Abstract
When the hazard quotient for ingestion (HQI) of a trace element in soil and dust particles is adjusted for the element’s bioaccessibility, the HQI is typically reduced as compared to its calculation using pseudo-total element concentration. However, those studies have mostly used bulk particles (<2 mm or <250 µm), and the reduction in HQI when expressed as bioaccessible metal may not be similar among particle size fractions, the possibility probed by the present study of street dusts and soils collected in Tehran. The highest Cu, Pb and Zn near-total concentrations occurred in the finest particles of dusts and soils. Bioaccessible concentrations of Cu, Pb and Zn in the particles (mg kg−1) were obtained using simple bioaccessibility extraction test (SBET). The bioaccessibility (%) did not vary much among near-total concentrations. In the bulk (<250 µm) sample, the bioaccessible concentration of Cu and Pb increased as the pH of sample increased, while Zn bioaccessibility (%) in the bulk particles was influenced by organic matter and cation exchange capacity. X-ray diffraction identified sulfide and sulfate minerals in all of the size-fractionated particles, which are insoluble to slightly soluble in acidic conditions and included most of the Cu and Pb in the samples. The only Zn-bearing mineral identified was hemimorphite, which would be highly soluble in the SBET conditions. The calculated HQI suggested potential non-carcinogenic health risk to children and adults from ingestions of soils and dusts regardless of particle size consideration, in the order of Zn > Pb ≥ Cu. The HQI calculated from near-total metal was not much different for particle size classes relative to bulk particles; however, the bioaccessibility percent-adjusted HQI for Pb was higher for the smaller particles than the bulk. This work is novel in its approach to compare HQI for a bulk sample of particles with its composite particle size fractions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban soil and street dust may be ‘reservoirs’ of environmental pollutants including potentially toxic metals (PTMs) from the surrounding areas (Tang et al. 2013). According to many published studies (Thornton et al. 1994; Lanphear et al. 1998; Mielke et al. 1999; Higgs et al. 1999; Abrahams 2002), ingestion of surface soil and street dust can represent a significant route of multiple contaminant exposure through which metals may exert their toxicity. Therefore, evaluation of the oral bioaccessibility of elements in urban soil and deposited urban dust can provide the information necessary to assess their aggregate risks to human health. Oral bioaccessibility of an element is defined as the fraction of the element that is soluble in the gastrointestinal environment and is available for absorption (Paustenbach 2000).
Selecting the particle size fraction for evaluation of oral bioaccessibility of metals in soils and dusts is crucially important for human health risk assessment. The first reason is the great influence of particle size fraction on total concentration of contaminants (Butte and Heinzow 2002; Lee and Dong 2011; Wensing et al. 2005). Adsorbed contaminants are mostly associated on a w/w basis with fine soil particles, due to higher surface area per unit mass of small particles (Duong and Lee 2011; Sutherland 2003). Finer particles may also contain more secondary minerals such as clay minerals, carbonates and oxy-hydroxides of Fe, Mn and Al (Hardy and Cornu 2006; Yu and Li 2011), which act as adsorbents for trace metals. Therefore, fine particles overall have a greater sorption capacity per unit mass than the large particles in heterogeneous environmental media (Sheppard and Evenden 1994).
Particles loaded with trace metals emitted from different anthropogenic sources may be of different sizes (Duzgoren-Aydin et al. 2006; Bi et al. 2013). In addition, resuspension of road dust, especially the finest particles (<10 µm in diameter) by traffic, often represents an important source of atmospheric particulate matter (Thorpe and Harrison 2008; Norman and Johansson 2006). This is a major inhalation risk in developing countries of the world (Mage et al. 1996). Several authors have identified soil and street dust as the main sources of fine particulate matter (PM10) in air of different cities of the world (Artíñano et al. 2003; Mossetti et al. 2005).
The second reason is that humans are not similarly exposed to all particle sizes, as adherence of dust on hand or skin and hence ingestion and/or dermal sorption may vary with particle size (Mercier et al. 2011). Ingestion of PTMs is a particularly important exposure route for children or the elderly, whose physiologies make them more vulnerable to environmental pollution (Rojas-Bracho et al. 2002; Leotsinidis et al. 2005). Children ingest soil through mouthing dirty hands and objects, eating dropped food and consuming soil directly (Ljung et al. 2006). Children also typically have moister hands, which increases the adherence of particulate, thus increasing the mass of particles ingested (Yamamoto et al. 2006).
Several physiologically based extraction tests (PBETs) have been proposed to estimate the fraction of the total PTMs that would be bioaccessible in the human gastrointestinal tract following accidental ingestion of soil and dust. Most PBETs (e.g., Madrid et al. 2008a; Ruby et al. 1996, 1999; Sialelli et al. 2010), which closely simulate human gastrointestinal tract, are difficult and time-consuming to carry out with large batches of samples. Wragg and Cave (2003) reviewed different PBETs and the Bioaccessibility Research Group of Europe (BARGE) compared these techniques (Oomen et al. 2002). Simple bioaccessibility extraction test (SBET), which is designed to be fast, easy and reproducible (Wragg and Cave 2003; US EPA 2008; LEAD 2007), was developed as a simplified PBET method (Kim et al. 2002). The SBET has been validated specifically for measuring the bioaccessibility of lead in soils (Medlin 1997) and has been modified (Mench et al. 2006; Kim et al. 2002; Geebelen et al. 2003) to accommodate different media and elements of interest. The bioaccessibility of Cu and Zn has been assessed in contaminated soils using the SBET extraction (Lamb et al. 2009; Madrid et al. 2008b). Several researchers have determined PTM concentrations in different particle sizes. Gunawardana et al. (2014) investigated the variation in metal content for different sizes of particles from urban road surfaces at the Gold Coast, Southeast Queensland. They found that parameters favorable for metal adsorption to solids such as organic carbon content, effective cation exchange capacity (CEC) and clay-forming mineral content decrease with increase in particle size. Abouelnasr (2010) investigated the relationship between soil particle size and pseudo-total lead concentration using data from four published studies. According to their results, increasing lead concentration with decreasing soil particle size is not simply explained by greater surface area of smaller particles; thus, characterizing the effects of internal surface area and resistance to mass transfer through the boundary layer surrounding the soil particles is needed. A few studies have focused on variation in bioaccessibility among particle size fractions for a single element only. As an example, Juhasz et al. (2011) evaluated impact of soil particle size and bioaccessibility on lead exposure in contaminated soils. They reported increasing Pb bioaccessibility with decreasing particle size fraction for 6 of the 16 soils tested and no difference (p > 0.05) in percentage Pb bioaccessibility among particle size fractions of the remaining samples. They also confirmed significant influence of the particle size and bioaccessibility on the contribution of incidental soil ingestion to daily Pb intake values.
To date, there has been no study of the oral bioaccessibility of multiple metals in soils and street dusts, separately for various particle size fractions, to assess health risks from metals individually and as a mixture, due to soil and dust ingestion. This is an important knowledge gap, because different particle sizes are more or less likely to stick to hands, and thus, the trace elements HQs for the bulk particles may over- or underestimate risk. The present study is based on our previous survey of pollutants in street dusts and surface soils from Tehran that determined zinc, copper and lead as highly accumulated elements in studied environments (Dehghani et al. 2017). In this study, the HQ of each element for which non-cancer end points are expected was determined for inhalation, ingestion and dermal adsorption separately; these exposures were then aggregated into a total HI for each metal. Therefore, the prime objectives of the current study were: (1) to investigate the distribution of Cu, Pb and Zn among different size fractions of street dust; (2) to evaluate oral bioaccessibility of Cu, Pb and Zn in different size fractions of surface soil and street dust samples from the central district of Tehran; (3) to investigate the influence of physiochemical parameters and mineralogy on oral bioaccessibility of elements; and (4) to calculate the hazard to human health expected for ingestion of these particle size fractions, both for the individual metals and for the mixture.
Study area
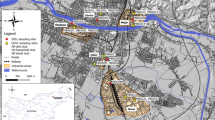
Tehran is the capital city of Iran and, with more than 8.2 million people, is the most densely populated area in Iran and Middle East at ~11.000 person km−2 (Statistical Center of Iran 2011) (Fig. 1). Tehran city is located on early Quaternary and Pliocene alluvial and moraine deposits, resulting from river activities and seasonal inundations, extending toward the south from the foothills of the Alborz Mountains range. The elevation in this city varies from 900 to 1700 meters above sea level.
Natural factors, urban structure layout, more than 4 million active vehicles (cars, trucks and motorcycles), around 17.4 million daily trips and consumption of 20% of country’s energy have turned this city to one of the most polluted cities in the world (Tehran Municipality 2015). Nowadays, around five thousand industrial units are active in Tehran, indicating high growth rate of industrial activities over the last decades. Central Tehran has been defined as permanent zone of traffic restriction in 2005 due to its dense population and high traffic load.
Tehran features a semiarid climate (Köppen climate classification: BSk) with continental climate characteristics and a Mediterranean climate precipitation pattern. The lowest and highest average annual temperatures are 0–37 °C, respectively, and average annual rainfall is 115 mm. The wettest month is March with an average of 50 mm of rain in study area (Iran Meteorological Organization 2015).
Materials and methods
Sampling
Five street dusts and five urban surface soils were chosen from 30 street dusts and 10 surface soils collected for our previous study based on their spatial distribution in the central district of Tehran (Fig. 1). Each street dust was a composite of four samples from road dust adjacent to the curb of the two sides of the street, collected by gently sweeping an area of about 30 m2 using a small plastic brush and shovel. Each composited sample was stored in a clean polyethylene bag. Each soil sample was a composite of at least two subsamples from the top 0–5 cm of 3 m2 area, collected with a plastic shovel and stored in a clean polyethylene bag. Street dust and surface soil samples no. 3 were collected from the same location.
Determination of chemical and physical properties and size fractionation
In the laboratory, extraneous matter such as paving stones and asphalt, small pieces of brick and concrete, leaves and other debris was removed from each sample. The samples were dried at 30 °C for 7 days. Soil and dust samples (<2 mm) were analyzed for pH, electrical conductivity (EC), cation exchange capacity (CEC), organic matter (OM) and carbonate content. Based on Heiri et al. (2001), OM and carbonate content of the samples were determined gravimetrically by weight loss of oven dried dust samples (12–24 h, 105 °C) after ignition at 550 and 950 °C, respectively. Electrical conductivity (EC) and pH were measured in 1:2.5 soil-to-distilled water w/w ratio, with a pH/EC meter (Cybershot PCD 6500 pH/EC meter, Eutech) (Ryan et al. 2007). Cation exchange capacity (CEC) of samples was measured as described in Ryan et al. (2007) using the ammonium acetate (NH4OAc)—sodium acetate (NaOAc) method. Dust and soil textures were determined by hydrometry (Hakanson 1980) and classified using the USDA classification system. X-ray powder diffraction patterns were taken using Bruker D8 Advance diffractometer.
Afterward, street dust and surface soil samples were sieved through a 250-µm stainless steel sieve. According to Kramer et al. (1994), stainless steel devices are not a source of contamination in trace element analyses, except for chromium or nickel, which were not included in current study.
In 2000, the US EPA concluded that 250 μm represents a reasonable upper bound for the size range of ingested soil particles. Hence, most studies of oral bioaccessibility for potentially toxic metals have used bulk soil with particle <250 μm. However, the critical size fraction is finer than this. Kissel et al. (1996) reported particle size fraction smaller than 65 μm as preferentially adhering to the hands. This size class is also close to upper threshold of silt-sized particles (63 µm in diameter) in USDA classification. Particles <10 µm were also selected as the fraction most likely to contribute to air pollution. To classify particles between 70 and 250 µm (wide range of size) into two size categories (fine and medium sand), 105-µm sieve was also chosen for this study. Moreover, coarser particles contribute mostly to pollution of urban runoff and are important to assess ecological risk of samples. Therefore, ~50 g of the dried dust and soil samples (<250 µm) was sieved into four size fractions of 250–105, 105–70, 70–10 and <10 µm using shaker sieving (nylon material) for 20 min. The weight percentages of street dust and topsoil samples composed of four size fractions were then determined for all samples.
Determination of near-total concentration of metals in different size fractions
According to Siaka et al. (1998), there is less chance of element loss through volatilization during digestion of environmental media with reverse aqua regia (HNO3/HCl, 3:1 v/v) than with other acids. Digestion using reverse aqua regia is safer than hydrofluoric acid or perchloric acid (Siaka et al. 1998), but leaves the more resistant silicates undissolved (Peltola and Åström 2003). One g of each particle size fraction of each dust and surface soil was digested in hotplate with 9 ml nitric and 3 ml hydrochloric acid, at 150 °C overnight. The digestion fluid from each vial was then filtered using Whatman No. 2 filter paper, brought to 25 ml with 18 mΩ water and stored in a Falcon tube at 4 °C until analysis. Concentrations of copper, lead and zinc in all extractions were determined using flame atomic absorption spectrometry (Varian SpectrAA 220). These concentrations are referred to as ‘near-total.’
Quality control was implemented using certified reference materials to assess accuracy, replicates to assess precision and method blanks to assess contamination. Each extraction batch was comprised of 40 samples including one blank and two different standard reference materials. Blanks were prepared as were the experimental samples and were routinely analyzed before each measurement. Two multi-element standards, SRM 2711a-Montana II Soil and SRM 2781-Domestic Sludge, from The National Institute of Standards and Technology (NIST) were used as the certified reference material. The recovery percent was in the range of 86–97%, which is within the specified limits. All measurements were performed by four replicates. The reported results are average values.
Grain size fraction load
In order to determine the relative contribution of each particle size fraction to the overall metal loads of the bulk samples, the grain size fraction load (GSF Load) of each sample was calculated (Sutherland 2003):
where C i is the trace metal concentration for a given grain size; GS i is the percentage by mass of the size fraction i in the total sample; and n is the number of grain size fractions.
Bioaccessibility test of Cu, Pb and Zn
The bulk (<250 µm) and sieved (<250, 250–105, 105–70, 70–10 and <10 µm size fractions) street dust and topsoil were subjected to bioaccessibility testing according to the standard operating procedure (SOP) developed by the Solubility and Bioavailability Research Consortium (SBRC) (Ruby et al. 1999; Birmingham and McLaughlin 2006). Glycine fluid (0.4 M at pH of 1.50 ± 0.05 and 37 °C) was prepared using ASTM type II deionized (DI) water (Birmingham and McLaughlin 2006). Five (±0.025) ml of the glycine fluid was transferred to 10-ml wide-mouth HDPE bottle containing 0.05 ± 0.0025 g of a street dust or surface soil. The HDPE bottle was placed into the toxicity characteristic leaching procedure (TCLP) extractor motor with temperature of 37 ± 2 °C and rotated end over end at 30 ± 2 rpm. After 1 h, the solution was filtered (0.45-μm cellulose acetate disk filter attached to the syringe) and stored at 4 °C until analysis. The pH of fluid remaining in the extraction bottles was measured, to evaluate whether it had remained within ±0.5 pH units of the starting pH (Birmingham and McLaughlin 2006); all extractions satisfied this QA/QC criterion. Filtrate fluids were analyzed for Cu and Pb using graphite furnace atomic absorption spectroscopy and for Zn using flame atomic absorption spectroscopy (Varian SpectrAA 220 equipment).
The bioaccessibility (%) of each element was calculated (Okorie et al. 2012):
To determine the controlling factors on bioaccessibility of Cu, Pb and Zn, in street dust and surface soil, stepwise multiple linear regression analysis (forward elimination of variables) was performed using the model:
Physicochemical parameters that were used as dependent variables (X i ) included percent of clay size fraction (as a representation for texture), pH, organic matter (OM), CEC, CaCO3 and EC. \(\beta_{0}\) is the expected value of bioaccessibility (%) when X i values all equal zero. For every unit increase or decrease in the value of X i , a \(\beta_{i}\) change in bioaccessibility (%) is predicted. The statistical analyses were performed using SPSS 16.
Quality control of bioaccessibility test
Certified reference materials, SRM 2711a-Montana II soil and SRM 2781-Domestic Sludge from National Institute of Standards and Technology (NIST), which are routinely used in our laboratory in bioaccessibility test of metals, were used to determine precision and accuracy of analysis as well as comparing the results to those prior values. The recovery of certified values ranged from 28% (SRM 2711a) to 73% (SRM 2781) for zinc, 41% (SRM 2711a) to 42% (SRM 2781) for lead and 42% (SRM 2711a) to 72% (SRM 2781) for copper. Contamination and bias of the analysis were determined by adding reagent blank and internal standards once per batch of samples (13% of frequency). No contamination was detected based on control limits of <10, <5, 70 µg l−1 for Cu, Pb and Zn, respectively. Extractions were conducted in triplicate and indicated that the average deviation between triplicate samples (n = 5) was 2%. The mean values were reported.
Potential health risk assessment
In order to quantify health risk due to oral ingestion of street dust and surface soil for children and adults, the average daily intakes of Cu, Pb, Zn and their risk characterization were calculated using hazard quotients (HQ) (US EPA 2010). Chemical daily intake (CDI) of given metals from incidental ingestion of samples was calculated (US EPA 2004):
where CDI is the chemical daily intake (mg kg−1 day−1); Tehran metropolis experienced 111 pollution days (with respect to suspended particulate matter) in 2016 (sampling year) as well as a severe dust storm in mid-August, which affected sampling on September. Hence, the upper percentile estimate of Özkaynak et al. (2011) (200 mg soil/dust day−1 for children and 100 mg day−1 for adults) was used as ingestion rate (IR) in this study (Staff 2001); the average of official working days per year was estimated; and the mean of non-active days was subtracted from it to find the modified exposure frequency (EF) of 200 days year−1 in this study (Zheng et al. 2010). ED is exposure duration (6 years for children and 24 years for adults) (Staff 2001); WAB is the average body weight, namely 15 kg for children and 70 kg for adults (Zheng et al. 2010); and TA is averaging time (for non-carcinogens, TA = ED × 365 days). C (exposure point concentration, mg kg−1) is the upper limit of the 95% confidence interval for the mean and was calculated using SPSS 16.0.
The potential non-carcinogenic risk of Cu, Pb and Zn was calculated with the following equation (US EPA 2007):
Oral reference doses (RfDo) were obtained from Regional Screening Levels (US EPA 2010) (Table S5). The reference dose of 0.0035 (mg kg−1 day−1) was determined for Pb by US EPA (2009) (Zhuang et al. 2013; Gilbert and Avenant-Oldewage. 2014). If the value of HQ is less than one, there is no significant risk of non-carcinogenic effects. If HQ exceeds one, non-carcinogenic effects may occur (Staff 2001).
If extensive mechanistic information is not available to assess the overall potential for non-carcinogenic effects posed by more than one toxic metal, hazard index (HI) is the recommended approach to assess health risk of mixtures of chemicals for which non-cancer endpoints are expected (US EPA 1989):
When the HI is less than 1, the combined risk is considered acceptable. HI calculation was applied in our previous study (Dehghani et al. 2017) to identify the total combined health hazard from each element from all exposure routes. GSF-adjusted HQs were calculated by multiplying HQ values in GSF loads.
Results and discussion
Physicochemical properties
The texture of the street dusts was mostly sandy loam (Table 1). Al-Chalabi and Hawker (1997) also reported high sand content and very low clay content in the road dust of Brisbane, Australia. The texture of the surface soils varied from sandy loam (sample 1S) to sandy clayey loam (sample 2S), loam (samples 3S and 4S) and loamy sand (sample 5S); pH ranged from 7.80 to 9.52. According to the classification of Ryan et al. (2007), all samples were ‘moderately alkaline’ except for sample 4D (pH = 9.52), which was ‘highly alkaline.’ CEC values ranged from moderate (12.2 meq/100 g) to extremely high (47.1 meq/100 g), based on the classification proposed by Metson (1957) (Table 1). The average organic matter content of street dusts (13.0%) was higher than that of surface soils (8.23%), while surface soils included higher carbonate content (5.56%, on average) than street dusts (4.96%, on average).
Metal concentrations in different particle sizes
The street dusts and surface soils were mainly composed of two particle size fractions, namely 250–105 and 70–10 µm (Fig. 2; Table S1). The difference between street dusts and surface soils in average load of each particle size fraction was negligible. The finest fraction (<10 µm) accounted for 2.90% and 1.17% (on average) of the total street dust and surface soil mass, respectively. There was inadequate mass in samples 2S and 3S of the <10 µm size fraction for analysis of metals.
Copper, Pb and Zn concentrations for various particle size classes of street dusts and surface soils demonstrate that mostly, the street dusts had higher metal concentrations than surface soils (Fig. 3). Concentrations of Pb and Zn increased as particle size decreased for street dust, but not for surface soils (Table S2). Concentrations of Cu, Pb and Zn ranged from 1.7 (for Zn) to 10.0 (for Cu) times higher in street dust sample 3D than in surface soil sample 3S, obtained from the same location. This suggests that anthropogenic sources, e.g., traffic activities, are important sources of elemental pollution to road dust.
The concentration of Pb did not vary much (8.4 ± 4.7 mg kg−1) among different particle size fractions of surface soil. However, differences were observed in concentrations of Zn (130 ± 39.5 mg kg−1 and 332 ± 58.3 mg kg−1) and Cu (53.3 ± 49.8 mg kg−1 and 125 ± 58.8 mg kg−1) between surface soils and street dusts, respectively. As particle size decreased, total Cu, Pb and Zn in street dust increased. Many researchers have shown an enrichment in Pb with decreasing particle size (Sutherland 2003; Madrid et al. 2008b; Juhasz et al. 2011). In the current study, Pb concentration in surface soils tended to be uniformly distributed among the four size fractions. In all street dusts, the highest mean concentrations of Cu, Pb and Zn were found in fine fractions (70–10 µm and <10 µm), except for Cu in 1D, which was 409.7 (± 14.5 mg kg−1) in the 105–70 µm fraction, higher than the finer fraction concentrations (372 ± 33.5 mg kg−1 in 70–10 µm fraction and 371 ± 67.0 mg kg−1 in <10 µm fraction) (Table S2). The highest concentrations of Cu and Pb were also found in the <10 and 70–10 µm fractions, respectively, of surface soils. For 1S and 2S, the highest mean concentration of Zn was found in the 250–105 µm fraction (510 ± 31.2 and 284 ± 44.3 mg kg−1, respectively), and for 3S and 4S, the highest mean concentration of Zn was in the 105–70 µm fraction (276 ± 24.6 and 482 ± 40.3 mg kg−1, respectively). The only surface soil with the highest zinc concentration (563 ± 51.9 mg kg−1) in the finest fraction (<10 µm) was 5S. Consequently, zinc is more likely to contribute disproportionately to pollution of urban runoff, as coarse particles are typically the first flush.
Grain size fraction (GSF) load of Cu, Pb and Zn
If the concentration of an element in a particle size fraction is very high but it forms only a small portion of the mass of the bulk material to which humans are exposed, the contribution of this fraction to the total sample PTM load is less than would be inferred from concentration, alone (Sutherland 2003). Expressing metal concentration data as ‘GSF load’ normalize the data. As an example, the highest concentration of Zn (563 ± 51.9 mg kg−1) in 5S was in the <10 µm fraction, but the GSF load was low (3.04%) because this particle size fraction formed only 2.34% of the bulk mass (Fig. 4; Table S3). Zinc in 18D had the highest GSF load (73.5%) in the 70–10 µm fraction, because of both high mass load (42.9%) and high Zn concentration (769 ± 129 mg kg−1). The GSF load was highly variable among metals, particle size fractions and locations from which soils and dusts were collected.
Previous studies confirmed that finer particles (i.e., <50 µm) tend to adhere more efficiently to the surface of skin than larger particles (Choate et al. 2006; Yamamoto et al. 2006; Siciliano et al. 2009); thus, GSF load is an important calculation for human health risk. Yamamoto et al. (2006) showed that soil particles with a modal size of 39 µm adhere to the hands of children and are potentially transferred to the mouth for ingestion. Sheppard and Evenden (1994) studied microscopic images of soil adhering to skin and found that clay-sized particles (<2 µm) are of the same scale as the surface roughness features of the skin and may be quite resistant to cleaning. Duggan et al. (1985) measured the size distribution of particles collected from children’s hands and found that they mostly were ≤10 μm in diameter. The current study demonstrates that the <10 µm particles generally had GSF loads that were too low to pose a potential health risk to human health through oral ingestion. However, particles <10 µm are resuspended by wind or traffic and contribute to the atmospheric particulate load (Ho et al. 2003), so the finest fraction still is useful to give indication about the likely contribution to PM2.5 and PM10 for inhalation.
Calculated GSF loads in the present study suggest a high contribution of the 70–10 µm fraction in overall load of street dust samples. In contrast, the surface soils show a different descending order of GSF load distribution, namely 250–105, 70–10, 105–70 µm, <10 µm, for the average concentrations of Cu, Pb and Zn. Coarse particles (250–105 µm) of surface soil samples with highest GSF among four size fractions have low toxicity due to the low likelihood of adhering to skin and being orally ingested.
Mineralogy of street dust and surface soil samples
X-ray diffraction (XRD) was used to identify crystalline phases in the particles, which had both amorphous and mineralogical constituents. Based on microscopic study of thin sections, all street dust and surface soils included much amorphous content that likely derives from partially weathered minerals, amorphous silicates and organic matter, as noted by Bish and Post (1989). Each sample also included complex mineral assemblages that reflect the mixed geological provenance of samples. For example, X-ray diffractograms of sample 3S in different size fractions are shown in Fig. S1. The major mineral components of street dust and surface soil samples were quartz, calcite, albite and anorthite. Minerals in the street dusts were similar to those found in surface soils, except for sanidine and microcline that were not observed in the street dusts. Thus, the mineralogy of the street dusts consisted of simpler assemblages than the surface soils, confirming that street dusts reflect the mineralogical composition of the nearby surface soils, but only some minerals are mobilized into the surrounding urban area. This is in agreement with findings of Xie et al. (2000) who noted that a significant component of road dust derives from urban surface soil. Sanidine and microcline, which are high- and low-temperature polymorphs of orthoclase feldspar, respectively, may derive from felsic volcanic rock in the vicinity of Tehran. This is in agreement with Norra et al. (2006) who demonstrated that urban surface soils, despite pollution, can still reflect the chemical composition of the source geological units.
Major cations (Ca and K) can be replaced by trace metal ions (Cu, Pb and Zn) of the same charge and of comparable size during the formation of secondary minerals. For example, albite and microcline, which were abundant in surface soils, have potential to form clay minerals. The metals of interest (Cu, Pb and Zn) are captured within the crystal structure, adsorbed to its surface or co-precipitated as metal-bearing carbonate minerals such as azurite (Cu3(CO3)2(OH)2), cerussite (PbCO3), malachite (Cu2(CO3)(OH)2) and shanonnite (Pb2OCO3). These minerals can become easily bioaccessible if the ambient pH condition changes. According to Saeedi and Pajooheshfar (2012), mean pH of rain and snow in Tehran is 5.9 and 5.1, respectively, which confirm acid rain occurrence. This pH is low enough to dissolve carbonate minerals.
Lead occurred as sulfate (PbSO4) and sulfite (PbSO3) in the surface soils and street dusts, both of which have low bioavailability due to low solubility (Dean 1990). Copper has a strong affinity for sulfur; thus, it principally occurred in bornite (Cu5FeS4), chalcocite (Cu2S), chalcopyrite (CuFeS2), chalcophyllite (Cu18Al2(AsO4)3(SO4)3(OH)27·33(H2O)), digenite (Cu9S5), spionkopite (Cu1.4S) and cubanite (CuFe2S3), in the street dust and surface soils. All of these phases are insoluble in water and HCl (Crawford 2009). Thus, these minerals of copper are expected not to be highly bioaccessible in human gastrointestinal tract. Some minor amount of oxidized copper minerals such as cuprite (Cu2O) and moolooite (Cu2CuO4·H2O), which are mainly formed during the weathering of copper sulfides exposed to erosion, were detected in soil and dust samples.
A trace amount of hemimorphite (Zn4Si2O7(OH)2·(H2O)), which is soluble in HCl with gel residue, was detected in samples as the only zinc mineral. Since the near-total concentration of zinc in samples was high, it could have occurred in an adsorbed form on the surface of other minerals. It is confirmed to be derived from anthropogenic sources. Moreover, based on these results, zinc is expected to be the most bioaccessible element in this study.
Bioaccessibility of Cu, Pb and Zn in particle size fractions
According to morphological studies, some of the studied elements are expected to be bioaccessible and may pose a threat to the receiving ecosystem. The expected oral bioaccessibility (%) of Cu, Pb and Zn in the road dust and surface soils of Tehran is in the following order: Zn > Pb ≥ Cu. In the current study, bioaccessibility was evaluated for the bulk surface soils and street dusts (i.e., <250 µm), as well as the four particle size fractions of these materials, using SBET extraction (Fig. 5; Table S4). The proportion of the near-total content found to be bioaccessible differed among samples and elements.
The oral bioaccessible concentration (BAC in mg kg−1) was regressed against the near-total concentration for Cu, Pb and Zn in surface soils and street dusts (Fig. 6). As the near-total concentrations of Cu, Pb and Zn increased, so did the bioaccessible concentrations of those metals. The coefficients of determination (R2) were +0.80, +0.91, and +0.81 for Cu, Pb and Zn, respectively. The trend is the same for Cu and Pb, as it is for Zn, given the R2 values for the linear regression.
Box plots of the BA concentration (mg kg−1) are similar to those for near-total metal concentrations, for both street dusts and surface soils. The bioaccessibility (mg kg−1) was greater in the <250 µm fraction for Zn (mean of 321 mg kg−1) than for Cu (mean of 71.1 mg kg−1) and Pb (mean of 56.7 mg kg−1), in the same size fraction of street dusts. The bioaccessible concentration of the <250 µm fraction in the surface soils decreased in following order: Cu (mean of 31.8 mg kg−1) > Zn (mean of 27.8 mg kg−1) > Pb (mean of 16.9 mg kg−1). Despite this trend (ZnBAC > CuBAC > PbBAC), bioaccessibility (%) was ordered differently: Zn > Pb > Cu for street dusts and Cu > Pb > Zn for surface soils (Fig. 7). The bioaccessibility (%) of Cu and Pb in surface soils was higher than street dusts (Fig. 7a, b). In contrast, bioaccessibility (%) for Zn in surface soils was lower than that for street dusts (Fig. 7c). There was a little variation in CuBAC (%) among the four particle size fractions of street dust (range of 2.30%) in contrast to ZnBAC (%) for surface soils, which ranged 23.7% (250–102 µm) and 42.0% (<10 µm). The distribution pattern of bioaccessible fraction (%) of metals among different size fractions was homogeneous in all street dusts and surface soils, except for Zn in surface soils, which displayed slight differences among bioaccessibility pattern of size fractions. In street dust, sample D4 showed highest Cu and Pb bioaccessibility percent, whereas highest bioaccessibility (%) of Zn was obtained in sample 5D. Among the surface soil samples, the highest Cu, Pb and Zn bioaccessibility values (%) were obtained from samples 1S, 4S and 5S, respectively. Of the three metals, Pb had the highest bioaccessibility (%) (median value 61.5%) in the 70–10 µm size fraction of surface dust. Zn had the highest bioaccessibility (%) (median value 60.2%) in the 70–10 µm fraction of surface soil. The highest Pb bioaccessibility (%) in street dusts was in the 105–70 µm fraction (Fig. 7b). This is in contrast to increasing Pb bioaccessibility (%) with decreasing particle size, demonstrated by Morrison and Gulson (2007).
Influence of physiochemical properties on metal bioaccessibilities
All three regressions of bioaccessible concentration (mg kg−1) were very strong, with pseudo-total concentrations explaining much of the variation in the dependent variable. But given what is known about the effect of physicochemical parameters on bioaccessibility, we tested whether additional independent variables would account for any of the unexplained variation in the dependent variable, using stepwise multiple regression with forward addition of variables on the bulk (<250 µm) samples. For all three metals, at least one physicochemical parameter of the particles further reduced unexplained variation (Table 2).
For every 1 unit increase in particle pH, CuBAC (mg kg−1) and PbBAC (mg kg−1) were expected to increase 19.1 and 13.6 mg kg−1, respectively. According to several authors (Grøn and Andersen 2003; Ellickson et al. 2001; Wragg et al. 2011), Pb and Cu behavior is strongly pH dependent, with higher solubility in acidic conditions. As the final pH of samples in SBET method was 1.5, greater increase in bioaccessibility (%) in elements in samples with higher pH was expected, due to greater change in pH of samples extracted under SBET. For each percent increase in OM, 19.1 mg kg−1 increase in ZnBAC (mg kg−1) was expected. Whereas ZnBAC (mg kg−1) was impacted negatively by CEC, each meq/100 g increase in CEC was predicted to result in 5.55 mg kg−1 decrease in ZnBAC (mg kg−1). In conclusion, bioaccessibility (mg kg−1) of Cu and Pb was impacted by pH. Among three studied metals, OM and CEC influenced ZnBAC (%) only.
Exposure estimates and implications for human health
Children have greater exposure to PTMs as they ingest more environmental particles through deliberate mouthing of dust or inadvertent mouthing of dirty hands or toys (Wragg and Cave 2003; Ruby et al. 1999). Therefore, daily intake of PTMs by children is higher than for adults and should be evaluated separately. Moreover, as reported by EPA (2007), presenting bioaccessibility data as bioaccessibility percent conceals the actual concentration of the elements in the extract. Therefore, bioaccessibility and near-total concentration results are combined in hazard quotient (HQ), which evaluates individual and aggregate health risk hazards to children and adults (Table S5). The Zn HQs to children and adults for street dusts (average 1.04+E04 and 1.11+E03, respectively) were significantly higher than those for Cu and Pb. Zinc and Cu HQs for surface soils increased with decreasing size fraction (Fig. 8). Zn in street dusts (1.32E+04 and 1.41E+03 to children and adults, respectively), Pb in surface soils (1.14E+03 and 1.22E+02 to children and adults, respectively) and Pb in street dusts (3.18E+03 and 3.41E+02 to children and adults, respectively) had the highest HQ values in 70–10 µm fraction.
HQs for Cu, Pb and Zn for children were also higher than those for adults. The HQ values of all three studied metals in all size fractions were greater than 1, suggesting non-carcinogenic risks to children and adults from these particles. According to SCHER et al. (2011), assessment groups can be formed by grouping mixture components based on their similarities in biological responses. The previous study on Cu, Pb and Zn in these street dusts and surface soils of Tehran (Dehghani et al. 2017) classified them as non-carcinogenic metals to human. The potential risk of non-cancer outcomes due to co-exposure to the three metals via the ingestion of street dust and surface soil was greater than 1, indicating that Tehran inhabitants were likely to experience adverse health effects, due to inadvertent ingestion of street dust/surface soil. However, when HI was expressed in terms of pseudo-total concentration of metals as would normally be the case for risk assessment, then the aggregate risk of co-exposure to these metals was up to 45 times greater than those expressed as bioaccessible proportion of metals (Table S5). Zn had greatest contribution to integrated risk of the metal mixture followed by Pb and Cu (Fig. 8. a). And the risk was greatest for the particle sizes most likely to stick to hands and fresh produce, namely less than 70 µm (Fig. 8). In comparison, our previously published work that examined HI for many metals individually but aggregated over three exposure routes (dermal, inhalation and ingestion), identified Pb but not Cu nor Zn as having an HI > 1. GSF-adjusted HQs (bioaccessibility percent base) displayed in Fig. 8b implied that among the studied metals, Zn posed the greatest non-carcinogen health risk to children via ingestion of street dust and surface soils.
Zinc had the highest concentration in street dusts and surface soils (556 and 347 mg kg−1, respectively) among three studied elements. This is in agreement with XRD results indicating that Zn occurred mostly in amorphous phases and trace amounts occurred in easily soluble crystalline phases. The highest Zn concentration was not necessarily associated with the smallest particles. Indeed, Zn was concentrated mostly in 105–70 µm fraction (432 mg kg−1) of the surface soils. Nevertheless, ZnBAC and ZnBAC (%) were increasing with decreasing size fraction. Bioaccessible concentration (343 mg kg−1) and ZnBAC (59.2%) in street dusts were higher than those of surface soils (27.8 mg kg−1 and 7.8%, respectively. OM in street dusts (13.0%) was higher than that of surface soils (8.23%). A strong positive correlation (r = 0.67) between ZnBAC (%) and OM in street dusts suggested that as OM increased, so did ZnBAC (%). This is in agreement with the most important Zn sorption mechanism in alkaline media, which is considered to be the chemisorption which is highly influenced by organic ligands (Kabata-Pendias and Mukherjee 2007). High ZnBAC (%) in surface soils resulted in high health hazard to children (HQ = 1.04E+04) and adults (HQ = 1.11E+03). Consequently, there is high potential health risk of Zn in the 70–10 µm and <10 µm particle size fractions in street dust and surface soil, respectively. Excess ingested Zn can cause system dysfunctions that result in impairment of growth and reproduction (Wilson 2009), liver failure, kidney failure and anemia (Fosmire 1990).
The Cu concentrations extracted from street dusts by the simulated gastric phase were higher than extracted from surface soils on average. However, they were still lower than Zn concentrations, likely due to Cu captivity in insoluble mineralogical phases such as sulfides. Since not all forms of a toxic element were likely to be solubilized by the gastric conditions (Reis et al. 2014; Wragg et al. 2007), the small observed variation in CuBAC (%) among the four particle size fractions of street dust suggested nearly similar soluble Cu content in SBRC extraction across particle sizes of street dusts. CuBAC (%) was strongly affected by pH. As a result of lower pH in soil samples (pH = 8.17) compared to street dust samples (pH = 8.48), Cu in soil was more bioaccessible. Carbonate content had also positive but weak correlation (r = 0.38) with PbBAC (%) in surface soil. Higher carbonate content of soil (5.56%) in comparison with street dust (4.96%) likely caused the higher PbBAC (%) in soil (45.3%) compared with street dust (36.1%). Lead in street dusts was concentrated mostly in smallest fraction (< 10 µm), but the highest PbBAC (%) was in the 105–70 µm fraction. It is noteworthy that even with lower near-total concentration of Cu and Pb in surface soils in comparison to Zn, their bioaccessibility was nearly as high (43.4 and 45.3% in <250 µm, respectively). This was reflected in the elevated HQs of Cu and Pb. Lead is one of the most well-known toxins among the PTMs. Many authors have found a connection between increased soil Pb levels and elevated blood Pb levels in children (Lanphear et al. 1998; Mielke et al. 1999; Berglund et al. 2000). Lead poisoning may cause serious health effects such as inhibition of the synthesis of hemoglobin, dysfunctions in the kidneys, joints, reproductive and cardiovascular system, acute and chronic damage to the central nervous system (CNS) and peripheral nervous system (PNS), and teratogenic effects (Duruibe et al. 2007; Ogwuegbu and Muhanga 2005). Copper is an essential element in the human body and a component of several proteins and metallo-enzymes. An excess of Cu has an adverse effect on several physiological processes such as impairment of membrane properties, impairment of DNA and enzyme functions, and cellular injury due to the generation of hydroxyl radicals which can lead to oxidative stress. An excess of Cu may also result in Wilson’s disease, mostly ending in death (Kabata-Pendias and Mukherjee 2007). Therefore, adverse health risk resulted due to street dust and surface soil exposure via ingestion.
Conclusion
The prime objectives of the current study were: (1) to investigate the distribution of Zn, Pb and Cu among different size fractions of street dust; (2) to evaluate oral bioaccessibility of Zn, Pb and Cu in different particle size fractions of surface soil and street dust samples from the central district of Tehran; (3) to investigate the influence of geochemistry and mineralogy on oral bioaccessibility of elements; and (4) to calculate the hazard to human health expected for ingestion of these particle size fractions, both for the individual metals and for the mixture. The data addressing these objectives were combined into estimates of HQ for each of Cu, Pb and Zn, and HI for aggregate risk of studied metals in street dusts and soils. All particle size fractions of street dusts and surface soils indicated high health risk of Cu, Pb and Zn to children and adults living in central district of Tehran. Among the studied metals, Zn had the highest potential to pose individual health risk to Tehran inhabitants. It is noteworthy that even when HI was expressed as bioaccessible content of elements, it is far above the safe level (HI = 1) for adults and children, indicating high non-carcinogenic risk from street dust and surface soil ingestion, especially from particles below 70 µm that are more likely to stick to hands.
This study highlights the need to determine particle size distribution of PTMs in studied environmental media before oral bioaccessibility assessment in order to obtain more realistic and precise results related to oral ingestion hazards. Furthermore, results demonstrated the necessity and importance of street sweeping and significant effect of high-quality hygiene standards to limit inadvertent ingestion of street dust, especially for children.
Although the SBRC is a simple method to simulate gastric conditions and is thought to overestimate the typical bioavailability of metals due to the aggressive pH of 1.5 in fasted conditions, it is a conservative approach to estimating risk of particle ingestion in areas with elevated concentrations of trace elements (Ettler et al. 2012). Although the <250 µm particle size fraction is recommended to calculate exposure to trace elements through inadvertent soil ingestion, using this fraction may underestimate potential hazard of elements exposure if metal concentration and/or bioaccessibility is dissimilar among particle size fractions, as there is no doubt that smaller particles preferentially adhere to the hands and are more susceptible to be ingested in comparison with coarser particles. Many researchers have reported increasing metal concentration with decreasing particle size that intensifies the hazardous effect of small size fractions on human health via their ingestion. The current study augments these observations, by determining that the highest element bioaccessibility (%) is not necessarily related to the finest particles, which is a more realistic indicator of exposure.
References
Abouelnasr, D. M. (2010). The relationship between soil particle size and lead concentration. In Proceedings of the annual international conference on soils, sediments, water and energy (Vol. 8).
Abrahams, P. W. (2002). Soils: Their implications to human health. Science of the Total Environment, 291, 1–32.
Al-Chalabi, A., & Hawker, D. (1997). Response of vehicular lead to the presence of street dust in the atmospheric environment of major roads. Science of the Total Environment, 206, 195–202.
Artíñano, B., Salvador, P., Alonso, D. G., Querol, X., & Alastuey, A. (2003). Anthropogenic and natural influence on the PM 10 and PM 2.5 aerosol in Madrid (Spain). Analysis of high concentration episodes. Environmental Pollution, 125, 453–465.
Berglund, M., Lind, B., Sörensen, S., & Vahter, M. (2000). Impact of soil and dust lead on children’s blood lead in contaminated areas of Sweden. Archives of Environmental Health: An International Journal, 55, 93–97.
Bi, X., Liang, S., & Li, X. (2013). A novel in situ method for sampling urban soil dust: Particle size distribution, trace metal concentrations, and stable lead isotopes. Environmental Pollution, 177, 48–57.
Birmingham, B., & McLaughlin, D. (2006). Soil investigation and human health risk assessment for nickel in community soils near a former nickel refinery in Southern Ontario, Canada. Journal of Toxicology and Environmental Health, Part A, 69, 845–892.
Bish, D. L., & Post, J. E. (1989). Modern powder diffraction. Washington, DC: Mineralogical Society of America.
Butte, W., & Heinzow, B. (2002). Pollutants in house dust as indicators of indoor contamination. Reviews of Environmental Contamination and Toxicology, 175, 1–46.
Choate, L. M., Ranville, J. F., Bunge, A. L., & Macalady, D. L. (2006). Dermally adhered soil: 1. Amount and particle-size distribution. Integrated Environmental Assessment and Management, 2, 375–384.
Crawford, J. (2009). Solubility data on 646 common and not so common minerals. Retrieved from http://www.mindat.org/article.php/553/.
Dean, J. A. (1990). Lange’s handbook of chemistry. Material and manufacturing process, 5(4), 687–688.
Dehghani, S., Moore, F., Keshavarzi, B., & Hale, B. A. (2017). Health risk implications of potentially toxic metals in street dust and surface soil of Tehran, Iran. Ecotoxicology and Environmental Safety, 136(2017), 92–103.
Duggan, M., Inskip, M., Rundle, S., & Moorcroft, J. (1985). Lead in playground dust and on the hands of schoolchildren. Science of the Total Environment, 44, 65–79.
Duong, T. T., & Lee, B.-K. (2011). Determining contamination level of heavy metals in road dust from busy traffic areas with different characteristics. Journal of Environmental Management, 92, 554–562.
Duruibe, J., Ogwuegbu, M., & Egwurugwu, J. (2007). Heavy metal pollution and human biotoxic effects. International Journal of Physical Sciences, 2, 112–118.
Duzgoren-Aydin, N. S., Wong, C. S., Aydin, A., Song, Z., You, M., & Li, X. D. (2006). Heavy metal contamination and distribution in the urban environment of Guangzhou, SE China. Environmental Geochemistry and Health, 28, 375–391.
Ellickson, K., Meeker, R., Gallo, M., Buckley, B., & Lioy, P. (2001). Oral bioavailability of lead and arsenic from a NIST standard reference soil material. Archives of Environmental Contamination and Toxicology, 40, 128–135.
EPA. (2007). In vitro bioaccessibility testing: Current science and way forward (environmental agency science update 2). http://www.environment-agency.gov.uk/.
Ettler, V., Kříbek, B., Majer, V., Knésl, I., & Mihaljevič, M. (2012). Differences in the bioaccessibility of metals/metalloids in soils from mining and smelting areas (Copperbelt, Zambia). Journal of Geochemical Exploration, 113, 68–75.
Fosmire, G. J. (1990). Zinc toxicity. The American Journal of Clinical Nutrition, 51, 225–227.
Geebelen, W., Adriano, D., van der Lelie, D., Mench, M., Carleer, R., Clijsters, H., et al. (2003). Selected bioavailability assays to test the efficacy of amendment-induced immobilization of lead in soils. Plant and Soil, 249, 217–228.
Geological Survey of Iran. (1986). http://www.gsi.ir/.
Gilbert, B. M., & Avenant-Oldewage, A. (2014). Arsenic, chromium, copper, iron, manganese, lead, selenium and zinc in the tissues of the largemouth yellowfish, Labeobarbus kimberleyensis (Gilchrist and Thompson, 1913), from the Vaal Dam, South Africa, and associated consumption risks. Water SA, 40(4), 739–748.
Grøn, C., & Andersen, L. (2003). Human bioaccessibility of heavy metals and PAH from soil. Environmental project, (840), 1–113.
Gunawardana, C., Egodawatta, P., & Goonetilleke, A. (2014). Role of particle size and composition in metal adsorption by solids deposited on urban road surfaces. Environmental Pollution, 184, 44–53.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14, 975–1001.
Hardy, M., & Cornu, S. (2006). Location of natural trace elements in silty soils using particle-size fractionation. Geoderma, 133, 295–308.
Heiri, O., Lotter, A. F., & Lemcke, G. (2001). Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. Journal of paleolimnology, 25(1), 101–110.
Higgs, F. J., Mielke, H. W., & Brisco, M. (1999). Soil lead at elementary public schools: Comparison between school properties and residential neighbourhoods of New Orleans. Environmental Geochemistry and Health, 21, 27–36.
Ho, K. F., Lee, S. C., Chow, J. C., & Watson, J. G. (2003). Characterization of PM10 and PM2.5 source profiles for fugitive dust in Hong Kong. Atmospheric Environment, 37, 1023–1032.
Iran Meteorological Organization. (2015). http://www.irimo.ir/.
Juhasz, A. L., Weber, J., & Smith, E. (2011). Impact of soil particle size and bioaccessibility on children and adult lead exposure in peri-urban contaminated soils. Journal of Hazardous Materials, 186, 1870–1879.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. New York: Springer.
Kim, J. Y., Kim, K. W., Lee, J. U., Lee, J. S., & Cook, J. (2002). Assessment of As and heavy metal contamination in the vicinity of Duckum Au–Ag mine, Korea. Environmental Geochemistry and Health, 24, 213–225.
Kramer, K. J., Brockmann, U. H., & Warwick, R. M. (1994). Tidal estuaries: Manual of sampling and analytical procedures. AA Balkema.
Kissel, J., Richter, K., & Fenske, F. (1996). Factors affecting soil adherence to skin in hand-press trials. Bulletin of Environmental Contamination and Toxicology, 56, 722–728.
Lamb, D. T., Ming, H., Megharaj, M., & Naidu, R. (2009). Heavy metal (Cu, Zn, Cd and Pb) partitioning and bioaccessibility in uncontaminated and long-term contaminated soils. Journal of Hazardous Materials, 171, 1150–1158.
Lanphear, B. P., Burgoon, D. A., Rust, S. W., Eberly, S., & Galke, W. (1998). Environmental exposures to lead and urban children’s blood lead levels. Environmental Research, 76, 120–130.
Lee, B.-K., & Dong, T. T. (2011). Toxicity and source assignment of polycyclic aromatic hydrocarbons in road dust from urban residential and industrial areas in a typical industrial city in Korea. Journal of Material Cycles and Waste Management, 13, 34–42.
Leotsinidis, M., Alexopoulos, A., & Kostopoulou-Farri, E. (2005). Toxic and essential trace elements in human milk from Greek lactating women: association with dietary habits and other factors. Chemosphere, 61, 238–247.
Ljung, K., Selinus, O., Otabbong, E., & Berglund, M. (2006). Metal and arsenic distribution in soil particle sizes relevant to soil ingestion by children. Applied Geochemistry, 21, 1613–1624.
Madrid, F., Biasioli, M., & Ajmone-Marsan, F. (2008a). Availability and bioaccessibility of metals in fine particles of some urban soils. Archives of Environmental Contamination and Toxicology, 55, 21–32.
Madrid, F., Diaz-Barrientos, E., & Madrid, L. (2008b). Availability and bio-accessibility of metals in the clay fraction of urban soils of Sevilla. Environmental Pollution, 156, 605–610.
Mage, D., Ozolins, G., Peterson, P., Webster, A., Orthofer, R., Vandeweerd, V., et al. (1996). Urban air pollution in megacities of the world. Atmospheric Environment, 30, 681–686.
Medlin EA. (1997). An In Vitro Method for Estimating the Relative Bioavailability of Lead in Humans, MS Thesis. University of Colorado, Boulder, CO.
Mench, M., Vangronsveld, J., Beckx, C., & Ruttens, A. (2006). Progress in assisted natural remediation of an arsenic contaminated agricultural soil. Environmental Pollution, 144, 51–61.
Mercier, F., Glorennec, P., Thomas, O., & Bot, B. L. (2011). Organic contamination of settled house dust, a review for exposure assessment purposes. Environmental Science and Technology, 45, 6716–6727.
Metson, A. J. (1957). Methods of chemical analysis for soil survey samples. Soil Science, 83, 245.
Mielke, H., Gonzales, C., Smith, M., & Mielke, P. (1999). The urban environment and children’s health: soils as an integrator of lead, zinc, and cadmium in New Orleans, Louisiana, USA. Environmental Research, 81, 117–129.
Morrison, A. L., & Gulson, B. L. (2007). Preliminary findings of chemistry and bioaccessibility in base metal smelter slags. Science of the Total Environment, 382, 30–42.
Mossetti, S., Angius, S. P., & Angelino, E. (2005). Assessing the impact of particulate matter sources in the Milan urban area. International Journal of Environment and Pollution, 24, 247–259.
Norman, M., & Johansson, C. (2006). Studies of some measures to reduce road dust emissions from paved roads in Scandinavia. Atmospheric Environment, 40, 6154–6164.
Norra, S., Lanka-Panditha, M., Kramar, U., & Stüben, D. (2006). Mineralogical and geochemical patterns of urban surface soils, the example of Pforzheim, Germany. Applied Geochemistry, 21, 2064–2081.
Ogwuegbu, M., & Muhanga, W. (2005). Investigation of lead concentration in the blood of people in the copper belt province of Zambia. J Environ, 1, 66–75.
Okorie, A., Entwistle, J., & Dean, J. R. (2012). Estimation of daily intake of potentially toxic elements from urban street dust and the role of oral bioaccessibility testing. Chemosphere, 86, 460–467.
Oomen, A. G., Hack, A., Minekus, M., Zeijdner, E., Cornelis, C., Schoeters, G., et al. (2002). Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants. Environmental Science and Technology, 36, 3326–3334.
Özkaynak, H., Xue, J., Zartarian, V. G., Glen, G., & Smith, L. (2011). Modeled estimates of soil and dust ingestion rates for children. Risk Analysis, 31(4), 592–608.
Paustenbach, D. J. (2000). The practice of exposure assessment: a state-of-the-art review. Journal of Toxicology and Environmental Health Part B: Critical Reviews, 3, 179–291.
Peltola, P., & Åström, M. (2003). Urban geochemistry: A multimedia and multielement survey of a small town in northern Europe. Environmental Geochemistry and Health, 25, 397–419.
Reis, A. P., Patinha, C., Wragg, J., Dias, A. C., Cave, M., Sousa, A. J., et al. (2014). Geochemistry, mineralogy, solid-phase fractionation and oral bioaccessibility of lead in urban soils of Lisbon. Environmental Geochemistry and Health, 36, 867–881.
Relative Bioavailability of Lead. (2007). Estimation of relative bioavailability of lead in soil and soil-like materials using in vivo and in vitro methods.
Rojas-Bracho, L., Suh, H. H., Oyola, P., & Koutrakis, P. (2002). Measurements of children’s exposures to particles and nitrogen dioxide in Santiago, Chile. Science of the Total Environment, 287, 249–264.
Ruby, M. V., Davis, A., Schoof, R., Eberle, S., & Sellstone, C. M. (1996). Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environmental Science and Technology, 30, 422–430.
Ruby, M., Schoof, R., Brattin, W., Goldade, M., Post, G., Harnois, M., et al. (1999). Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environmental Science and Technology, 33, 3697–3705.
Ryan, J., Estefan, G., & Rashid, A. (2007). Soil and plant analysis laboratory manual. Beirut: ICARDA.
Saeedi, M., & Pajooheshfar, S. P. (2012). Acid rain examination and chemical composition of atmospheric precipitation in Tehran, Iran. EnvironmentAsia, 5(1). doi:10.14456/ea.2012.5
SCHER, SCENIHR, SCCS. (2011). Toxicity and assessment of chemical mixtures. http://ec.europa.eu/health/scientific_committees/consultations/public_consultations/scher_consultation+06_en.tm.
Sheppard, S., & Evenden, W. (1994). Contaminant enrichment and properties of soil adhering to skin. Journal of Environmental Quality, 23, 604–613.
Siaka, M., Owens, C., & Birch, G. (1998). Evaluation of some digestion methods for the determination of heavy metals in sediment samples by flame-AAS. Analytical Letters, 31, 703–718.
Sialelli, J., Urquhart, G. J., Davidson, C. M., & Hursthouse, A. S. (2010). Use of a physiologically based extraction test to estimate the human bioaccessibility of potentially toxic elements in urban soils from the city of Glasgow, UK. Environmental Geochemistry and Health, 32, 517–527.
Siciliano, S. D., James, K., Zhang, G., Schafer, A. N., & Peak, J. D. (2009). Adhesion and enrichment of metals on human hands from contaminated soil at an Arctic urban brownfield. Environmental Science and Technology, 43, 6385–6390.
Staff, E. (2001). Supplemental guidance for developing soil screening levels for superfund sites, peer review draft (pp. 9355.4–9355.24). Washington, DC: US Environmental Protection Agency Office of Solid Waste and Emergency Response, OSWER.
Statistical Center of Iran (SCI). (2011). http://www.amar.org.ir.
Sutherland, R. A. (2003). Lead in grain size fractions of road-deposited sediment. Environmental Pollution, 121, 229–237.
Tang, R., Ma, K., Zhang, Y., & Mao, Q. (2013). The spatial characteristics and pollution levels of metals in urban street dust of Beijing, China. Applied Geochemistry, 35, 88–98.
Tehran Municipality (2015). Annual Internal Report. http://www.tehran.ir.
Thornton, I., Watt, J., Davies, D., Hunt, A., Cotter-Howells, J., & Johnson, D. (1994). Lead contamination of UK dusts and soils and implications for childhood exposure: An overview of the work of the Environmental Geochemistry Research Group, Imperial College, London, England 1981–1992. Environmental Geochemistry and Health, 16, 113–122.
Thorpe, A., & Harrison, R. M. (2008). Sources and properties of non-exhaust particulate matter from road traffic: A review. Science of the Total Environment, 400, 270–282.
US EPA. (1989). Risk assessment guidance for superfund. Human health evaluation manual (part A). Interim Final (Vol. 1). Washington (DC): United States Environmental Protection Agency, 540, 1–89.
US EPA. (2004). Integrated risk information system. http://www.epa.gov/iris. Accessed 28 November 2004.
US EPA (2007). Estimation of relative bioavailability of lead in soil and soil-like materials using in vivo and in vitro methods. US Environmental protection agency, office of solid waste and emergency response. OSWER 9285.7–77. Washington, DC.
US EPA. (2007). Guidance for evaluating the oral bioavailability of metals in soils for use in human health risk assessment. US Environmental Protection Agency, Office of solid waste and emergency response. OSWER 9285.7 80, Washington, DC.
US EPA. (2009). Risk-based concentration table Environmental Protection Agency, Philadelphia, PA, Washington, DC.
US EPA. (2010). Region 9, regional screening levels. http://www.epa.gov/region9/superfund/prg/index.html.
US Environmental Protection Agency. (2008). Standard operating procedure for an in vitro bioaccessibility assay for lead in soil. Office of Solid Waste and Emergency Response, US Environmental Protection Agency. EPA Washington, DC.
Wensing, M., Uhde, E., & Salthammer, T. (2005). Plastics additives in the indoor environment—Flame retardants and plasticizers. Science of the Total Environment, 339, 19–40.
Wilson, L (2009). Copper toxicity syndrome. Center for Development, 1.
Wragg, J., & Cave, M. (2003). In-vitro methods for the measurement of the oral bioaccessibility of selected metals and metalloids in soils: A critical review. Bristol: Environment Agency.
Wragg, J., Cave, M., Basta, N., Brandon, E., Casteel, S., Denys, S., et al. (2011). An inter-laboratory trial of the unified BARGE bioaccessibility method for arsenic, cadmium and lead in soil. Science of the Total Environment, 409, 4016–4030.
Wragg, J., Cave, M., & Nathanail, P. (2007). A study of the relationship between arsenic bioaccessibility and its solid-phase distribution in soils from Wellingborough, UK. Journal of Environmental Science and Health Part A, 42, 1303–1315.
Xie, S., Dearing, J. A., & Bloemendal, J. (2000). The organic matter content of street dust in Liverpool, UK, and its association with dust magnetic properties. Atmospheric Environment, 34, 269–275.
Yamamoto, N., Takahashi, Y., Yoshinaga, J., Tanaka, A., & Shibata, Y. (2006). Size distributions of soil particles adhered to children’s hands. Archives of Environmental Contamination and Toxicology, 51, 157–163.
Yu, S., & Li, X.-D. (2011). Distribution, availability, and sources of trace metals in different particle size fractions of urban soils in Hong Kong: implications for assessing the risk to human health. Environmental Pollution, 159, 1317–1326.
Zheng, N., Liu, J., Wang, Q., & Liang, Z. (2010). Health risk assessment of heavy metal exposure to street dust in the zinc smelting district, Northeast of China. Science of the Total Environment, 408, 726–733.
Zhuang, P., Li, Z. A., McBride, M. B., Zou, B., & Wang, G. (2013). Health risk assessment for consumption of fish originating from ponds near Dabaoshan mine, South China. Environmental Science and Pollution Research, 20(8), 5844–5854.
Acknowledgements
The authors are grateful for financial support from BH from Canada’s Natural Sciences and Engineering Research Council (NSERC), from Vale Canada Ltd. and from Glencore Integrated Operations. The authors also greatly acknowledge technical and analytical support provided by the School of Environmental Sciences, University of Guelph. We are grateful to Ms. M. Saadati for assisting in the sampling and University of Shiraz research council to provide analytical support for physiochemical properties of samples. The authors also greatly thank Mr. Peter Smith, instrument laboratory manager at University of Guelph, for assistance in analytical projects and Dr. M. Thierry, University of Montreal, for XRD analysis of samples.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dehghani, S., Moore, F., Vasiluk, L. et al. The influence of physicochemical parameters on bioaccessibility-adjusted hazard quotients for copper, lead and zinc in different grain size fractions of urban street dusts and soils. Environ Geochem Health 40, 1155–1174 (2018). https://doi.org/10.1007/s10653-017-9994-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-9994-6