Abstract
One of the most important environmental problems in the world is micro-pollutants. The aim of this study was to investigate the antioxidant responses of Gammarus pulex to Bisphenol A (BPA), an endocrine-disrupting agent. For this purpose, sublethal concentrations of BPA were applied to G. pulex and biochemical responses were studied. Enzymatic antioxidants superoxide dismutase (SOD) and catalase (CAT) activities and nonenzymatic antioxidants glutathione (GSH) and thiobarbituric acid reagents (TBARS) levels in G. pulex were determined in four different groups during 24 and 96 h. Biochemical biomarkers were measured using commercial kits in a microplate reader. When we compared with control, SOD enzyme activity increased in all groups during both administration periods and CAT enzyme activity decreased in all groups. GSH and TBARS levels were increased after 24 and 96 h of application periods in all groups when compared with control. For changes in SOD and CAT activities and GSH, TBARS levels have been determined to be useful as biomarkers against BPA in G. pulex tissues. It has also been proven that G. pulex is an effective bioindicator that shows BPA pollution in water. The different results of biochemical biomarkers can be evaluated as a marker of possible metabolic processes, and the biochemical response of G. pulex can reveal to some extent the environmental consequences of BPA pollution resulting from industrial waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is a raw material mostly used in the plastic industry and an endocrine disruptor. The wide range of use increases the likelihood of human exposure to this chemical (Kang et al. 2006). BPA synthesized in 1891 firstly is one of the most widely used industrial chemicals (Dodds and Lawson 1938). The half-life of BPA in water is between 3 and 5 days and it is sufficient to affect the aquatic organisms (Fürhacker et al. 2000). BPA may cause toxic effects to aquatic organisms even with low concentrations (Tatar et al. 2018).
There is a significant balance between the formation of reactive oxygen species (ROS) and their removal by the antioxidant defense system. ROS are removed by enzymatic and nonenzymatic antioxidants (Serdar 2019). ROS formation in organisms exposed to BPA has also been shown in previous studies (Kabuto et al. 2003; Wu et al. 2011; Jemec et al. 2012). When cells are under oxidative stress, they often increase levels of antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) (Valavanidis and Vlachogianni, 2010; Serdar et al. 2018). SOD is a very important enzyme that catalyzes the conversion of superoxides into O2 and H2O (Ding et al. 2008). The main function of CAT is to remove hydrogen peroxide, synthesized in some stages of metabolism in the presence of molecular oxygen (Keha and Küfrevioğlu 2001). When oxidative stress increases in the organism, the formation of free radicals increases. GSH is responsible for protecting cells against the destructive effects of these free radicals (Meister and Anderson 1983). Lipid peroxidation occurs when the levels of free radicals increase and exceed the antioxidant capacity of the cells. Lipid peroxidation is defined as conversion of lipid hydroperoxide to aldehydes and other carbonyl compounds. One of these compounds is malondialdehyde (MDA) and can be used to determine the level of lipid peroxides (Khoschsorur et al. 2000). MDA reacts with thiobarbituric acid and forms TBARS. Lipid peroxidation and antioxidant defense mechanisms were used as biomarkers of oxidative stress in order to determine pollutant effects in water (Livingstone 2001; Valavanidis and Vlachogianni, 2010).
Gammarus sp. is an appropriate organism for the determination of the ecotoxicological effects of pollutants (Kunz et al. 2010; Yildirim and Yaman 2019).
This study was designed to determine some enzymatic (SOD and CAT) and nonenzymatic (GSH and MDA) antioxidants that are biomarkers of oxidative stress in G. pulex individuals exposed to BPA.
Materials and methods
Experimental setup
The model organisms were collected by hand nets from The Munzur River in Tunceli, Turkey, and were quickly brought to a laboratory at 18 °C and 12:12 light/dark period. It was placed in 20-L aerated aquariums filled with water and sediment taken from the natural environment. The organisms were fed with willow leaves during 2 weeks before starting the experimental studies (De Lange et al. 2006).
The lethal concentration of BPA to G. pulex was obtained by probit analysis and the value was calculated as 249.97 ± 1.4 μg/l BPA. Synthetic solutions containing three sublethal doses of BPA were applied to G. pulex (n: 10 per group) for 24 and 96 h. Four application groups were designed as A (group containing 25 μg BPA); B (group containing 12.5 μg BPA); C (group containing 6.25 μg BPA), and control group. G. pulex individuals were not fed during the experimental applications and were controlled per 24 h. Inactivity was accepted as the death criterion.
Determination of biochemical response
G. pulex organisms were weighed. Phosphate buffered saline (PBS buffer) was added at 1/5 w/v and homogenized with ice by using a homogenizer. These homogenized samples were centrifuged for 15 min at 17000 rpm in a refrigerated centrifuge and the obtained supernatants were stocked in the − 86 °C freezer until measurement was complete.
Biochemical biomarkers were determined by using the ELISA kits (catalog numbers of SOD, CAT, GSH, and TBARS: 706002; 707002; 703002 and 10009055, respectively) purchased from The CAYMAN Chemical Company.
Statistical analyses
SPSS 18 software was used for statistical analysis. One-way ANOVA and Duncan’s multiple range tests were employed to evaluate the statistical differences in three application groups (A, B, C) in the same exposure time (abcp < 0.05). Two-tailed independent T test was used to compare the differences between exposure times in the same application group (*p < 0.05).
Results
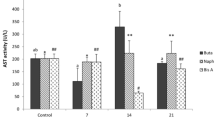
SOD activities increased in all groups compared with the control group after 24 h (p < 0.05). However, no statistically significant changes were found in all groups compared with the control group after 96 h (p > 0.05). When the exposure times were compared, a significant difference was not found in all application groups (p > 0.05; Fig. 1a).
a SOD activities (U/ml), b CAT activities (nmol/min/ml), c GSH levels (μM) and d TBARS levels (μM) of G. pulex exposed to BPA. The asterisk shows statistical differences according to the two-tailed independent T test between different exposure times in the same group; *p < 0.05. The letters (a, b, c) show statistical differences of Duncan’s multiple range test between all application groups in the same exposure time; abcp < 0.05. Values represent mean ± SE; n = 10
CAT activities decreased in all groups compared with that in the control group after 24 and 96 h (p < 0.05). This decrease was not statistically significant only in group A after 24 h (p > 0.05; Fig. 1b).
GSH levels increased in all groups compared with the control group after 24 and 96 h (p < 0.05). When the exposure times were compared, significant differences were found in A and B groups (p > 0.05; Fig. 1c).
TBARS levels increased in all groups compared with the control group after 24 and 96 h (p < 0.05). When the exposure times were compared, significant differences were found in A and C groups (p > 0.05; Fig. 1d).
Discussion
BPA, which is an endocrine disrupter, is a raw material in the plastics industry. BPA has become a critical environmental problem due to its widespread use in the sector. According to the latest estimates, worldwide BPA production is annually 6.8 million tons (Jandegian et al. 2015). Estrogenic activity of BPA has been confirmed in many toxicological studies using animals. Physiological functions in the reproductive organs and endocrine system can be impaired (Crain et al. 2007; Zhang et al. 2016). In this study, some enzymatic and nonenzymatic antioxidant parameters were investigated in G. pulex exposed to various concentrations of BPA (Watts et al. 2001; Schirling et al. 2006). In our study, the enzymatic antioxidants SOD and CAT and nonenzymatic antioxidants MDA and GSH levels were used as biochemical biomarkers to determine the effects of BPA application.
SOD and CAT enzyme activities are the first defense systems against oxygen toxicity (Zagal and Mazmancı 2011). SOD converts reactive and toxic superoxide radicals to hydrogen peroxide (H2O2) (Duman and Kar 2015). CAT enzyme reduces H2O2 to nontoxic H2O and O2, which are innocuous to cells (Li et al. 2012). These enzyme activities can be used as early indicators against xenobiotics that is the source of oxidative stress. In our study, the SOD activity increased after 24 h in all groups compared with the control group and the highest activity was found in group A. After 96 h, an increase was observed only in group A and no change was observed in groups B and C compared with the control group. It is thought that the increase in the SOD activity may lead to H2O2 accumulation and this will cause the inhibition of the CAT activity (Li et al. 2012). Previous studies have reported that ROS may inhibit the CAT activity (Kono and Fridovich 1982; Escobar et al. 1996). The effect of BPA on the antioxidant defense system in Arabidopsis thialiana was investigated and an increase in SOD activity was detected (Imran et al. 2017). In our study, CAT activity decreased after 24 and 96 h of application compared with the control group. Oxidative stress caused by BPA may have led to depletion of SOD and CAT activities, which may be indicative of weakening of the antioxidant system (Li et al. 2012). Özaydın et al. (2018) investigated the effect of BPA on antioxidant defense system in rats and they found that SOD and CAT activities were decreased significantly at the end of the application. Faheem and Parvez Lone (2017), in their study on Ctenopharyngodon idella, reported that BPA reduces CAT activity. In another study that applied BPA to chicken embryos, SOD activity was found to be increased (Sravani et al. 2015).
It is suggested that increased GSH levels have been reported to be a response to adaptation to increasing stress conditions (Moreno et al. 2010; Serbes 2011). The use of GSH in elimination of detoxified peroxide products formed by lipid peroxidation caused to decrease the GSH levels (El-Gendy et al. 2010). In our study, the duration of exposure was found to affect GSH levels in groups A and B. The highest GSH level was found in group B after 96 h, and GSH levels in groups A and C increased after 24 and 96 h compared with the control group. Therefore, the increases observed in G. pulex may be due to adaptation mechanisms developed against stress. Özaydın et al. (2018) found that GSH levels decreased significantly at the end of BPA exposure in their study on rats. In a study with Ctenopharyngodon idella, BPA was found to significantly reduce GSH levels (Faheem and Parvez Lone 2017).
The increase in MDA levels, which is an indicator of lipid peroxidation, suggests that BPA causes oxidative stress in G. pulex. Xenobiotics can increase the amount of MDA by increasing free radical formation or by reducing the cell’s defend capacity against peroxidation reactions (Lackner 1998). In our study, the duration of exposure was found to affect MDA levels in groups A and C. The highest levels of MDA were found in group A after 96 h of administration and MDA levels in groups B and C increased after 24 and 96 h compared with the control group. MDA levels were found to be increased in a study in which BPA was applied to chicken embryos (Sravani et al. 2015). In a study conducted on freshwater fish, MDA levels were found to be increased at the end of the application similar to our study (Faheem and Parvez Lone 2017).
Conclusion
According to the data obtained in the present study, it is believed that BPA, which is mostly used in the plastics industry, has a toxic effect on G. pulex and pollution caused by BPA may pose a great risk to the environment. MDA levels increased in G. pulex exposed to BPA compared with the control group. This is an indicator of lipid peroxidation. Changes in lipid peroxidation and increased GSH levels indicate antioxidant defense. Changes in the level of GSH suggest that there is a tendency to adapt to stress. Partial reductions in the level of GSH are due to the use of GSH in removal of ROS.
Furthermore, the differences in exposure time and application dose according to the obtained data changed the biochemical responses of G. pulex. For changes in the antioxidant enzymes and GSH, MDA levels showed that stresses caused by BPA metabolism are modifiable. It has been indicated that the toxicity of metabolites induced the changes in the antioxidant defense system.
As a compensatory response to low levels of oxidative stress, the antioxidant enzyme system can be induced. When severe oxidative stress occurs, the antioxidant enzyme activities can inhibit and cause oxidative damage. It was concluded that the biomarkers we used to investigate the toxic effects of BPA on G. pulex were useful biomarkers.
References
Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette LJ (2007) An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol 24(2):225–239
De Lange HJ, Noordoven W, Murk AJ et al (2006) Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat Toxicol 78(3):209–216
Ding F, Song WH, Guo J, Gao ML, Hu WX (2008) Oxidative stress and structure activity relationship in the zebrafish (Danio rerio) under exposure to paclobutrazol. J Environ Sci Health Part B 44(1):44–50
Dodds E, Lawson W (1938) Molecular structure in relation to oestrogenic activity. Compounds without a phenanthrene nucleus. Proceedings of the royal society of London, Series B, Biological sciences 125:222–232
Duman F, Kar M (2015) Evaluation of effects of exposure conditions on the biological responses of Gammarus pulex exposed to cadmium. Int J Environ Sci Technol 12:437–444
El-Gendy KS, Aly NM, Mahmoud FH, Kenawy A, El-Sebae AKH (2010) The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol 48:215–221
Escobar JA, Rubio MA, Lissi EA (1996) SOD and catalase inactivation by singlet oxygen 4 and peroxyl radicals. Free Radic Biol Med 20:285–290
Faheem M, Parvez Lone K (2017) Oxidative stress and histopathologic biomarkers of exposure to bisphenol-A in the freshwater fish. Ctenopharyngodon idella Braz J Pharm Sci. https://doi.org/10.1590/s2175-97902017000317003
Fürhacker M, Scharf S, Weber H (2000) Bisphenol A: emissions from point sources. Chemosphere 41:751–756
Imran A, Mehmood J, Abdul W, Azizullah A, Bohan L, Faisal I, Abid A, Daud MK, Yihua L, Yinbo G (2017) Biochemical responses and ultrastructural changes in ethylene insensitive mutants of Arabidopsis thialiana subjected to bisphenol A exposure. Ecotoxicol Environ Saf 144:62–71
Jandegian CM, Deem SL, Bhandari RK, Holliday CM, Nicks D, Rosenfeld CS, Selcer KW, Tillitt DE, Vom Saal FS, Velez-Rivera V, Yang Y, Holliday DK (2015) Developmental exposure to bisphenol A (BPA) alters sexual differentiation in painted turtles (Chrysemys picta). Gen Comp Endocrinol 216:77–85
Jemec A, Tisler T, Erjavec B, Pintar A (2012) Antioxidant responses and whole-organism changes in Daphnia magna acutely and chronically exposed to endocrine disruptor bisphenol A. Ecotoxicol Environ Saf 86:213–218
Kabuto H, Hasuike S, Minagawa N, Shishibori T (2003) Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ Res 93:31–35
Kang J, Kondo F, Katayama Y (2006) Human exposure to bisphenol A. Toxicol 226:79–89
Keha EE, Küfrevioğlu Öİ (2001) Biyokimya. İstanbul, Turkey
Khoschsorur GA, Winklhofer-Roob BM, Rab PH (2000) Evaluation of a sensitive HPLC method for the determination of malondialdehyde and application of the method to different biological materials. Chromatogr A 52:181–184
Kono Y, Fridovich I (1982) Inhibition of catalase by superoxide radicals. Fed Proc 257(10):2571–5754
Kunz PY, Kienle C, Gerhardt A (2010) Gammarus spp. in aquatic ecotoxicology and water quality assessment: toward integrated multilevel tests. Rev Environ Contam T 205:1–76
Lackner R (1998) Oxidative stress in fish by environmental pollutants. Fish Ecotoxicol 203–224
Li Y, Shi JQ, Qu RJ, Feng MB, Liu F, Wang M, Wang ZY (2012) Toxicity assessment on three direct dyes (D-BLL,D-GLN,D-3RNL) using oxidative stress bioassay and quantum parameter calculation. Ecotoxicol Environ Saf 86:132–140
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42(8):656–666
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Moreno DH, Soler F, Míguez MP, Pérez-López M (2010) Brain acetylcholinesterase, malondialdehyde and reduced glutathione as biomarkers of continuous exposure of tench, Tinca tinca, to carbofuran or deltamethrin. Sci Total Environ 408:4976–4983
Özaydın T, Öznurlu Y, Sur E, Çelik I, Uluışık D, Dayan MO (2018) Effects of bisphenol A on antioxidant system and lipid profile in rats. Biotech Histochem. https://doi.org/10.1080/10520295.2017.1420821
Schirling M, Jungmann D, Ladewig V, Ludwichowski KU, Nagel R, Kohler HR, Triebskorn R (2006) Bisphenol A in artificial indoor streams: II. Stress response and gonad histology in Gammarus fossarum (Amphipoda). Ecotoxicology 15:143–156
Serbes D (2011) Cyfluthrin, imidacloprid ve karişim uygulamalarinin Cyprinus Carpio’da beyin ve karaciğer dokularinda glutatyon, malondialdehit ve protein karbonil düzeylerine etkileri. University of Çukurova, Dissertation
Serdar O (2019) The effect of dimethoate pesticide on some biochemical biomarkers in Gammarus pulex. Environ Sci Pollut R 26:21905–21914
Serdar O, Yildirim NC, Tatar S, Yildirim N, Ogedey A (2018) Antioxidant biomarkers in Gammarus pulex to evaluate the efficiency of electrocoagulation process in landfill leachate treatment. Environ Sci Pollut R 25:12538–12544
Sravani J, Padmaja K, Eswara Prasad P, Punya Kumari B (2015) Effect of bisphenol-A on antioxidant enzymes and lipid peroxidation in liver of chick embryos. Int J Meat Sci 6:1–5
Tatar S, Cikcikoglu Yildirim N, Serdar O, Yildirim N, Ogedey A (2018) The using of Gammarus pulex as a biomonitor in ecological risk assessment of secondary effluent from municipal wastewater treatment plant in Tunceli, Turkey. Hum Ecol Risk Assess 24(3):819–829
Valavanidis A, Vlachogianni T (2010) Integrated biomarkers in aquatic organisms as a tool for biomonitoring environmental pollution and improved ecological risk assessment. Science advances on environmental chemistry, toxicology and ecotoxicology issues, www.chem-tox-ecotox.org
Watts M, Pascoe D, Carroll K (2001) Survival and precopulatory behaviour of Gammarus pulex (L.) exposed to two xenoestrogens. Water Res 35(10):2347–2352
Wu M, Xu H, Shen Y, Qiu W, Yang M (2011) Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environ Toxicol Chem 30:2335–2341
Yildirim NC, Yaman M (2019) The usability of oxidative stress and detoxification biomarkers in Gammarus pulex for ecological risk assessment of textile dye methyl orange. Chem Ecol 35(4):319–329
Zagal A, Mazmancı B (2011) Oxidative stress response in Nile tilapia (Oreochromis niloticus) exposed to textile mill effluent. Toxicol Ind Health 27(1):81–85
Zhang JZ, Li XY, Zhou L, Wang LH, Zhou Q, Huang XH (2016) Analysis of effects of a new environmental pollutant, bisphenol A, on antioxidant systems in soybean roots at different growth stages. Sci Rep 6:23782
Funding
This study was financially supported by the Scientific Research Projects Coordination Unit of Munzur University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tatar, Ş., Türkmenoğlu, Y. Investigation of antioxidant responses in Gammarus pulex exposed to Bisphenol A. Environ Sci Pollut Res 27, 12237–12241 (2020). https://doi.org/10.1007/s11356-020-07834-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07834-0