Abstract
Imazalil (IMZ), a fungicide containing imidazole group, is extensively used for the prevention and treatment of fungal diseases in plants. Current study was performed to examine cyto-genotoxic potential of IMZ on Allium cepa roots by following Allium ana-telophase and single cell gel electrophoresis (comet) assays. The concentration which reduced the growth of the root tips of IMZ by 50% compared to the negative control group (EC50) was found to be 1 μg/mL by Allium root growth inhibition test. 0.5, 1, and 2 μg/mL concentrations of IMZ were exposed to Allium roots for intervals of 24, 48, 72, and 96 h. 10 μg/mL of methyl methane sulfonate (MMS) and distilled water were used as control groups, both positive and negative. Statistical analysis was performed by using one-way ANOVA with Duncan’s multiple comparison tests at p ≤ 0.05 and Pearson correlation test at p = 0.01. IMZ showed cytotoxic effect by statistically decreasing root growth and mitotic index (MI) and also genotoxic effect by statistically increasing chromosomal aberrations (CAs) and DNA damage compared to the negative control group. With these cyto-genotoxic effects, it should be used carefully and further cyto-genotoxic mechanisms should be investigated along with other toxicity tests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungicides have been widely used for economic benefit in agriculture and industry for the last few decades, due to their high efficiency and low toxicity (Jin et al. 2017; Zhang et al. 2018). The global fungicide market was worth approximately USD 13.4 billion in 2018 and is forecast to be worth USD 15.7 billion in 2024 (Garside 2019). Imazalil (IMZ; 1-[2-(2,4-dichlorophenyl)-2-(2-propenyloxy) ethyl]-1H-imidazole) is a type of fungicide which is highly beneficial for the prevention and cure of fungal diseases in many plants such as cucumber, tomatoes, citrus fruits, tomato, barley, wheat, and bananas in post-harvest treatments, and it is also used as antimycotic drug in human and veterinary medicine and as a biocide in the formulation of wood and building materials (Bossche et al. 2003; USEPA 2005; Bylemans and Thys 2007; Smilanick et al. 2006; Sepulveda et al. 2015). The derivatives of imidazole and triazole such as IMZ inhibit fungal cell wall synthesis by the inhibition of ergosterol biosynthesis and thus interrupting mechanism of cytochrome P450 enzyme called CYP51 (lanosterol-14-α-demethylase), present in several organisms which acts on cell membrane homeostasis, fluidity, and permeability (USEPA 2005; Correia and de Montellano 2005; Zega et al. 2009; Kuhlmann et al. 2019). They consist of about 25% of fungicides worldwide (Saxena et al. 2015). According to the World Health Organization, extreme allowable residue levels of IMZ in citrus fruits and in bananas should not be more than 5 mg/kg and 2 mg/kg, respectively (Tanaka et al. 2013). In addition to its pest-reducing effects, IMZ has been also detected in soil/sediment, water, and aquatic organisms (Gilbert-López et al. 2012; Belenguer et al. 2014; Masiá et al. 2015; Ruiz-Rodríguez et al. 2015; Xu et al. 2015; Ccanccapa et al. 2016). As IMZ is not specific to fungi, it can interact and inhibit the cytochrome P450 enzymes in non-target organisms like other azoles and may have undesirable effects (Walker 2008; Gottardi and Cedergreen 2019; Kuhlmann et al. 2019). So, cyto-genotoxic assessment of IMZ is inevitable to find its hazards on non-target species.
Allium test is considered as the most frequently used plant cytogenetic assessment method for revealing the cyto-genotoxic effects of fungicides. Different parameters like root growth, mitotic index (MI), and chromosomal aberrations (CAs) can be easily observed. It is a quick, easy, highly accurate, and easily reproducible. It is also accepted as a standard assay by the United Nations Environmental Programme, the United States Environmental Protection Agency, and the World Health Organization (Grant 1994; Rank and Nielsen 1994; Teixeira et al. 2003; Leme and Marine-Morels 2009; Liman et al. 2011; Silveira et al. 2016; Fatma et al. 2018; Bernardes et al. 2019). Root meristems of A. cepa–based comet assay have been widely used to determine DNA damage of pesticides at the level of individual cells, because this technique is quite simple, sensitive, and reliable. A small number of cells are required, and it is also relatively inexpensive compared to other test systems (Türkoğlu 2012; Karaismailoglu 2015; Liman et al. 2015; Silveira et al. 2017; Özkan and Liman 2019).
Despite the mentioned superior properties of the IMZ, its potential adverse effects on non-target plants are not yet known sufficiently. The current study was designed to determine cyto-genotoxic effects of IMZ on A. cepa roots by observing root growth, MI, mitotic phase changes, CAs in ana-telophase cells, and DNA damage.
Materials and methods
Chemicals
The chemicals used in this study were supplied by Sigma Aldrich, Munich, Germany, including IMZ (CAS No 35554-44-0), MMS (CAS No 67-27-3), glacial acetic acid, basic fuchsin hydrochloric acid, potassium disulfite, potassium chloride, sodium chloride, trizma hydrochloride, disodium hydrogen phosphate, potassium phosphate monobasic, normal melting point agarose, low melting point agarose, trizma base, magnesium chloride hexahydrate, triton X-100, sodium hydroxide, di-sodium salt of ethylene diamine tetra acetic acid (EDTA), and ethidium bromide.
Allium root growth inhibition test
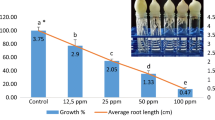
Healthy and equal-sized A. cepa bulbs were obtained from local market which were 25–30 mm in diameter. The growth inhibition test was performed according to the modified method proposed by Fiskesjö (1985) as described in Küçük and Liman (2018). Bulbs cleaned from dried roots and brown outer shells were directly exposed to different concentrations of IMZ (0.5, 1, 2.5, 5, 10, 25, 50, and 100 μg/mL) for 96 h which were kept in dark at room temperature as well as distilled water for negative control. To calculate the EC50 value, the average lengths were determined by taking 10 roots from one bulb (50 roots from 5 bulbs) for each application after the exposure time.
Allium ana-telophase test
The Allium ana-telophase test was performed by following the protocol proposed by Rank and Nielsen (1993) with slight modifications. 0.5, 1, and 2 μg/mL of IMZ were exposed to Allium roots whose lengths varied from 2 to 3 cm at the room temperature in the dark for several time intervals (24, 48, 72, and 96 h) as well as distilled water for negative control and 10 μg/mL of MMS for positive control. Three onions were used for each concentration. Five to 8 root tips from each onion were cut about 1 cm in length and fixed for 1 day at 4 °C in Farmer’s solution (1 glacial acetic acid: 3 ethanol, v/v) and then stored in alcohol (70%) at the same temperature. After hydrolysis of roots with 1 N HCl at 60 °C for 8–10 min, Feulgen dye was employed to smear of the root tips at room temperature for 25–30 min. Following rinsing with distilled water thrice for 5 min, squash preparations of the dark stained root tips were prepared with one drop of 45% acetic acid and sealed with finger nail polish. For each application, 5000–5150 cells (1000–1040 cells one slide per bulb) and 500 ana-telophase cells (100 ana-telophase cells one slide per bulb) were counted for MI and CA frequencies using a trinocular light microscope according to Saxena et al. (2005). The following formulas were used in the calculation of MI, phase index, and CA.
Comet assay
Genotoxic potential of IMZ on A. cepa roots was assessed with the alkaline comet assay by the instructions of Tice et al. (2000) with slight modifications as stated by Küçük and Recep (2018). The treated root tips as mentioned above were gently sliced to isolate nuclei in 600 μL cold nuclear isolation buffer (0.5% w/v Triton X-100, 2 M Tris 4 mM, MgCl2-6H2O; pH 7.5) and then filtered through 60-μm meshes Nylon filter. At 1200 rpm, the solution was centrifuged, at 4 °C for 7 min. Fifty-microliter 1.5% low melting point agarose was mixed with the pellet of nuclei suspension (50 μL), and then the mixture was smeared onto slides that pre-coated with 1% normal melting agarose and kept on ice for 5 min. After removing cover slips, the slides were placed in a horizontal electrophoresis tank having cold 1 mM EDTA+ 300 mM NaOH buffer (pH ≥ 13) for 20 min at 4 °C and then electrophoresed 300 mA/24 V for 20 min under the same conditions. The slides were neutralized with 0.4 M Tris (pH 7.5) thrice for 5 min. After neutralization, staining was done with ethidium bromide solution (20 μg/mL). A BAB fluorescence microscope (TAM-F, Turkey) was used to analyze on randomly selected 50 comets per slide (150 comets per sample) for evaluation of DNA damage expressed as arbitrary unit at × 400 magnification. The comets were classified in five classes [0 (no damage) to 4 (maximum damage)], according to Kocyigit et al. (2005) as shown in Fig. 1.
Data analysis
Duncan’s multiple range tests were used for statistical evaluation of the results (mean ± standard deviation) using SPSS 23.0 version (SPSS Inc., Chicago, USA) at p ≤ 0.05. Pearson correlation analysis was also used to determine dose-response and dose-time relationships at p = 0.01 significance level.
Results and discussion
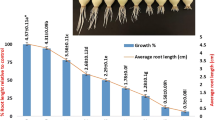
The EC50 of IMZ was found to be 1 μg/mL (50.72%) by Allium root growth inhibition test (Fig. 2). IMZ decreased root growth within the range of 35.4 to 96.07% compared to the control group in a dose-dependent manner (r = −0.969). The factors affecting root growth are generally related to suppression of apical meristematic activity (Webster and Macleod 1996), cell cycle during differentiation (Fusconi et al. 2006), or enzymes that are related to cell division (Silveira et al. 2017). IC50 of IMZ for Chironomus riparius was found to be 0.11 ± 0.01 and 0.09 ± 0.01 μmol/L for R- and S-imazalil, respectively (Kuhlmann et al. 2019). EC50 of IMZ for Pseudokirchneriellasubcapitata was found to be 0.623 μg/mL for 72 h. LC50 of IMZ was found to be 0.882 μg/mL for Daphnia magna and 2.324 μg/mL for Danio rerio (Li et al. 2019), 541 μg/g in the artificial soil test and 12.8 μg/cm2 for Eisenia foetida (Van Leemput et al. 1989), and 173.7 μM for Phallusia mammillata (Pennati et al. 2006).
Table 1 shows MI and mitotic phase indices of IMZ obtained using Allium test. All concentrations of IMZ statistically decreased MI compared to the control group not only dose-dependently (for 24 h r = − 0.885, for 48 h r = − 0.924, for 72 h r = − 0.855, and for 96 r = −0.831) but also time-dependently (for 0.5 μg/mL r = − 0.943, for 1 μg/mL r = − 0.94, and for 2 μg/mL r = − 0.905). The reduction in MI for IMZ was not lower than MMS. IMZ statistically decreased the prophase index but increased the telophase index compared to the control group. It also increased metaphase and anaphase indices but was not found statistically significant except for the anaphase index at 96 h. A significant decrease in MI by IMZ was indicative of cytotoxic damage and may occur due to inhibition of DNA polymerase by the inhibition of specific proteins (Hidalgo et al. 1989), the changes of cell durations (El-Ghamery et al. 2000; Sudhakar et al. 2001; Rajeshwari et al. 2016), or mitotic stage duration changes (Tkalec et al. 2009) or mitodepressive regulation of chemicals (Sharma and Vig 2012), and/or ROS disturbance homeostasis (Livanos et al. 2012). IMZ showed also toxicity on isolated rat hepatocytes at 0.75 mM (Nakagawa and Moore 1995), on the mouse fibroblast L929 cells at 50 μM (Maruyama et al. 2007), and on Danio rerio embryos at 10 mM and above (Şişman and Türkez 2010). Hatch ability in chickens was inhibited by in ovo exposure of IMZ at 2 mg/egg (Matsushita et al. 2006). IMZ induced acute cell death higher than 7 μg/mL on Scaphechinus mirabilis and Strongylocentrotus nudus (Hosoya and Mıkamı 2008). IMZ was found as toxic in vivo but nontoxic or moderate toxic in vitro by Ames and micronucleus test (Ilyushina et al. 2019). Unlike our result, 2.5 μg/mL of IMZ did not show toxicity in the human intestinal Caco-2 cells after 48-h incubation (Sergent et al. 2009). Leaf chlorophyll content and growth in Phragmites australi were also found to be similar between the control group and 10 μg/mL of the IMZ exposed groups after 24 days (Lv et al. 2017).
The types and rates of CAs caused by the IMZ in A. cepa root meristem cells are given in Figs. 3 and 4. IMZ prompted total CAs by showing the disturbed ana-telophase, stickiness, anaphase bridges, chromosome laggards, and polyploidy in ana-telophase cells of A. cepa in a dose-dependent manner compared to the control group, suggesting genotoxic effect of IMZ. However, the increases between IMZ groups were not statistically significant. Stickiness (at 0.5 μg/mL for 24 h, 2.6%) was the most common CAs, and the least seen CAs was polyploidy (at 0.5 μg/mL for 24 h, 1.4%). Disturbed ana-telophase (Fig. 4a) and chromosome laggards (Fig. 4c) may result from degraded microtubules or inhibition of movement of chromosomes to opposite poles (Evseeva et al. 2005; Kumari et al. 2009; Rajeshwari et al. 2016; Singh and Roy 2017). Stickiness (Fig. 4b), an indicator of toxicity, may be caused by formation cross-linking of DNA-DNA or DNA-protein (Amin 2002; Barbério et al. 2011). Anaphase bridges (Fig. 4d) may cause chromosome laggards by showing clastogenic effect due to the formation of dicentric chromosomes, stickiness, changes in replication enzyme activity, breakage or fusion of chromosomes, and unequal chromatid exchange (El-Ghamery et al. 2000; Luo et al. 2004; Dutta et al. 2018). Polyploidy (Fig. 4e) may result from abnormal segregation of chromosomes during the cell division (Nefic et al. 2013; Palsikowski et al. 2018). In addition to ana-telophase anomalies, c-metaphase and binuclear cells in other cells were also observed. Cytokinesis inhibition at any cell cycle control point (Ateeq et al. 2002) may induce binuclear cells (Fig. 4f). C-metaphase (Fig. 4g) may occur due to spindle failure or an imbalance in amount of proteins responsible for the formation of nuclear chromatin (Odeigah et al. 1997; Mesi and Kopliku 2013). IMZ also induced micronucleus frequency and CAs in human peripheral lymphocytes (Şişman and Türkez 2010).
The genotoxic effects of IMZ in the A. cepa root meristematic cells were evaluated by comet assay (Fig. 5). There was more DNA damage by the IMZ groups compared to the control group. A dose-dependent (for 24 h r = 0.94, for 48 h r = 0.971, for 72 h r = 0.954, and for 96 r = 0.946) and time-dependent (for 0.5 μg/mL r = 0.961, for 1 μg/mL r = 0.943, and 2 μg/mL r = 0.92) increases of DNA damage between 95.67 ± 6.03 and 153 ± 2.65 were observed after IMZ applications. Similarly, IMZ induced DNA damage observed by the alkaline comet assay in mouse hepatocytes (Đikić et al. 2012) and in human lymphocytes (Ramirez and Cuenca 2002; Vindas et al. 2004). After chronic IMZ exposure in mice for 15 weeks, reactive oxygen species (ROS) were also increased in mouse hepatocytes, resulting to oxidative stress (Jin et al. 2018). Oxidative stress produced by IMZ at high concentrations in different organisms was linked to cell death by damaging cell membranes according to previous studies (Heusinkveld et al. 2013; Prado et al. 2015; Pereira et al. 2019).
Conclusions
IMZ showed not only cytotoxic effect by decreasing inhibition of root growth and MI but also genotoxic effect by increasing CAs and DNA damage in A. cepa roots. Further studies are therefore needed to clarify IMZ’s cyto-genotoxic mechanisms on plants.
References
Amin AW (2002) Cytotoxicity testing of sewage water treatment using Allium cepa chromosome aberration assay. Pak J Biol Sci 5(2):184–188
Ateeq B, Farah MA, Ali MN, Ahmad W (2002) Clastogenicity of pentachlorophenol, 2, 4D and butachlor evaluated by Allium root tip test. Mutat Res 514:105–113. https://doi.org/10.1016/S1383-5718(01)00327-8
Barbério A, Voltolini JC, Mello MLS (2011) Standardization of bulb and root sample sizes for the Allium cepa test. Ecotoxicology 20:(4)927–935. https://doi.org/10.1007/s10646-011-0602-8
Belenguer V, Martinez-Capel F, Masiá A, Picó Y (2014) Patterns of presence and concentration of pesticides in fish and waters of the Júcar River (Eastern Spain). J Hazar Mater 265:271–279. https://doi.org/10.1016/j.jhazmat.2013.11.016
Bernardes PM, Andrade-Vieira LF, Aragão FB, Ferreira A, da Silva Ferreira MF (2019) Toxicological effects of comercial formulations of fungicides based on procymidone and iprodione in seedlings and root tip cells of Allium cepa. Environ Sci Pollut R 1-9. https://doi.org/10.1007/s11356-019-04636-x
Bossche HV, Engelen M, Rochette F (2003) Antifungal agents of use in animal health–chemical, biochemical and pharmacological aspects. J Vet Pharmacol Ther 26:(1)5-29. https://doi.org/10.1046/j.1365-2885.2003.00456.x
Bylemans DLJ, Thys APM (2007) Biocidal combinations comprising imazalil. Google Patents
Ccanccapa A, Masiá A, Navarro-Ortega A, Picó Y, Barceló D (2016) Pesticides in the Ebro River basin: occurrence and risk assessment. Environ Pollut 211:414–424. https://doi.org/10.1016/j.envpol.2015.12.059
Correia MA, de Montellano PRO (2005) Inhibition of cytochrome P450 enzymes. In cytochrome P450. Springer 247-322. https://doi.org/10.1007/0-387-27447-2_7
Đikić D, Mojsović-Ćuić A, Čupor I, Benković V, Horvat-Knežević A, Lisičić D, Oršolić N (2012) Carbendazim combined with imazalil or cypermethrin potentiate DNA damage in hepatocytes of mice. Hum Exp Toxicol 31(5):492–505. https://doi.org/10.1177/0960327111417910
Dutta J, Ahmad A, Singh J (2018) Study of industrial effluents induced genotoxicity on Allium cepa L. Caryologia 71:139–145. https://doi.org/10.1080/00087114.2018.1447631
El-Ghamery AA, El-Nahas AI, Mansour MM (2000) The action of atrazine herbicide as an indicator of cell division on chromosomes and nucleic acid content in root meristems of Allium cepa and Vicia faba. Cytologia 65:277–287. https://doi.org/10.1508/cytologia.65.277
Evseeva TI, Geras’kin SA, Shuktomova II, Taskaev AI (2005) Genotoxicity and cytotoxicity assay of water sampled from the underground nuclear explosion site in the north of the Perm region (Russia). J Environ Radioactiv 80:59–74. https://doi.org/10.1016/j.jenvrad.2004.08.014
Fatma F, Verma S, Kamal A, Srivastava A (2018) Monitoring of morphotoxic, cytotoxic and genotoxic potential of mancozeb using Allium assay. Chemosphere 195:864–870. https://doi.org/10.1016/j.chemosphere.2017.12.052
Fiskesjö G (1985) The Allium test as a standard in environmental monitoring. Hereditas 102(1):99–112. https://doi.org/10.1111/j.1601-5223.1985.tb00471.x
Fusconi A, Repetto O, Bona E, Massa N, Gallo C, Dumas-Gaudot E, Berta G (2006) Effect of cadmium on meristem activity and nucleus ploidy in roots of Pisum sativum L. cv. Frisson seedlings. Environ Exp Bot 58:253–260. https://doi.org/10.1016/j.envexpbot.2005.09.008
Garside M (2019) Global fungicide market revenue 2016–2024. Available online at: https://www.statista.com/statistics/586532/fungicide-market-value-worldwide (accessed January 18, 2020)
Gilbert-López B, Jaén-Martos L, García-Reyes JF, Villar-Pulido M, Polgar L, Ramos-Martos N, Molina-Díaz A (2012) Study on the occurrence of pesticide residues in fruit-based soft drinks from the EU market and Morocco using liquid chromatography–mass spectrometry. Food control 26:(2)341-346. https://doi.org/10.1016/j.foodcont.2012.01.025
Gottardi M, Cedergreen N (2019) The synergistic potential of azole fungicides does not directly correlate to the inhibition of cytochrome P450 activity in aquatic invertebrates. Aqua Toxicol 207:187–196. https://doi.org/10.1016/j.aquatox.2018.12.010
Grant WF (1994) The present status of higher plant bioassays for the detection of environmental mutagens. Mutat Res 310(2):175–185. https://doi.org/10.1016/0027-5107(94)90112-0
Heusinkveld HJ, Molendijk J, Berg MVD, Westerink RH (2013) Azole fungicides disturb intracellular Ca2+ in an additive manner in dopaminergic PC12 cells. Toxicol Sci 134(2):374–381. https://doi.org/10.1093/toxsci/kft119
Hidalgo A, Gonzales-Reyes JA, Navas P, Garcia-Herdugo G (1989) Abnormal mitosis and growth inhibition in Allium cepa roots induced by propham and chlorpropham. Cytobios 57:7–14
Hosoya N, Mıkamı T (2008) Effects of fungicide imazalil on the early development of sea urchin eggs. Tiss Cult Res Commun 27:117–123. https://doi.org/10.11418/jtca.27.117
Ilyushina N, Egorova O, Rakitskii V (2019) Limitations of pesticide genotoxicity testing using the bacterial in vitro method. Toxicol in Vitro 57:110–116. https://doi.org/10.1016/j.tiv.2019.02.018
Jin CY, Luo T, Zhu ZH, Pan ZH, Yang JJ, Wang WC, Fu ZW, Jin YX (2017) Imazalil exposure induces gut microbiota dysbiosis and hepatic metabolism disorder in zebrafish. Comp Biochem Physiol C-Toxicol Pharmacol 202:85–93. https://doi.org/10.1016/j.cbpc.2017.08.007
Jin C, Luo T, Fu Z, Jin Y (2018) Chronic exposure of mice to low doses of imazalil induces hepatotoxicity at the physiological, biochemical, and transcriptomic levels. Wit TR Biomed Health 33(6):650–658. https://doi.org/10.1002/tox.22550
Karaismailoglu MC (2015) Investigation of the potential toxic effects of prometryne herbicide on Allium cepa root tip cells with mitotic activity, chromosome aberration, micronucleus frequency, nuclear DNA amount and comet assay. Caryologia 68(4):323–329. https://doi.org/10.1080/00087114.2015.1109927
Kocyigit A, Keles H, Selek S, Guzel S, Celik H, Erel O (2005) Increased DNA damage and oxidative stress in patients with cutaneous leishmaniasis. Mutat Res-Gen Tox En 585(1):71–78. https://doi.org/10.1016/j.mrgentox.2005.04.012
Küçük D, Liman R (2018) Cytogenetic and genotoxic effects of 2-chlorophenol on Allium cepa L. root meristem cells. Envıron Sci Pollut R 25(36):36117–36123. https://doi.org/10.1007/s11356-018-3502-0
Kuhlmann J, Kretschmann AC, Bester K, Bollmann UE, Dalhoff K, Cedergreen N (2019) Enantioselective mixture toxicity of the azole fungicide imazalil with the insecticide α-cypermethrin in Chironomus riparius: investigating the importance of toxicokinetics and enzyme interactions. Chemosphere 225:166–173. https://doi.org/10.1016/j.chemosphere.2019.03.023
Kumari M, Mukherjee A, Chandrasekaran N (2009) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407(19):5243–5246. https://doi.org/10.1016/j.scitotenv.2009.06.024
Leme DM, Marine-Morels MA (2009) Allium cepa test in environmental monitoring: a review on its applications. Mutat Res 682(1):71–81. https://doi.org/10.1016/j.mrrev.2009.06.002
Li R, Pan X, Tao Y, Jiang D, Chen Z, Dong F, Zheng Y (2019) Systematic evaluation of chiral fungicide imazalil and its major metabolite R14821 (imazalil-m): stability of enantiomers, enantioselective bioactivity, aquatic toxicity and dissipation in greenhouse vegetables and soil. J Agr Food Chem 67:(41)11331–11339. https://doi.org/10.1021/acs.jafc.9b03848
Liman R, Ciğerci İH, Akyıl D, Eren Y, Konuk M (2011) Determination of genotoxicity of fenaminosulf by Allium and comet tests. Pestic Biochem Phys 99(1):61–64. https://doi.org/10.1016/j.pestbp.2010.10.006
Liman R, Ciğerci İH, Öztürk NS (2015) Determination of genotoxic effects of imazethapyr herbicide in Allium cepa root cells by mitotic activity, chromosome aberration, and comet assay. Pestic Biochem Phys 118:(38)-42. https://doi.org/10.1016/j.pestbp.2014.11.007
Livanos P, Galatis B, Quader H, Apostolakos P (2012) Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana. Cytoskeleton 69(1):1–21. https://doi.org/10.1002/cm.20538
Luo LZ, Werner KM, Gollin SM, Saunders WS (2004) Cigarette smoke induces anaphase bridges and genomic imbalances in normal cells. Mutat Res-Fund Mol M 554(1):375–385. https://doi.org/10.1016/j.mrfmmm.2004.06.031
Lv T, Carvalho PN, Casas ME, Bollmann UE, Arias CA, Brix H, Bester K (2017) Enantioselective uptake, translocation and degradation of the chiral pesticides tebuconazole and imazalil by Phragmites australis. Enviro Pollut 229:362–370. https://doi.org/10.1016/j.envpol.2017.06.017
Maruyama T, Komatsu C, Michizoe J, Sakai S, Goto M (2007) Laccase-mediated degradation and reduction of toxicity of the postharvest fungicide imazalil. Process Biochem 42(3):459–461. https://doi.org/10.1016/j.procbio.2006.09.011
Masiá A, Vásquez K, Campo J, Picó Y (2015) Assessment of two extraction methods to determine pesticides in soils, sediments and sludges. Application to the Túria River Basin J Chromatogr A 1378:19–31. https://doi.org/10.1016/j.chroma.2014.11.079
Matsushita S, Yamashita J, Iwasawa T, Tomita T, Ikeda M (2006) Effects of in ovo exposure to imazalil and atrazine on sexual differentiation in chick gonads. Poult Sci 85(9):1641–1647. https://doi.org/10.1093/ps/85.9.1641
Mesi A, Kopliku D (2013) Cytotoxic and genotoxic potency screening of two pesticides on Allium cepa L. Proc Tech 8:19–26. https://doi.org/10.1016/j.protcy.2013.11.005
Nakagawa Y, Moore GA (1995) Cytotoxic effects of postharvest fungicides, ortho-phenylphenol, thiabendazole and imazalil on isolated rat hepatocytes. Life Sci 57(15):1433–1440. https://doi.org/10.1016/0024-3205(95)02106-S
Nefic H, Musanovic J, Metovic A, Kurteshi K (2013) Chromosomal and nuclear alterations in root tip cells of Allium cepa L. induced by alprazolam. Medical archives 67(6):388-392. https://doi.org/10.5455/medarh.2013.67.388-392
Odeigah PGC, Nurudeen O, Amund OO (1997) Genotoxicity of oil field wastewater in Nigeria. Hereditas 126:161–167. https://doi.org/10.1111/j.1601-5223.1997.00161.x
Özkan S, Liman R (2019) Cytotoxicity and genotoxicity in Allium cepa L. root meristem cells exposed to the herbicide penoxsulam. Celal Bayar Üniversitesi Fen Bilimleri Dergisi 15(2):221–226. https://doi.org/10.18466/cbayarfbe.533466
Palsikowski PA, Roberto MM, Sommaggio LR, Souza PM, Morales AR, Marin-Morales MA (2018) Ecotoxicity evaluation of the biodegradable polymers PLA, PBAT and its blends using Allium cepa as test organism. J Polym Environ 26:938–945. https://doi.org/10.1007/s10924-017-0990-9
Pennati R, Groppelli S, Zega G, Biggiogero M, De Bernardi F, Sotgia C (2006) Toxic effects of two pesticides, imazalil and triadimefon, on the early development of the ascidian Phallusia mammillata (Chordata,Ascidiacea). Aquat Toxicol 79(3):205–212. https://doi.org/10.1016/j.aquatox.2006.05.012
Pereira PCG, Soares LOS, Júnior SFS, Saggioro EM, Correia FV (2019) Sub-lethal effects of the pesticide imazalil on the earthworm Eisenia andrei: reproduction, cytotoxicity, and oxidative stress. Envıron Sci Pollut R:1–12. https://doi.org/10.1007/s11356-019-05440-3
Prado R, García R, Rioboo C, Herrero C, Cid A (2015) Suitability of cytotoxicity endpoints and test microalgal species to disclose the toxic effect of common aquatic pollutants. Ecotoxicol Environ Saf 114:117–125. https://doi.org/10.1016/j.ecoenv.2015.01.021
Rajeshwari A, Suresh S, Chandrasekaran N, Mukherjee A (2016) Toxicity evaluation of gold nanoparticles using an Allium cepa bioassay. RSC Adv 6(29):24000–24009. https://doi.org/10.1039/c6ra04712b
Ramirez V, Cuenca P (2002) DNA damage in female workers exposed to pesticides in banana plantations at Limon, Costa Rica. Rev Biol Trop 50(2):507–518
Rank J, Nielsen MH (1993) A modified Allium test as a tool in the screening of the genotoxicity of complex mixtures. Hereditas 118(1):49–53. https://doi.org/10.1111/j.1601-5223.1993.t01-3-00049.x
Rank J, Nielsen MH (1994) Evaluation of Allium anaphase–telophase test in relation to genotoxicity screening of industrial wastewater. Mutat Res 312:17–24. https://doi.org/10.1016/0165-1161(94)90004-3
Ruiz-Rodríguez L, Aguilar A, Díaz AN, Sánchez FG (2015) Enantioseparation of the fungicide imazalil in orange juice by chiral HPLC. Study on degradation rates and extractive/enrichment techniques. Food Chem 178:179–185. https://doi.org/10.1016/j.foodchem.2015.01.004
Saxena PN, Chauhan LKS, Gupta SK (2005) Cytogenetic effects of commercial formulation of cypermethrin in root meristem cells of Allium sativum: spectroscopic basis of chromosome damage. Toxicology 216(2–3):244–252. https://doi.org/10.1016/j.tox.2005.08.008
Saxena AK, Devillers J, Bhunia SS, Bro E (2015) Modelling inhibition of avian aromatase by azole pesticides. SAR QSAR Environ Res 26(7–9):757–782. https://doi.org/10.1080/1062936X.2015.1090749
Sepulveda M, Cuevas II, Smilanick JL, Cerioni L, Rapisarda VA, Ramallo J (2015) Improvement in imazalil treatments in commercial packinglines to control green mold on lemon fruit. Sci Hortic 192:387–390. https://doi.org/10.1016/j.scienta.2015.06.021
Sergent T, Dupont I, Jassogne C, Ribonnet L, Van Der Heiden E, Scippo ML, Schneider YJ (2009) CYP1A1 induction and CYP3A4 inhibition by the fungicide imazalil in the human intestinal caco-2 cells—comparison with other conazole pesticides. Toxicol Lett 184(3):159–168. https://doi.org/10.1016/j.toxlet.2008.11.009
Sharma S, Vig AP (2012) Genotoxicity of atrazine, avenoxan, diuron and quizalofop-P-ethyl herbicides using the Allium cepa root chromosomal aberration assay. Terr Aquat Environ Toxicol 6(2):90–95
Silveira MAD, Ribeiro DL, dos Santos TA, Vieira GM, Cechinato CN, Kazanovski M, d’Arce LPG (2016) Mutagenicity of two herbicides widely used on soybean crops by the Allium cepa test. Cytotechnology 68(4):1215–1222. https://doi.org/10.1007/s10616-015-9881-x
Silveira GL, Lima MGF, dos Reis GB, Palmieri MJ, Andrade-Vieria LF (2017) Toxic effects of environmental pollutants: comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere 178:359–367. https://doi.org/10.1016/j.chemosphere.2017.03.048
Singh D, Roy BK (2017) Evaluation of malathion-induced cytogenetical effects and oxidative stress in plants using Allium test. Acta Physiol Plant 39(4):92–102. https://doi.org/10.1007/s11738-017-2391-z
Şişman T, Türkez H (2010) Toxicologic evaluation of imazalil with particular reference to genotoxic and teratogenic potentials. Toxicol Ind Health 26(10):641–648. https://doi.org/10.1177/0748233710375951
Smilanick JL, Brown GE, Eckert JW (2006) The biology and control of postharvest diseases. Fresh citrus fruits, Florida Science Source, Florida
Sudhakar R, Ninge Gowda KN, Venu G (2001) Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia 66(3):235–239. https://doi.org/10.1508/cytologia.66.235
Tanaka T, Ogata A, Inomata A, Nakae D (2013) Effects of maternal exposure to imazalil on behavioral development in F1-generation mice. Birth Defects Res B 98(4):334–342. https://doi.org/10.1002/bdrb.21070
Teixeira RDO, Camparoto ML, Mantovani MS, Vicentini VEP (2003) Assessment of two medicinal plants, Psidium guajava L. and Achillea millefolium L., in vitro and in vivo assays. Genet Mol Biol 26(4):551–555. https://doi.org/10.1590/S1415-47572003000400021
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35(3):206–221. https://doi.org/10.1002/(SICI)1098-2280(2000)35:3<206::AID-EM8>3.0.CO;2-J
Tkalec M, Malarić K, Pavlica M, Pevalek-Kozlina B, Vidaković-Cifrek Ž (2009) Effects of radiofrequency electromagnetic fields on seed germination and root meristematic cells of Allium cepa L. Mutat Res 672:76–81. https://doi.org/10.1016/j.mrgentox.2008.09.022
Türkoğlu Ş (2012) Determination of genotoxic effects of chlorfenvinphos and fenbuconazole in Allium cepa root cells by mitotic activity, chromosome aberration, DNA content, and comet assay. Pestic Biochem Phys 103(3):224–230. https://doi.org/10.1016/j.pestbp.2012.06.001
USEPA (United States Environmental Protection Agency) (2005) Registration document for −Imazalil (EPA-738-F-04-011)
Van Leemput L, Swysen E, Woestenborghs R, Michielsen L, Meuldermans W, Heykants J (1989) On the terrestrial toxicity of the fungicide imazalil (enilconazole) to the earthworm species Eisenia foetida. Ecotox Environ Safe 18(3):313–320. https://doi.org/10.1016/0147-6513(89)90025-0
Vindas R, Ortiz F, Ramirez V, Cuenca P (2004) Genotoxicity of three pesticides used in Costa Rican banana plantations. Revista de Biologia Tropical 52(3):601–609
Walker CH (2008). Organic pollutants: an ecotoxicological perspective (second ed.) CRC Press/Taylor & Francis, Boca
Webster PL, Macleod RD (1996) The root apical meristem and its magrin, in: Waishel Y, Eshel a, Kafkafi U (Eds.) Plant roots. The hidden half (second ed.), Marcel Dekker, New York, ss 51–76
Xu L, Luan F, Liu H, Gao Y (2015) Dispersive liquid–liquid microextraction combined with non-aqueous capillary electrophoresis for the determination of imazalil, prochloraz and thiabendazole in apples, cherry tomatoes and grape juice. J Sci Food Agr 95(4):745–751. https://doi.org/10.1002/jsfa.6834
Zega G, De Bernardi F, Groppelli S, Pennati R (2009) Effects of the azole fungicide imazalil on the development of the ascidian Ciona intestinalis (Chordata, Tunicata): morphological and molecular characterization of the induced phenotype. Aquat Toxicol 91(3):255–261. https://doi.org/10.1016/j.aquatox.2008.11.015
Zhang R, Pan Z, Wang X, Shen M, Zhou J, Fu Z, Jin Y (2018) Short-term propamocarb exposure induces hepatic metabolism disorder associated with gut microbiota dysbiosis in adult male zebrafish. Acta Biochim Biophys Sin 51(1):88–96. https://doi.org/10.1093/abbs/gmy153
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Çıldır, D.S., Liman, R. Cytogenetic and genotoxic assessment in Allium cepa exposed to imazalil fungicide. Environ Sci Pollut Res 27, 20335–20343 (2020). https://doi.org/10.1007/s11356-020-08553-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08553-2