Abstract
The pollution assessment and the evaluation of potential risks in the Atlantic Coastal Region of the Democratic Republic of the Congo are still very limited. Consequently, the present study investigates for the first time the concentrations of heavy metals and persistent organic pollutants (organochlorine pesticides (OCPs), polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and polycyclic aromatic hydrocarbons (PAHs)) in river, estuary, and marine sediments from this area. The results highlighted high concentrations of Cr, Zn, As, and Pb exceeding the probable effect level (PEL) on aquatic life. Zn was the most dominant element detected at a range of 180–480 mg kg−1 in marine sediment, 132–382 mg kg−1 in estuary sediment, and 121–687 mg kg−1 in river sediment. Total PCBs (∑7 × 4.3) ranged from 1995 to 20,156 μg kg−1, 2013–12,058 μg kg−1, and 1861–36,417 μg kg−1 in marine, estuary, and river sediments, respectively. Total PCBs (∑7 × 4.3) were above PEL for all sediments, suggesting potential adverse effects on benthic organisms. The OCP, PBDE, and PAH levels were low to moderate for all sediments. Taking into consideration, the concentrations of Zn, Pb, PCBs, and DDTs, probable environmental risks, are present.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contamination of aquatic ecosystems by persistent organic pollutants (POPs) including organochlorine pesticides (OCPs), polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), polycyclic aromatic hydrocarbons (PAHs), or heavy metals is of a great environmental concern globally. These pollutants have been intensively investigated in different environmental compartments such as soils, sediments, water, and biota worldwide (Doong et al. 2002; Fu and Wu 2006; Fair et al. 2018). Due to their high stability and resistance to degradation in the aquatic environment, these contaminants can accumulate in sediments over time and enter into food webs presenting high risks to the public health (Davis et al. 2007; Miglioranza et al. 2013; Montuori et al. 2016; Kayembe et al. 2018; Combi et al. 2016). Many of these studies demonstrated that POPs can persist in coastal and freshwater sediments for a long time and affect the benthic organisms by causing adverse biological effects. POPs and PAHs may cause human ailments including cancer, damage the nervous system, and retard the growth in infants and children due to toxic, carcinogenic, and mutagenic effects (UNEP 2012; IARC 2014). Given the associated ecological and human health risks, developed countries have banned the utilization of OCPs in agriculture. However, developing countries are still extensively using OCPs to prevent pest attacks on crops due to low cost (Zhao et al. 2009; Yuan et al. 2015). In the same manner, DDTs is also used in sanitation in developing countries (Zhang et al. 2013; Kilunga et al. 2017; Verhaert et al. 2013; UNDP 2009). POPs are generally hydrophobic, having low degradation rates with high chemical stability, and can effectively adsorb onto sediments (Ribeiro et al. 2016; Montuori et al. 2016). Several studies have shown that physicochemical properties may influence the distributions of POPs, PAHs, and heavy metals in different bays (Poté et al. 2008; Yang et al. 2011; Jiang et al. 2013; Alegria et al. 2016). Sediments can act as a reservoir for organic and inorganic pollutants for an extended period. In this context, the assessment of the spatial distribution of POPs, PAHs, and heavy metals in sediments can help to validate the ecological and human-associated risks.
The rivers in Congo generally receive pollutants from untreated urban, industrial and hospital effluents, agriculture runoff, solid wastes, and landfills (Laffite et al. 2016; Kilunga et al. 2017; Kayembe et al. 2018). The pollutants traverse the river by substantial anthropogenic activities lead to the gradual deterioration of the quality of the estuaries. The coastal environments are the final receptacle for xenobiotics such as POPs and heavy metals (Mayon et al. 2006; Maanan 2008; Carro et al. 2010). The Atlantic Coast of the Democratic Republic of the Congo (DRC) is located to the West of the DRC, in the province of Kongo Central, district of Boma, Muanda territory. The coast is considered as a geographical area of great economic and environmental importance. It constitutes a significant source of fish production in DRC. There also have been enhanced oil exploitation activities in the region, fuel traffic, buffer storage of imported petroleum products, and tankers navigation. These activities could also have contributed to the pollution of Muanda’s marine environment (Mbomba 2007; Suami et al. 2018). Despite these several potential sources of contamination, to our best knowledge, the studies on the assessment of this coastal pollution are still scarce. Quantitative data on the coastal contamination by organic and inorganic pollutants are not present in the scientific literature. The levels of heavy metals and POPs (OCPs, PCBs, and PBDEs, and PAHs) in sediments are good indicators to evaluate the environmental quality of aquatic systems. Consequently, the objective of this study was to determine the concentrations, degree of contamination and possible sources of heavy metals (Cr, Co, Ni, Cu, Zn, As, Cd, Pb, and Hg), and POPs (including OCPs, PCBs, PBDEs, and PAHs) in the surface sediments from Atlantic Coast of Muanda in Democratic Republic of the Congo. The assessment was performed also on sediments from Congo and Tonde rivers, surrounding estuaries and the coast.
Materials and methods
Study site description and sampling procedure
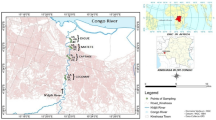
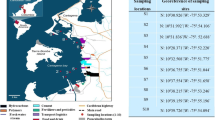
This research was performed in the Congolese Atlantic Coastal basin located to the West of the DRC, in the province of Kongo Central, district of Boma, Muanda territory (Fig. 1). The GPS location of sampling sites is given in Table 1S (Supplementary Data, Table 1S).
The sediments sampling took place in February 2018 from river (Fig. 2a), estuary (Fig. 2b), and ocean (Fig. 2c). The surface sediments (0–5-cm layer) were collected from 8 sites (Fig. 1) namely (1) Yacht-Socir in the estuary (S1, n = 2, labeled: S1A, S1B), (2) Banana in the ocean (S2, n = 2, labeled: S2A, S2B), (3) Marana in the ocean (S3, n = 1, labeled: S3A), (4) Nsiamfumu in the ocean (S4, n = 2, labeled: S4A, S4B), (5) Muanda Village in the ocean (S5, n = 2, labeled: S5A, S5B), (6) Kimuabi (Kikaka Kiaku) in the Congo River (S6, n = 2, labeled: S6A, S6B), (7) Baku Yanga in the estuary (S7, n = 2, labeled: S7A, S7B), and (8) Tonde River (S8, n = 2, labeled: S8A, S8B). These sites were selected for different reasons according to our previous study (Suami et al. 2018). The Atlantic Coast of Congo DR is situated in the tropical zone. In recent years, this coast has witnessed major socioeconomic and rapid industrial developments. The Congo River is highly impacted by both the direct pollution and diffusion sources during its course and primarily with upstream pressure. Riverine input to the estuary and coastal regions during its course most likely caused accumulation of pollutants (Suami et al. 2018). Approximately 200–300 g of surface sediments was taken from each site in triplicates. The sediment samples from all sites were collected manually using indigenous canoes (Fig. 2d) and transferred into autoclaved glasses for POP and PAH analyses and into the clean polyethylene bottles for other analyses. All samples were stored in an icebox at 4 °C and transported to the Department F.-A. Forel of the University of Geneva for analysis.
Sediment grain size, organic matter, and total carbon
Measurement of particle size in sediments was carried out in a Laser Coulter® LS-100 diffractometer (Beckman Coulter, Fullerton, CA, USA), following 5-min ultrasonic dispersal in degassed and deionized water. The sediment total organic matter content was obtained from mass loss on ignition at 550 °C during 1 h in a Salvis oven (Salvis AG, Luzern, Switzerland). The percentage of TC was measured with an Elemental Analyzer (Perkin Elmer CHNS/O PE 2400 Series II, Waltham, MA, USA) by the following conditions: combustion temperature, 975 °C; reduction temperature, 500 °C; detection oven, 82.2 °C; pressure, 514.0 mmHg (Poté et al. 2008).
Metal analysis in sediment samples
Before analysis, the sediment samples were lyophilized, grounded into a fine and homogenized powder and sieved through a 63-μm mesh size sieve, and digested according to the previous method as described by (Poté et al. 2008). The digested samples were subjected to analysis by inductive coupled plasma-mass spectroscopy (Agilent 7700 × series ICP-MS developed for complex matrix analysis, Santa Clara, CA, USA). A collision/reaction cell (helium mode) and interference equations were utilized to remove spectral interferences that might otherwise bias results. This is sufficient for many routine applications. Multi-element standard solutions at different concentrations (0, 0.2, 1, 5, 20, 100, and 200 μg L−1) were used for calibration. The certified sediment reference materials LKSD-2 and LKSD-4 were used for lake and river sediment analysis in order to verify the sensibility of the instrument and the reliability of the results, respectively. Concentrations are in mg kg−1 (ppm) dry weight (dw). The standard deviations of 3 replicate measurements were below 5%, and chemical blanks for the procedure were less than 1% of the sample signal.
Hg analysis in sediment samples was carried out using atomic absorption spectrophotometry for mercury determination (Advanced Mercury Analyzer; AMA 254, Altec s.r.l., Czech Rep.) following the procedure described by (Bravo et al. 2011). This method is based on sample combustion, gold amalgamation, and atomic absorption spectrometry. It is important to compare the levels of determined metals in the sediments with the sediment quality guideline values. In our study, we used Canadian Sediment Quality for the Protection of Aquatic Life (EPC-98E, CCME 1999) to assess the potential ecotoxicological risk (MacDonald et al. 2000a; Long et al. 2006; Poté et al. 2008; Haller et al. 2011).
Geo-accumulation index and enrichment factor
The enrichment factor (EF) and geo-accumulation index (Igeo) in sediment samples were calculated as described by Maanan (2008), Thevenon et al. (2013), and Varol (2011). The Igeo accumulation index is defined by the following equation:
Cn is the concentration of metals (n) examined in the sediment samples; Bn is the concentration of the metal (n) geochemical background; and 1.5 is the lithospheric effect background correlation matrix factor.
Enrichment factor is a useful tool to determine the degree of anthropogenic heavy metal pollution. EF is calculated using the following equation, and according to our previous study, Scandium (Sc) was used for the geochemical normalization (Kilunga et al. 2017; Montuori et al. 2016; Mwanamoki et al. 2014).
Chlorinated pesticide, PCB, PAH, and PBDE analyses
Chlorinated pesticide, PCB, PAH, and PBDE analyses were performed according to our previous paper (Kilunga et al. 2017; Mwanamoki et al. 2014; Thevenon et al. 2013). Briefly, all glassware was rinsed with acetone and hexane and tested before use. Blanks did not present any quantifiable amount of contaminants or interfering compounds. After addition of surrogate standards (13C-labeled for all halogenated compounds and deuterated-labeled compounds for all PAHs), about 5 g of dry sediment was subjected to extractant reflux system composed of 20% of acetone in 80% of hexane (v/v) for 4 h into a Soxhlet system (Buchi B-811, Flawil, Switzerland). Interfering sulfur compounds were removed by addition of activated copper to the extract. Then, the organic extract was concentrated to 1 mL in a vacuum rotary evaporator (Buchi Rotavapor, Flawil, Switzerland). The chemicals were measured by gas chromatography with triple mass spectrometry detection (GC-MS/MS, Thermo Scientific, TSQ Quantum XLS Ultra, Waltham, MA, USA). The detailed extraction procedure and analysis are described in Supplementary Data section S1. The methods used to identify the sources of PAHs and DDTs were performed as described previously (Yunker et al. 2002; Mitra et al. 2019). In this study, ratios of Fluo/(Fluo + Pyr), IDP/(IDP + BghiP), BaA/(BaA + Chry), and LMW/HMW were employed to identify the sources of PAHs. The results for POP and PAH analyses are expressed in μg kg−1 of dry weight.
Data analysis
Triplicate measurements were performed for all analyses. Statistical treatment of data (Spearman’s rank order correlation) has been realized using SigmaStat 12.5 (Systat Software, Inc., USA). Principal component analysis (PCA), a multivariate statistical analysis, was performed using R (R Core Team 2015) in order to understand the relationship among analyzed compounds and their potential sources. Prior to performing PCA analysis, data were centered in order to maximize the dispersion.
Results and discussion
Physicochemical characteristics of sediments
The physicochemical characteristics of the sediments including particle grain size, sediment organic matter (OM) contents, total carbon (TC), and TN are given in Table 1S (Supplementary Data, Table 1s). The sediments OM range in marine, estuary, and river sediments is 7–18%, 6–27%, and 8–19% respectively. The results reveal that there are significant spatial variations (p < 0.05) among the study sites and a few sites in marine, estuary, and rivers relatively have high OM contents. In general, the site S7A for the estuary has the highest OM content (27%), which might be attributed to the mixing of river water carrying industrial and agricultural runoff. The median grain size distribution in the marine sediments ranged from 13 to 23 μm. All of the marine sediment samples were mainly composed of fine particles with a high proportion of silt. Estuary sediment samples had high percentage of silt (62–70%) followed by sand 27–34% indicating muddy sediments. The level of TC contents had not demonstrated any significant spatial variations among the study sites ranging between 3 and 4%, 0.5 and 2.7%, and 0.8 and 1.0% for marine, estuary, and river sediments, respectively. The marine sediments relatively present high level of TC while the river sediments had the lowest. The high TC level in coastal marine sediments may be attributed to the flow of Tonde and Congo River waters into the Muanda coastal regions which receive various industrial effluents, seepage of estuary water to the coast, and fishing activities (Mbomba 2007; Suami et al. 2018). Grain size had displayed a strong positive correlation with OM (r = 0.127, p < 0.05, n = 15) and TC (r = 0.112, p < 0.01, n = 15), indicating that accumulation of OM and TC can be influenced by sediment grain size (Hong et al. 2012; Poté et al. 2008; Yuan et al. 2015).
Metal concentrations in the surface sediments
The results of the heavy metal (Sc, Cr, Cu, Ni, Zn, As, Cd, Pb, and Hg) analysis are presented in Table 1. Heavy metal concentrations in sediment samples followed the order of Zn (687 mg kg−1 dw) > Cu (217 mg kg−1 dw) > Cr (187 mg kg−1 dw) > Pb (125 mg kg−1 dw) > Ni (30 mg kg−1 dw) > As (24 mg kg−1 dw) > Co (18 mg kg−1 dw) > Sc (24 mg kg−1 dw) > Cd (0.30 mg kg−1 dw) > Hg (0.14 mg kg−1 dw). In general, the concentrations of Cr, Zn, As, and Pb for most of the sediment samples were higher than the reference limit values established by Sediment Quality Guidelines for the Protection of Aquatic Life (EPC-98E 1999). Zn was found to be the highest contaminant metal in all sediments. The highest concentration of Zn was recorded in site S6A of the Congo River. The higher concentrations of Zn observed in all study sites might be attributed to intense anthropic activities, disposal of petroleum wastes, untreated industrial effluents, dumping of urban wastes, wastes from automobile industries, ship activities, and wastes derived from the fishing port on the Muanda coast (Mbomba 2007; Suami et al. 2018).
The concentrations of Cr in estuary sediments vary considerably with sampling site (p < 0.05). The site S1B recorded the highest level (140.74 mg kg−1 dw). Unlike marine and estuary sediments, the concentrations of Cr in river sediments were just above the recommended value of 37.30 mg kg−1 dw (EPC-98E 1999). The levels of Cu in all sediments were generally lower than that of recommended value (35.7 mg kg−1 dw) except for site S3A marine sediment which had the highest values of 216.84 mg kg−1 dw. This site is impacted with intense anthropogenic activities including oil exploration and petrochemical activities (Suami et al. 2018). Hg and Cd concentrations at all the sites were lower than the recommended values. The concentration of Pb had shown considerable variation among the sampling sites. The Pb level ranged from 9 to 122, 8–125, and 7–124 mg kg−1 dw for marine, estuary, and river sediments, respectively. The high levels of Pb at the marine sites S2A, S2B, and S4B were probably due to the mixing of wastes from petroleum refinery activities, untreated industrial and transport vehicle oil wastes, ship movement, and fuel drum deposits following traffic activities of fuel between Soyo (Angola) and Yacht. The sites S3A, S5A, and S5B are located on the high seas and downstream of the Congo River which is an ultimate receptacle of pollutants. A fishing port is present at S6A and S6B sites located on Kimuabi Kikala Kiaku where a large number of pirogues accost and important commercial activities of fish and seafood take place.
The heavy metal contaminations of the estuary sediments may be attributed with the confluence of the river and the estuary. The site S8A seems to be less polluted than the other sites. Indeed, this site is far from places where there are significant human activities and does not receive any discharges that would come from the Congo River generated in the big cities (Matadi, Boma); it is located to the north on the Atlantic coast of Muanda away from the Congo River. The results of this study were comparable with consensus-based guidelines for the sediment quality (Long et al. 2006; MacDonald et al. 2000a, b) which present the hazard of sediments that can affect the local biota. Threshold effect concentration (TEC) is a level above which some effects (or responses) might be produced in a living organism and below it, effects are not likely to occur. Probable effect level (PEL) is a contamination level that is likely to cause an adverse effect on biota (Poté et al. 2008). The levels of Cu, Zn, and Pb for most of the sediment samples from marine, estuary, and rivers were above the TEC values. The Cr concentrations at S2A, S2B, S3A, S4B, and S5B were above the PEC level. The site S3A showed higher Cu level than the PEL. The Zn concentrations at S2B, S6A, and S6B were also higher than that of the PEL level. These results suggest that higher concentrations of Cu, Cr, Zn, and Pb are likely to have a harmful effect on aquatic organisms.
Geo-accumulation index and enrichment factor
The EF and Igeo results are shown in Table 2. The Igeo values of marine sediments were in the range of − 1.66–2.05 (Cu), 2.91–4.32 (Zn), 3.32–3.98 (As), and − 0.15–3.54 (Pb). The Igeo values for Cu in all marine sediment samples fell into class 0 indicating no contamination except for the site S3A which displayed moderate pollution with Igeo value of 2.05. Zn in most of the marine sediments had the Igeo values between class 4 and 5 indicating that the marine sediments are heavy to extremely contaminated by Zn. It is to be noted that all of the Igeo values for As in marine sediment were in class 4, suggesting they are heavily polluted by As. The Igeo values of Pb in marine sediments showed significant variation and fell between class 0 and 4, meaning these sediments range between unpolluted and heavily polluted by Pb. The mean Igeo values for Cu in all the sites from estuary sediments were less than zero, suggesting that these sites were uncontaminated by Cu, whereas the Igeo values of Zn uniformly fell in class 4 for estuary sediments except at S1A indicating heavy pollution with Zn in sediments. The As Igeo values of estuary sediments varied greatly with site S7A had the high value 2.16 indicating moderate contamination of As. In contrast, the Igeo values of Pb in all estuary sediments were falling in class 4 except at the site S7B which had the lowest value in class 0. The results suggest that estuary sediments were also heavily polluted by Pb. The Igeo values of Cr, Cu, As, and Pb for river sediments were in the range of 0 to 2 except at site S8B which had the Igeo value of class 4 for both Zn and Pb. The results indicate that river sediments were not contaminated by Cr and As. However, they are moderate to heavily polluted by Zn and Pb. In general, according to the Igeo average values, the pollution status of heavy metals in sediments was in the order of Zn > As > Pb > Cr > Cu.
The EF results were interpreted according to previous studies (Kilunga et al. 2017; Mavakala et al. 2016; Sakan et al. 2009; Thevenon et al. 2013). The value of EF for a specific heavy metal can be used for the identification of it likely source. It has been proposed that when EF ≤ 0.5 is indicative of bioturbation in the upper mixed layers and dilution with less contaminated sediment components; 0.5 ≤ EF ≤ 1.5 is indicative of metals from crustal sources or natural sources; EF ≥ 1.5 is indicative of specific metal inputs, e.g., from anthropogenic sources (Feng et al. 2004; Zhang and Liu 2002). Except for the site S1B for Cu (moderate enrichment, EF = 3.53), no enrichment was observed for Cu and Cr in the sediment samples from marine, estuary, and two rivers, respectively. Moderate enrichment (EF > 3) was observed for Zn in many marine sediment samples. EF > 3 for As showed moderate enrichment at S4A and S5A. Other sites exhibited minor enrichment as indicated by EF values ranging from 1 to 3 for As. The EF values for Pb in marine sediments ranged from 0.3 to 2, indicating no enrichment to minor enrichment. The EF values of the sites S1A (13), S1B (21), and S7B (7) in estuary sediment samples exhibited severe enrichment and moderate enrichment for Zn. The EF values for Pb in estuary sediments ranged between 0.4 and 27 indicating no enrichment to severe enrichment. Furthermore, the EF values for Pb showed significant variation among the sampling sites. Remarkably, no enrichment was observed for Cr, Cu, and As in river sediments as indicated by their EF values below 1, whereas Zn showed moderate to severe enrichment with the EF values ranging from 3 to 16. The EF values for Pb ranged from 0.5 to 6, indicating no enrichment to moderately severe enrichment. On the whole, according to the average EF values, the enrichment of heavy metals in this investigation was in this order Pb > Zn > As > Cr > Cu. Data on heavy metals in sediments from the Atlantic coast of Congo DR are relatively limited. According to the study sites, the physicochemical properties of sediments and human linked anthropogenic activities caused varying degrees of metal enrichments. Therefore, the results of this investigation provide insight to local government and policymakers on identifying the sources and prevention of pollutants.
Spatial distribution of persistent organic pollutants in sediments
Table 3 presents the concentration of persistent organic pollutants (POPs) including organochlorine pesticides (OCPs), polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the investigated sites. Overall, PCBs and PAHs were detected in all sediment samples, while PDBEs were not detected in any of the sediments (under quantification limit). PCBs were the predominant compounds detected among the POPs. It should be noted that 12 PCB congeners (PCBs 28, 52, 101, 105, 118, 128, 138, 149, 153, 156, 170, and 180) were detected and quantified in all surface sediment samples analyzed. The sum of 12 PCBs (∑12 PCBs) ranged from 604 to 6235 μg kg−1, 588 to 3699 μg kg−1, and 531 to 11,864 μg kg−1 in marine, estuary, and river sediments, respectively. The most dominant individual PCB congeners were CB 153 (21% of ΣPCB), CB 149 (16%), CB 101 (15%), CB 52 (12%), CB 138 (12%), and CB 180 (9%) in the decreasing order concentration. The sum of ∑12 PCBs detected in river surface sediments in this investigation shows an extreme increase in comparison with those previously reported in the Congo River (Kilunga et al. 2017; Mwanamoki et al. 2015; Verhaert et al. 2013). In general, PCB concentrations showed a decreasing trend from the river to marine as expected in the downstream watercourse. PCB levels at site S7B in estuary sediments present high concentrations. The high levels of PCBs in the upstream region in the river might be attributed to dumping and incineration of municipal wastes, landfills, combustion of urban and industrial wastes, and atmospheric deposition from ash due to the burning of biomass and from old electronic devices (Mwanamoki et al. 2014; Rabodonirina et al. 2015). Based on the levels of PCBs in the present study, all of the sediments are heavily contaminated by PCBs.
The levels of PCBs in marine sediments in this study were higher than those in marine sediments from the State of Kuwait (Lyons et al. 2015), Jobos Bay (Puerto Rico) (Alegria et al. 2016), the Atlantic Coast (Andalusia, SW Spain) (Pintado-Herrera et al. 2017), and the Bahiret el Bibane lagoon (Tunisia, Southwestern Mediterranean Sea) (Barhoumi et al. 2014). Regarding estuary sediments, their concentrations were extremely higher than those that of Hooghly estuary (∑7 PCBs × 4.3) (0.28–7.7 ng g−1 dry weight) (Mitra et al. 2019). For river sediments, PCB concentrations were found to be extremely higher than reported in the previous study in the tropical river Niger (Unyimadu et al. 2018) and Soan River, Pakistan (27.9–116 μg kg−1) (Malik et al. 2014). Remarkably, the concentration of ∑7PCBs in all sediment samples in this investigation was several folds higher than TEC and PEC implying the cause of adverse biological effects on sediment-dwelling organisms is very likely (MacDonald et al. 2000a). The levels of individual PAHs detected in this investigation are shown in Table 3. All the tested sediments presented detectable concentration of PAHs. The sum of Σ16 PAHs varied significantly among the sampling sites. The concentrations of ΣPAH16 ranged from 23 to 195, 99 to 780, and 13 to 347 μg kg−1 dw for marine, estuary, and river sediments, respectively. The result exhibited a decreasing trend of Σ16 PAH levels from the estuary to marine sediments. The concentrations of PAHs in the present study were found to be in relatively low to moderate levels which indicate that sediments were slightly to moderately polluted by PAHs. As shown in Table 3, the sites S5A, S1B, and S6B were found to be moderately contaminated by PAHs. Other sites are slightly polluted by PAHs. The site S6B had the highest PCB and PAH levels, which might be related to the intense anthropogenic activities and discharge of untreated effluents from highly populated urban areas. Among the study sites, S1B (780 μg kg−1 dw) estuary sediment had the highest levels for ΣPAH16. The high concentration of PAHs in this site could be due to persistence and inputs from watershed to the estuary.
The higher concentrations of PAHs in estuary sediments were consistent with a previous report by Santos et al. (2018) who reported that shallow water of estuary showed a higher concentration of PAHs than river ecosystem. The levels of PAHs in this investigation were comparable with those from other marine environments. The sum of Σ16 PAHs was found to be higher than in sediments previously reported by Hassan et al. (2018), Liu et al. (2012), and Sojinu et al. (2010) from the marine sediments (Qatar), continental shelf sediments (China), and in sediments from oil exploration areas of the Niger Delta (Nigeria). In terms of total PAHs in river sediments, this investigation reported higher levels compared with other rivers from Congo DR (Kilunga et al. 2017; Mwanamoki et al. 2014). The concentrations of ∑13PAHs in all marine, estuary, and river sediments were below the TEC (1610 μg kg−1) and PEC (22,800 μg kg−1), suggesting no adverse effect on sediment-dwelling organisms (MacDonald et al. 2000b). The more common four diagnostic ratios of PAHs reported in earlier studies were used in this investigation to identify the possible sources of PAHs. Concentration ratios of Fluo/(Fluo + Pyr) or IDP/(IDP + BghiP) were the principal investigation methods. If in the first case the ratio is less than 0.4, the source is petrogenic; if it is between 0.4 and 0.5, the source is the burning of oil; and if this ratio is higher than 0.5, the source is the burning of grass, wood, or coal. In the second case, the source will be petrogenic when the ratio is less than 0.2. If this ratio is between 0.2 and 0.5, the source is the combustion of oil and if this ratio is higher than 0.5, the source is the combustion of grass, wood, or coal (De La Torre-Roche et al. 2009; Yunker et al. 2002). For the LMW/HMW ratio, the source will be pyrogenic when it is less than 1, and it will be petrogenic when it is higher than 1 (Budzinski et al. 1997; Zhang et al. 2008). In addition, when the ratio BaA/(BaA + Chry) is less than 0.2, the source is considered petrogenic, if this ratio is between 0.2 and 0.35, the source is the combustion of coal, whereas when this ratio is higher than 0.35, the source is pyrolytic (Akyuz and Cabuk 2010). Table 4 presents the diagnostic ratios Fluo/(Fluo + Pyr), IDP/(IDP + BghiP), BaA/(BaA + Chry), and LMW/HMW. The results indicate that PAHs from marine sediments might be derived from petrogenic, petroleum combustion, and pyrolytic origin as shown in Table 4. A similar trend was observed for estuary sediments, whereas river PAHs were derived from all sources including petrogenic, petroleum combustion, pyrogenic processes, and grass, wood, or coal combustion (Table 4).
Table 2s (Supplementary Data, Table 2s) presents the measured concentrations of the OCPs in marine, estuary, and river sediments. Only hexachlorobenzene, p,p′-DDE, o,p′-DDD, p,p′-DDD, and p,p′-DDT were detected from the 35 OCPs studied. No other compounds were detected in sediments. Hexachlorobenzene was detected in all sediments with the concentrations ranged from 4.91 to 29.36, 3.26 to 21.71, and 9.30 to 29.47 μg kg−1 in marine, estuary, and river sediments, respectively. Among the OCPs, hexachlorobenzene and DDTs were the most predominant compounds and the result could be due to extensive use in agriculture and from urban effluents (Lin et al. 2016). Levels of hexachlorobenzene in the present investigation were higher than those reported in Hugli estuary sediments from India (< 0.05–0.98 μg kg−1) and river sediments (< 0.05–1.44 μg kg−1) (Guzzella et al. 2005; Kilunga et al. 2017).
From the analysis, DDTs and p,p′-DDE were detected in most of the sediments, whereas p,p′-DDD was detected in all sediments except at site S8B. o,p′-DDD was detected only in two sediments (S7A and S6B) of the analyzed samples. Similarly, p,p′-DDT was less frequently detected which occurred only at three sites (S1B, S7A, and S6A). o,p′-DDE and o,p′-DDT were below their detection limits in all sediments analyzed. In all sediments, the predominant isomers were in the decreasing trend of p,p′-DDD > p,p′-DDE > o,p′-DDD > p,p′-DDT. Corroborated with previous reports in sediments, DDE was the predominant compound among the DDTs as the result of degradation of DDT to DDE and DDT into DDD by aerobic conditions and later was through the process of dechlorination under anaerobic conditions (Oliveira et al. 2016; Syed et al. 2014). The highest concentrations of ∑6 DDTs were recorded at the site S6B river sediment (8.99 μg kg−1), while the lowest values detected at the site S8A (0.11 μg kg−1). The higher concentrations observed in river sediments in this study could be attributed to the most common use of DDTs in agriculture and household appliances for the sanitary purpose in Congo DR. The results are comparable with previous studies. The DDT levels in this investigation were higher than those in sediments from rivers in Congo DR (Mwanamoki et al. 2014, 2015; Verhaert et al. 2013) and lower than reported by (Kilunga et al. 2017) in urban rivers in Congo. The contamination of DDTs remained low except at the S6A (7.82) and S6B (8.99) river sediments as compared with TEL value (6.15 μg kg−1) and PEL value (20.03 μg kg−1). The results suggest that these compounds will likely cause no adverse effects on sediment-dwelling organisms (CCME 2002; EPC-98E 1999). The diagnostic ratios of DDT/DDD and DDT/(DDD + DDE) were used to identify the DDT used in regard to its recent or past in the environment, if the ratio in both cases is < 1 indicating the past use and > 1 implying its recent use (Mitra et al. 2019). Note that the site S6B river sediment had the ratio above 1 suggesting current use and the site S5A marine sediment showed the ratio of higher than 1 indicating its current use as well. Other sites in this investigation showed past use of DDTs as their DDT/DDD level were below 1. Previous studies especially performed in our laboratory showed an increasing presence of DDTs in urban rivers (Kilunga et al. 2017). It is noteworthy that the lower levels of DDTs observed in marine environments might be due to the downstream river effects through its course. The data of this investigation are of great significance for monitoring POPs by government and non-governmental organizations to put forward effective management strategies to control these toxic pesticides into environments.
Correlation between toxic metals, OM, TOC, median grain size, PCBs, PAHs, and DDTs
The Spearman’s rank order correlation values are shown in Table 3S (Supplementary Data, Table 3S). Strong positive correlations between Cr and Cu, As and Pb, and Pb and Cu were observed (p < 0.01, n = 15) indicating similar sources and transport pathways of these contaminants (Haller et al. 2009; Poté et al. 2008). On the other hand, no correlation was found between Zn and other heavy metals (Cr, Cu, As, and Pb) suggesting it originates from a different source. Cr had displayed a strong positive correlation with OM (r = 0.1000, p < 0.01) and TC (r = 0.677, p < 0.01) indicating Cr accumulation in sediments was influenced by TC and OM. A positive correlation between As and TC (r = 0.833, p < 0.01) was also found. Besides, OM showed a strong positive correlation with TC (r = 0.112, p < 0.01) implying that grain size could have influenced TOC accumulation. Heavy metals Cr, As, Pb, and Cu exhibited a significant negative correlation with grain size. This result implies that the transportation and accumulation of Cr, As, Pb, and Cu did not depend on the grain size of the sediments. A strong negative correlation was found between grain size and TC (r = 0.715, p < 0.01). PCBs showed significant negative correlations with grain size (r = − 0.207, p < 0.05), TC (r = 0.061, p < 0.01), and with Cr (r = − 0.093, p < 0.01) indicating that the sources of PCBs are not mainly from surface runoff and likely more from atmospheric deposition (Yuan et al. 2015), whereas PAHs showed significant positive correlations with grain size (r = 0.121, p < 0.05) and TC (r = 0.007, p < 0.01). The result suggests that grain size and TC may have influenced the accumulation of PAHs on sediment particles. On the other hand, PAHs have displayed a strong negative correlation with As (r = − 0.257, p < 0.01) and Cu (r = − 0.139, p < 0.01). The result indicates that PAH origin is a different source. On the other hand, PCA analysis based on PCB concentration corresponds to 97.7% of the total variance. This suggests that all the PCBs hold the same fate and behave very similarly in all sampling environments, which is expected due to the similarity in their chemical composition. The concentrations of the PCBs varied considerably in the river environment compared with the ocean and estuary environment, which could be attributed to the active dynamics of the river (Fig. 3a). Similarly, the PCA analysis of PAH corresponds to the total variance of 94.0%. The ocean’s concentrations of PAHs change less frequently compared with the estuary and the river environments. The estuary environment concentrations of PAHs seem to vary significantly between sites (Fig. 3b). Overall, principal component analysis suggests that the origin of PCB congeners in the marine environments was probably from atmospheric deposition with large loadings in the same group. The PCB distribution in the river was mainly associated with both point and non-point sources. Based on the PAH source identification results, PCA results indicated that PAH’s origin most likely associated with various contributions as reflected by different clusters.
Conclusion
This is the first investigation of heavy metals, OCPs, PCBs, PBDEs, and PAHs in surface sediment from Atlantic Coastal Region of the Democratic Republic of the Congo. The results highlighted that the concentrations of toxic metals (Zn, As, Pb, Cr, Cu) are in many cases, higher than SQGs. The PCB values were higher than TEC and PEC in many investigated sediment samples, indicating the potential environmental risks. The levels of PAHs were lower than the recommended values suggesting no environmental risk but showed site-specific increase could pose a possible hazard by accumulation onto sediments over time. DDT inputs were found to be aged inputs for most of the sediment samples with the exception of river sediments which show DDT input. The PBDEs were not detected in investigated sediment samples. The correlation results suggest that heavy metals, Cr, Cu, and Pb, and PCBs are likely from similar sources, whereas PAHs are probably from a different source. The study warrants that further research on heavy metals and POPs should be conducted at regular intervals in this marine environment and biota. Based on the results, the authors ascertained that to implement suitable measures and efforts by policymakers, to reduce these contaminants, to improve the quality of this economic zone, and to minimize future risks.
References
Akyuz M, Cabuk H (2010) Gas-particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci Total Environ 408:5550–5558
Alegria H, Martinez-Colon M, Birgul A, Brooks G, Hanson L, Kurt-Karakus P (2016) Historical sediment record and levels of PCBs in sediments and mangroves of Jobos Bay, Puerto Rico. Sci Total Environ 573:1003–1009
Barhoumi B, LeMenach K, Devier MH, El Megdiche Y, Hammami B, Ben Ameur W, Ben Hassine S, Cachot J, Budzinski H, Driss MR (2014) Distribution and ecological risk of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in surface sediments from the Bizerte lagoon, Tunisia. Environ Sci Pollut Res 21:6290–6302
Bravo AG, Bouchet S, Amouroux D, Pote J, Dominik J (2011) Distribution of mercury and organic matter in particle-size classes in sediments contaminated by a waste water treatment plant: Vidy Bay, Lake Geneva, Switzerland. J Environ Monit 13:974–982
Budzinski H, Jones I, Bellocq J, Pierard C, Garrigues P (1997) Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Mar Chem 58:85–97
Carro N, Garcia I, Ignacio M, Mouteira A (2010) Spatial and temporal trends of PCBs (polychlorinated biphenyls) in mussel from Galician coast (1998-2008). Environ Int 36:873–879
CCME (2002) Canadian sediment Quality Guidelines for the Protection of Aquatic Life, Canadian Environmental Quality Guidelines. In: Canadian Council of Ministers of the Environment
Combi T, Miserocchi S, Langone L, Guerra R (2016) Polychlorinated biphenyls (PCBs) in sediments from the western Adriatic Sea: sources, historical trends and inventories. Sci Total Environ 562:580–587
Davis JA, Hetzel F, Oram JJ, McKee LJ (2007) Polychlorinated biphenyls (PCBs) in San Francisco Bay. Environ Res 105:67–86
De La Torre-Roche RJ, Lee WY, Campos-Diaz SI (2009) Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: analysis of a potential problem in the United States/Mexico border region. J Hazard Mater 163:946–958
Doong RA, Sun YC, Liao PL, Peng CK, Wu SC (2002) Distribution and fate of organochlorine pesticide residues in sediments from the selected rivers in Taiwan. Chemosphere 48:237–246
EPC-98E, CCME (1999) Canadian Sediment Quality for the Protection of Aquatic Life. In: Canada: Canadian Council of Ministers of the Environment
Fair PA, White ND, Wolf B, Arnott SA, Kannan K, Karthikraj R, Vena JE (2018) Persistent organic pollutants in fish from Charleston Harbor and tributaries, South Carolina, United States: a risk assessment. Environ Res 167:598–613
Feng H, Han XF, Zhang WG, Yu LZ (2004) A preliminary study of heavy metal contamination in Yangtze River intertidal zone due to urbanization. Mar Pollut Bull 49:910–915
Fu CT, Wu SC (2006) Seasonal variation of the distribution of PCBs in sediments and biota in a PCB-contaminated estuary. Chemosphere 62:1786–1794
Guzzella L, Roscioli C, Vigano L, Saha M, Sarkar SK, Bhattacharya A (2005) Evaluation of the concentration of HCH, DDT, HCB, PCB and PAH in the sediments along the lower stretch of Hugli estuary, West Bengal, Northeast India. Environ Int 31:523–534
Haller L, Poté J, Loizeau JL, Wildi W (2009) Distribution and survival of faecal indicator bacteria in the sediments of the Bay of Vidy, Lake Geneva, Switzerland. Ecol Indic 9:540–547
Haller L, Tonolla M, Zopfi J, Peduzzi R, Wildi W, Poté J (2011) Composition of bacterial and archaeal communities in freshwater sediments with different contamination levels (Lake Geneva, Switzerland). Water Res 45:1213–1228
Hassan HM, Castillo AB, Yigiterhan O, Elobaid EA, Al-Obaidly A, Al-Ansari E, Obbard JP (2018) Baseline concentrations and distributions of polycyclic aromatic hydrocarbons in surface sediments from the Qatar marine environment. Mar Pollut Bull 126:58–62
Hong XQ, Li RZ, Liu WJ, Zhang XS, Ding HS, Jiang H (2012) An investigation on reuse of Cr-contaminated sediment: Cr removal and interaction between Cr and organic matter. Chem Eng J 189:222–228
IARC (2014) Monographs on the identification of carcinogenic hazards to humans. https://monographs.iarc.fr/wp-content/uploads/2018/08/14-002.pdf. Accessed 4 Mar 2020
Jiang M, Zeng GM, Zhang C, Ma XY, Chen M, Zhang JC, Lu LH, Yu Q, Hu LP, Liu LF (2013) Assessment of heavy metal contamination in the surrounding soils and surface sediments in Xiawangang River, Qingshuitang District. PLoS One 8
Kayembe JM, Sivalingam P, Salgado CD, Maliani J, Ngelinkoto P, Otamonga JP, Mulaji CK, Mubedi JI, Pote J (2018) Assessment of water quality and time accumulation of heavy metals in the sediments of tropical urban rivers: case of Bumbu River and Kokolo Canal, Kinshasa City, Democratic Republic of the Congo. J Afr Earth Sci 147:536–543
Kilunga PI, Sivalingam P, Laffite A, Grandjean D, Mulaji CK, de Alencastro LF, Mpiana PT, Pote J (2017) Accumulation of toxic metals and organic micro-pollutants in sediments from tropical urban rivers, Kinshasa, Democratic Republic of the Congo. Chemosphere 179:37–48
Laffite A, Kilunga PI, Kayembe JM, Devarajan N, Mulaji CK, Giuliani G, Slaveykova VI, Pote J (2016) Hospital effluents are one of several sources of metal, antibiotic resistance genes, and bacterial markers disseminated in sub-Saharan urban rivers. Front Microbiol 7
Lin T, Nizzetto L, Guo Z, Li Y, Li J, Zhang G (2016) DDTs and HCHs in sediment cores from the coastal East China Sea. Sci Total Environ 539:388–394
Liu LY, Wang JZ, Wei GL, Guan YF, Zeng EY (2012) Polycyclic aromatic hydrocarbons (PAHs) in continental shelf sediment of China: implications for anthropogenic influences on coastal marine environment. Environ Pollut 167:155–162
Long ER, Ingersoll CG, Macdonald DD (2006) Calculation and uses of mean sediment quality guideline quotients: a critical review. Environ Sci Technol 40:1726–1736
Lyons BP, Barber JL, Rumney HS, Bolam TP, Bersuder P, Law RJ, Mason C, Smith AJ, Morris S, Devlin MJ, Al-Enezi M, Massoud MS, Al-Zaidan AS, Al-Sarawi HA (2015) Baseline survey of marine sediments collected from the State of Kuwait: PAHs, PCBs, brominated flame retardants and metal contamination. Mar Pollut Bull 100:629–636
Maanan M (2008) Heavy metal concentrations in marine molluscs from the Moroccan coastal region. Environ Pollut 153:176–183
MacDonald DD, Dipinto LM, Field J, Ingersoll CG, Long ER, Swartz RC (2000a) Development and evaluation of consensus-based sediment effect concentrations for polychlorinated biphenyls. Environ Toxicol Chem 19:1403–1413
MacDonald DD, Ingersoll CG, Berger TA (2000b) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Malik RN, Mehboob F, Ali U, Katsoyiannis A, Schuster JK, Moeckel C, Jones KC (2014) Organo-halogenated contaminants (OHCs) in the sediments from the Soan River, Pakistan: OHCs(adsorbed TOC) burial flux, status and risk assessment. Sci Total Environ 481:343–351
Mavakala BK, Le Faucheur S, Mulaji CK, Laffite A, Devarajan N, Biey EM, Giuliani G, Otamonga JP, Kabatusuila P, Mpiana PT, Pote J (2016) Leachates draining from controlled municipal solid waste landfill: detailed geochemical characterization and toxicity tests. Waste Manag 55:238–248
Mayon N, Bertrand A, Leroy D, Malbrouck C, Mandiki SN, Silvestre F, Goffart A, Thome JP, Kestemont P (2006) Multiscale approach of fish responses to different types of environmental contaminations: a case study. Sci Total Environ 367:715–731
Mbomba NB (2007) Rapport Scientifique et technique sur le monitoring du littoral marin de Muanda en R.D.CONGO en rapport avec la pollution et la biologie marine de la cote congolaise. In, edited by Universite De Kinshasa, 57. Kinshasa, DRC: UNIKIN
Miglioranza KSB, Gonzalez M, Ondarza PM, Shimabukuro VM, Isla FI, Fillmann G, Aizpun JE, Moreno VJ (2013) Assessment of Argentinean Patagonia pollution: PBDEs, OCPs and PCBs in different matrices from the Rio Negro basin. Sci Total Environ 452:275–285
Mitra S, Corsolini S, Pozo K, Audy O, Sarkar SK, Biswas JK (2019) Characterization, source identification and risk associated with polyaromatic and chlorinated organic contaminants (PAHs, PCBs, PCBzs and OCPs) in the surface sediments of Hooghly estuary, India. Chemosphere 221:154–165
Montuori P, Aurino S, Garzonio F, Triassi M (2016) Polychlorinated biphenyls and organochlorine pesticides in Tiber River and estuary: occurrence, distribution and ecological risk. Sci Total Environ 571:1001–1016
Mwanamoki PM, Devarajan N, Thevenon F, Birane N, de Alencastro LF, Grandjean D, Mpiana PT, Prabakar K, Mubedi JI, Kabele CG, Wildi W, Pote J (2014) Trace metals and persistent organic pollutants in sediments from river-reservoir systems in Democratic Republic of Congo (DRC): spatial distribution and potential ecotoxicological effects. Chemosphere 111:485–492
Mwanamoki PM, Devarajan N, Niane B, Ngelinkoto P, Thevenon F, Nlandu JW, Mpiana PT, Prabakar K, Mubedi JI, Kabele CG, Wildi W, Poté J (2015) Trace metal distributions in the sediments from river-reservoir systems: case of the Congo River and Lake Ma Vallée, Kinshasa (Democratic Republic of Congo). Environ Sci Pollut Res 22:586–597
Oliveira AHB, Cavalcante RM, Duavi WC, Fernandes GM, Nascimento RF, Queiroz M, Mendonça KV (2016) The legacy of organochlorine pesticide usage in a tropical semi-arid region (Jaguaribe Ri-ver, Ceará, Brazil): implications of the influence of sediment parameters on occurrence, distribution and fate. Sci Total Environ 542:254–263
Pintado-Herrera MG, Combi T, Corada-Fernandez C, Gonzalez-Mazo E, Lara-Martin PA (2017) Occurrence and spatial distribution of legacy and emerging organic pollutants in marine sediments from the Atlantic coast (Andalusia, SW Spain). Sci Total Environ 605:980–994
Poté J, Haller L, Loizeau JL, Bravo AG, Sastre V, Wildi W (2008) Effects of a sewage treatment plant outlet pipe extension on the distribution of contaminants in the sediments of the Bay of Vidy, Lake Geneva, Switzerland. Bioresour Technol 99:7122–7131
Rabodonirina S, Net S, Ouddane B, Merhaby D, Dumoulin D, Popescu T, Ravelonandro P (2015) Distribution of persistent organic pollutants (PAHs, Me-PAHs, PCBs) in dissolved, particulate and sedimentary phases in freshwater systems. Environ Pollut 206:38–48
R Core Team (2015) R: A language and environment for statistical computing. Vienne, Austria, R Foundation for Statistical Computing
Ribeiro C, Ribeiro AR, Tiritan ME (2016) Occurrence of persistent organic pollutants in sediments and biota from Portugal versus European incidence: a critical overview. J Environ Sci Health B 51:143–153
Sakan SM, Dordevic DS, Manojlovic DD, Predrag PS (2009) Assessment of heavy metal pollutants accumulation in the Tisza river sediments. J Environ Manag 90:3382–3390
Santos E, Souza MRR, Vilela Junior AR, Soares LS, Frena M, Alexandre MR (2018) Polycyclic aromatic hydrocarbons (PAH) in superficial water from a tropical estuarine system: distribution, seasonal variations, sources and ecological risk assessment. Mar Pollut Bull 127:352–358
Sojinu OSS, Wang JZ, Sonibare OO, Zeng EY (2010) Polycyclic aromatic hydrocarbons in sediments and soils from oil exploration areas of the Niger Delta, Nigeria. J Hazard Mater 174:641–647
Suami RB, Sivalingam P, Kabala CD, Otamonga JP, Mulaji CK, Mpiana PT, Pote J (2018) Concentration of heavy metals in edible fishes from Atlantic Coast of Muanda, Democratic Republic of the Congo. J Food Compos Anal 73:1–9
Syed JH, Malik RN, Li J, Chaemfa C, Zhang G, Jones KC (2014). Status, distribution and ecological risk of organochlorines (OCs) in the surface sediments from the Ravi River, Pakistan. Sci Total Environ 472:204–211
Thevenon F, de Alencastro LF, Loizeau JL, Adatte T, Grandjean D, Wildi W, Pote J (2013) A high-resolution historical sediment record of nutrients, trace elements and organochlorines (DDT and PCB) deposition in. A drinking water reservoir (Lake Bret, Switzerland) points at local and regional pollutant sources. Chemosphere 90:2444–2452
UNDP (United Nations Development) (2009) Plan National de Mise en Oeuvre de la Convention de Stockholm sur les Polluants Organiques Persistants (POP)/RDC. In: 279. Ministère de l’environnement Conservation de la Nature et Tourisme
UNEP (2012) Persistent organic pollutants review committee. UNEP/POPS/POPRC.8/16/Add.2. Geneva, 15–19
Unyimadu JP, Osibanjo O, Babayemi JO (2018) Polychlorinated biphenyls (PCBs) in River Niger, Nigeria: occurrence, distribution and composition profiles. Toxicol Ind Health 34:54–67
Varol M (2011) Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater 195:355–364
Verhaert V, Covaci A, Bouillon S, Abrantes K, Musibono D, Bervoets L, Verheyen E, Blust R (2013) Baseline levels and trophic transfer of persistent organic pollutants in sediments and biota from the Congo River Basin (DR Congo). Environ Int 59:290–302
Yang HY, Xue B, Jin LX, Zhou SS, Liu WP (2011) Polychlorinated biphenyls in surface sediments of Yueqing Bay, Xiangshan Bay, and Sanmen Bay in East China Sea. Chemosphere 83:137–143
Yuan XT, Yang XL, Na GS, Zhang AG, Mao YZ, Liu GZ, Wang LL, Li XD (2015) Polychlorinated biphenyls and organochlorine pesticides in surface sediments from the sand flats of Shuangtaizi Estuary, China: levels, distribution, and possible sources. Environ Sci Pollut Res 22:14337–14348
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China—weathering features, anthropogenic impact and chemical fluxes. Estuar Coast Shelf Sci 54:1051–1070
Zhang W, Zhang SC, Wan C, Yue DP, Ye YB, Wang XJ (2008) Source diagnostics of polycyclic aromatic hydrocarbons in urban road runoff, dust, rain and canopy throughfall. Environ Pollut 153:594–601
Zhang K, Wei YL, Zeng EY (2013) A review of environmental and human exposure to persistent organic pollutants in the Pearl River Delta, South China. Sci Total Environ 463:1093–1110
Zhao ZH, Zhang L, Wu JL, Fan CX (2009) Distribution and bioaccumulation of organochlorine pesticides in surface sediments and benthic organisms from Taihu Lake, China. Chemosphere 77:1191–1198
Acknowledgments
This research presents the collaboration between University of Geneva (Department F. A. Forel), University of Kinshasa and Pedagogic National University of Congo (The Democratic Republic of the Congo).
Funding
We are grateful to financial support from the Swiss National Science Foundation (grant no. 31003A_173281/1) and Academy of Research and Higher Education of Wallonia-Brussels (ARES).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Christian Gagnon
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Suami, R.B., Sivalingam, P., Al Salah, D.M. et al. Heavy metals and persistent organic pollutants contamination in river, estuary, and marine sediments from Atlantic Coast of Democratic Republic of the Congo. Environ Sci Pollut Res 27, 20000–20013 (2020). https://doi.org/10.1007/s11356-020-08179-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08179-4