Abstract
This study evaluated the mutagenic effects of two herbicides: Clorimurom Nortox® and Imazaquim Ultra Nortox® widely used on soybean crops in Brazil. As a test system, Allium cepa assay was used, which analyzes the frequency of micronuclei (MN), chromosomal aberrations (CA) and the mitotic index (MI). Four concentrations of each herbicide (50, 75, 100 and 125 %) were tested in triplicate using distilled water (negative control) and methyl methanesulfonate (positive control) as controls. Three experimental repetitions were realized. Clorimurom Nortox® showed a significantly lower MI than the negative control for the concentrations of 75, 100 and 125 %, but the CA was significantly increased at all concentrations. There was no recovery for CA or MI. The 125 % concentration of Imazaquim Ultra Nortox® was cytotoxic and also exerted an effect on the other parameters. The concentration of 100 % showed a statistically increased MN and there was no recovery, while the 75 % concentration significantly affected CA, with recovery observed. The two herbicides showed mutagenic damage in Allium cepa cells, which implies a careful handling of these products, to minimize the risk of human and environmental contamination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organic synthetic pesticides were introduced to Brazil in 1943 with the use of the DDT insecticide (Spadotto 2006). Currently, Brazil is the largest consumer of pesticides in the world and has occupied this position since 2010 (Abrasco 2012). This is due to a strong agricultural economy, primarily soybeans, which are grown in several states.
The herbicide Clorimurom Nortox® is classified as slightly toxic, and Imazaquim Ultra Nortox® as moderately toxic in the toxicological classification defined by the Brazilian Health Surveillance Agency (ANVISA). However, these two herbicides are classified as dangerous to the environment with respect to environmental hazard standards (ANVISA 2012).
The Allium cepa test shows a great correlation with the results obtained from mammalian test (Grant 1982; Chaparro et al. 2010), with an 82 % greater sensitivity compared to rodents (Rank and Nielsen 1994), is relatively inexpensive, and has a wide analytical range. This test has been widely used in toxicity, mutagenicity and genotoxicity studies for diverse hazardous contaminants such as pesticides, dyes, food preservatives, and hydrocarbons (Fatima and Ahmad 2006; Mitteregger et al. 2007; Feretti et al. 2007; Türkoğlu 2007; Leme and Marin-Morales 2008; Mustafa and Arikan 2008; Ashraf and Husain 2010), and is one of the most established test systems used to determine toxicity in several laboratories (Rank et al. 2002; Chandra et al. 2005; Yıldız et al. 2009). This assay demonstrates alterations in all phases of the cell cycle, which are considered evidence for mutagenic effects induced by clastogenic or aneugenic agents (classified according to the type of alteration induced) (Vidakovié-cifrek et al. 2002). Some of these alterations, such as chromosomal breaks and asynchronous micronuclei (MN), are chromosomal aberrations (CA) used to evaluate mutagenicity (Sobral et al. 2013). Recovery assays reveal ‘cell cycle delay’ effects which lead to late cell responses, and even though the cells are no longer subjected to direct toxic exposure, they continue to express genotoxic effects (Kirkland 1998; Komissarova et al. 2005).
The increased frequency of MN and CA in the A. cepa assay are strong evidence for mutagenicity of the substance evaluated (Ribeiro 2003), and analysis of these parameters is a simple and efficient way to assess the mutagenic effect promoted by the chemical(s) of interest (Leme and Marin-Morales 2009). The mitotic index (MI) is an indicator of cell proliferation (Gadano et al. 2002) and can be used to evaluate the level of cytotoxicity of an agent, as it is decreased or increased (Fernandes et al. 2007). Furthermore, the Allium test can be more sensitive than the Ames test, detecting some carcinogens which are negative in the Ames test (Rank and Nielsen 1994). Liman et al. (2015), in a recent study, showed that an AHAS inhibiting pesticide of the imidazolinone class (Imazetapyr), like Imazaquim Ultra Nortox®, caused cytotoxicity and mutagenic damage in Allium cepa roots.
This study is aimed to evaluate the mutagenic effects of two herbicides (Clorimurom Nortox® and Imazaquim Ultra Nortox®) widely used on soybeans in Brazil. These herbicides may be overused due to their hazard classification and because there is no specific legislation that recommends reliable mutagenic test before the product can be commercialized.
Materials and methods
The Herbicides
Clorimurom Nortox® (Nortox S.A, Arapongas/Brazil) has Clorimurom-ethyl as the active ingredient (Ethyl 2-(4-chloro-6-methoxypyrimidin-2 ylcarbamoylsulfamoyl) benzoate) and is part of the sulfonylurea chemical group. Imazaquim Ultra Nortox® (Nortox S.A, Arapongas/Brazil) has Imazaquin as the active ingredient ((RS)-2-(4-isopropyl-4-methyl-5-oxo-2 imidazolin-2-yl) quinoline-3-carboxylic) and is a member of the Imidazolinone group.
Dilution of the herbicides
The indicated dilution/concentration (used in soybean cultivation) on the label for each herbicide was taken as 100 % (Clorimurom Nortox—60 grams/hectare (g/ha), Imazaquim Ultra Nortox—1 Liters per cent/ha (L.p.c/ha)), which was further diluted to the 75 and 50 % concentrations. The 125 % concentration is an extrapolation (on the label) for soybeans, and was included because all the tested pesticides are known to be slightly or moderately toxic, which often leads to a lesser dilution of the same by farmers attempting to potentiate the action of the herbicides. The seeds were treated (1 mL) every 8 h, to avoid the filter paper on the petri dishes from drying, first with distilled water until the root reaches 1 cm length, and later with the respective concentration of the herbicide.
Procedure
Seeds of A. cepa were germinated at room temperature (25 ± 5 °C) covered with filter paper in petri dishes. The sprouts were kept moist with distilled water until they reached 1 cm in length. After this, the filter paper was replaced and the treatment with each herbicide was started. After 72 h of treatment, some of the seedlings were fixed while the remaining underwent recovery, which consisted of treatment with distilled water for an additional 48 h (fresh filter paper) before being fixed. Fixation was accomplished with Carnoy for 24 h after which the slides were stained based on the protocol of Grant (1982), with modifications, using Schiff’s reagent for 1 h and Acetic Carmine in the slides for counter staining.
Each treatment comprises analysis of 5000 meristematic cells in five slides (1000 cells per slide). Three biological replicates were evaluated, totalizing 15,000 meristematic cells analyzed per sample. Three genetic parameters were evaluated, the mitotic index (MI) the micronuclei frequency (MN) and chromosomal aberrations (CA). Images were captured with the aid of an Olympus DP 71 camera mounted onto an Olympus BX 60 using the DP manager software (version 3.1.1.208). Distilled water was used as the negative control and MMS (methyl methane sulfonate, 4 × 10−4 M, ACROS, Geel, Belgium) was used as the positive control.
Chromosomal aberrations analyzed
In this study were evaluated C-metaphases, anaphase-telophase bridges, chromosomal losses, chromosomal breaks, laggards and as other CA (multipolar anaphase, metaphase delay and nuclear buds).
Statistical analysis
Statistical analyses were performed comparing all groups (experimental and control) by One-Way ANOVA. When significant variations were observed, the Dunnett’s test was used, comparing all groups to the negative control. Recovery data were analyzed in a similar manner by comparing between groups after recovery (One-Way ANOVA and Dunnett’s test). SigmaStat 3.5 (Systat Software, Inc., Chicago, Il, USA) was used to perform the statistical analyses.
Results
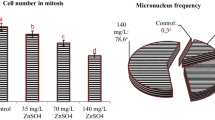
Treatment with Clorimurom Nortox® showed that the MI was significantly lower at concentrations of 75, 100 and 125 % compared to the negative control and with no recovery. The frequency of CA was significantly high at all concentrations. There was no recovery for CA in the roots after the treatment. The MN was not significant at any concentration studied, and was attributed to the low MI (Table 1).
Treatment with Imazaquim Ultra Nortox® showed that the 125 % concentration was cytotoxic to the roots of A. cepa. In addition to affecting the MI, there was deregulation of other parameters at this concentration. With regards to the other concentrations, the 100 % concentration significantly increased (p < 0.05) the frequency of MN with no recovery observed, while the 75 % concentration had a statistically significant effect on CA frequency (as compared to the negative control), but recovery was observed (Table 2).
Discussion
According to the literature, some herbicides are known to induce clastogenic and aneugenic effects in the Allium cepa test (Ateeq et al. 2002; Bolle et al. 2004; Fernandes et al. 2007). Others herbicides have the ability to directly interfere in plant cell division, elongation and/or cell differentiation, causing the disruption of the vascular tissue of roots (Linck 1979; Srivastava and Mishra 2009). In animals, herbicides can affect different tissues or organs and are often associated with the tumorigenic process (Natarajan 2002). It is also known that DNA damage caused by mutagenic compounds depends on the intensity, duration of exposure and the efficiency of DNA repair activated by the exposure (Pedrazzini et al. 2012).
Clorimurom Nortox® and Imazaquim Ultra Nortox® are herbicides of the sulfonylurea and imidazolinone chemical groups, and the mode of action affects the synthesis of the ALS/AHAS enzyme (ANVISA 2012). It is known that the toxicity levels of an agent can be determined by increases or decreases in MI. Having an MI that is significantly lower than the negative control indicates inhibition in cell proliferation (by the action of chemical substance) (Fernandes et al. 2007).
The trend in the data indicated that the MI was affected in some concentrations of both herbicides indicating an inhibition in cell proliferation, which is associated to a lower frequency with chromosomal aberration and micronuclei. According to Cotelle et al. (1999), there is evidence of severe inhibition in mitotic cells of A. cepa subjected to various soil contaminants such as heavy metals and certain herbicides. Bolle et al. (2004) showed an increase in the frequencies of CA and an inhibition of MI in a study that evaluated the genotoxicity of an atrazine herbicide, indicating atrazine was also a clastogenic agent due to the presence of a significant frequency of chromosomal breaks. Liman et al. (2015) showed a similar result, but with an imidazolinone herbicide called Imazetapyr. The same authors also showed a cytotoxic activity, due to a decrease in MI frequencies, and an induction of CA and DNA damage, concluding that this herbicide should be used under control in agricultural fields. Our study corroborates these results, confirming that Clorimurom Nortox® and Imazaquim Ultra Nortox® have some toxic chemicals in their composition that affects the cell cycle at higher concentrations, causing cytotoxicity. Masood and Malik (2013) found similar results from contaminated soils where cytotoxicity was associated with a decreased mitotic index.
In addition, the ALS enzyme, a common substrate for imidazolinones, sulfonylureas and sulphonanilide triazolopyrimidine herbicide classes, is not present in animals (ANVISA 2012). Therefore, in theory, the toxicity of these chemicals should be specific for plants and appears to be one of the main reasons for the use of these herbicide classes. Results obtained in vivo with rat experiments have shown that these herbicides are rapidly excreted before they can accumulate in the tissues or blood (Oliveira and Constantin 2001), which is an advantage for the use of these herbicides. However, Koutros et al. (2009) found a correlation between the increase of bladder and colon cancer in farmers and the use of an imidazolinone class herbicide with imazethapyr as the active ingredient. This demonstrates that the toxicity of these herbicides is not specific for plants (opposed to what ANVISA has proposed) and indicates a problem in the inordinate use of these herbicides. This has implications for new pesticides that are released without specific legislation requiring appropriate mutagenic tests before they are sent to open market.
Some of the cellular changes found during the analysis of these herbicides were chromosomal breaks in metaphase and/or anaphase, lagging chromosomes and bridges in anaphase or telophase (Fig. 1). It is known that anaphase bridges are formed during an unequal exchange of chromatids or by breakage and fusion of chromosomes and chromatids, and these bridges can cause structural chromosomal mutations (El-Ghamery et al. 2000). Chromosome stickiness has generally been inflicted by highly toxic agents and could lead cells to death (Barbério et al. 2011). Usually, chromosome bridges are found when A. cepa suffers from clastogenic damage. For example, Herrero et al. (2012) observed anaphase and telophase bridges, suggesting disturbances in the mitotic spindle, with three contaminants of emerging concern: di (2-ethylhexyl) phthalate, 5-chloro-2-(2,4-dichlorophenoxy) phenol (triclosan) propyl and -p-hydroxybenzoate (propylparaben). Our data corroborate this study, since we found common chromosomal aberrations and/or disturbances in the cell cycle, but with great impact to evaluate damage, all in the same test system.
Geras’kin et al. (2011) showed that the A. cepa test system is effective for evaluating the potential risks to human health and biological components of the natural ecosystem, detecting genotoxic damage. Thus, these results are of great concern in a world with a large demand for crop pesticides, which directly or indirectly affect humans, leading to food poisoning and other diseases. Additionally, few farmers read and follow the information on the herbicide label before handling it, and sometimes the information is incorrect even on the product label. Beside this, mutagenicity testing may not have been done prior to releasing the product to the market. Finally, there is a lack of specific legislation on the subject, further aggravating the situation.
Conclusions
The chromosomal aberrations (multipolar anaphases, anaphases bridges, chromosomal breakage and loss, C-metaphases and micronuclei) observed in this assay (Fig. 1) suggest that the two herbicides exert a mutagenic/cytotoxic effect. The concentrations/dilutions indicated on the product label caused mutagenic damage in A. cepa, supporting the view that this bioassay works well for evaluating the mutagenicity of chemical compounds that affect the environment. In addition, 48 h in distilled water was often insufficient to restore the cells to normalcy, indicating that the product may be bioaccumulative and can affect man via the diet. Therefore, the handling and use of these noxious products must follow all appropriate precautionary procedures, as there are several cases in literature regarding correlation between diseases, especially cancer, and occupational exposure to these non-biodegradable compounds.
References
ANVISA: Brazilian Health Surveillance Agency. SIA—system of information about herbicides (2012) http://www4.anvisa.gov.br/AGROSIA/asp/frm_pesquisa_agrotoxico.asp. Access in April 2012
Ashraf H, Husain Q (2010) Studies on bitter gourd peroxidase catalyzed removal of p-bromophenol from wastewater. Desalination 262:267–272
Ateeq MB, Farah MA, Ali N, Ahmad W (2002) Clastogenicity of pentachlorophenol, 2,4-D and butachlor evaluated by Allium root tip test. Mutat Res 514:105–113
Barbério A, Voltolini JC, Mello MLS (2011) Standardization of bulb and root numbers for the Allium cepa test. Ecotoxicology 20(4):927–935
Bolle P, Mastrangelo S, Tucci P, Maria G, Evandri MG (2004) Clastogenicity of atrazine assessed with the Allium cepa test. Environ Mol Mutagen 43:137–141
Chandra S, Chauhan LKS, Murthy RC, Saxena PN, Pande PN, Gupta SK (2005) Comparative biomonitoring of leachates from hazardous solid waste of two industries using Allium test. Sci Total Environ 347:46–52
Chaparro TR, Botta CM, Pires EC (2010) Biodegradability and toxicity assessment of bleach plant effluents treated anaerobically. Water Sci Tecnol 62:1312–1319
Cotelle S, Masfaraud JF, Fe´rard JF (1999) Assessment of the genotoxicity of contaminated soil with the Allium/Vicia-micronucleus and the Tradescantia-micronucleus assays. Mutat Res 426:167–171
Dôssie ABRASCO – Um alerta sobre os impactos dos agrotóxicos na saúde, ABRASCO, Rio de Janeiro, junho de 2012. 2ª Parte. pp 1–137
El-Ghamery AA, El-Nahas AI, Mansour MM (2000) The action of atrazine herbicide as an indicator of cell division on chromosomes and nucleic acid content in root meristems of Allium cepa and Vicia faba. Cytology 65:277–287
Fatima RA, Ahmad M (2006) Allium cepa derived EROD as a potential biomarker for the presence of certain pesticides in water. Chemosphere 62:527–537
Feretti D, Zerbini I, Zani C, Ceretti E, Moretti M, Monarca S (2007) Allium cepa chromosome aberration and micronucleus test applied to study genotoxicity of extracts from pesticide-treated vegetables and grades. Food Addit Contam 24:561–572
Fernandes TCC, Mazzeo DEC, Marin-Morales MA (2007) Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pestic Biochem Physiol 88:252–259
Gadano A, Gurni A, Lopez P, Ferraro G, Carballo M (2002) In vitro genotoxic evaluation of the medicinal plant Chenopodium ambrosioides L. J Ethnopharmacol 81:11–16
Geras’kin S, Oudalova A, Michalik B, Dikareva N, Dikarev V (2011) Geno-toxicity assay of sediment and water samples from the Upper Silesia post-mining areas, Poland by means of Allium-test. Chemosphere 83:1133–1146
Grant WF (1982) Chromosome aberration assays in Allium, a reporto of U.S. Environmental protection agency gene-tox program. Mutat Res 99:273–291
Herrero O, Martin JMP, Freire PF, Lopez LC, Peropadre A, Hazen MJ (2012) Toxicological evaluation of three contaminants of emerging concern by use of the Allium cepa test. Mutat Res 743:20–24
Kirkland D (1998) Chromosome aberration testing in genetic toxicology: past, present and future. Mutat Res Fundam Mol Mech Mut 404:173–185
Komissarova EV, Saha SK, Rossman TG (2005) Dead or dying: the importance of time in cytotoxicity assays using arsenite as an example. Toxicol Appl Pharmacol 202:99–107
Koutros S, Lynch CF, Ma X, Lee WJ, Hoppin JA, Christensen CH, Andreotti G, Freeman LB, Rusiecki JA, Hou L, Sandler DP, Alavanja MC (2009) Heterocyclic aromatic amine pesticide use and human câncer risk: results from the U.S, Agricultural Health Study. Int J Cancer 124:1206–1212
Leme DM, Marin-Morales MA (2008) Chromosome aberration and micronucleus frequencies in Allium cepa cells exposed to petroleum polluted water: a case study. Mutat Res 650:80–86
Leme DM, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res 682:71–81
Liman R, Ciğerci IH, Ozturk NS (2015) Determination of genotoxic effects of Imazethapyr herbicide in Allium cepa root cells by mitotic activity, chromosome aberration, and comet assay. Pestic Biochem Physiol 118:38–42
Linck AJ (1979) Effects on the cytology and fine structure of plant cells. Herbicides 1:83–121
Masood F, Malik A (2013) Cytotoxic and genotoxic potential of tannery waste contaminated soils. Sci Total Environ 444:153–160
Mitteregger H Jr, da Silva J, Arenzon A, Portela CS, Ferreira ICFS, Henriques JAP (2007) Evaluation of genotoxicity and toxicity of water and sediment samples from a Brazilian stream influenced by tannery industries. Chemosphere 67:1211–1217
Mustafa Y, Arikan ES (2008) Genotoxicity testing of quizalofop-P-ethyl herbicide using the Allium cepa anaphase-telophase chromosome aberration assay. Caryologia 61:45–52
Natarajan AT (2002) Chromosome aberrations: past, present and future. Mutat Res 504:3–16
Oliveira RS Jr, Constantin J (2001) Mecanismo de ação de herbicidas. Plantas daninhas e seu manejo. Agropecuária, Guaíba, pp 207–260
Pedrazzani R, Ceretti E, Zerbini I, Casale R, Gozio E, Bertanza G, Gelatti U, Donato F, Feretti D (2012) Biodegradability, toxicity and mutagenicity of detergents: integrated experimental evaluations. Ecotoxicol Environ Saf 84:274–281
Rank J, Nielsen MH (1994) Evaluation of Allium anaphase–telophase test in relation to genotoxicity screening of industrial wastewater. Mutat Res 312:17–24
Rank J, Lopez LC, Nielsen MH, Moretton J (2002) Genotoxicity of maleic hydrazide, acridine and DEHP in Allium cepa root cells performed by two different laboratories. Hereditas 136:13–18
Ribeiro LR (2003) Teste de Micronúcleo em Medula Óssea de Roedores In vivo. In: Ribeiro LR, Salvadori DMF, Marques EK, Canoas, Mutagênese Ambiental, p 174
Sobral O, Marin-Morales MA, Ribeiro R (2013) Could contaminant induced mutations lead to a genetic diversity overestimation? Ecotoxicol. doi:10.1007/s10646-013-1079-4
Spadotto CA (2006) Abordagem interdisciplinar na avaliação ambiental de agrotóxicos, Petrópolis, Vozes
Srivastava K, Mishra KK (2009) Cytogenetic effects of commercially formulated atrazine on the somatic cells of Allium cepa and Vicia faba. Peste Biochem Physiol 93:8–12
Turkoglu S (2007) Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat Res 626:4–14
Vidakovic-Cifrek Z, Pavlica M, Regula I, Papes D (2002) Cytogenetic damage in shallot (Allium cepa) root meristems induced by oil industry “High-Density Brines”. Arch Environ Contam Toxicol 43:284–291
Yıldız M, Cigerci IH, Konuk M, Fidan AF, Terzi H (2009) Determination of genotoxic effects of copper sulphate and cobalt chloride in Allium cepa root cells by chromosome aberration and comet assays. Chemosphere 75:934–938
Acknowledgments
Supported by Universidade Estadual do Oeste do Paraná (UNIOESTE), Fundação Araucária, Mr. Luiz Carlos Ribeiro and Herbioeste LTDA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silveira, M.A.D., Ribeiro, D.L., dos Santos, T.A. et al. Mutagenicity of two herbicides widely used on soybean crops by the Allium cepa test. Cytotechnology 68, 1215–1222 (2016). https://doi.org/10.1007/s10616-015-9881-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-015-9881-x