Abstract
In this presented study, it was aimed to determine the effects of pesticides on non-target organisms on the freshwater amphipod, Gammarus pulex, by biochemical responses. Acute toxicity value (LC50) in G. pulex of the dimethoate pesticide was determined. The superoxide dismutase (SOD), glutathione S-transferaz (GST), glutathione peroxidase (GPx), and catalase (CAT) activities and malondialdehyde (MDA), glutathione (GSH) levels of the G. pulex organism exposed to the subletal concentrations were analyzed by ELISA for 24 and 96 h. In conclusion, the present study demonstrated the abilities of dimethoate pesticide induce to oxidative stress. The results revealed that MDA, GSH levels SOD, CAT, GPx, and GST activities of G. pulex can be used as an effective biomarkers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater ecosystems are affected by a large number of human activities pesticides, nutrients, etc. that lead to water pollution (Malaj et al. 2014; Sarriquet et al. 2006; UNEP 2010; Vörösmarty et al. 2010; Münze et al. 2015). Pesticides used in agriculture are chemical substances or biological substances which produced to prevent the growth of pest animals and plants to repel these harms or to reduce the number of these harms (US Environmental Protection Agency 2007; Uğurlu et al. 2015). Pesticides applications for plant protection was contaminated to water ecosystems to via surface waters (Iwakuma et al. 1993), drainage (Bennett et al. 2005) drifting with precipitation (Schulz et al. 2001; Bundschuh et al. 2013).
Organophosphorus (OP) insecticides have been replaced by organochlorine pesticides due to their rapid degradation in the water and low environmental persistence (Lundebye et al. 1997). However, their widespread use has resulted in high pollution risks for water environments (Gabrielides 1995). Dimethoate is a dithiophosphate insecticide with a wide range of applications worldwide in various fruits and vegetables and various products (Hassall 1990; Sorensen et al. 1995).
Reactive oxygen species (ROS), the antioxidant system, induce oxidative stress, lipid peroxidation, and oxidation of proteins during metabolism (Sturve et al. 2008; Almroth et al. 2008; Tatar et al. 2018). There is an important balance between the production of ROS and the removal of antioxidant defense systems by organisms. ROS are scavenged by antioxidant enzymes and non-enzymatic antioxidants (Hermes-Lima 2004; Halliwell and Gutteridge 2007; Serdar et al. 2018). Lipid peroxidation is a chain reaction in which oxidants break down membrane phospholipids with polyunsaturated fatty acids. Lipid peroxidation causes damage to bio-membranes, which can lead to significant consequences for living organisms (Hermes-Lima 2004; Jemec et al. 2012; Serdar et al. 2018). The reactive aldehydes produced during lipid peroxidation can spread from the production site damage intracellular targets (Esterbauer et al. 1990). The induction of high ROS production can lead to oxidative damage to important cellular macromolecules (lipids, proteins, nucleic acids, etc.) (Bergamini et al. 2004; Yonar and Sakin 2011). Glutathione (GSH) is an important antioxidant that acts as a direct scavenger of oxidants as well as an antioxidant enzyme substrate (Della et al. 1994; Ferrari et al. 2007). Depletion leads to an imbalance in the redox state and the ability to cope with organic xenobiotics metabolized by glutathione S-transferase (GST) and glutathione peroxidases (GPx). The biological role of superoxide dismutase (SOD) antioxidant defense is to defend cells against the toxic effects of O2 by catalyzing dismutation reactions (Prasad 2004). Aerobic organisms have an antioxidant defense system against various stress factors (Sies 1986). GPx reduces reactive lipid hydroperoxides to prevent malondialdehyde (MDA) formation (Flohe 1989; Vijayavel et al. 2004; Tatar et al. 2018). An increase in the catalase (CAT) and the SOD activity is generally observed in response to environmental pollutants since the first defense line against the oxidative stress represents the SOD-CAT system (McCord 1996).
Assessments of the biomarkers as an early warning of adverse changes and damage have been shown to be a suitable tool in G. pulex. Environmental pollutions such as pesticide possibly play a role in the generation of oxidative stress in cells (Dat et al. 2000; Achary et al. 2008; Yildirim et al. 2018).
Crustaceans are used as bioindicators in most aquatic ecosystems (Rinderhagen et al. 2000). With their abundance in fresh water, their high ecological relevance, and their important role in the food chain, the amphipods of the gammarus genus are often used in ecotoxicological studies (Kunz et al. 2010; Adam et al. 2010; Serdar et al. 2018). Among freshwater species, Gammarus sp. is a suitable organism for ecotoxicological assessment of environmental pollutants at a large scale, mostly because gammarid are present in most river in Europe (Jażdżewski 1980). Gammarids are known to be sensitive to pollutants and can easily be used for laboratory and field studies (Kunz et al. 2010). Hence, many ecotoxicological studies were carried out using gammarids to evaluate toxic impact of xenobiotics (Sornom et al. 2010; Khan et al. 2011; Sroda and Cossu-Leguille 2011; Gismondi et al. 2012). Bioassays and biomarkers have attracted much attention since they are an attractive alternative to chemical determinations and complementary to the assessment of the environmental impacts of wastes (Petala et al. 2009). One of the methods to determine pollution in the aquatic environment is to determine the effects of the environment on aquatic organisms.
This present study addressed the changes on some oxidative stress biomarkers in G. pulex exposed to sub-lethal concentrations of dimethoate. For this purpose, antioxidant enzyme activities like SOD, GPx, GST, and CAT as well as level of MDA linked to lipid peroxidation and non-enzymatic parameter GSH level were measured as biomarkers of biochemical responses of non-target organisms exposed to dimethoate.
Material methods
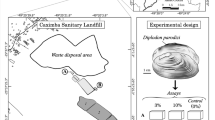
Organisms for experimental procedure were collected with handnets (kick sample) in the Munzur River (39.156820 N, 39.499640 E) in Tunceli, Turkey (Fig. 1). The organisms were then transferred to laboratory in a ventilated plastic container. Organisms were stored in an 80-L aquarium, which oxygenated with air pump in a room controlled by air condition at 18 °C±0.5 temperature and 12:12 h light-dark circles in laboratory conditions. Willow leaves used for fed of organisms. They were at least 15 days to this adaption conditions before they were used for experiments. Organisms are similar, healthy, active, and adult male individuals (W=0.0314±0.0076 g; L=10.23±0.55 mm) were selected for the study (De Lange et al. 2006). The organisms were checked per 24 h.
Area where G. pulex organisms are collected (URL-1 2018)
Acute toxicity (LC50)
Static non-renewal tests were used in this study (Weber 1991), after the interval was determined, organisms were exposed to different dimethoate concentrations (0 (control), 20, 40, 80, 160, and 320 μg L−1 dimethoate). At all stages of the LC50 experiment, ten organisms were exposed to dimethoate concentrations determined in 1-L glass aquariums. Organisms did not feed during the 96-h LC50 trials. Organisms were checked per 24 h. Dead individuals were counted and removed from the experimental environment. Inactive organisms were considered dead.
LC50 experiments were repeated three times. The obtained data were calculated by probit analysis and LC50 values were determined for 96 h (Weber 1991).
Experimental design
The experimental study was designed into four groups:
-
Group 1;
(Control (C)), not contained in the concentration of dimethoate, kept in the water taken from the natural environment of organisms.
-
Group 2;
(D1) was applied 10 μg/L concentration of dimethoate, about 1/16 of LC50 concentration.
-
Group 3;
(D2) was applied 20 μg/L concentration of dimethoate, about 1/8 of LC50 concentration.
-
Group 4;
(D3) was applied 40 μg/L concentration of dimethoate, about 1/4 of the LC50 concentration.
The living beings used in the study were selected from similar sized and healthy individuals (De Lange et al. 2006). The study was repeated three times. Ten organisms were used in each 1-L aquarium. Two time groups (24 and 96 h) for four groups and a total of 240 organisms were used in three subletal dimethoate groups.
Biochemical analyses
The samples were weighed and homogenized by adding PBS buffer (salt solution buffered with phosphate) at a rate of 1/5 w/v and using a homogenizer with ice to measure antioxidant parameters. The samples were centrifuged at 17,000 rpm for 15 min; the supernatants were kept in deep freeze at −70 °C until their measurements were done. The concentration of GSH was performed by the method of Beutler et al. (1963) and expressed as nanomoles per gram tissue. MDA level, SOD, CAT, and GPx activities were conducted by using ELISA kit. The activities of SOD, CAT, and GPx were determined by ELISA kits (catalog numbers, CAT 707002, SOD 706002, and GPx 703102) purchased from the CAYMAN Chemical Company.
Statistical analysis
In this study, the LC50 value has been calculated using probit analysis. The SPSS 24.0 package program one-way ANOVA (Duncan 0.05) has been used for the evaluation of biochemical analyzes.
Results
Acute toxicity (LC50) value
In this study, the average of the LC50 value of G. pulex, exposed to three repetitive dimethoate pesticides, was found to be 167 μg L−1.
The GSH levels
The GSH levels were significantly reduced in D1, D2, and D3 groups at 24 and 96 h compared to the control group (p>0.05; Fig. 2).
The MDA levels
The MDA levels were significantly increased in D1, D2, and D3 groups at 24 and 96 h compared to the control group (p>0.05) (Fig. 3).
The SOD activity
The SOD enzyme activity was found to be statistically significantly reduced in the D1 and D2 groups compared to that the control group at 24 h (p<0.05). At the 96 h, the SOD enzyme activity was found to be statistically significantly reduced (p<0.05) in the D3 group compared to that in the control group (Fig. 4).
The GPx activity
The GPx enzyme activity was not statistically significantly reduced in D1, D2, and D3 groups compared to that in the control group (p>0.05). At the 96 h, the GPx enzyme activity was found to be statistically significantly reduced (p<0.05) in the D3 group compared to that in the control group (p<0.05) (Fig. 5).
The GST activity
The GST enzyme activity was found to be statistically significantly reduced (p<0.05) in the D3 group compared to that in the control group at 24 h. At the 96 h, the GST enzyme activity was not statistically significantly reduced (p>0.05) in D1, D2, and D3 groups compared to that in the control group (Fig. 6).
The CAT enzyme activity
The CAT enzyme activity was found to be statistically significantly reduced (p<0.05) in the D3 group compared to that in the control group at 24 h. At the 96 h, the CAT enzyme activity was statistically significantly reduced in D1, D2, and D3 groups compared to that in the control group (Fig. 7).
Discussion
GSH; MDA levels; and SOD, GPX, GST, and CAT enzyme activities of G. pulex exposed to dimethoate pesticide for 24 and 96 h were determined. The present study demonstrates that dimethoate pesticides cause to oxidative stress in G. pulex.
The GSH, an important component of antioxidant defense, is a non-enzymatic scavenger. Glutathione reacts with free radicals and peroxides and protects the cells against oxidative damage (Meister and Anderson 1983). GSH levels in organisms exposed to dimethoate pesticide decreased in both 24 and 96 h compared to the control group. Similarly to this study, a decrease in GSH content produced by anticholinesterase agents has also been reported in diverse aquatic organisms (Venturino et al. 2001; Hai et al. 1997a, b; Della et al. 1994; Ferrari et al. 2007; Serdar et al. 2018; Yildirim et al. 2018). GSH depletion has been associated with oxidation of glutathione peroxidases due to an increase in free radicals and/or the direct oxidation of these compounds (Hai et al. 1997a, b; Almar et al. 1998; Ferrari et al. 2007). In addition, the decrease in GSH content may be associated with its role as a GST substrate in detoxification reactions.
Lipid peroxidation, considered as a valuable indicator of oxidative damage of cellular components known as the first step in cellular membrane damage, is caused by pesticides, metals, and other xenobiotics (Gamble et al. 1995; Regoli et al. 1998). It is likely that most components of cellular structure and function are potentially oxidative damage targets, and the most susceptible substrates for auto-oxidation are polyunsaturated fatty acids of the cell membrane undergoing rapid eradication. Considering that the typical reaction during ROS-induced damage involves the peroxidation of unsaturated fatty acids, our results clearly showed that dimethoate exposure for 24 and 96 h led to oxidative stress, with significant increases of MDA values in organisms when compared to the control group. In this study, increased hydroperoxide lipid production suggests that ROS-induced oxidative damage may be one of the main toxic effects of dimethoate. It has been reported that MDA can be induced by various environmental pollutants in studies conducted on various aquatic organisms (Ploch et al. 1999; Ahmad et al. 2000; Wilhelm Filho and Marcon 1996; Oakes and Van der Kraak 2003; Oakes et al. 2004; Mensah et al. 2012; Yildirim et al. 2018). However, in the D1 group (lowest concentration) at 96 h, MDA level was found to be the highest. Similarly, Oliveira et al. (2013) observed the same effect in the shrimp Palaemon serratus which exposed to fenitrothion. In another study, Lavarías and García (2015) Macrobrachium borellii in organophosphate fenitrothion exposure were similar results. For these results, the probability of increased of the level of MDA in the concentration said in this study may be that CAT activities were inhibited at 96 h of dimethoate exposure.
The SOD, which is a group of metalloenzymes, is a primary defense against the toxic effects of superoxide radicals in aerobic organisms and catalyzes the transformation of superoxide radicals to H2O2 and O2, which play an important role in the antioxidant system (Kappus 1985; Kohen and Nyska 2002; Yonar and Sakin 2011). It is not a general rule that an increase in xenobiotic concentrations induces antioxidant activity (Cheung et al. 2001). In some cases, O2− by itself or after its transformation to H2O2 causes a strong oxidation of the cysteine in the enzyme and decrease the SOD activity (Dimitrova et al. 1994; Durmaz et al. 2006). In the presence of xenobiotic, an initial decreased response in the antioxidant system may be followed by an induction. Thus, the existence of an inducible antioxidant system may reflect an adaptation of organism (Doyotte et al. 1997; Oruç and Usta 2007). The response of antioxidant system to oxidative stress in various tissues shows differences from one species to another due to the differences in antioxidant potential of these tissues (Ahmad et al. 2000; Oruç and Usta 2007). In this study, SOD activity has been decreased after dimethoate exposure in G. pulex. Similar to this study, studies have reported that SOD activity is also reduced in some aquatic organisms exposed to various pollutants (Durmaz et al. 2006; Tatar et al. 2018; Serdar et al. 2018).
The GPx, considered as a protective enzyme against lipid peroxidation at the expense of GSH, catalyzes the reduction of hydrogen peroxide and lipid peroxides (Moreno et al. 2005). Monteiro et al. (2006) have shown that the activity of this enzyme is reduced by negative feedback resulting from the excess of the substrate or the damage caused by the oxidative modification. Inhibition of the GPx activity may reflect the failure of the antioxidant system in contact with pesticides (Ballesteros et al. 2009) or may be related to the direct effect of superoxide radicals or pesticides on enzyme synthesis (Bainy et al. 1993; Yonar et al. 2014). The GPx activity in G. pulex exposed to dimethoate is significantly lower than that obtained for control organisms in this study. This reduction may show that the antioxidant capacity is exceeded by the amount of hydroperoxide product synthesized through lipid peroxidation (Remacle et al. 1992). In this context, the GPx inhibition observed in the present study might reflect a possible antioxidant defense failure responsible for the observed increase in MDA levels. Similar to the present study, many studies have reported that GPx activity is triggered by pollutants (Oliveira et al. 2012; Tatar et al. 2018; Almeida et al. 2002; Sayeed et al. 2003; Zhang et al. 2004; Fatima et al. 2000).
The GSTs are a family of enzymes that play an important role in the biotransformation of endogenous substances of the xenobiotics. GSTs function in both antioxidant and detoxification processes, catalyzing the conjugation of various electrophilic compounds with glutathione and convert these compounds to water soluble compounds (Osten et al. 2005; Yildirim et al. 2018). Increased GST activity may indicate the development of a defensive mechanism to counteract the effects of exposure and may reflect the possibility of a more efficient protection against pesticide toxicity. In this study, GST enzyme activity in freshwater amphipod G. pulex decreased with pesticide exposure in only high concentration group in 24 h. In the present study, GST activity was inhibited, suggesting the failure of adaptive response and indicating an oxidative stress status in the organisms or may indicate that the high concentration pesticide induces interactions leading to greater toxicity and thus induction of the GST enzyme. Similarly to the present study, GST activity has been reported to decrease in various aquatic organisms exposed to pollutants (Yildirim et al. 2018; Demirci et al. 2018; Mosleh et al. 2005; Hamed et al. 1999).
The CAT is a very common enzyme found in virtually all living organisms that use oxygen. It acts in water and oxygen formation by catalyzing the decomposition of hydrogen peroxide (Chelikani et al. 2004). CAT activity may increase or decrease in contaminated environments depending on the substance (Sobjak et al. 2017). In this study, the CAT enzyme activity is inhibited by the organisms under stress with dimethoate exposure. Similar to the present study, decreases in CAT activity have been reported in aquatic organisms exposed to various pollutants (Thomas and Murthy 1976; Hasspieler et al. 1994; Sayeed et al. 2003; Zhang et al. 2004; Crestani et al. 2007; Yildirim et al. 2018). According to the results found in the literature related to the activity of this enzyme, potential antioxidant changes by species, habitat, etc. can be explained (Glusczak et al. 2007).
Conclusion
The increased use of dimethoate pesticide increases the risk of environmental contamination with toxic effects on non-target organisms in aquatic ecosystems. In this study, MDA, GSH levels, GPx, SOD, GST, and CAT enzyme activities of oxidative stress parameters were determined on G. pulex of dimethoate pesticide. In this study, it can be concluded that stress condition provided by dimethoate exposure at sublethal concentrations evoked specific responses in G. pulex. Therefore, the above results indicate that dimethoate, an environmental pollutant, shows toxic effects due to oxidative stress.
References
Achary VM, Jena S, Panda KK, Panda BB (2008) Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol Environ Saf 70(2):300–310
Adam O, Degiorgi F, Crini G, Badot PM (2010) High sensitivity of Gammarus sp. juveniles to deltamethrin: outcomes for risk assessment. Ecotoxicol Environ Saf 73(6):1402–1407
Ahmad I, Hamid T, Fatima M, Chand HS, Jain SK, Athar M, Raisuddin S (2000) Induction of hepatic antioxidants in freshwater catfish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochim Biophys Acta Gen Subj 1523(1):37–48
Almar M, Otero L, Santos C, Gallego JG (1998) Liver glutathione content and glutathione-dependent enzymes of two species of freshwater fish as bioindicators of chemical pollution. J Environ Sci Health B 33(6):769–783
Almeida JA, Diniz YS, Marques SFG, Faine LA, Ribas BO, Burneiko RC, Novelli ELB (2002) The use of the oxidative stress responses as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environ Int 27(8):673–679
Almroth BC, Albertsson E, Sturve J, Förlin L (2008) Oxidative stress, evident in antioxidant defences and damage products, in rainbow trout caged outside a sewage treatment plant. Ecotoxicol Environ Saf 70(3):370–378
Bainy ACD, Arisi ACM, Azzalis LA, Simizu K, Barros SBM, Videla LA, Junqueira VBC (1993) Differential effects of short-term lindane administration on parameters related to oxidative stress in rat liver and erythrocytes. J Biochem Mol Toxicol 8(4):187–194
Ballesteros ML, Wunderlin DA, Bistoni MA (2009) Oxidative stress responses in different organs of Jenynsia multidentata exposed to endosulfan. Ecotoxicol Environ Saf 72(1):199–205
Bennett ER, Moore MT, Cooper CM, Smith S, Shields FD, Drouillard KG, Schulz R (2005) Vegetated agricultural drainage ditches for the mitigation of pyrethroid-associated runoff. Environ Toxicol Chem 24:2121–2127
Bergamini CM, Gambetti S, Dondi A, Cervellati C (2004) Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des 10(14):1611–1626
Beutler E, Duron O, Kelly BM et al (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bundschuh M, Zubrod JP, Klemm P, Elsaesser D, Stang C, Schulz R (2013) Effects of peak exposure scenarios on Gammarus fossarum using field relevant pesticide mixtures. Ecotoxicol Environ Saf 95:137–143
Chelikani P, Fita I, Loewen PC (2004) Diversity of structures and properties among catalases. Cell Mol Life Sci 61(2):192–208
Cheung CCC, Zheng GJ, Li AMY, Richardson BJ, Lam PKS (2001) Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis. Aquat Toxicol 52(3–4):189–203
Crestani M, Menezes C, Glusczak L, dos Santos Miron D, Spanevello R, Silveira A et al (2007) Effect of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere 67(11):2305–2311
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
De Lange HJ, Noordoven W, Murk AJ, Lürling MFLLW, Peeters ETHM (2006) Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat Toxicol 78(3):209–216
Della RM, Villani GR, Di EM, Squillacioti C, De LM, Vuotto P et al (1994) Glutathione depletion induced in rat liver fractions by seven pesticides. Boll Soc Ital Biol Sper 70(8–9):185–192
Demirci Ö, Güven K, Asma D, Öğüt S, Uğurlu P (2018) Effects of endosulfan, thiamethoxam, and indoxacarb in combination with atrazine on multi-biomarkers in Gammarus kischineffensis. Ecotoxicol Environ Saf 147:749–758
Dimitrova MS, Tishinova V, Velcheva V (1994) Combined effect of zinc and lead on the hepatic superoxide dismutase-catalase system in carp (Cyprinus carpio). Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 108(1):43–46
Doyotte A, Cossu C, Jacquin MC, Babut M, Vasseur P (1997) Antioxidant enzymes, glutathione and lipid peroxidation as relevant biomarkers of experimental or field exposure in the gills and the digestive gland of the freshwater bivalve Unio tumidus. Aquat Toxicol 39(2):93–110
Durmaz H, Sevgiler Y, Üner N (2006) Tissue-specific antioxidative and neurotoxic responses to diazinon in Oreochromis niloticus. Pestic Biochem Physiol 84(3):215–226
Esterbauer H, Zollner H, Schaur RJ (1990) Aldehydes formed by lipid peroxidation: mechanisms of formation, occurrence, and determination. In: Vigo-Pelfrey C (ed) Membrane lipid oxidation. CRC Press, Boca Raton, pp 239–268
Fatima M, Ahmad I, Sayeed I, Athar M, Raisuddin S (2000) Pollutant-induced over-activation of phagocytes is concomitantly associated with peroxidative damage in fish tissues. Aquat Toxicol 49(4):243–250
Ferrari A, Venturino A, de D’Angelo AMP (2007) Effects of carbaryl and azinphos methyl on juvenile rainbow trout (Oncorhynchus mykiss) detoxifying enzymes. Pestic Biochem Physiol 88(2):134–142
Flohe L (1989) Structure and catalytic mechanism of glutathione peroxidase. In: Glutathione Centennial: Molecular Perspectives and Clinical Implications. pp 103–114
Gabrielides GP (1995) Pollution and the Mediterranean Sea. Water Sci Technol 32:1–10
Gamble SC, Goldfarb PS, Porte C, Livingstone DR (1995) Glutathione peroxidase and other antioxidant enzyme function in marine invertebrates (Mytilus edulis, Pecten maximus, Carcinus maenas and Asterias rubens). Mar Environ Res 39(1–4):191–195
Gismondi E, Beisel JN, Cossu-Leguille C (2012) Influence of gender and season on reduced glutathione concentration and energy reserves of Gammarus roeseli. Environ Res 118:47–52
Glusczak L, dos Santos Miron D, Moraes BS, Simões RR, Schetinger MRC, Morsch VM, Loro VL (2007) Acute effects of glyphosate herbicide on metabolic and enzymatic parameters of silver catfish (Rhamdia quelen). Comp Biochem Physiol C Toxicol Pharmacol 146(4):519–524
Hai DQ, Varga SI, Matkovics B (1997a) Organophosphate effects on antioxidant system of carp (Cyprinus carpio) and catfish (Ictalurus nebulosus). Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 117(1):83–88
Hai DQ, Varga S, Matkovics B (1997b) Effects of diethyl-dithiocarbamate on antioxidant system in carp tissue. Acta Biol Hung 48(1):1–8
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine. Oxford University Press, New York
Hamed RR, Elawa SE, Farid NM, Ataya FS (1999) Evaluation of detoxification enzyme levels in Egyptian catfish, Clarias lazera, exposed to dimethoate. Bull Environ Contam Toxicol 63(6):789–796
Hassall KA (1990) The biochemistry and uses of pesticides. Macmillan Press, London
Hasspieler BM, Behar JV, Carlson DB, Watson DE, Di Giulio RT (1994) Susceptibility of channel catfish (Ictalurus punctatus) and brown bullhead (Ameriurus nebulosus) to oxidative stress: a comparative study. Aquat Toxicol 28(1–2):53–64
Hermes-Lima M (2004) Oxygen in biology and biochemistry: role of free radicals. In: Storey B (ed) Functional metabolism: regulation and adaptation. Wiley, Hoboken
Iwakuma T, Shiraishi H, Nohara S, Takamura K (1993) Runoffpropertiesand change in concentrations of agricultural pesticides in a river system during a rice cultivation period. Chemosphere 27:677–691
Jażdżewski K (1980) Range extensions of some gammaridean species in European inland waters caused by human activity. Crustaceana Supplement 84–107
Jemec A, Tisler T, Erjavec B, Pintar A (2012) Antioxidant responses and whole-organism changes in Daphnia magna acutely and chronically exposed to endocrine disruptor bisphenol A. Ecotoxicol Environ Saf 86:213–218
Kappus H (1985) Lipid peroxidation: mechanisms, analysis, enzymology and biological relevance. Oxidative Stress 273
Khan FR, Irving JR, Bury NR, Hogstrand C (2011) Differential tolerance of two Gammarus pulex populations transplanted from different metallogenic regions to a polymetal gradient. Aquat Toxicol 102(1–2):95–103
Kohen R, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods of their quantification. 30(6):620–650 PMID Google Scholar
Kunz PY, Kienle C, Gerhardt A (2010) Gammarus spp. in aquatic ecotoxicology and water quality assessment: toward integrated multilevel tests. In: Reviews of Environmental Contamination and Toxicology, vol 205. Springer, New York, pp 1–76
Lavarías S, García CF (2015) Acute toxicity of organophosphate fenitrothion on biomarkers in prawn Palaemonetes argentinus (Crustacea: Palaemonidae). Environ Monit Assess 187(3):65
Lundebye AK, Curtis TM, Braven J, Depledge MH (1997) Effects of the organophosphorous pesticide, dimethoate, on cardiac and acetylcholinesterase (AChE) activity in the shore crab Carcinus maenas. Aquat Toxicol 40(1):23–36
Malaj E, Peter C, Grote M, Kühne R, Mondy CP, Usseglio-Polatera P et al (2014) Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc Natl Acad Sci 111(26):9549–9554
McCord JM (1996) Effects of positive iron status at a cellular level. Nutr Rev 54(3):85–88
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Mensah PK, Palmer CG, Muller WJ (2012) Lipid peroxidation in the freshwater shrimp Caridina nilotica as a biomarker of Roundup® herbicide pollution of freshwater systems in South Africa. Water Sci Technol 65(9):1660–1666
Monteiro DA, De Almeida JA, Rantin FT, Kalinin AL (2006) Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion). Comp Biochem Physiol C Toxicol Pharmacol 143(2):141–149
Moreno I, Pichardo S, Jos A, Gomez-Amores L, Mate A, Vazquez CM, Camean AM (2005) Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin-LR administered intraperitoneally. Toxicon 45(4):395–402
Mosleh YY, Paris-Palacios S, Couderchet M, Biagianti-Risbourg S, Vernet G (2005) Metallothionein induction, antioxidative responses, glycogen and growth changes in Tubifex tubifex (Oligochaete) exposed to the fungicide, fenhexamid. Environ Pollut 135(1):73–82
Münze R, Orlinskiy P, Gunold R, Paschke A, Kaske O, Beketov MA, Hundt M, Bauer C, Schüürmann G, Möder M, Liess M (2015) Pesticide impact on aquatic invertebrates identified with Chemcatcher® passive samplers and the SPEARpesticides index. Sci Total Environ 537:69–80
Oakes KD, Van Der Kraak GJ (2003) Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat Toxicol 63(4):447–463
Oakes KD, McMaster ME, Van Der Kraak GJ (2004) Oxidative stress responses in longnose sucker (Catostomus catostomus) exposed to pulp and paper mill and municipal sewage effluents. Aquat Toxicol 67(3):255–271
Oliveira C, Almeida J, Guilhermino L, Soares AM, Gravato C (2012) Acute effects of deltamethrin on swimming velocity and biomarkers of the common prawn Palaemon serratus. Aquat Toxicol 124:209–216
Oliveira C, Almeida JR, Guilhermino L, Soares AM, Gravato C (2013) Swimming velocity, avoidance behavior and biomarkers in Palaemon serratus exposed to fenitrothion. Chemosphere 90(3):936–944
Oruç EÖ, Usta D (2007) Evaluation of oxidative stress responses and neurotoxicity potential of diazinon in different tissues of Cyprinus carpio. Environ Toxicol Pharmacol 23(1):48–55
Osten RV, Ortiz-Arana A, Guilhermino L, Soares A (2005) In vivo evaluation of three biomarkers in the mosquitofish (Gambusia yucatana) exposed to pesticides. Chemosphere 58:627–636
Petala M, Kokokiris L, Samaras P, Papadopoulos A, Zouboulis A (2009) Toxicological and ecotoxic impact of secondary and tertiary treated sewage effluents. Water Res 43(20):5063–5074
Ploch SA, Lee YP, MacLean E, Di Giulio RT (1999) Oxidative stress in liver of brown bullhead and channel catfish following exposure to tert-butyl hydroperoxide. Aquat Toxicol 46(3–4):231–240
Prasad MNV (2004) Heavy metal stress in plants: from biomolecules to ecosystems. Springer, Berlin Heidelberg
Regoli F, Nigro M, Orlando E (1998) Lysosomal and antioxidant responses to metals in the Antarctic scallop Adamussium colbecki. Aquat Toxicol 40(4):375–392
Remacle J, Lambert D, Raes M, Pigeolet E, Michiels C, Toussaint O (1992) Importance of various antioxidant enzymes for cell stability. Confrontation between theoretical and experimental data. Biochem J 286(Pt 1):41
Rinderhagen M, Ritterhoff J, Zauke GP (2000) Crustaceans as bioindicators. In: Biomonitoring of Polluted Water-Reviews on Actual Topics. Trans Tech Publications-Scitech Publications, Environmental Research Forum vol 9, pp 161–194
Sarriquet PE, Delettre YR, Marmonier P (2006) Effects of catchment disturbance on stream invertebrates: comparison of different habitats (vegetation, benthic and interstitial) using bio-ecological groups. Ann Limnol Int J Limnol 42(4):205–219 EDP Sciences
Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Raisuddin S (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf 56(2):295–301
Schulz R, Peall SK, Dabrowski JM, Reinecke AJ (2001) Spray deposition of two insecticides into surface waters in a South African orchard area. Zoological Institute, Technical Univ., Fasanenstrasse 3, D-38092 Braunschweig, Germany. J Environ Qual 30(3):814–822
Serdar O, Yildirim NC, Tatar S, Yildirim N, Ogedey A (2018) Antioxidant biomarkers in Gammarus pulex to evaluate the efficiency of electrocoagulation process in landfill leachate treatment. Environ Sci Pollut Res 1–7
Sies H (1986) Biochemistry of oxidative stress. Angew Chem Int Ed Eng 25(12):1058–1071
Sobjak TM, Romão S, do Nascimento CZ, dos Santos AFP, Vogel L, Guimarães ATB (2017) Assessment of the oxidative and neurotoxic effects of glyphosate pesticide on the larvae of Rhamdia quelen fish. Chemosphere 182:267–275
Sorensen FF, Bayley M, Baatrup E (1995) The effects of sublethal dimethoate exposure on the locomotor behavior of the collembolan Folsomia candida (Isotomidae). Environ Toxicol Chem 14(9):1587–1590
Sornom P, Felten V, Médoc V, Sroda S, Rousselle P, Beisel JN (2010) Effect of gender on physiological and behavioural responses of Gammarus roeseli (Crustacea Amphipoda) to salinity and temperature. Environ Pollut 158(5):1288–1295
Sroda S, Cossu-Leguille C (2011) Effects of sublethal copper exposure on two gammarid species: which is the best competitor? Ecotoxicology 20(1):264–273
Sturve J, Almroth BC, Forlin L (2008) Oxidative stress in rainbow trout (Oncorhynchus mykiss) exposed to sewage treatment plant effluent. Ecotoxicol Environ Saf 70(3):446–452
Tatar Ş, Cikcikoglu Yildirim N, Serdar O, Yildirim N, Ogedey A (2018) The using of Gammarus pulex as a biomonitor in ecological risk assessment of secondary effluent from municipal wastewater treatment plant in Tunceli, Turkey. Hum Ecol Risk Assess Int J 1–11
Thomas PC, Murthy TL (1976) Studies on the impact of a few organic pesticides on certain fish enzymes. Indian J Anim Sci 46(11):619–624
Uğurlu P, Ünlü E, Satar EI (2015) The toxicological effects of thiamethoxam on Gammarus kischineffensis (Schellenberg 1937) (Crustacea: Amphipoda). Environ Toxicol Pharmacol 39(2):720–726
UNEP (2010) Clearing the waters - a focus on water quality solutions. United Nations Environment Programme (UNEP), Division of Environmental Policy Implementation, Nairobi, Kenya. Available from: http://www.unep.org/PDF/Clearing_the_Waters.pdf. Accessed 19 March 2018
URL-1 (2018) https://www.google.com.tr/maps/@39.0917745,39.7985657,9.25z?hl=tr. Accessed 19 March 2018
US Environmental Protection Agency (2007) What is a pesticide? https://www.epa.gov/pesticides. Accessed 19.03.2018
Venturino A, Anguiano OL, Gauna L, Cocca C, Bergoc RM, de D’Angelo AMP (2001) Thiols and polyamines in the potentiation of malathion toxicity in larval stages of the toad Bufo arenarum. Comp Biochem Physiol C Toxicol Pharmacol 130(2):191–198
Vijayavel K, Gomathi RD, Durgabhavani K, Balasubramanian MP (2004) Sublthal effect of naphthalene on lipid peroxidation and antioxidant status in the edible marine crab Scylla serrata. Mar Pollut Bull 48(5–6):429–433
Vörösmarty CJ, McIntyre PB, Gessner MO, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn SE, Sullivan CA, Liermann CR, Davies PM (2010) Global threats to human water security and river biodiversity. Nature 467:555–561
Weber CI (ed) (1991) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. Environmental Monitoring Systems Laboratory, Office of Research and Development, US Environmental Protection Agency, Cincinnati, p 197
Wilhelm Filho D, Marcon JL (1996) Antioxidant defenses in fish of the Amazon. In: Physiology and Biochemistry of the Fishes of the Amazon. INPA, Manaus, pp 299–312
Yildirim NC, Tanyol M, Yildirim N, Serdar O, Tatar S (2018) Biochemical responses of Gammarus pulex to malachite green solutions decolorized by Coriolus versicolor as a biosorbent under batch adsorption conditions optimized with response surface methodology. Ecotoxicol Environ Saf 156:41–47
Yonar ME, Sakin F (2011) Ameliorative effect of lycopene on antioxidant status in Cyprinus carpio during pyrethroid deltamethrin exposure. Pestic Biochem Physiol 99(3):226–231
Yonar SM, Ural MŞ, Silici S, Yonar ME (2014) Malathion-induced changes in the haematological profile, the immune response, and the oxidative/antioxidant status of Cyprinus carpio carpio: protective role of propolis. Ecotoxicol Environ Saf 102:202–209
Zhang J, Shen H, Wang X, Wu J, Xue Y (2004) Effects of chronic exposure of 2, 4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere 55(2):167–174
Funding
This study was supported by the Scientific Research Projects Coordination Unit of Munzur University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Serdar, O. The effect of dimethoate pesticide on some biochemical biomarkers in Gammarus pulex. Environ Sci Pollut Res 26, 21905–21914 (2019). https://doi.org/10.1007/s11356-019-04629-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04629-w