive compound interference and sample matrix effects, resulting in increased signal to noise (S/N) ratio and ultimately robust limits of detection. GC-MS/MS.
Concentrations of pesticides
The concentrations of the pesticides detected in each sample are shown in Table 3. Thirty out of the forty-nine targeted pesticides were detected at least in one of the samples. Total pesticide concentrations ranged from 0.83-33.67 ng/g dry weight, with the highest levels found at the mouth of Canal del Dique (S1, S2, S3, and S4), and at S9. Correspondingly, these locations exhibited the highest number of pesticides detected with a significant contribution from herbicides such as atrazine, bromacil, and butachlor. Organophosphorus (OPPs) and OCPs were the main contributors to the total concentrations of pesticides detected at the remaining locations. Thiocarbamates have more affinity with water than with sediment, as indicated by their slow Kow values, and were not detected in most samples, except for vernolate at S1 (28.93 ng/g) and molinate (< 0.1 ng/g) at S8 and S9.
Total OPPs concentrations ranged from 0.39 to 3.06 ng/g dw with S1, S2, and S3 showing the highest frequencies of detection for OPPs. Chlorpyrifos (0.28–1.43 ng/g) and diazinon (< 0.08–0.27 ng/g) were detected in all the samples. Chlorpyrifos exhibits a strong affinity for aquatic sediments that correlates with organic content (Etchegoyen et al. 2017; Gebremariam et al. 2012). While diazinon is a moderately mobile pesticide, its adsorption into sediments also increases with organic matter alongside organic carbon contents (Aggarwal et al. 2013), which could explain its detection in the collected sediments. These two pesticides are among the OPPs of main use in Colombia (Aguirre-Rubí et al. 2019).
The presence of chlorpyrifos at Cartagena Bay is affected by the input from Canal del Dique (intensive agriculture in the Magdalena River basin) and by the agrochemical industry based near the seaport (Vivas-Aguas et al. 2010). In 2011, chlorpyrifos was detected at the bay (Vivas-Aguas et al. 2014) with a toxic level at a concentration of 0.024 μg/L, higher than the reference value for acute effects on aquatic life (0.011 μg/L) (Buchman 2008). Menzies et al. (2013) found Ʃcyclodiene pesticides, including chlorpyrifos, in seawater from Colombia (Cartagena Bay = 3.7 ng/L, Rosario Island = 20.5 ng/L, Santa Martha bay = 6.6 ng/L, and Cienaga = 22.2 ng/L), Panama (Panama Canal, Gatun locks = 11.7 ng/L), Costa Rica (Santa Elena = 32.2 ng/L), and Galapagos Island (Santacruz = 12.1 ng/L).
One study, on the content of chlorpyrifos in mangrove oysters from Nicaragua and Colombia, shows that chlorpyrifos was only detected in Nicaraguan oysters within the range of 5–11 ng/g dry-weight; it was not detected in oysters from Colombian localities (Aguirre-Rubí et al. 2019). Total OCP concentrations ranged from 0.39 to 1.02 ng/g in the sediments with the highest concentrations found at S2, S6, and S10. Although 13 out of the 19 OCPs targeted were detected in the samples, most of them were below the MQL. DDT-related compounds were the main OCP contributors with 4,4′-DDE (0.26–0.84 ng/g) and 4,4′-DDD (0.10–0.27 ng/g) detected in all the samples. DDT was present in all the samples but largely fell below the MQL (< 0.21–0.31 ng/g). Higher concentrations of DDE and DDD can be attributed to the degradation of DDT, which can be degraded under anaerobic and aerobic conditions to DDD and DDE, respectively (Kang et al. 2016). A DDE/DDD ratio of less than one was observed in all the sites, suggesting aerobic degradation (Table S4). The residence time of DDT can be estimated using the ratio of DDT to DDE + DDD. If DDT/(DDE + DDD) < 1, the loadings of DDT are historical, whereas a ≥ 1 ratio indicates new loadings of DDT (Kang et al. 2016). In this study, DDTs/(DDE + DDD) were in the range of 0.21–0.57, indicating that DDT residues in the bay are historical and not due to a recent introduction. Total DDT levels found in the samples were below the TEL (3.89 ng/g) for marine sediments, indicating that adverse effects to aquatic organisms are potentially rare (MacDonald et al. 1996).
The presence of pesticides in the waters, sediments, and biota of Cartagena Bay has been reported (INVEMAR 2016; Jaramillo-Colorado et al. 2015; Jaramillo et al. 2010). Previous studies have accounted for the presence of PCBs and OCPs in the Cartagena Bay water with total concentrations of 75.5 ng/L and 23.9 ng/L, respectively (Menzies et al. 2013). Residual amounts in the muscle of M. incilis such as β-HCH (0.00638 ng/g), γ-HCH (0.00851 ng/g), heptachlor (0.0725 ng/g), endosulfan (0.00415 ng/g), and 4,4-DDE (0.00401 ng/g), among others, were identified, indicating that the fauna of Cartagena Bay is exposed to pollutants (Jaramillo-Colorado et al. 2015).
Although OCPs have been banned in Colombia since 1993 (ICA 2004), the country still has a stock of about 160.000 kg of OCPs (García Ubaque et al. 2015), which constitute a latent risk. In Nicaragua, DDTs and their derivatives were recorded in oyster tissues in all the samples analyzed; interestingly, the highest tissue concentrations of DDT derivatives such as 4,4-DDE were recorded in Punta Lora during the rainy season (ƩDDT = 1082) (Aguirre-Rubi, et al., 2019).
Concentrations of PBDEs and PCBs
Table 4 shows the concentrations of dioxin-like PCB congeners (77, 105, 114, 126, 156, 157, 167, 169, 170, 180, 189) and PBDE congeners (47, 99, 100, 153, 154) in the Cartagena Bay sediment from the 10 locations sampled. Total levels of PBDEs ranged from 0.02 to 0.0.40 ng/g dw. PBDE-47 and PBDE-99 were the most abundant PBDEs present in each sample, with detection frequencies of 100% and 70%, respectively. Meanwhile, in another study conducted in Colombia by Barón et al., (2013), in the Atlantic coastal area of influence of the Magdalena River, BDE-209 was detected. In the same study, marine sediments from Chile (Bio-Bio region) were analyzed. PBDEs were detected in all sediment samples from Chile at concentrations ranging from 0.03 to 2.43 ng g−1 dw.
BDE-47 and BDE-99 were also the most abundant PBDE congeners in settled particulates from an estuary in Argentina, but average levels of total PBDEs were 10-fold greater at 1.8 ng/g dw (Cappelletti et al. 2015). The occurrence of PBDE can occur due to the debromination of brominated congeners by biotic or photolytic pathways (La Guardia et al. 2006).
Polybrominated diphenyl ethers (PBDEs) are added to foam, textiles, plastics, television casings, computers, furniture, and carpets, all of which contain PBDEs as flame retardants and can become a source of pollution (Anim et al. 2017; Tombesi et al. 2018). Direct leaching, poor disposal of disused appliances, automotive scrap shredding, and indirect waste streams are some of the pathways of PBDE contamination in estuarine systems (Anim et al. 2017).
The total PCB concentrations in the ten sampling sites at Cartagena Bay ranged from 0.09 to 20 ng/g dw. Levels of PCBs were highest in samples 6, 8, and 10. The PCB congener with the most significant detection in the Cartagena Bay samples was PCB-180, followed by PCB-126 and PCB-170, with median levels of 1.45, 0.45, and 0.81 ng/g dw, respectively. Total levels of PCBs were far below TEL in marine (21.6 ng/g dw) and freshwater sediment (34.1 ng/g dw) in most of the samples (MacDonald et al. 1996, 2000). The concentrations of dioxin-like PCBs found in this study were similar to the sediment sample from Argentina Bay, where total concentrations reached 6.8 ng/g dry-weight (Cappelletti et al. 2015). PCBs were widely used as dielectric fluids from transformer hydraulic tools, capacitors and heat-exchange liquid, lubricants, cutting oils, surface coatings, plasticizers, carbonless copy paper, ink, waxes, dyes, and adhesives (Souza et al. 2018). PCBs reach the marine environment via dry and wet deposition, sewage sludge used as fertilizer, and leaching from landfills, river discharge, and continental runoff (Ruiz-Fernández et al. 2019).
Aguirre-Rubí et al. (2019) made a study of PCBs and PBDEs content in oyster from the Caribbean Coasts of Nicaragua and Colombia. Low concentrations of PCBs and PBDEs were found in Nicaragua. Only PCB levels were occasionally exceeded in Colombia, while PBDEs were not detected.
Spearman’s correlation analysis demonstrated significant positive correlations (p < 0.05) between OCPS-PBDEs (p = 0.044) (Table S5). Commendatore et al. (2018) reported a strong positive correlation both between total PBDEs and OCPs detected in sediment, crab, and gull samples. Their bioaccumulation was observed in marine biota, suggesting risks to upper trophic level predators (Commendatore et al. 2018).
Table 4 shows organochlorine and related compounds in sediments, water, and biota from other Colombian coastal regions, and Latinomerican bays or estuaries.
Trace metals
pH and TOC and heavy metal content of collected sediments are shown in Table 5. The pH of the samples indicates the neutral nature of the sediments, varying within a narrow range along the bay (7.2 to 8.1). Total organic content in the sediments ranged mainly from 4.18 to 8.15%, except for S6, which contained 21.64% organic carbon. These results are consistent with TOC values reported in some coastal areas around the world. However, they are above the median value (1.5%) (Seiter et al. 2004), suggesting both terrestrial and anthropogenic sources. The high sedimentation rate found in the bay is influenced by the discharge from Canal del Dique (Restrepo et al. 2017), which could be the primary source for organic carbon accumulation, particularly during the moderate rainy season when the samples were collected. Additionally, Cartagena Bay has mangrove ecosystems, which could provide organic-rich sediments as well (Kennedy et al. 2004).
The metal concentrations in the sampled sediments are shown in Table 6. All metals evaluated in the marine sediments were found in S6, thus exceeding the accepted values of threshold effect levels (TEL) used as indicator of their potential on marine life (Restrepo et al. 2017).
Strontium was found in all sediments from the sampling sites (32.9–959.6 mg/kg). Aghadadashi et al. (2019) report that high concentrations of Sr could be related to shell fragments, which are mostly carbonated structures. Studies show that the presence of strontium in marine sediments represents a natural geochemical footprint (Delgado et al. 2010; Sondi et al. 2017).
This trend was followed by S1 and S2. Concentrations of As, Pb, and Cr were highest in the central part of the bay (station S6), which may be associated with activities of the chemical and metallurgical industries around the Mamonal area. Besides, for Cd, the highest concentrations in the sediments were found at stations S1 and S4, near the outlet of Canal del Dique (2.0 and 2.4 mg/kg, respectively); Cu, Cr, As, and Sr also were found. Some of these are accumulated through the Magdalena River basin upstream of Canal del Dique, which indicates that Cartagena’s anthropogenic activities are not the only sources of contamination at Cartagena Bay (Tosic et al. 2019). Therefore, the presence of metals in marine sediments may be due to mining and intensive agricultural activities, being the river’s discharge the main input areas of pollutants to the coastal areas (Vallejo Toro et al. 2016).
When we compared our results with other studies, it can be seen the Pb, Cu, and Cr values were higher than the ones registered in other studies on the Colombian Caribbean coast (Table 6); Cd was lower than those reported by Tosic et al. (2019) and Restrepo et al. (2017), but higher than those reported by Burgos-Núñez et al. 2017, in the Cispata Bay, and Caballero-Gallardo et al. (2015) in Santa Martha Bay in Colombia. In the same Table, the results of metals in sediments from other bays of Latin America can be observed.
Physicochemical parameters in sediments are known to affect the bioavailability of metals in the environment (Benson et al. 2016). This is the degree of freedom in which an element or compound is found from a potential source to be captured by an organism (ingested or adsorbed). The accumulation of heavy metals depends on the geochemical properties of sediments and significant variations of their concentration are related to habitats. Kadhum et al. (2017) and Dehghani et al. (2018), among others, found the bioaccessibility of heavy metals influenced by pH, particle size fraction, and cation exchange capacity (CEC).
Spearman’s correlation analysis demonstrated significant positive correlations (p < 0.05) between the metals Pb-Cr (p = 0.026), Pb-Cd (p = 0.032), TOC-Pb (p = 0.031), and TOC-Cr (Table S6). The results could suggest that Pb, Cr, and Cd have possible common sources and that TOC played an important role in the adsorption of Cd and Pb into the sediments collected. High organic content is related to a significant sedimentary metal affinity for humic substances, which could decrease trace metal bioavailability through complexation (Benson et al. 2016), thus explaining the positive correlation found between TOC with Cd and Pb concentrations. However, other parameters such as grain size, ligand type, presence of chelating agents, the oxidation state of mineral components, and the system’s redox potential were not determined in this study, which may also influence the solubility of metals and their detected levels at the Bay (Gundersen and Steinnes 2003).
Evaluation of metal pollution in sediments and comparison to sediment quality guidelines
The results of sediment contamination indices calculated for the collected sediments and their sediment qualification description are presented in Fig. 2. The contamination factor (CF) was used to evaluate pollution by single metals in the sediments. CF values found for As indicated considerable to very high contamination (CF > 5) in eight of the ten locations. CF values for Pb and Cu indicated moderate contamination in seven and nine out of the ten sampled locations, respectively, except for Cu at location S9, where a high degree of pollution was found. This point is near beaches and neighborhoods from Cartagena city.
Contamination factors for Cr and Cd indicated moderated pollution at a few locations, while CF values for Sr indicated low contamination in nine of the ten samples. The geo-accumulation index (Igeo) was used to determine contamination by comparing current concentrations with pre-industrial levels. For most of the metals, all sampled locations indicated unpolluted conditions (Igeo < 0), except for As which exhibited values between 1 and 3 for nine of the locations, indicating moderate to heavy pollution in sediments.
The pollution load index (PLI) was used for assessing the integral level of metal pollution at each sampling site (Tomlinson et al. 1980). PLI values ranged from 0.5 to 2.0 in the samples, with six out of the ten locations exhibiting overall metal pollution. In general, sample S6 showed the highest level of pollution for most of the tested metals and pollution indices calculated. Copper was found to be the main contributor to sediment pollution in S9. Further, Cu and As presented moderate and high degrees of pollution across the bay sediments, respectively, with an elevated anthropogenic input.
Eco-toxicological indices (SQGs) were used to assess the contamination levels of the sediments. The metal concentration values obtained were compared with the TEL and PEL of SQGs for marine sediments (Buchman 2008) (see Table 6).
In this study, most metal concentrations were within the TEL–PEL range, indicating that this can be linked to occasional adverse biological effects (Pena-Icart et al. 2017). Pb concentrations were mostly below TEL, showing that adverse effects to aquatic organisms were not expected, except at location S6 (Hubner et al. 2009). Chromium was found at concentrations above TEL at S1, S2, S4, S6, S7, S10, which is associated with occasional adverse effects. The presence of Cr at the Bay can be influenced by the tannery industry. Copper levels were found above PEL at S9 (429 mg/kg), which is associated with adverse biological effects (Pena-Icart et al. 2017).
Multivariate analysis
Principal component
The levels of POPs and metals in estuary sediments are considered a local source to the marine environment, which may affect the fish and mammals directly exposed. Therefore, there is a need to investigate contaminant relationships to identify common trends between sampling sites and potential sources of investigated compounds. Principal component analysis yielded eight significant PCs characterizing a total of 98.5% of the total variance between contaminants. The first four PCs account for approximately 80% of the total variance indicating four primary sources among the sample locations with minor contributions from other sources. Sample locations were separated organically into three groups based on sample scores from PCs 1, 2, and 4 (Fig. 3). While significant, PC3 was dominated by four of the analyzed contaminants limiting its use to visualize the reduced dataset.
Each group of related sites roughly corresponds to a different geographical area of the bay. Site S1, located at the distal end of the canal, exhibited positive loading by PC1 alone, which consists of all the analyzed pesticides except DDT, DDE, DDD, and cis-chlordane, and suggests that the contamination at S1 mainly originates from the application and runoff of pesticides upstream. Sites S2 and S3, located in the middle part of the inner bay, exhibit positive loadings in PCs 1, 2, and 4, and a negative contribution from PC3, suggesting mixed inputs from the industrial application of pesticides. PC2, which consists mainly of chloroacetanilide herbicides, Pb, Cr, As, and PBDEs, accounts for approximately 44–46%, while the remaining pesticides contribute ~ 30% to each site, and the PCBs, Cu, and Sr, pitches in with ~ 10% with all other sources making up the remainder. Sites S4, S5, and S6 demonstrated minimal contribution from the pesticides in PC1 with marked contributions from Cu, PCBs 77, 105, and 169 and DDT in PC3 (53%, 57%, and 61% for S4, S5, and S6, respectively). Sites S7, S8, and S9 each exhibit positive inputs from PC3 (64–72%) and PC4 (28–36%), indicating a source contribution from the release of PCBs, Cu, Sr, and BDEs 153 and 154. Site S10 is loaded positively only with PC 4, indicating significant supplies from a single source emitting a mixture of PCBs, PBDEs, pesticides, and Cu.
Cluster analysis
In the current study, the nearest neighbor hierarchical (HCA) method was applied for the bulk sediments. The examined data matrix involves the concentrations of heavy metals and organic pollutants in the bulk sediments against the site (Marrugo-Negrete et al. 2017; Birch et al. 2001).
Cluster analysis (CA) suggests a total number of 4 groups, incorporating, from smaller to larger, group 4 (includes one site: S6) site was located in a region, which receives chromiun wastewater discharges from a tannery factory, group 2 (include one site:S10) site was in a moderate pollution region, group 3 (include three sites: S1, S2, S3, S4) were in front of Canal del Dique discharges (Fig. 4).
Environmental implications
Metals such as Cd, Cu, Cr, Pb, and As could become a threat to the marine ecosystem due to their persistence, inherent ecotoxicological characteristics, and tendency for biomagnification. Elevated concentrations of such elements in sediments or water are a potential threat for human health through the food chain (Pinzón-Bedoya et al. 2020; Marrugo-Negrete et al. 2017; Pereira et al. 2015b; Fernandez-Cadena et al. 2014). Colombia does not have regulations about metals in marine sediments. Nevertheless, resolution No. 122 of 2012 establishes the physicochemical, microbiological, and chemical contaminant requirements that must be met by fishery products, particularly fish, mollusks, and crustaceans for human consumption (Ministry of Health and Social Protection. 2012). Sediment quality guidelines (SQGs) are used as a screening tool to evaluate the biological significance of sediment-bound contaminants in the absence of direct biological effects data (Simpson and Batley 2016; Casado-Martínez et al. 2006,). The results obtained in this study were also compared with SQGs from other countries as can been in Table 7. This shows, for example, that Cd concentrations were greater than Spain and Hong Kong SQGs, and for Cu were higher than the USA, Australia, Canada, and Hong Kong SQGs.
As, Pb, Cd, and Hg have all been included on the top 20 list of hazardous substances by the United States Environmental Protection Agency (EPA) and the Agency for Toxic Substances and Disease Registry (ATSDR) (Kumar Rai et al. 2019). The ingestion of metals through food causes serious damage to human health, i.e., lead contamination reduces mental development and produces neurological and cardiovascular diseases (El-Kady and Abdel-Wahhab 2018). Several studies have reported that Cd, Pb, As, and Cr (VI) have carcinogenic effects and can affect the bones and nervous system, among others (Kumar Rai et al. 2019).
When comparing the concentrations of the OC pesticides obtained in this study with the different sediment quality guidelines of different countries presented in Table 7, it can be seen that at no point are the pesticide thresholds exceeded in the sediments from Cartagena Bay. OCPs, PCBs, and PBDE are ubiquitous contaminants, all of which have a high accumulation potential in marine environments, i.e., PCB concentrations in R. mangle tissue from Jobo Bay in Puerto Rico (roots, leaves, and seeds) indicate bioaccumulation in these sensitive coastal plants (Alegria et al. 2016).
Organochlorine compounds can be adsorbed in marine sediment, and bio-accumulated in fatty tissues of living organisms (Jaramillo-Colorado et al. 2015). They can produce harmful effects on aquatic organisms, such as impaired reproduction, immune suppression, and endocrine disruption and/or high occurrence of chronic lesions such as tumors in the liver and spleen (Magalhães et al. 2017; Tham et al. 2019). PCBs and PBDEs are considered potent hormonal disruptors that cause neurodevelopmental deficit, adverse reproductive effects, and possibly cancer (de Miranda et al. 2016; Taiwo 2019).
Concerning pesticides, PCBs, and PBDE, in Colombia, there is no regulation on these compounds in marine sediments, but it does exist on water and seafood. The Ministry of Health and Social Protection is the entity that ensures these regulations for fish and fishery products that are consumed in Colombia and which are intended for export, whereby the threshold is established by Colombian Resolution No 122 of 2012 (4.0 pg WHO-TEQ PCDD/F g− 1 for PCDD/Fs and 8.0 pg WHO-TEQ g− 1 for PCDD/Fs and dl-PCBs) (Ministry of Health and Social Protection 2012; Pemberthy et al. 2016). On the other hand, European regulation set the maximum established levels at 3.500 pg g− 1 of dry weight WHO-TEQ PCDD/Fs and 6.500 pg g− 1 of dry weight WHOTEQ PCDD/Fs and dl-PCBs as maximum levels for fish (shrimp) (EU 1259 2011). According to the Food and Drug Administration (FDA), the tolerance level for PCB in edible tissue of seafood is two ppm, and the action level for pesticides DDT, TDE, and DDE is five ppm (FDA 2018). In Colombia, regulations regarding the use of OCPs began in 1974, and they were completely banned in 2001 (Ministry of the Environment of Colombia 2010).
Food items must comply with the maximum residue limits of pesticides—LMR—of Codex Alimentarius, by resolution No. 2906 of 2007 (Ministry of Agriculture and Rural Development-Ministry of Social Protection 2007).
On the other hand, chlorpyrifos is a broad-spectrum, chlorinated, organophosphate pesticide used to control insect, tick, and mite populations. It is widely employed in Colombia in the control of insects in crops (Varela-Martíneza et al. 2019), and is being reviewed for its harmful effects (Centner 2018). This pesticide is moderately hazardous to humans (WHO 2010; Ventura et al. 2015). However, in 2014 and 2016, scientific evidence considered by the EPA showed that existing tolerances do not protect persons from dangerous levels of chlorpyrifos (EPA 2014, 2016; Centner 2018).
Our results of Ʃ-PCB, p,p′-DDE, and total DDT were compared with other sediment quality guidelines from other countries (Table 7). Concentrations were below the lowest concentration associated with adverse biological effects.
Conclusions
In this study, Cartagena Bay sediments exhibited the occurrence of OCPs, PCBs, and PBDEs; their concentrations were lower than those reported in NOAA Screening Quick Reference Tables. DDTs and PCBs are banned organochlorine compounds since, even at low levels, their presence in sediments represents a hazard to aquatic organisms and subsequently to human health through the trophic chain. The prominence of Ʃdichlorodiphenyldichloroethylene (DDT) among pesticides in sediments is the result of its past use in the region and in the Magdalena River basin. Chlorpyrifos was the pesticide found in the highest level in the marine sediments from Cartagena Bay, which has not been banned yet, despite the fact that currently there are several studies on its harmfulness.
Surface sediments from Cartagena Bay have shown the presence of metals. All metals evaluated in the marine sediments were found in the S6 sampling point; this was near the tannery and hydrocarbon industries (Pb 37.1 mg/kg, Cr 137.2 mg/kg, Cd 1.7 mg/kg, Cu 64.4 mg/kg, As 13.1 mg/kg, Sr 318.9 mg/kg); these results exceeded the accepted values of threshold effect levels (TEL) used as an indicator of their potential risk on marine life which represents a potential ecotoxicological risk according to sediment quality guidelines. Metals in the bay are mainly attributed to anthropogenic activities, such as port and shipping operations, as well as the industrial sector that currently includes over seventy industries with domestic and agricultural activities on the margins of the Magdalena river that discharge into the bay. Cu and Pb are a well-known marker element of agricultural activities, mainly fertilizer application.
Data availability
The majority of the data generated or analyzed during this study are included in this published article (and its supplementary information files), but if some are missing, data are available from the corresponding author on reasonable request.
References
Abreu IM, Cordeiro RC, Soares-Gomes A, Abessa DMS, Maranho LA, Santelli RE (2016) Ecological risk evaluation of sediment metals in a tropical Euthrophic Bay, Guanabara Bay, Southeast Atlantic. Mar Pollut Bull 109(1):435–445. https://doi.org/10.1016/j.marpolbul.2016.05.030
Aggarwal V, Deng X, Tuli A, Goh KS (2013) Diazinon-chemistry and environmental fate: a california perspective. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology Volume 223. Springer, New York
Aghadadashi V, Neyestani MR, Mehdinia A et al (2019) Spatial distribution and vertical profile of heavy metals in marine sediments around Iranʼs special economic energy zone; Arsenic as an enriched contaminant. Mar Pollut Bull 138:437–450. https://doi.org/10.1016/j.marpolbul.2018.11.033
Aguirre-Rubí JR, Ortiz-Zarragoitia M, Izagirre U, Etxebarria N, Espinoza F, Marigómez I (2019) Prospective biomonitor and sentinel bivalve species for pollution monitoring and ecosystem health disturbance assessment in mangrove–lined Nicaraguan coasts. Sci Total Environ 649:186–200. https://doi.org/10.1016/j.scitotenv.2018.08.269
Alegria H, Martinez-Colon M, Birgul A, Brooks G, Hanson L, Kurt-Karakus P (2016) Historical sediment record and levels of PCBs in sediments and mangroves of Jobos Bay, Puerto Rico. Sci Total Environ 573:1003–1009. https://doi.org/10.1016/j.scitotenv.2016.08.165
Alonso D, Pineda P, Olivero J, Gonzalez H, Campos N (2000) Mercury levels in muscle of two fish species and sediments from the Cartagena Bay and the Cienaga Grande de Santa Marta, Colombia. Environ Pollut 109:157–163. https://doi.org/10.1016/S0269-7491(99)00225-0
Anim AK, Drage DS, Goonetilleke A, Mueller JF, Ayoko GA (2017) Distribution of PBDEs, HBCDs and PCBs in the Brisbane River estuary sediment. Mar Pollut Bull 120:165–173. https://doi.org/10.1016/j.marpolbul.2017.05.002
Barón E, Gago-Ferrero P, Gotga M, Rudolph I (2013) Occurrence of hydrophobic organic pollutants (BFRs and UV-filters) in sediments from South America. Chemosphere 92:309–316
Beltrame M, De Marco S, Marcovecchio J (2009) Dissolved and particulate heavy metals distribution in coastal lagoons. A case study from Mar Chiquita Lagoon, Argentina. Estuar Coast Shelf Sci 85:45–56. https://doi.org/10.1016/j.ecss.2009.04.027
Benson NU, Asuquo FE, Williams AB, Essien JP, Ekong CI, Akpabio O, Olajire AA (2016) Source evaluation and trace metal contamination in benthic sediments from equatorial ecosystems using multivariate statistical techniques. PLoS One 11:e0156485. https://doi.org/10.1371/journal.pone.0156485
Besis A, Samara C (2012) Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments-a review on occurrence and human exposure. Environ Pollut 169:217–229. https://doi.org/10.1016/j.envpol.2012.04.009
Birch GF, Taylor SE, Matthai C (2001) Small-scale spatial and temporal variance in the concentration of heavy metals in aquatic sediments: a review and some new concepts. Environ Pollut (Barking, Essex : 1987) 113(3):357–372. https://doi.org/10.1016/s0269-7491(00)00182-2
Buchman MF (2008) NOAA Screening Quick Reference Tables, NOAA OR&R Report 08-1, Seattle WA, Office of Response and Restoriation Division, National Oceanic and Atmospheric Administration, 34 pages. In: https://repository.library.noaa.gov/view/noaa/9327. Accesed 30 april 2019
Burgos-Núñez S, Navarro-Frómeta A, Marrugo-Negrete J, Enamorado-Montesa G, Urango-Cárdenas I (2017) Polycyclic aromatic hydrocarbons and heavy metals in the Cispata Bay, Colombia: a marine tropical ecosystem. Mar Pollut Bull 120:379–386. https://doi.org/10.1016/j.marpolbul.2017.05.016
Caballero-Gallardo K, Guerrero-Castilla A, Johnson-Restrepo B, de la Rosa J, Olivero-Verbel J (2015) Chemical and toxicological characterization of sediments along a Colombian shoreline impacted by coal export terminals. Chemosphere 138:837–846. https://doi.org/10.1016/j.chemosphere.2015.07.062
Canadian Council of Ministers of the Environment (1999) Protocolo for derivation of Canadian sediment quality guideline for the protection for aquatic life. Winnipeg, Canada. http://ceqg-rcqe.ccme.ca/download/en/226. Accessed 30 of june of 2020
Cappelletti N, Speranza E, Tatone L, Astoviza M, Migoya MC, Colombo JC (2015) Bioaccumulation of dioxin-like PCBs and PBDEs by detritus-feeding fish in the Rio de la Plata estuary, Argentina. Environ Sci Pollut Res 22:7093–7100. https://doi.org/10.1007/s11356-014-3935-z
Cárdenas O, Silva E, Ortiz JE (2010) Uso de plaguicidas inhibidores de acetilcolinesterasa en once entidades territoriales de salud en Colombia, 2002-2005. Biomedica 30:95–106. https://doi.org/10.7705/biomedica.v30i1.157
Casado-Martínez MC, Buceta JL, Belzunce MJ, DelValls TA (2006) Using sediment quality guidelines for dredged material management in commercial ports from Spain. Environ Int 32:388–396. https://doi.org/10.1016/j.envint.2005.09.003
Celis-Hernandez O, Rosales-Hoz L, Cundy B, Carranza-Edwards A, Croudace I, Hernandez-Hernandez H (2018) Historical trace element accumulation in marine sediments from the Tamaulipas shelf, Gulf of Mexico: an assessment of natural vs anthropogenic inputs. Sci Total Environ 622–623:325–336. https://doi.org/10.1016/j.scitotenv.2017.11.228
Centner TJ (2018) Canccelling pesticide registrations and revokig tolerances: the case of chlorpyrifos. Environ Toxicol Pharmacol 57:53–61. https://doi.org/10.1016/j.etap.2017.11.009
Cogua P, Campos-Campos NH, Duque G (2012) Concentration de mercurio total y metilmercurio en sedimento y seston de la bahia de Cartagena, Caribe Colombiano. Bol Invest Mar Cost 41:267–285 ISSN 0122-9761
Combi T, Taniguchi S, Figueira R, Mahiques M, Martins C (2013) Spatial distribution and historical input of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in sediments from a subtropical estuary (Guaratuba Bay, SW Atlantic). Mar Pollut Bull 70:247–252. https://doi.org/10.1016/j.marpolbul.2013.02.022
Combi T, Martins C, Taniguchi S, Leonel J et al (2017) Depositional history and inventories of polychlorinated biphenyls (PCBs) in sediment cores from an Antarctic Specially Managed Area (Admiralty Bay, King George Island). Mar Pollut Bull 118:447–451
Commendatore M, Yorio P, Scenna L, Ondarza PM, Suárez N, Marinao C, Miglioranza KSB (2018) Persistent organic pollutants in sediments, intertidal crabs, and the threatened Olrogʼs gull in a northern Patagonia salt marsh, Argentina. Mar Pollut Bull 136:533–546. https://doi.org/10.1016/j.marpolbul.2018.09.010
D’agostino RBR, Russell HK (2005) Scree test, in: Colton, P.A.A.T. (Ed.), Encyclopedia of biostatistics. John Wiley & Sons Ltd. https://doi.org/10.1002/0470011815
de Miranda AS, Kuriyama SN, da Silva CS, do Nascimento MS, Parente TE, Paumgartten FJ (2016) Thyroid hormone disruption and cognitive impairment in rats exposed to PBDE during postnatal development. Reprod Toxicol 63:114–124. https://doi.org/10.1016/j.reprotox.2016.05.017
Defew LH, Mair JM, Guzman HM (2005) An assessment of metal contamination in mangrove sediments and leaves from Punta Mala Bay, Pacific Panama. Mar Pollut Bull 50:547–552. https://doi.org/10.1016/j.marpolbul.2004.11.047
Dehghani S, Moore F, Vasiluk L, Hale BA (2018) The influence of physicochemical parameters on bioaccessibility-adjusted hazard quotients for copper, lead and zinc in different grain size fractions of urban street dusts and soils. Environ Geochem Health 40:1155–1174. https://doi.org/10.1007/s10653-017-9994-6
Delgado J, Nieto JM, Boski T (2010) Analysis of the spatial variation of heavy elements in the Guadiana Estuary sediments (SW Iberian Peninsula) based on GISmapping techniques. Estuar Coast Shelf Sci 88:71–83. https://doi.org/10.1016/j.ecss.2010.03.011
El-Kady AA, Abdel-Wahhab MA (2018) Occurrence of trace metals in foodstuffs and their health impact. Trends Food Sci Technol 75:36–45. https://doi.org/10.1016/j.tifs.2018.03.001
Elnar AA, Diesel B, Desor F, Feidt C, Bouayed J, Kiemer AK, Soulimani R (2012) Neurodevelopmental and behavioral toxicity via lactational exposure to the sum of six indicator non-dioxin-like-polychlorinated biphenyls (∑6 NDL-PCBs) in mice. Toxicology 299:44–54. https://doi.org/10.1016/j.tox.2012.05.004
EPA (2014) Revised human health risk assessment for registration review. https://www.regulations.gov/document?D=EPA-HQ-OPP-2008-0850. Accessed 29 December 2014
EPA (2016) Chlorpyrifos: Revised Human Health Risk Assessment for Registration Review. https://www.regulations.gov/document?D = EPA-HQOPP- 2015-0653-0454. 0195. Accessed 3 November 2016
Espitia N (2014) Determinación de metales pesados en sedimentos superficiales en cuerpos de agua del Canal del Dique en las poblaciones de Gambote y Soplaviento (Bolívar). Revista del Instituto de Investigación (RIIGEO), FIGMMG-UNMSM. 17:91-100. file:///D:/Downloads/11389-Texto%20del%20art%C3%ADculo-39870-1-10-20151107.pdf. Accessed 6 June 2019.
Etchegoyen MA, Ronco AE, Almada P, Abelando M, Marino DJ (2017) Occurrence and fate of pesticides in the Argentine stretch of the Paraguay-Paraná basin. Environ Monit Assess 189:63. https://doi.org/10.1007/s10661-017-5773-1
EU (European Union Commission Regulation) 1259/2011 (2011) Amending regulation (EC) No. 1881/2006. Off J Eur Union L320:18–23
FDA (2018) Compliance Policy Guide Sec. 575.100 Pesticide Residues in Food and Feed -Enforcement Criteria (Compliance Policy Guide 7141.01). In: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/compliance-policy-guide-sec-575100-pesticide-residues-food-and-feed-enforcement-criteria-compliance. Accessed 14 April 2019
Fernandes VC, Domingues VF, Mateus N, Delerue-Matos C (2012) Pesticide residues in Portuguese strawberries grown in 2009–2010 using integrated pest management and organic farming. Environ Sci Pollut Res 19:4184–4192. https://doi.org/10.1007/s11356-012-0934-9
Fernandez-Cadena JC, Andrade S, Silva-Coello CL, De la Iglesia R (2014) Heavy metal concentration in mangrove surface sediments from the north-west coast of South America. Mar Pollut Bull 82:221–226. https://doi.org/10.1016/j.marpolbul.2014.03.016
Ferré DM, Quero AAM, Hernández AF, Hynes V, Tornello MJ, Lüders C, Gorla NBM (2018) Potential risks of dietary exposure to chlorpyrifos and cypermethrin from their use in fruit/vegetable crops and beef cattle productions. Environ Monit Assess 190:292. https://doi.org/10.1007/s10661-018-6647-x
García Ubaque CA, García Ubaque JC, Vaca Bohórquez ML (2015) Compuestos orgánicos persistentes en Colombia: cuantificación y diagnóstico para pesticidas organoclorados. Tecnura 19:157–163. https://doi.org/10.14483/udistrital.jour.tecnura.2015.1.a011
García EM, Bastidas C, Cruz-Motta J, Farina O (2011) Metals in waters and sediments of the Morrocoy National Park, Venezuela: increased contamination levels of cadmium over time. Water Air Soil Pollut 214:609–621. https://doi.org/10.1007/s11270-010-0450-9
Gaudette HE, Flight WR, Toner L, Folger DW (1974) An inexpensive titration method for the determination of organic carbon in recent sediments. J Sediment Res 44:249–253. https://doi.org/10.1306/74D729D7-2B21-11D7-8648000102C1865D
Gebremariam SY, Beutel MW, Flury M, Harsh JB, Yonge DR (2012) Nonsingular adsorption/desorption of chlorpyrifos in soils and sediments: experimental results and modeling. Environ Sci Technol 46:869–875. https://doi.org/10.1021/es203341b
Gonzalez-Mille DJ, Espinosa-Reyes G, Rivero-Pérez NE, Trejo-Acevedo A, Nava-Montes AD, Ilizaliturri-Hernández CA (2013) Persistent organochlorine pollutants (POPs) and DNA damage in giant toads (Rhinella marina) from an industrial area at Coatzacoalcos, Mexico. Water Air Soil Pollut 224:1781. https://doi.org/10.1007/s11270-013-1781-0
Gundersen P, Steinnes E (2003) Influence of pH and TOC concentration on Cu, Zn, Cd, and Al speciation in rivers. Water Res 37:307–318. https://doi.org/10.1016/S0043-1354(02)00284-1
Hubner R, Brian Astin K, Herbert RJH (2009) Comparison of sediment quality guidelines (SQGs) for the assessment of metal contamination in marine and estuarine environments. J Environ Monit 11:713–722. https://doi.org/10.1039/b818593j
ICA (2004) Restricciones, prohibiciones y suspension de registro de plaguicidas de uso agricola en Colombia. Instituto Colombiano agropecuario. https://www.ica.gov.co/getdoc/b2e5ff99-bd80-45e8-Jaa7a-e55f0b5b42dc/plaguicidas-prohibidos.aspx. Accesed 10 July 2018
INVEMAR ( 2016) Diagnóstico y Evaluación de la Calidad de las Aguas Marinas y Costeras en el Caribe y Pacífico Colombianos. http://www.invemar.org.co/documents/10182/14479/Informe+REDCAM+2015_mayo2016.pdf. Accessed 10 July 2018
Jaramillo BE, Marrugo MP, Duarte E (2010) Monitoreo de residuos de pesticidas organoclorados en camaron (Penaeus vannamei) del area costera de la Bahía de Cartagena (Colombia). Biotecnología en el Sector Agropecuario y Agroindustrial 8:66-71. https://revistas.unicauca.edu.co/index.php/biotecnologia/article/view/738/365. Accesed 16 July 2018.
Jaramillo-Colorado BE, Arroyo-Salgado B, Ruiz-Garcés LC (2015) Organochlorine pesticides and parasites in Mugil incilis collected in Cartagena Bay, Colombia. Environ Sci Pollut Res 22:17475–17485. https://doi.org/10.1007/s11356-015-4986-5
Jaramillo-Colorado BE, Aga DS, Noguera-Oviedo K (2016) Heavy metal contamination of estuarine sediments from Cartagena Bay, Colombia. Toxicol Lett 259:s170. https://doi.org/10.1016/j.toxlet.2016.07.405
Kadhum SA, Ishak MY, Zulkifli SZ, Hashim RB (2017) Investigating geochemical factors affecting heavy metal bioaccessibility in surface sediment from Bernam River Malaysia. Environ Sci Pollut Res 24:12991–13003. https://doi.org/10.1007/s11356-017-8833-8
Kalender L, Çiçek Uçar S (2013) Assessment of metal contamination in sediments in the tributaries of the Euphrates River, using pollution indices and the determination of the pollution source, Turkey. J Geochem Explor 134:73–84. https://doi.org/10.1016/j.gexplo.2013.08.005
Kang L, He Q-S, He W, Kong X-Z, Liu WX, Wu WJ, Li YL, Lan XY, Xu FL (2016) Current status and historical variations of DDT-related contaminants in the sediments of Lake Chaohu in China and their influencing factors. Environ Pollut 219:883–896. https://doi.org/10.1016/j.envpol.2016.08.072
Kennedy H, Gacia E, Kennedy DP, Papadimitriou S, Duarte CM (2004) Organic carbon sources to SE Asian coastal sediments. Estuar Coast Shelf Sci 60:59–68. https://doi.org/10.1016/j.ecss.2003.11.019
Klanova J, Matykiewiczová N, Máčka Z, Prosek P, Láska K, Klán P (2008) Persistent organic pollutants in soils and sediments from James ROSS Island, Antarctica. Environ Pollut 152:416–423. https://doi.org/10.1016/j.envpol.2007.06.026
Kraugerud M, Doughty RW, Lyche JL, Berg V, Tremoen NH, Alestrøm P, Aleksandersen M, Ropstad E (2012) Natural mixtures of persistent organic pollutants (POPs) suppress ovarian follicle development, liver vitellogenin immunostaining and hepatocyte proliferation in female zebrafish (Danio rerio). Aquat Toxicol 116–117:16–23. https://doi.org/10.1016/j.aquatox.2012.02.031
Kumar Rai P, Soo Lee S, Zhang M, Tsang YF, Kim KH (2019) Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Int 125:365–385. https://doi.org/10.1016/j.envint.2019.01.067
La Guardia MJ, Hale RC, Harvey E (2006) Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol 40:6247–6254. https://doi.org/10.1021/es060630m
Lide D (2008) CRC Handbook of chemistry and physics, geophysics, astronomy, and acoustics. Section 14. In: Abundance of elements in the Earthʼs crust and in the sea, 89th edn. CRC Press, Boca Raton
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Long ER, MacDonald DD, Severn CG, Hong CB (2000) Classifying probabilities of acute toxicity in marine sediments with empirically derived sediment quality guidelines. Environ Toxicol Chem 19:2598–2601
MacDonald DD, Carr SC, Calder FD, Long ER, Ingersoll CG (1996) Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 5:253–278. https://doi.org/10.1007/BF00118995
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31. https://doi.org/10.1007/s002440010075
Macías-Zamora JV, Ramírez-Álvarez N, Hernández-Guzmán FA, Mejía-Trejo A (2016) On the sources of PBDEs in coastal marine sediments of Baja California, Mexico. Sci Total Environ 571:59–66. https://doi.org/10.1016/j.scitotenv.2016.07.142
Magalhães CA, Taniguchi S, Lourenço RA, Montone RC (2017) Organochlorine pesticides, PCBs, and PBDEs in liver and muscle tissues of Paralonchurus brasiliensis, Trichiurus lepturus and Cathorops spixii in Santos Bay and surrounding area, São Paulo, Brazil. Reg Stud Mar Sci 16:42–48. https://doi.org/10.1016/j.rsma.2017.08.010
Marine Water Quality of Hong Kong (2018) Environmental Protection Department The Government of the Hong Kong Special Administrative Region. In: https://www.epd.gov.hk/epd/sites/default/files/epd/english/environmentinhk/water/hkwqrc/files/waterquality/annual-report/marinereport2018.pdf. Accesed 30 july 2020
Marrugo-Negrete JL, Pinedo-Hernández J, Diez S (2017) Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ Res 154:380–388. https://doi.org/10.1016/j.envres.2017.01.021
Menzies R, Soarez-Quinete N, Gardinali P, Seba D (2013) Baseline occurrence of organochlorine pesticides and other xenobiotics in the marine environment: Caribbean and Pacific collections. Mar Pollut Bull 70:289–295
Ministry of Agriculture and Rural Development-Ministry of Social Protection (2007) Resolution 2906 of 2007 In:https://normograma.info/invima/docs/pdf/resolucion_minagricultura_2906_2007.pdf. Accesed 6 May 2019.
Ministry of Health and Social Protection (2012) Colombian Resolution No. 122/2012 of January 26 of 2012b. In:Https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/resolucion-0122-de-2012.pdf. Accessed 4 April 2019
Ministry of the Environment of Colombia (2010) In: http://quimicos.minambiente.gov.co/index.php/contaminantes-organicos-persistentes/la-convencion-de-estocolmo/plan-nacional-de-aplicacion. Accessed 6 May 2019
Mohammed A, Peterman P, Echols K, Feltz K, Tegerdine G, Manoo A, Maraj D, Agard J, Orazio C (2011) Polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in harbor sediments from Sea Lots, Port-of-Spain, Trinidad and Tobago. Mar Pollut Bull 62:1324–1332. https://doi.org/10.1016/j.marpolbul.2011.03.043
Montone RC, Taniguchi S, Weber RR (2001) Polychlorinated biphenyls in marine sediments of Admiralty Bay, King George Island, Antarctica. Mar Pollut Bull 42(7):611–614. https://doi.org/10.1016/s0025-326x(01)00092-3
Muniz P, Marrero A, Brugnoli E, Kandratavicius N, Rodríguez M, Bueno C, Venturini N, Figueira RCL (2019) Heavy metals and As in surface sediments of the north coast of the Río de la Plata estuary: spatial variations in pollution status and adverse biological risk. Reg Stud Mar Sci 28:100625. https://doi.org/10.1016/j.rsma.2019.100625
Parra S, Bravo MA, Quiroz W, Querol X, Paipa C (2015) Distribution and pollution assessment of trace elements in marine sediments in the Quintero Bay (Chile). Mar Pollut Bull 99(1–2):256–263. https://doi.org/10.1016/j.marpolbul.2015.07.066
Payá P, Anastassiades M, Mack D, Sigalova I, Tasdelen B, Oliva J, Barba A (2007) Analysis of pesticide residues using the Quick Easy Cheap Effective Rugged and Safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection. Anal Bioanal Chem 389:1697–1714. https://doi.org/10.1007/s00216-007-1610-7
Pemberthy D, Quintero A, Martrat MG, Parera J, Ábalos M, Abad E, Villa AL (2016) Polychlorinated dibenzo-p-dioxins, dibenzofurans and dioxin-like PCBs in commercialized food products from Colombia. Sci Total Environ 568:1185–1191
Pena-Icart M, Rodrigues Pereira-Filho E, Lopes Fialho L, Nobrega JA, Alonso-Hernandez C, Bolanos-Alvarez Y, Pomares-Alfonso MS (2017) Combining contamination indexes, sediment quality guidelines and multivariate data analysis for metal pollution assessment in marine sediments of Cienfuegos Bay, Cuba. Chemosphere 168:1267e1276. https://doi.org/10.1016/j.chemosphere.2016.10.053
Pereira LC, de Souza AO, Bernardes MFF, Pazin M, Tasso MJ, Pereira PH, Dorta DJ (2015a) A perspective on the potential risks of emerging contaminants to human and environmental health. Environ Sci Pollut Res 22:13800–13823. https://doi.org/10.1007/s11356-015-4896-6
Pereira TS, Moreira ITA, de Oliveira OMC, Rios MC, Filho WACS, de Almeida M, de Carvalho GC (2015b) Distribution and ecotoxicology of bioavailable metals and As in surface sediments of Paraguaçu estuary, Todos os Santos Bay, Brazil. Mar Pollut Bull 99:166–177. https://doi.org/10.1016/j.marpolbul.2015.07.031
Phyu YL, Warne MSJ, Lim RP (2006) Toxicity and bioavailability of atrazine and molinate to the freshwater fish (Melanotenia fluviatilis) under laboratory and simulated field conditions. Sci Total Environ 356:86–99. https://doi.org/10.1016/j.scitotenv.2005.04.003
Pinzón-Bedoya CH, Pinzon-Bedoya ML, Pinedo-Hernandez J, Urango-Cardenas I, Marrugo-Negrete J (2020) Assessment of potential health risks associated with the intake of heavy metals in fish harvested from the largest estuary in Colombia. Int J Environ Res Public Health 17:2921. https://doi.org/10.3390/ijerph17082921
Restrepo JC, Franco D, Escobar J, Correa ID, Otero L, Gutiérrez J (2013) Bahía de Cartagena (Colombia): distribución de sedimentos superficiales y ambientes sedimentarios. Lat Am J Aquat Res 41:99–112. https://doi.org/10.3856/vol41-issue1-fulltext-8
Restrepo JD, Park E, Aquino S, Latrubesse EM (2016) Coral reefs chronically exposed to river sediment plumes in the southwestern Caribbean: Rosario Islands, Colombia. Sci Total Environ 553:316e329. https://doi.org/10.1016/j.scitotenv.2016.02.140
Restrepo JC, Escobar J, Otero L, Franco D, Pierini J, Correa I (2017) Factors Influencing the distribution and characteristics of surface sediment in the Bay of Cartagena, Colombia. J Coast Res 33:135–148. https://doi.org/10.2112/JCOASTRES-D-15-00185.1
Riba I, Casado-Martínez MC, Forja JM, DelValls TA (2004) Sediment quality in the Atlantic coast of Spain. Environ Toxicol Chem 23:271–282. https://doi.org/10.1897/03-146
Rizzi J, Taniguchi S, Martins CC (2017) Polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in sediments from an urban- and industrial-impacted subtropical estuary (Babitonga Bay, Brazil). Mar Pollut Bull 119(1):390–395. https://doi.org/10.1016/j.marpolbul.2017.03.032
Rodrigues SK, Abessa DMS, Rodrigues APS et al (2017) Sediment quality in a metal-contaminated tropical bay assessed with a multiple lines of evidence approach. Environ Pollut 228:265e276. https://doi.org/10.1016/j.envpol.2017.05.045
Ruiz-Fernández AC, Sanchez-Cabeza JA, Pérez-Bernal LH, Gracia A (2019) Spatial and temporal distribution of heavy metal concentrations and enrichment in the southern Gulf of Mexico. Sci Total Environ 651:3174–3186
Seiter K, Hensen C, Schröter J, Zabel M (2004) Organic carbon content in surface sediments—defining regional provinces. Deep Sea Res Part I Oceanogr Res Pap 51:2001–2026. https://doi.org/10.1016/j.dsr.2004.06.014
Simpson SL, Batley GE (editors) (2016) Handbook for Sediment. Quality Assessment. CSIRO, 2nd Edition. Australia.
Sondi I, Mikac N, Vdovic N, Ivanic M, Furdek M, Skapin SD (2017) Geochemistry of recent aragonite-rich sediments in Mediterranean karstic marine lakes: trace elements as pollution and palaeoredox proxies and indicators of authigenic mineral formation. Chemosphere 168:786e797. https://doi.org/10.1016/j.chemosphere.2016.10.134
Souza AC, Combi T, da Silva J, Martins CC (2018) Occurrence of halogenated organic contaminants in estuarine sediments from a biosphere reserve in Southern Atlantic. Mar Pollut Bull 133:436–441. https://doi.org/10.1016/j.marpolbul.2018.05.052
Su PH, Lv BY, Tomy GT, Xu JX, Tian W, Hou CY, Yin F, Li YF, Feng DL (2017) Occurrences, composition profiles and source identifications of polycyclic aromatic hydrocarbons (PAHs), polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in ship ballast sediments. Chemosphere 168:1422–1429. https://doi.org/10.1016/j.chemosphere.2016.11.09
Suárez R, Clavijo S, González A, Cerdà V (2018) Determination of herbicides in environmental water samples by simultaneous in-syringe magnetic stirring-assisted dispersive liquid-liquid microextraction and silylation followed by GC-MS. J Sep Sci 41:1096–1103. https://doi.org/10.1002/jssc.201700875
Taiwo AM (2019) Review. A review of environmental and health effects of organochlorine pesticide residues in Africa. Chemosphere 220:1126–1140. https://doi.org/10.1016/j.chemosphere.2019.01.001
Tejeda-Benitez L, Flegal R, Odigie K, Olivero-Verbel J (2016) Pollution by metals and toxicity assessment using Caenorhabditis elegans in sediments from the Magdalena River, Colombia. Environ Pollut 212:238–250. https://doi.org/10.1016/j.envpol.2016.01.057
Tejeda-Benítez L, Noguera-Oviedo K, Aga DS, Olivero-Verbel J (2018) Toxicity profile of organic extracts from Magdalena River sediments. Environ Sci Pollut Res Int 25:1519–1532. https://doi.org/10.1007/s11356-017-0364-9
Tham TT, Anh HQ, Trinh LT et al (2019) Distributions and seasonal variations of organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in surface sediment from coastal areas of central Vietnam. Mar Pollut Bull 144:28–35. https://doi.org/10.1016/j.marpolbul.2019.05.009
Tombesi N, Pozo K, Arias A, Alvarez M, Pribylova P, Audy O, Klánová J (2018) Records of organochlorine pesticides in soils and sediments on the southwest of Buenos Aires Province, Argentina. Environ Earth Sci 77:403. https://doi.org/10.1007/s12665-018-7582-4
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol Meeresunters [ZDB] 33:566–575. https://doi.org/10.1007/BF02414780
Tosic M, Restrepo JD, Lonin S, Izquierdo A, Martins F (2019) Water and sediment quality in Cartagena Bay, Colombia: seasonal variability and potential impacts of pollution. Estuar Coast Shelf Sci 16:187–203. https://doi.org/10.1016/j.ecss.2017.08.013
USEPA. US Environmental Protection Agency (1996) Acid digestion of sediments, sludges, and soils, 3rd edn. Method 3050B. SW-846 Test methods for evaluating solid waste physical/chemical methods, Washington
Vaca Bohórquez ML, García Ubaque CA, García Ubaque JC (2014) Cuantificación de existencias de compuestos bifenilos policlorados (PCB) en Colombia. Tecnura 18:39–44. https://doi.org/10.14483/udistrital.jour.tecnura.2014.SE1.a03
Vaclavik L, Shippar JJ, Koesukwiwat U, Mastovska K (2018) Method development and validation for low-level propineb and propylenethiourea analysis in baby food, infant formula and related matrices using liquid chromatography-tandem mass spectrometry. Food Addit Contam Part A Chem 35(12):2387–2399. https://doi.org/10.1080/19440049.2018.1539529
Vallejo Toro PP, Vásquez Bedoya LF, Correa ID, Bernal Franco GR, Alcántara-Carrió J, Palacio Baena JA (2016) Impact of terrestrial mining and intensive agriculture in pollution of estuarine surface sediments: spatial distribution of trace metals in the Gulf of Urabá, Colombia. Mar Pollut Bull 111:311–320. https://doi.org/10.1016/j.marpolbul.2016.06.093
Varela-Martíneza DA, González-Curbelo MA, González-Sálamo J, Hernández-Borges J (2019) High-throughput analysis of pesticides in minor tropical fruits from Colombia. Food Chem 280:221–230. https://doi.org/10.1016/j.foodchem.2018.12.045
Velisek J, Kouba A, Stara A (2013) Acute toxicity of triazine pesticides to juvenile signal crayfish (Pacifastacus leniusculus). Neuro Endocrinol Lett 34(Suppl.2):31–36
Ventura C, Venturino A, Miret N, Randi A, Rivera E, Núñez M, Cocca C (2015) Chlorpyrifos inhibits cell proliferation through ERK1/2 phosphorylation in breast cancer cell lines. Chemosphere 120:343–350. https://doi.org/10.1016/j.chemosphere.2014.07.088
Vivas-Aguas L, Tosic M, Sánchez J, Narváez S, Cadavid B, Bautista P, Betancourt J, Parra J, Echeverri L, Espinosa L (2010) Diagnóstico y evaluación de la calidad ambiental marina en el Caribe y Pacifico colombiano. REDCAM Inf Tec 2010. pMAR. Santa Marta
Vivas-Aguas L, Sánchez J, Betancourt J, Quintero M,Moreno Y, Santana C, Cuadrado I, Ibarra K, Rios M, Obando P, Sánchez D (2014) Diagnóstico y evaluación de la calidad ambiental marina en el Caribe y Pacifico colombiano. REDCAM Inf Téc 2014. INVEMAR. Santa Marta
Vodopivez C, Curtosi A, Villaamil E, Smichowski P, Pelletier E, Mac Cormack WP (2015) Heavy metals in sediments and soft tissues of the Antarctic clam Laternula elliptica: more evidence as a possible biomonitor of coastal marine pollution at high latitudes? Sci Total Environ 502:375–384. https://doi.org/10.1016/j.scitotenv.2014.09.031
WHO (2010) The WHO recommended classification of pesticides by hazard and guidelines to classification. Geneva
Zhang H, Bayen S, Kelly BC (2015) Multi-residue analysis of legacy POPs and emerging organic contaminants in Singaporeʼs coastal waters using gas chromatography–triple quadrupole tandem mass spectrometry. Sci Total Environ 523:219–232. https://doi.org/10.1016/j.scitotenv.2015.04.012
Acknowledgments
The authors would like to thank the Research Groups Support Program and Research Project Support Program, sponsored by the University of Cartagena’s Vice-Presidency for Research (Grant No. 023-2015), and the internship mobility Program of Beatriz Jaramillo-Colorado and Edisson Duarte-Restrepo, sponsored by the Vice-Presidency for Research at the University of Cartagena. To Fulbright Colombia-Colciencias Program for the financial support to Katia Noguera-Oviedo. The authors would also like to thank Diana Aga’s group at the University at Buffalo.
Funding
This study was financially supported by the Research Groups Support Program and Research Project Support Program, sponsored by the University of Cartagena’s Vice-Presidency for Research (Grant No. 023-2015). Katia Noguera-Oviedo received financial support from the Fulbright Colombia-Colciencias Program.
Author information
Authors and Affiliations
Contributions
Conceptualization: Edisson Duarte-Restrepo, Beatriz E. Jaramillo-Colorado.
Formal analysis: Edisson Duarte-Restrepo, Katia Noguera Oviedo, Deena Butryn
Funding acquisition: Beatriz E. Jaramillo-Colorado
Data curation: Katia Noguera, Deena Butryn, Edisson Duarte-Restrepo
Methodology: Beatriz E. Jaramillo C, Diana Aga, Edisson Duarte-Restrepo, Katia Noguera, Deena Butryn
Software: Edisson Duarte-Restrepo and Joshua S. Wallace
Project administration: Beatriz E. Jaramillo-Colorado
Resources: Beatriz E. Jaramillo-Colorado and Diana S. Aga
Supervision: Beatriz E. Jaramillo-Colorado and Diana S. Aga
Writing—original draft: Beatriz E. Jaramillo C, Diana Aga, Katia Noguera, Deena Butryn, Edisson Duarte-Restrepo
Writing—review and editing: Edisson Duarte-Restrepo, Beatriz E. Jaramillo-Colorado
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Vedula VSS Sarma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Duarte-Restrepo, E., Noguera-Oviedo, K., Butryn, D. et al. Spatial distribution of pesticides, organochlorine compounds, PBDEs, and metals in surface marine sediments from Cartagena Bay, Colombia. Environ Sci Pollut Res 28, 14632–14653 (2021). https://doi.org/10.1007/s11356-020-11504-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11504-6