Abstract
Biosorption efficacy of Bacillus strain DPAML065, isolated from the tannery sludge, was appraised for the removal of toxic hexavalent chromium (VI) ions from synthetic wastewater. Effects of the process variable on biosorbent surface by variation in pH, metal Cr(VI) concentration and retention time were examined using batch experiments. The isolated Bacillus strain biosorbent was studied for its morphology and surface chemistry through FE-SEM, EDX and FTIR. It discloses that, the reduction mechanism of Cr(VI) during the process is mainly attributed to precipitation in addition to the functional groups (such as –COOH, –OH, C–O, P=O) present on the cellular matrix of Bacillus. Biochemical tests and 16s rRNA sequencing were also performed to identify the biosorbent at the genus level. A 95% Cr(VI) removal efficiency was procured by Bacillus strain DPAML065 biosorbent at pH 6, incubation period 24 h, 80 mg/L initial feed concentration and operational temperature 35 °C. Equilibrium behaviour of chromium binding follows the Langmuir isotherm model (R2 = 0.968) with an adsorption capacity of 106.38 mg/g. Kinetic modelling disseminates that biosorption of Cr(VI) ions by Bacillus strain DPAML065 obeyed pseudo-second-order model (R2 = 0.984) rather than the pseudo-first-order model. Concisely, the results indicate that the Bacillus strain DPAML065 is a potential, economically feasible and eco-friendly biosorbent which can be effectively used for removal of chromium (VI) from wastewater.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the various anthropogenic activities, untreated discharge of heavy metals effluent from the industries is one of the substantial problems (Gupta et al. 2000; Ghiasi et al. 2020; Prabhakar and Samadder 2020). The major problem is its magnitude and has been perused for its effect on the environment including genetic and developmental defects in humans as well as on aquatic life (Huang et al. 2013; Singh and Kumar 2020; Sharma and Malaviya 2016; Sanjay et al. 2018; Uniyal et al. 2013). The most common heavy metals like chromium, cadmium, lead, arsenic and mercury have a nearly meagre biological role and are detrimental to human health even at a minimal concentration (Khan et al. 2013; Singh and Kumar 2020; Lata et al. 2019). The effluent discharge having chromium has diverse use in industries like steel, cleaning agents and electroplating and also in the production of chromic acid which is extensively employed in leather industries in India (Bharagava and Mishra 2018; Sanjay et al. 2018; Singh et al. 2018; Torab-Mostaedi et al. 2013). Among these industries, leather and its allied industries consume nearly 35 L of water per 100 kg of skin hides during the process. Thus, the discharged effluent consists of chromium, which is the most pernicious contaminant emancipated in an enormous quantity into the environment. Furthermore, it is documented as one of the most abhorrent anthropogenic contaminants released from the industries (Nandy et al. 1999; Ryan et al. 2002; Kumari et al. 2016). Chromium exists in multiple oxidation states, with trivalent Cr(III) and hexavalent Cr(VI) species being the most prevalent forms. Out of it, Cr(VI) is more prevalent and mutagenic compared to Cr(III), the latter being more stable. Chromium is the most lethal constituent classified by the International Agency for Research on Cancer (IARC 1990) as a group-1 human carcinogen due to its high solubility and fast permeability in biological cell membrane as it interacts with intracellular proteins and nucleic acids leading to structural modifications (Beukes et al. 2017; Cardoso et al. 2017; Vinod et al. 2010). The convergence of chromium in leather effluent is about 2 to 5 g/L, which significantly is sky high when compared to the suggested allowed limit of 2 mg/L. If the concentration of Cr(VI) surpass > 0.05 ppm, it predominantly affects the human health, causing damage to internal organs like the kidney, lungs, liver and pancreas and even leading to bioaccumulation in organisms (Vinod et al. 2010; Cheung and Gu 2007; Belay 2010).
Conventional methods such as filtration, precipitation, coagulation, reverse osmosis, solvent extraction, ion exchange electrochemical treatments, adsorption, evaporation recovery, kaolinite and activated carbon were considered and were reported successful for the removal of hexavalent chromium (Balasubramanian et al. 2019; Arukula et al. 2018; Deepa et al. 2019; Zambrano and Min 2019; Oves et al. 2013; Rathinam et al. 2010; Xiao et al. 2010; Saxena et al. 2016; Ahluwalia and Goyal 2007). As these technologies involve high capital and generate metal-rich sludge which results in environmental pollution, it shifts the focus towards some economic and environment-friendly approaches (Tsekova et al. 2010; Green-Ruiz et al. 2008). Chromium-tolerant bacteria can be considered as the most practical approach and is even eco-friendly to reduce hexavalent chromium to trivalent chromium. Cr(VI) detoxification and bioremediation due to the presence of high surface charge and contact area of the bacteria could help effectively during an interchange with the metals that alter physiologically and stay dynamic in the environment (Cervantes and Campos-García 2007; Dash et al. 2014). Bacteria can bind to a high concentration of heavy metals by some of the most common biosorption mechanisms such as physical adsorption, ion exchange, coordination, chelation, precipitation and complexation. Various familiar functional groups were incorporated in biosorption, which includes hydroxyl, carboxyl and amine groups resulting in the binding of metal ions in bacteria (Deepa and Mishra 2020; Volesky 2003; Bharagava and Mishra 2018).
The main objective of the present study was to isolate and identify the Cr(VI)-resistant bacteria from the tannery sludge and to understand the performance of bacteria on the fate and mobility of chromium. The biosorptive capacity of Cr(VI) in response to initial concentration, pH and contact time was evaluated. Morphological variations prior to biosorption treatment and after biosorption of Cr(VI) on Bacillus strain DPAML065 were investigated by XRD analysis. Different functional groups involved in Cr(VI) biosorption were spotted using FTIR analysis. FE-SEM and EDX were employed to clarify the mechanism of Bacillus species in the removal of chromium through biosorption. Factors affecting biosorption isotherms and kinetic studies were also deliberated. Thus, this biosorption study offers a simple and economical solution in the reduction of hexavalent chromium.
Materials and methods
Isolation of the bacteria and culture conditions
The microorganisms were screened out in the tannery sludge from the leather industry located in Kanpur, India. The isolated bacteria were characterized through microscopic, biochemical characteristic study and 16s rRNA sequencing analysis, as shown in Table 1. The stock solution of the bacterial culture contains sodium chloride 5.0 g/L, meat extract 1.5 g/L, yeast extract 1.5 g/L and peptone 5.0 g/L. The bacterial cells used in the experimental study were harvested in nutrient broth medium for 24 h at 35 °C and then centrifuged at 10000 rpm for 12 min. Further, the pellets were washed twice with 0.1 M phosphate buffer solution.
Primary screening of chromium-resistant bacterial strain and growth conditions
The metal-resistant microbe was isolated from the tannery sludge samples by serial dilution method, in which 1 mL of the sample was serially diluted in a phosphate buffer solution. Desired diluted samples (10−3 to 10−10) of 0.1 mL were plated on the duplicates of the appropriate media amended with varying concentrations of Cr(VI) using spread plate method and were incubated at 37 °C for 24–48 h. After incubation, the isolated strain was repeatedly sub cultured on the appropriate medium to obtain a pure culture with maximum Cr(VI)-tolerant bacterial colonies and then subjected to biochemical characterization, identification by 16s rRNA gene sequencing analysis and other studies.
Chemicals and reagents
Nutrient Agar and Nutrient Broth were provided by Hi-Media Laboratories Pvt. Ltd., India. Nitric acid (HNO3), hydrochloric acid (HCl), sodium hydroxide (NaOH), potassium dichromate (K2Cr2O7) and 1, 5-diphenyl carbazide (DCP) were purchased from Loba Chemie and Merck, India. All the reagents used in this experiment were of analytical grade with high purity levels (about 99%), without further purification.
Biosorption experiments
The biosorption experimental studies were carried out at room temperature, and the experimental protocol was accomplished. Preparation of chromium stock solution of 1000 ppm was done by adding 2.835 g of potassium dichromate (K2Cr2O7) salt to 1000 mL de-ionized water. The working solution (20, 40, 60, 80, 100 and 120 mg/L) was prepared by diluting the stock solution with Millipore distilled water (Merck, Germany). All the experiments were conducted in triplicates. Two hundred fifty milligrams (wet weight) of bacterial cells was added to the varying concentrations of the chromium mentioned above, and batch study was done. Then, the flasks were incubated in a rotary shaker with 120 rpm at 35 °C for 24 h (REMI CIS-24 PLUS, India). Control was maintained with Cr(VI) solution without bacterial culture. After, 24 h of growth, bacterial culture from each test concentration was centrifuged at 10000 rpm for 12 min (REMI R 24, India). The optical density (OD600) was monitored using UV Spectrophotometer (Lab-Tech, China). Further evaluation of Cr(VI) concentration reduction was done by the DPC method at 540 nm as per the method described by (APHA 2012) and was calculated through the calibrated curve developed from K2Cr2O7. Cr(VI) was measured by taking 5 mL of the sample in a beaker with an adjusted pH of 1.0 which was done by using 0.1 N HNO3; the sample was made up to 100 mL using Millipore water. Further 2 mL of DPC (1,5-diphenylcarbazide (C13H14ON4)) solution was added in the sample (prepared by dissolving 500 mg in 100 mL of acetone) and left to stand for 5 min to develop a pink colour. The absorbance was noted down in a UV spectrophotometer. Removal percentage of Cr(VI) biosorption (R %) and adsorption capacity (Qmg/g) was calculated from the following Eqs. (1) and (2)
where C0 = initial metal concentration (mg/L), C = final concentration of the metal (mg/L), V = volume of the solution (litres) and W is the weight of the bacterial cells (grams).
Determination of minimum inhibitory concentration of heavy metals (MIC)
The Cr(VI) minimum inhibitory concentration of Bacillus species strain DPAML065 was determined. A stock solution of heavy metal was prepared at increasing concentrations (20, 40, 60, 80, 100 and 120 mg/L); the solutions were incubated with separate batches of the cultured bacterial strain. The result was then measured by agar dilution method for the complete inhibition zone. Thus, MIC was noted when no growth was observed in the petri dishes even after 48 h of incubation, and the results are given in Table S1.
FE-SEM and EDX analysis
Figure 4 depicts the morphological and surface characterization of the metal precipitates of chromium-loaded and chromium-unloaded Bacillus species. Quantification of chromium in the Bacillus species was obtained from energy-dispersive X-ray analysis and field emission scanning electron microscopy (FE-SEM) (Hitachi S-4300). For FE-SEM and EDX analysis, the chromium-loaded and chromium-unloaded samples were prepared by using 2.5% (W/V) of glutaraldehyde in sodium phosphate buffer having pH 7.0 with 0.1 molarity. Further, the pellets were washed thrice with the phosphate buffer and then were fixed for 1 h at 4 °C in the buffer solution before dehydration. Different concentration of ethanol (W/V) was used in turn to dehydrate the samples; further, the samples were fixed on the cover slip, and these cover slips were supported on the copper grids for further morphological studies (Nwinyi and Owolabi 2019).
FTIR and XRD analysis
Fourier transform infrared spectroscopy (FTIR) analysis for Bacillus strain which was grown in the nutrient broth medium was recovered through centrifugation (10,000 rpm for 10 min); further, the cells were washed with Millipore water and lyophilized. Chromium-loaded and chromium-unloaded bacterial cells were finally grounded with solid KBr, and then, the infrared spectra were recorded at 500–4000 cm−1 using FTIR spectrometer (Perkin Elmer) which is represented in Table 3. X-ray diffraction studies (XRD) have been carried out with anhydrous chromium-treated and non-treated chromium pellets and were examined with Bruker (Model: Kappa APEX II).
Results and discussion
Identification of the isolated bacterial species
In the present study, morphological and biochemical characteristics were carried out for the isolated bacterium from the tannery sludge and are listed in Table 1. A 16s rRNA gene analysis of the stain DPAML065 was carried out by DNA sequencing facility, Yazz Xenomics Pvt. Ltd., Chennai, Tamil Nadu using universal primers, 8F (5′AGAGTTTGATCCTGGCTCAG3′) and drive back primer; 1541R (5′AAGGAGGTGATCCAGCCGCA3′). Further, nucleotide sequence data were deposited in the Gen-Bank sequence database. The online program BLAST was used to find out the related sequences with known taxonomic information in the databank at the NCBI website (http://www.ncbi.nlm.nih.gov/BLAST) to accurately identify the strain, as shown in Fig. 1; the phylogenetic bonding of the bacterial isolate was identified. The results revealed that the isolated Bacillus species DPAML065 bacteria was homogenous with 99% of 16s rRNA gene sequencing. Further, the strain was identified as Bacillus species, and the gene sequence was deposited in the Gene bank under accession number MT250939.1.
Effect of pH on Cr(VI) reduction
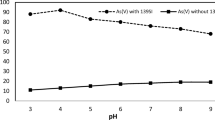
A pivotal role in the biosorption of chromium ions is manifested by pH (Lata et al. 2015). The pH of the medium primarily influences the biosorption ability as it affects the valency of metals in solution and functional groups present on the biomass. Moreover, pH also affects the network of negative charges on the surface of the biosorbing cells (especially in case of Gram-positive bacteria) and the chemistry of the walls, as well as physicochemistry and hydrolysis of the metal (Kalola and Desai 2019). The pH optimization of chromium by Bacillus species in the range of (pH 1–9) at an initial concentration of Cr(VI) 20 mg/L with a contact time of 24 h at 35 °C was studied. As shown in Fig. 2, the biosorption of Cr(VI) increased gradually with an increase in pH value from 1 to 5 and maximum Cr(VI) biosorption of 97% was observed at pH 6. Whereas in the case of pH values < 6, chromium biosorption was less due to the decreased growth of cell in the medium and the functional anionic active sites protonate at the surface of biomass and attract a large number of anionic groups. Depending on the pH, chromium exists in the form of chromate (CrO42−), dichromate (Cr2O72−) and bichromate (HCrO4−) (Acharya et al. 2020; Babel and Kurniawan 2004). At low acidic pH < 6, chromium is in the form of Cr2O72−.. The stable form of Cr(VI) existing at pH 6 is HCrO4−which possess negative charge; hence, an electrostatic attraction between chromium species and the positively charged functional groups present on the bacterial cell surface of the biomass occurs (Anah and Astrini 2017). In addition to it, at pH 6, favoured cell growth in the medium takes place in which the propinquity of positively charged ions of the utility group available on the cell of the biomass results in the electrostatic attraction, precipitation, ion exchange and complex formation between the anionic Cr(VI) and biomass (Nguema et al. 2014; Jobby et al. 2019; Hu et al. 2012). Researchers also reported that B. coagulans and Halomonas species have also reduced Cr(VI) at an optimum pH 6–7 with an initial concentration of chromium at 60 mg/L with 96.7% of Cr(VI) reduction (Murugavelh and Mohanty 2013; QuiIntana et al. 2001). At pH > 6, chromium exists in the form of CrO42−; hence, the reduction capacity of Cr(VI) was drastically decreased which might be due to the development of negative charge on the cell surface developed as a result of deprotonation of carboxylate groups. Even metals start precipitating at high pH, which reduces the solubility and affinity of metal ions leading to reduced biosorption (Vijayaraghavan and Balasubramanian 2015).
Biosorption behaviour of Cr(VI) on Bacillus sp. strain DPAML065
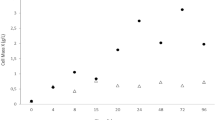
The initial metal concentration effect of Cr(VI) on Bacillus strain DPAML065 was examined at an optimum pH of 6.0. Figure 3 shows the optimum (pH 6) with varied concentration of Cr(VI) in the range from (20–120 mg/L). Results revealed that in low concentration, the reduction was nearly high. As the concentration increased, the biosorption efficiency significantly decreased to 120 mg/L, which might be due to the increased toxic effect of cell growth in the medium. Additionally, elevated mass transfer with aggravating kinetic energy due to the collision between the metal ions and biosorbents could also be responsible for it. (Uniyal et al. 2013). Besides this, it can also be observed that the concentration of Cr(VI) was drastically reduced within 24 h by Bacillus species. Similar studies have been documented (QuiIntana et al. 2001) in Thiobacillus ferroxidans in which the concentration of chromium was considerably decreased from 60 to 1.89 mg/L within 48 h. Thus, the results conclude that the biosorption of Cr(VI) by isolated culture Bacillus strain DPAML065 is a time-dependent activity which removed the maximum concentration of chromium ions, i.e., 80 mg/L within 24 h, and depicted a biosorption efficiency of 95.4%. Besides this, the potential strain was compared with the other Bacillus species of live biomass in the removal of Cr(VI) and is represented in Table 2.
The minimum inhibitory concentration of heavy metals (MIC)
The Cr(VI) MIC for Bacillus strain DPAML065was provoked by agar dilution method in solid media which ranged from 20 to 160 ppm of hexavalent chromium and showed a maximal degree of resistance to chromium in 80 ppm when compared with other increased concentrations as shown in the Table S1.
FE-SEM and EDX analysis
Field emission scanning electron microscopy (FE-SEM) and energy-dispersive X-ray analysis (EDX) were used to determine the quantification of chromium ions on the biosorption surface. In Fig. 4d, the spectral image clearly exhibit that chromium was adsorbed on the surface of the Bacillus strain DPAML065. Similar quantification and accumulation studies of chromium was also documented with the help of FE-SEM and EDX by other researchers in fungal and bacterial strains (Aspergillus niger and Serratia sp.; Halomonas sp. (Srivastava and Thakur 2006, 2012; Kalola and Desai 2019).
The FE-SEM and EDX analysis of Bacillus sp. strain DPAML065. A(i) Microscopic image of rod-shaped bacteria, A(ii) morphological features and surface characteristics of biosorbent before Cr(VI) adsorption and (B) after Cr(VI) adsorption. C, D EDX analysis of Cr(VI)-unloaded and Cr(VI)-loaded Bacillus species during biosorption
High biosorption of chromium was observed in the isolated potential microbe, as shown in Fig. 4d and was in good agreement with the reports of Guo et al. 2012. However, chromium biosorption of Bacillus species from various sources was below the optimal conditions (Sultan et al. 2012; Al-Daghistani 2012). In the present study, very high biosorption of chromium was remarked when compared with different experimental conditions performed by other researchers (Sultan et al. 2012; Al-Daghistani 2012). This phenomenon can be assigned to facts that the functional groups present on the surface of the bacterial cell wall hold polysaccharides as building blocks of proteins and lipids which perform ion exchange properties as well as precipitation mechanism. Thus, as a result, these functional groups are proficient in the binding of chromium ions (Hu et al. 2012; García-Mendieta et al. 2012).
FTIR analysis
The infrared spectra were performed for control (without chromium) and chromium-loaded sample in the range of 500–4000 cm−1 at room temperature. The analysis was done to investigate predominant groups present on the cell wall of Bacillus strain DPAML065, which is responsible for chromium biosorption shown in Table 3. Figure 5 represents the FTIR spectra of before and after biosorption of chromium peaks which showed different bonds due to the interactive action of bacteria on the mineral surface with copious functional groups. In the control sample, a broad peak was observed at 3432.95 cm−1 that designates the presence of amines (N–H) bonded group with a stretching and medium intensity. But in case of a chromium-loaded sample, a sharp peak was observed at 3426.75 cm−1 which shifted the functional group, indicating a secondary amide protein representing the participation of membrane proteins in the binding of chromium. The peak at 2960.18 cm−1 in control (Bacillus microorganism) represents the presence of aldehyde or other =C–H with strong stretching vibration. After treatment, the band appeared at 2942.07 cm−1 in a reduced form which revealed that symmetric or asymmetric C–H stretching vibrations of aliphatic acids involved in the reduction of chromium ions. The band at 2925.77 cm−1 in control biomass (C–H) bond with stretching vibration along with a strong intensity has been shifted after biosorption of chromium to 2914.07 cm−1 depicting that C–H covalent bond participated in the reduction of chromium ions (Ramrakhiani et al. 2011). The existence of spectral peaks has been observed in chromium-loaded biomass at 2850.76 cm−1 and 2355.28 cm−1 on the surface of Bacillus strain DPAML065 that might be due to the attraction of positive electrons donated by chromium species which are favourable for the reduction of chromium ions during the biosorption process (Han et al. 2007). The bands appeared in a raw sample at 1722.17 cm−1, 1652.67 cm−1 and 1640.97 cm−1 depicting the presence of (C=O) carbonyl acid groups with strong and stretching vibrations. While in chromium-loaded biomass, the peaks were observed at 1629.96 cm−1 which are attributed to –NH bending of primary and secondary amides from the peptide bond coupled with –COO anion. Several other peaks in the treated sample at 1474.43 cm−1 and 1439.34 cm−1 wavelengths designate the presence of aromatic functional groups which mainly participated in the reduction of Cr(VI) ions (Sun et al. 2014). The FTIR spectral band observed at 1329.92 cm−1 in control has been shifted to 1399.42 cm−1 indicating that aromatic amine (CN stretching) exhausted the chromium ions during the biosorption process. The peak present at 1191.60 cm−1 and 1059.47 cm−1 in control biomass has been shifted to 1082.18 in the chromium-loaded biomass which suggests that reduction of chromium ions with the involvement of polysaccharides P=O and (C–O) groups of alcohol with strong and stretching vibration (Sun et al. 2014). A small and sharp peak appeared at 978.96 cm−1 and 794.53 cm−1 that corresponds to bending aromatic compounds which affect revelation and suppression of functional groups on the microbial cell wall (Arivalagan et al. 2014; Rajesh et al. 2014). Therefore, these results suggest that chromium binding on to the bacterial biomass is because of the involvement of carboxyl, hydroxyl and amine groups, which is accountable for the electrostatic attraction and complexation mechanisms while reducing chromium ions (Ramrakhiani et al. 2011; Chakravarty and Banerjee 2012; Javanbakht et al. 2014).
XRD analysis
Figure 6 shows the powder X-ray diffraction patterns of (A) control biomass and (B) chromium-loaded biomass of Bacillus strain DPAML065 grown in nutrient broth medium, supplemented with chromium, and the results were analysed using Jade 5 software. In Fig. 6, it can be noticed that the XRD pattern of control biomass (A) revealed the significant peak around (2ө) = 27.53, 45.47 and 56.73 which attributes to the presence of fatty acids and some polysaccharides in the cell wall of Bacillus strain DPAML065 (Das et al. 2014; McLean et al. 2000). Additionally, the spectrum showed several other peaks of (2ө) at 31.69 and 45.45 with a d- spacing values of 2.82 and 1.99 and was referred with ICCD-00-005-0628 as well as through the peak information from JCPDS file. It revealed the presence of sodium crystals that might be due to the existence of NaCl in the nutrient broth medium. In Fig. 6b, the Cr(VI)-loaded biomass showed some peaks which appeared in the XRD pattern of the bacterial cell, which corresponds with the precipitation of reduced Cr(III) species. Various low-intensity peaks at 27.53, 56.65 and 66.45 are responsible for the existence of Cr (III) in the bacterial cell after the chromium biosorption process (Dhal et al. 2010; Karthik et al. 2017).
Biosorption isotherm
Biosorption isotherm curve can be used for better understanding the equilibrium distribution of metal ions between the aqueous and solid phase, as well as the capacity of biosorption at a constant temperature. The most frequently used models are Langmuir and Freundlich, which illustrate the biosorption process (Sonal et al. 2020; Joo et al. 2010; Volesky 2003; Sala et al. 2002; Volesky and Holan 1995). Langmuir isotherm is the supreme model that explained the mechanism in which the maximum adsorption capacity corresponding to homogeneous monolayer biosorption of metal ions on the surface of the biomass, by electrostatic attraction. The mathematical formula of non-linear Langmuir equation can be expressed as Eq. (3):
where qmax (mg/g) is the maximum adsorption capacity of the chromium ions per unit weight of biomass to form a complete monolayer, Ce (mg/L) represents equilibrium concentration of metal, qeq (mg/g) represents the amount of metal uptake occurs at equilibrium and b (L/mg) is the Langmuir constant, which also reflects the affinity of the chromium for binding sites on the biosorbent.
The Freundlich isotherm is the earliest known to describe non-ideal and reversible adsorption, but later its empirical model used to explain the multilayer adsorption with non-uniform distribution of adsorption heat and affinities over heterogeneous. The mathematical formula of non-linear Freundlich equation was represented in Eq. (4)
where Kf (mg/L)n represents the Freundlich constant and n is the dimensionless Freundlich intensity parameter that defines the magnitude of adsorption driving force. Fig. S1 delineates the biosorption of chromium by Langmuir and Freundlich isotherm.
The parameters of the Langmuir and Freundlich were estimated by non-linear analysis is presented in Table 4. Based on the obtained results of the correlation coefficient (R2), it has been concluded that the Langmuir isotherm model gave an acceptable fit to the experimental data than the Freundlich. Hence, the monolayer biosorption mechanism occurs during the biosorption of chromium on the surface of Bacillus DPAML065.
Biosorption rate kinetics
The kinetic study is important to understand the mechanism and rate of the adsorption process. The kinetics designates solute uptake, as well as its dominant contact period of the biosorbent in the solid medium of the interface. The adsorption of metal ions on the surface of biosorbent was evaluated by pseudo-first-order, pseudo-second-order and intraparticle diffusion model. Pseudo-first and pseudo-second-order kinetics models are the two kinetic models which were used for experimental biosorption of chromium on the surface of Bacillus strain DPAML065.
The pseudo-first-order model is expressed as:
where qe and qt represent the quantity of Cr(VI) absorbed in the active biomass (mg/g) at equilibrium and at time t (min) and k1 is the first-order rate constant (min−1).
Similarly, the pseudo-second-order model can be expressed as:
The amount of metal adsorbed on the active sites of adsorbent at equilibrium is denoted by qeq(mg/g), or at any time t, the adsorption capacity of adsorbent is denoted by qt and t is the contact time (minute), k2 (g mg−1 min−1) is the rate constant of pseudo-second-order kinetics (Senthilkumar et al. 2006; Lutke et al. 2019). A straight line was obtained by plotting t/qt as the function of t (min) as shown in Fig. S2. The intraparticle diffusion model (Weber and Morris 1962a, b) is expressed as:
where ki (mg g−1 min−1/2) represents the intraparticle diffusion model’s initial rate constant and C (mg/g) is the intercept representing a constant related with the thickness of the boundary layer. The values of qe, k2 and correlation coefficient (R2) were calculated using the slope and a straight-line intercept perceived in the graph and are listed in Table 5.
The outcome values are in good agreement and best fits with pseudo-second-order model as coefficient values, R2 = 0.984, which has a higher correlation than the pseudo-first-order model (R2 = 0.921) is given in Fig. S2(A) and (B), indicating the chemisorption behaviour of Cr(VI) on the surface of Bacillus strain DPAML065 (Sinha et al. 2018). Similarly, numerous reports have been published on the chemisorption behaviour of heavy metals like chromium, cadmium, zinc, arsenic and copper along with lead by the Klebsiella, Arthrobacter and Rhodococcusopacus species, respectively (Bueno et al. 2008; Muñoz et al. 2015; Prasad et al. 2013). Microbial biomass generously authoritative on the metal ions for their dynamic site, which could be better understood by chemisorption nature of the adsorbent surface (Munoz et al. 2015). Also, in Fig. S2(C), it has been found that in the intraparticle diffusion model (R2 = 0.8124), the straight lines did not pass through the origin, implying that the process of adsorption is controlled by multistep mechanism (Sonal et al. 2020). Hence, it can be concluded that the pseudo-second-order adsorption model would be the most appropriate to describe the adsorption kinetic model.
Generally, there are two distinct mechanisms which explain the Cr(VI) biosorption on the bacterial biomass: (i) underlying binding of Cr(VI) ions (i.e., adsorption of Cr(VI)) at the protonated active sites on the surface of the biosorbent through electrostatic interactions and (ii) through a complex Cr(VI) reduction step, by contact with the electron-donor groups of the biomass, resulting in binding of reduced chromium form to the biomass (Albadarin et al. 2011; Park et al. 2006). In our present study, the integrated analysis of models and the experimental and instrumentational data, it has been observed that biosorption of Cr(VI) on the biomass adsorbent has been accomplished through more than one step mechanism, including active electrostatic attractions of Cr(VI) with the protonated sites (such as amino, carboxyl) along with the reduction of Cr(VI) by reductive groups (such as hydroxyl, carbonyl ) on the biomass surface. Ramrakhiani et al. 2011 studied the biosorption mechanism of Cr(VI) by heat-inactivated fungal biomass of Termitomycesclypeatus and reported that biosorption of Cr(VI) on the biomass involved more than one mechanism.
Conclusion
In the present study, we have explored chromium biosorption and the involved mechanism between chromium and Bacillus strain DPAML065 derived from the tannery sludge. Maximum Cr(VI) reduction (95%) was observed at pH 6.0 with Cr(VI) concentration of 80 mg/L at a retention time of 24 h and operational temperature of 35 °C. Biosorption of Cr(VI) by Bacillus strain was well fitted with Langmuir adsorption isotherm, and the maximum adsorption capacity (qmax) was calculated to be 106.38 mg/g. The biosorption kinetics of Cr(VI) was represented with pseudo-second-order kinetics (R2 = 0.984). FE-SEM and EDX analysis were carried out to find out the biosorption competence of the Bacillus strain DPAML065 as well as to discover the surface adsorption of the metals on the bacterial cell surface. FTIR spectra revealed the participation of carboxyl, hydroxyl and amine groups involved in binding of chromium ions and suggested that ion exchange, precipitation and electrostatic attraction following complex reduction steps are the possible mechanisms during the biosorption of Cr(VI) by the isolated Bacillus strain DPAML065. XRD results confirmed that the peaks at 27.53, 56.65 and 66.45 depict Cr (III) on the surface of the bacterial cell, the plausible reason being precipitation of Cr(VI) during the biosorption process. Hence, this study concludes that Bacillus strain DPAML065 can be used as a low cost and highly efficient biosorbent to decontaminated wastewaters from the leather industries. Additionally, it can be utilized as a promising biosorbent in bioremediation strategies.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Acharya S, Sahoo S, Sonal S, Lee JH, Mishra BK, Nayak GC (2020) Adsorbed Cr (VI) based activated carbon/polyaniline nanocomposite: a superior electrode material for asymmetric supercapacitor device. Compos B Eng 193:107913
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98(12):2243–2257
Albadarin AB, Ala’a H, Al-Laqtah NA, Walker GM, Allen SJ, Ahmad MN (2011) Biosorption of toxic chromium from aqueous phase by lignin: mechanism, effect of other metal ions and salts. Chem Eng J 169(1–3):20–30
Al-Daghistani H (2012) Bio-remediation of Cu, Ni and Cr from rotogravure wastewater using immobilized, dead, and live biomass of indigenous thermophilic Bacillus species. Internet J Microbiol 10(1):1–10
American Public Health Association, American Water Works Association, Water Pollution Control Federation, Water Environment Federation (2012) In: Clesceri LS, Greenberg AE, Eaton AD (eds) Standard methods for the examination of water and wastewater, 22nd ed. American Public Health Association, Washington, DC
Anah L, Astrini N (2017) Influence of pH on Cr (VI) ions removal from aqueous solutions using carboxymethyl cellulose-based hydrogel as adsorbent. IOP Conf Ser Earth Environ Sci 60(1):012010
Arivalagan P, Singaraj D, Haridass V, Kaliannan T (2014) Removal of cadmium from aqueous solution by batch studies using Bacillus cereus. Ecol Eng 71:728–735
Arukula D, Prem P, Tanwi P, Hariraj S, Vijay LM, Brijesh KM (2018) Treatment of tannery wastewater using aluminium formate: influence of the formate over sulphate-based coagulant. Global NEST J 20(3):458–464
Babel S, Kurniawan TA (2004) Cr (VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54(7):951–967
Balasubramanian UM, Murugaiyan SV, Marimuthu T (2019) Enhanced adsorption of Cr (VI), Ni (II) ions from aqueous solution using modified Eichhornia crassipes and Lemna minor. Environ Sci Pollut Res 27(17):20648–20662
Belay AA (2010) Impacts of chromium from tannery effluent and evaluation of alternative treatment options. J Environ Prot 1(01):53–58
Beukes JP, Du Preez SP, Van Zyl PG, Paktunc D, Fabritius T, Päätalo M, Cramer M (2017) Review of Cr (VI) environmental practices in the chromite mining and smelting industry–relevance to development of the Ring of Fire, Canada. J Clean Prod 165:874–889
Bharagava RN, Mishra S (2018) Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol Environ Saf 147:102–109
Bueno BYM, Torem ML, Molina FALMS, De Mesquita LMS (2008) Biosorption of lead (II), chromium (III) and copper (II) by R. opacus: equilibrium and kinetic studies. Miner Eng 21(1):65–75
Cardoso SL, Costa CSD, Nishikawa E, da Silva MGC, Vieira MGA (2017) Biosorption of toxic metals using the alginate extraction residue from the brown algae Sargassum filipendula as a natural ion-exchanger. J Clean Prod 165:491–499
Cervantes C, Campos-García J (2007) Reduction and efflux of chromate by bacteria. In: Molecular microbiology of heavy metals. Springer, Berlin, Heidelberg, pp 407–419
Chakravarty R, Banerjee PC (2012) Mechanism of cadmium binding on the cell wall of an acidophilic bacterium. Bioresour Technol 108:176–183
Cheung KH, Gu JD (2007) Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int Biodeterior Biodegradation 59(1):8–15
Das S, Mishra J, Das SK, Pandey S, Rao DS, Chakraborty A, Thatoi H (2014) Investigation on mechanism of Cr (VI) reduction and removal by Bacillus amyloliquefaciens, a novel chromate tolerant bacterium isolated from chromite mine soil. Chemosphere 96:112–121
Dash HR, Mangwani N, Das S (2014) Characterization and potential application in mercury bioremediation of highly mercury-resistant marine bacterium Bacillus thuringiensis PW-05. Environ Sci Pollut Res 21(4):2642–2653
Deepa A, Mishra BK (2020) Microbial biotransformation of hexavalent chromium [Cr (VI)] in tannery wastewater. In: Microbial bioremediation & biodegradation. Springer, Singapore, pp 143–152
Deepa A, Prakash P, Mishra BK (2019) Performance of biochar-based filtration bed for the removal of Cr (VI) from pre-treated synthetic tannery wastewater. Environ Technol:1–13. https://doi.org/10.1080/09593330.2019.1626912
Dhal B, Thatoi H, Das N, Pandey BD (2010) Reduction of hexavalent chromium by Bacillus sp. isolated from chromite mine soils and characterization of reduced product. J Chem Technol Biotechnol 85(11):1471–1479
García-Mendieta A, Olguín MT, Solache-Ríos M (2012) Biosorption properties of green tomato husk (Physalis philadelphica Lam) for iron, manganese and iron–manganese from aqueous systems. Desalination 284:167–174
Ghiasi B, Niksokhan MH, Mahdavi Mazdeh A (2020) Effect of bentonite particles’ presence on two-dimensional chromium transmission. Environ Sci Pollut Res 27(17):1–10
Green-Ruiz C, Rodriguez-Tirado V, Gomez-Gil B (2008) Cadmium and zinc removal from aqueous solutions by Bacillus jeotgali: pH, salinity and temperature effects. Bioresour Technol 99(9):3864–3870
Guo J, Zheng XD, Chen QB, Zhang L, Xu XP (2012) Biosorption of Cd (II) from aqueous solution by Pseudomonas plecoglossicida: kinetics and mechanism. Curr Microbiol 65(4):350–355
Gupta R, Ahuja P, Khan S, Saxena RK, Mohapatra H (2000) Microbial biosorbents: meeting challenges of heavy metal pollution in aqueous solutions. Curr Sci 78(8):967–973
Han X, Wong YS, Wong MH, Tam NFY (2007) Biosorption and bioreduction of Cr (VI) by a microalgal isolate, Chlorella miniata. J Hazard Mater 146(1–2):65–72
Hu JL, He XW, Wang CR, Li JW, Zhang CH (2012) Cadmium adsorption characteristic of alkali modified sewage sludge. Bioresour Technol 121:25–30
Huang F, Dang Z, Guo CL, Lu GN, Gu RR, Liu HJ, Zhang H (2013) Biosorption of Cd (II) by live and dead cells of Bacillus cereus RC-1 isolated from cadmium-contaminated soil. Colloids Surf B: Biointerfaces 107:11–18
IARC C (1990) Nickel and welding. International Agency for Research on Cancer, Lyon
Javanbakht V, Alavi SA, Zilouei H (2014) Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci Technol 69(9):1775–1787
Jobby R, Jha P, Gupta A, Gupte A, Desai N (2019) Biotransformation of chromium by root nodule bacteria Sinorhizobium sp. SAR1. PLoS One 14(7):e0219387
Joo JH, Hassan SH, Oh SE (2010) Comparative study of biosorption of Zn2+ by Pseudomonas aeruginosa and Bacillus cereus. Int Biodeterior Biodegradation 64(8):734–741
Kalola V, Desai C (2019) Biosorption of Cr (VI) by Halomonas sp. DK4, a halotolerant bacterium isolated from chrome electroplating sludge. Environ Sci Pollut Res 27:27330–27344
Karthik C, Barathi S, Pugazhendhi A, Ramkumar VS, Thi NBD, Arulselvi PI (2017) Evaluation of Cr (VI) reduction mechanism and removal by Cellulosimicrobiumfunkei strain AR8, a novel haloalkaliphilic bacterium. J Hazard Mater 333:42–53
Khan TA, Chaudhry SA, Ali I (2013) Thermodynamic and kinetic studies of As (V) removal from water by zirconium oxide-coated marine sand. Environ Sci Pollut Res 20(8):5425–5440
Kumari V, Yadav A, Haq I, Kumar S, Bharagava RN, Singh SK, Raj A (2016) Genotoxicity evaluation of tannery effluent treated with newly isolated hexavalent chromium reducing Bacillus cereus. J Environ Manag 183:204–211
Lata S, Singh PK, Samadder SR (2015) Regeneration of adsorbents and recovery of heavy metals: a review. Int J Environ Sci Technol 12(4):1461–1478
Lata S, Prabhakar R, Adak A, Samadder SR (2019) As (V) removal using biochar produced from an agricultural waste and prediction of removal efficiency using multiple regression analysis. Environ Sci Pollut Res 26(31):32175–32188
Lutke SF, Igansi AV, Pegoraro L, Dotto GL, Pinto LA, Cadaval TR Jr (2019) Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J Environ Chem Eng 7(5):103396
McLean JS, Beveridge TJ, Phipps D (2000) Isolation and characterization of a chromium-reducing bacterium from a chromated copper arsenate-contaminated site. Environ Microbiol 2(6):611–619
Munoz AJ, Espínola F, Moya M, Ruiz E (2015) Biosorption of Pb (II) ions by Klebsiella sp. 3S1 isolated from a wastewater treatment plant: kinetics and mechanisms studies. Biomed Res Int. https://doi.org/10.1155/2015/719060
Murugavelh S, Mohanty K (2013) Isolation, identification and characterization of Cr (VI) reducing Bacillus cereus from chromium contaminated soil. Chem Eng J 230:1–9
Nandy T, Kaul SN, Shastry S, Manivel U, Deshpande CV (1999) Wastewater management in cluster of tanneries in Tamil Nadu through implementation of common effluent treatment plants+. NISCAIR-CSIR, India, pp 475–516
Nguema PF, Luo Z, Lian J (2014) The biosorption of Cr (VI) ions by dried biomass obtained from a chromium-resistant bacterium. Front Chem Sci Eng 8(4):454–464
Nwinyi OC, Owolabi TA (2019) Scanning electron microscopy and Fourier transmission analysis of polyhydroxyalkanoates isolated from bacteria species from abattoir in Ota, Nigeria. J King Saud Univ Sci 31(3):285–298
Oves M, Khan MS, Zaidi A (2013) Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil. Saudi J Biol Sci 20(2):121–129
Park D, Yun YS, Park JM (2006) Mechanisms of the removal of hexavalent chromium by biomaterials or biomaterial-based activated carbons. J Hazard Mater 137(2):1254–1257
Prabhakar R, Samadder SR (2020) Use of adsorption-influencing parameters for designing the batch adsorber and neural network–based prediction modelling for the aqueous arsenate removal using combustion synthesised nano-alumina. Environ Sci Pollut Res 27(7). https://doi.org/10.1007/s11356-020-08975-y
Prasad KS, Ramanathan AL, Paul J, Subramanian V, Prasad R (2013) Biosorption of arsenite (As+ 3) and arsenate (As+ 5) from aqueous solution by Arthrobacter sp. biomass. Environ Technol 34(19):2701–2708
QuiIntana M, Curutchet G, Donati E (2001) Factors affecting chromium (VI) reduction by Thiobacillusferrooxidans. Biochem Eng J 9(1):11–15
Rajesh V, Kumar ASK, Rajesh N (2014) Biosorption of cadmium using a novel bacterium isolated from an electronic industry effluent. Chem Eng J 235:176–185
Ramrakhiani L, Majumder R, Khowala S (2011) Removal of hexavalent chromium by heat inactivated fungal biomass of Termitomycesclypeatus: surface characterization and mechanism of biosorption. Chem Eng J 171(3):1060–1068
Rathinam A, Maharshi B, Janardhanan SK, Jonnalagadda RR, Nair BU (2010) Biosorption of cadmium metal ion from simulated wastewaters using Hypneavalentiae biomass: a kinetic and thermodynamic study. Bioresour Technol 101(5):1466–1470
Ryan MP, Williams DE, Chater RJ, Hutton BM, McPhail DS (2002) Why stainless steel corrodes. Nature 415(6873):770–774
Sala Cossich E, Granhen Tavares CR, Kakuta Ravagnani TM (2002) Biosorption of chromium (III) by Sargassum sp. biomass. Electron J Biotechnol 5(2):6–7
Sanjay MS, Sudarsanam D, Raj GA, Baskar K (2018) Isolation and identification of chromium reducing bacteria from tannery effluent. J King Saud Univ Sci 32(1):265–271
Saxena G, Chandra R, Bharagava RN (2016) Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. In: Reviews of environmental contamination and toxicology, 240. Springer, Cham, pp 31–69
Senthilkumar R, Vijayaraghavan K, Thilakavathi M, Iyer PVR, Velan M (2006) Seaweeds for the remediation of wastewaters contaminated with zinc (II) ions. J Hazard Mater 136(3):791–799
Sharma S, Malaviya P (2016) Bioremediation of tannery wastewater by chromium resistant novel fungal consortium. Ecol Eng 91:419–425
Singh S, Kumar V (2020) Mercury detoxification by absorption, mercuric ion reductase, and exopolysaccharides: a comprehensive study. Environ Sci Pollut Res 27(22):27181–27201
Singh H, Sonal S, Mishra BK (2018) Hexavalent chromium removal by monopolar electrodes based electrocoagulation system: optimization through box–Behnken design. J Water Supply Res Technol AQUA 67(2):147–161
Sinha S, Behera SS, Das S, Basu A, Mohapatra RK, Murmu BM, Dhal NK, Tripathy SK, Parhi PK (2018) Removal of Congo red dye from aqueous solution using Amberlite IRA-400 in batch and fixed bed reactors. Chem Eng Commun 205(4):432–444
Sonal S, Prakash P, Mishra BK, Nayak GC (2020) Synthesis, characterization and sorption studies of a zirconium (iv) impregnated highly functionalized mesoporous activated carbons. RSC Adv 10(23):13783–13798
Srivastava S, Thakur IS (2006) Biosorption potency of Aspergillus niger for removal of chromium (VI). Curr Microbiol 53(3):232–237
Srivastava S, Thakur IS (2012) Biosorption and biotransformation of chromium by Serratia sp. isolated from tannery effluent. Environ Technol 33(1):113–122
Sultan S, Mubashar K, Faisal M (2012) Uptake of toxic Cr (VI) by biomass of exo-polysaccharides producing bacterial strains. Afr J Microbiol Res 6:3329–3336
Sun Y, Yue Q, Mao Y, Gao B, Gao Y, Huang L (2014) Enhanced adsorption of chromium onto activated carbon by microwave-assisted H3PO4 mixed with Fe/Al/Mn activation. J Hazard Mater 265:191–200
Torab-Mostaedi M, Asadollahzadeh M, Hemmati A, Khosravi A (2013) Equilibrium, kinetic, and thermodynamic studies for biosorption of cadmium and nickel on grapefruit peel. J Taiwan Inst Chem Eng 44(2):295–302
Tsekova K, Todorova D, Ganeva S (2010) Removal of heavy metals from industrial wastewater by free and immobilized cells of Aspergillus niger. Int Biodeterior Biodegradation 64(6):447–451
Uniyal S, Rawat M, Rai JPN (2013) Cadmium biosorption by Stenotrophomonas humi and Micrococcus luteus: kinetics, equilibrium and thermodynamic studies. Desalin Water Treat 51(40–42):7721–7731
Vijayaraghavan K, Balasubramanian R (2015) Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J Environ Manage 160:283–296
Vinod VTP, Sashidhar RB, Sreedhar B (2010) Biosorption of nickel and total chromium from aqueous solution by gum kondagogu (Cochlospermumgossypium): a carbohydrate biopolymer. J Hazard Mater 178(1–3):851–860
Volesky B (2003) Biosorption process simulation tools. Hydrometallurgy 71(1–2):179–190
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11(3):235–250
Weber WJ, Morris JC (1962a) Proceedings of the international conference on water pollution symposium, vol 2. Pergamon Press, Oxford, pp 235–266
Weber WJ, Morris JC (1962b) Advances in water pollution research. In: Proceedings of the First international conference on water pollution research, vol 2. Pergamon Press, Oxford, p 231
Xiao X, Luo S, Zeng G, Wei W, Wan Y, Chen L, Xi Q (2010) Biosorption of cadmium by endophytic fungus (EF) Microsphaeropsis sp. LSE10 isolated from cadmium hyperaccumulator Solanum nigrum L. Bioresour Technol 101(6):1668–1674
Zambrano J, Min B (2019) Comparison on efficiency of electrochemical phenol oxidation in two different supporting electrolytes (NaCl and Na2SO4) using Pt/Ti electrode. Environ Technol Innov 15:100382
Acknowledgements
The authors are very thankful for the financial support from the Indian Institute of Technology (Indian School of Mines), Dhanbad under a Junior Research Fellowship scheme funded by Ministry of Human Resource Development (MHRD), Government of India, New Delhi, to carry out this research work.
Funding
Funding information is not applicable.
Author information
Authors and Affiliations
Contributions
Arukula Deepa did all the experiments and the analysis and interpreted the results. Astha Singh interpreted the isotherm study. Aakansha Singh interpreted the biosorption study. Brijesh Kumar Mishra formulated the experimental design.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Bacillus species strain DPAML065 was explored for chromium biosorption.
2. The biosorbent showed Cr(VI) removal efficiency of 95% under optimal conditions.
3. Maximum biosorption capacity of 106.38 mg/L was estimated for Bacillus strain DPAML065 biosorbent.
4. Chromium biosorption on Bacillus strain DPAML065 obeyed Langmuir isotherm and pseudo-second-order.
5. The study confirmed the biosorption of Cr(VI) on the bacterial cell surface through multistep mechanism process.
Electronic supplementary material
ESM 1
(DOCX 219 kb).
Rights and permissions
About this article
Cite this article
Deepa, A., Singh, A., Singh, A. et al. An experimental approach for the utilization of tannery sludge-derived Bacillus strain for biosorptive removal of Cr(VI)-contaminated wastewater. Environ Sci Pollut Res 28, 9864–9876 (2021). https://doi.org/10.1007/s11356-020-11284-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11284-z