Abstract
Zinc is one of the important essential trace minerals to human health due to its antioxidant properties. The present study was conducted to elucidate its potential protective role against maneb-induced nephrotoxicity. For this purpose, animals were randomly divided into four groups of six each. Mice of group I (negative controls) have received daily 0.5 ml of distilled water, a solvent of maneb. Mice of group II (MB) have received 30 mg/kg bw of maneb daily by intraperitoneal way. Mice of group III (MB + Zn) have received the same dose of maneb as group II, along with ZnSO4 (30 mg/kg bw) daily. Mice of group IV (Zn), considered as positive controls, have received the same dose of ZnSO4 as group III daily. Our results revealed that ZnSO4 co-administration to maneb-treated mice decreased kidney levels of malondialdehyde, hydrogen peroxide, protein carbonyls, and advanced oxidation protein products; the levels of non-enzymatic antioxidants like vitamin C, glutathione, and metallothionein. It recovered the alteration of antioxidant enzyme activities (catalase, superoxide dismutase, and glutathione peroxidase) and attenuated DNA fragmentation. Furthermore, this essential trace element was also able to alleviate kidney biomarkers’ alterations by lowering plasma levels of creatinine, urea, uric acid, and lactate dehydrogenase. In addition, the histopathological changes induced by maneb were improved following zinc administration. Our results indicated that zinc might be beneficial against maneb-induced renal oxidative damage in mice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Essential trace minerals play a pivotal role in the normal development and the protection of human body and health (Prashanth et al. 2015). Most of them mediate crucial biochemical reactions by acting as enzyme cofactors or catalysts. In particular, zinc (Zn) is a micronutrient, as well as an essential trace mineral obtained from the diet. It is essential for a wide range of biological activities except when used at high doses (Salgueiro et al. 2000). In trace amounts, this mineral is important in cellular function because it is an integral component of many proteins, including metalloenzymes, structural proteins, and transcriptional factors (Zhou et al. 2007). Many studies have revealed that Zn has antioxidant properties and acts by several different mechanisms. Indeed, Zn binds to thiol groups of biomolecules, allowing their stabilization against oxidation (Bettger 1993). Moreover, it antagonizes transition metal-catalyzed reactions by competing with redox active metals, such as iron and copper, for binding to cell membranes as well as to some proteins (Powell 2000). Zn is also a co-factor of copper zinc superoxide dismutase (Cu/Zn-SOD), which transforms superoxide anion radicals (O2−) to the less harmful species: oxygen (O2) and hydrogen peroxide (H2O2), as reported by Mariani et al. (2008). This mineral has been proven to upregulate the nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor regulating the expression of genes encoding the antioxidant molecules superoxide dismutase (SOD), glutathione S-transferase (GST), heme oxygenase 1 (HO-1), and glutathione (GSH) (Cortese et al. 2008; Smith and Loo 2012; Jarosz et al. 2017). Besides, Zn has been demonstrated to increase the expression of the antioxidant enzyme catalase (Tate Jr et al. 1997). In addition, this trace element can retard oxidative processes through inducing the biosynthesis of metallothioneins, cysteine-rich proteins which can bind pro-oxidant metals, such as cadmium, and provide thiol groups to scavenge reactive oxygen species (ROS) (Dondero et al. 2005). Interestingly, previous experimental studies on animal models have confirmed Zn efficiency in protecting against oxidative injury, associated with ROS-generating xenobiotics exposure, to a variety of tissues and organs, including the kidneys, and this effect was related to the antioxidant capacity of this mineral. For instance, Ghabaee et al. (2017) have reported that Zn administration to rats during gestation and lactation protects against arsenic-induced oxidative stress in kidney tissue. Likewise, Babaknejad et al. (2016) have demonstrated that Zn treatment reverses the toxic renal alterations induced by cadmium in rats. Zn showed also an ameliorating effect against oxidative stress induced by the pesticide fipronil in the renal tissue of rats (Swelam et al. 2017). In this context, it is well documented that pesticides, in general, have numerous negative effects including dermatological, respiratory, gastrointestinal, neurological, carcinogenic, reproductive, and endocrine changes (Mnif et al. 2011; Sanborn et al. 2007; Thakur et al. 2014). Among pesticides, maneb, a manganese-containing dithiocarbamate fungicide, is extensively employed in agriculture to treat a variety of crop pathologies, because of its low acute toxicity and short environmental persistence. At large amounts, maneb produces adverse effects in non-target organisms, including human beings, like headache, nausea, weight loss, confusion, respiratory paralysis, and even death (Edwards et al. 1991). It has been demonstrated that maneb acts on the thyroid gland (Mallem et al. 2007) as well as on the central and peripheral nervous systems (Domico et al. 2006) and possesses genotoxic effects (Bertini et al. 2000). Moreover, maneb exerts an antithyroid effect due to ethylene thiourea (ETU), its main metabolite, which also causes liver toxicity. Recently, we have demonstrated the beneficial role of vanillin against maneb-induced oxidative stress, DNA damage, and liver histological changes in mice (Sefi et al. 2019). According to Jaballi et al. (2017), maneb used at graded doses (1/8, 1/6, 1/4, and 1/2) of LD50 led to oxidative stress generation, provoking renal cell damages due to lowered defense systems’ capacities.
To our knowledge, no investigations have reported the potential ability of Zn to alleviate maneb toxicity in the kidney. Therefore, the aim of the present work was focused, for the first time, on the renoprotective role of Zn against maneb-induced kidney damages in adult mice.
Materials and methods
Animals
Seven-week-old male adult mice of Swiss strain, with an initial body weight of 30 ± 1 g, were obtained from the Central Pharmacy (SIPHAT, Tunisia). They were kept in a controlled temperature (22 ± 2 °C), humidity (40%), and photoperiod (12 h light-dark cycle). Mice were acclimated for 2 weeks prior to testing. They had free access to a commercial standard pellet diet (SNA, Sfax, Tunisia) and tap water. The content of Zn in the standard diet corresponded to 65 mg/kg of pellet according to the manufacturer of Animal Nutrition Society (SNA, Sfax, Tunisia). All the experimental procedures were performed according to the International Guidelines for Animal Care (Council of European Communities 1986). The test protocol was approved by the Ethical Committee of Sciences Faculty of Sfax, with 1204 as an ethics approval number.
Experimental design
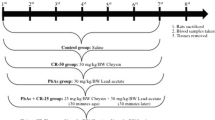
After acclimatization, animals were randomly divided into four groups of six each. Mice of group I, considered as negative controls, have received daily by intraperitoneal way 0.5 ml of distilled water, a solvent of maneb. Mice of group II (MB) have received 30 mg/kg bw of maneb daily by intraperitoneal way. Mice of group III (MB + Zn) have received the same dose of maneb as group II, along with ZnSO4 (30 mg/kg bw) daily. Mice of group IV (Zn), considered as positive controls, have received the same dose of ZnSO4 as group III daily. Maneb dose and the manner of administration, used in the current study, were chosen on the basis of the previous findings of Yadav et al. (2012). Concerning zinc, we have used the dose 30 mg/kg bw, according to Ding et al. (2016).
After the treatment period of 10 days, mice were fasted overnight and then sacrificed by cervical decapitation to avoid stress condition. Blood samples were collected from the trunk into heparinized tubes and centrifuged at 2200×g for 10 min. Plasma samples were drawn and stored at − 80 °C until analysis. Kidney tissues were dissected out, cleaned from adipose tissue, and weighed. Some samples were rinsed, homogenized in ice-cold phosphate buffer (0.1 M; pH 7.4), and centrifuged. The resulting supernatants were collected and kept at − 80 °C until biochemical analysis. Other samples were either fixed in 10% buffered formalin solution and embedded in paraffin for histological examination or taken for DNA assay.
Biochemical analysis
Protein quantification
Total protein content in kidney homogenates was estimated according to the Lowry et al. (1951) method, and bovine serum albumin was used as a standard.
Kidney lipid peroxidation
The kidney content in malondialdehyde (MDA), index of lipid peroxidation, was spectrophotometrically determined at 532 nm by Draper and Hadley (1990) method, and the values were expressed as nmoles MDA/mg of protein.
Hydrogen peroxide measurement
Measurement of hydrogen peroxide (H2O2) in the kidney was carried out by the ferrous ion oxidation xylenol orange method (Ou and Wolff 1996). Values were expressed as nmoles/mg of protein.
Protein carbonyl content
Kidney protein carbonyls (PCO) content was determined according to the method of Reznick and Packer (1994). The carbonyl content was determined based on the molar extinction coefficient of DNPH (휺 = 2.2 × 104 cm−1 M−1) and expressed as nmoles/mg of protein.
Kidney advanced oxidation protein product
Kidney advanced oxidation protein product (AOPP) levels were assayed according to the method described by Kayali et al. (2006). For each sample, AOPP concentration was calculated using the extinction coefficient 261 cm−1 mM−1 and the results were expressed as nmoles/mg of protein.
Kidney non-enzymatic antioxidant (GSH and vitamin C) levels
Reduced glutathione (GSH) levels in the kidney tissue were analyzed at 412 nm by Ellman (1959) method modified by Jollow et al. (1974), and the values were expressed as nmoles/mg of protein.
Kidney ascorbic acid (vitamin C) content was determined by dinitrophenyl hydrazine method, according to Jacques-Silva et al. (2001). The data were presented as nmoles/mg of protein.
Metallothionein content in kidney
Kidney metallothionein (MT) content was estimated based on the method described by Viarengo et al. (1997) and modified by Petrovic et al. (2001). Absorbance was measured at 412 nm. The data were expressed as μmoles of GSH/mg of protein.
Kidney enzymatic antioxidant activities
Catalase (CAT) activity in the renal tissue was determined at 240 nm following the Aebi (1984) method, and the values were expressed as μmoles H2O2 consumed/min/mg of protein.
Superoxide dismutase (SOD) activity was analyzed at 560 nm according to Beauchamp and Fridovich method (1971), and the values were expressed as U/mg of protein.
Glutathione peroxidase (GPx) activity was assayed by following the method described by Flohe and Gunzler (1984). Enzyme activity was expressed as nmoles of GSH oxidized/min/mg of protein.
Assays of creatinine, urea, and uric acid levels in plasma
Creatinine, urea, and uric acid plasma levels were quantified spectrophotometrically using commercial diagnostic kits (Ref 20151, 20143, 20091), purchased from Biomaghreb.
Determination of lactate dehydrogenase activities in plasma and kidney
Plasma and kidney activities of lactate dehydrogenase (LDH) were estimated using a commercially available reagent kit purchased from Biomaghreb (Ref: 20012) and they were expressed as units/L and units/g of tissue, respectively.
Qualitative DNA fragmentation assay by agarose gel electrophoresis
Genomic DNA was isolated from the kidney tissue using a commercial kit and electrophoresed on a 1% agarose gel stained with ethidium bromide (Pure Link Genomic DNA Invitrogen ref. K 182001). The gel was then observed under ultraviolet lamp and photographed.
Histological studies
Some portions of the kidney were placed in 10% of buffered formalin solution for 48 h. The specimens were washed and dehydrated through an ascending series of ethanol. Then they were embedded in paraffin. Blocks were made, sectioned at a thickness of 5 μm, and stained with hematoxylin and eosin. The obtained slides were examined under light microscopy (Suvarna et al. 2013) and fitted with a Canon Power Shot camera (A640) to record digitally the required images for histological studies. The severity of histopathological changes (glomeruli fragmentation, Bowman’s space enlargement, hypercellularity and increased nuclear size of glomeruli, leukocytes infiltration, and bleeding area) was scored using the blind study methodology, based on the following scale: 0: no injury, 1: injury to 25% of the field, 2: injury to 50% of the field, 3: injury to 75% of the field, 4: diffuse injury.
Statistical analysis
The data of the present work were analyzed using the statistical package program Stat view 5 Software for Windows (SAS Institute, Berkley, CA). Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) test as a post hoc test for multiple comparison tests. When comparison between two groups was required, the student unpaired t test was also used. All values were presented as means ± S.D. A p value of 0.05 was considered as the point of minimal statistical significance.
Results
Relative kidney weight
During 10 days of treatment, no death was observed in any group; and there were no significant changes in body weights of control and treated mice. Our results showed that the relative kidney weight was significantly (P < 0.001) increased by 56% in MB and by 30% in MB + Zn groups, when compared to those of controls (Table 1).
Lipid peroxidation and hydrogen peroxide generation in the kidney
Administration of maneb resulted in a significant increase (P < 0.001) in MDA concentrations as well as in H2O2 levels in kidney tissues compared to those of control group. However, Zn administration to maneb-treated mice induced a significant reduction in the levels of these parameters compared to those of the maneb-treated group. Exposure of mice to Zn alone did not induce any changes in MDA and H2O2 levels (Table 2).
Protein oxidative markers in the kidney
Our data revealed a significant increase (P < 0.001) in PCO and AOPP levels of maneb-treated mice compared to those of control group. Interestingly, co-administration of Zn with maneb significantly reduced the levels of these parameters (Table 2).
Non-enzymatic antioxidant status in the kidney
Maneb exposure caused a significant increase (P < 0.001) of GSH, vitamin C, and MT levels in the kidney when compared to those of control group. These modifications were alleviated following Zn administration to maneb-treated mice as indicated by a significant decrease (P < 0.001) of the above parameters when compared to those of MB group (Table 2).
Enzymatic antioxidant status in the kidney
In maneb-treated mice, CAT and GPx activities in the kidney were significantly decreased (P < 0.001) when compared to those of control group. In contrast, there was a significant increase (P < 0.001) in SOD activity. Co-treatment with Zn induced a total recovery in SOD and GPx activities when compared to those of maneb-treated mice. Meanwhile, CAT activity was moderately (P < 0.05) ameliorated. The activities of these antioxidant enzymes in kidney of mice treated only with Zn were near to those of negative controls (Fig. 1).
Biomarkers of renal toxicity
As shown in Table 3, the levels of plasma creatinine, urea, and uric acid were significantly (P < 0.001) increased in maneb-treated mice when compared to those of controls. In mice co-treated with Zn, a recovery of these parameters was observed.
LDH activities in plasma and kidney
Maneb-treated mice showed a significant increase (P < 0.001) in the activities of LDH in plasma associated with its significant decrease (P < 0.001) in the kidney compared to those of controls. Co-treatment with Zn improved LDH activities in plasma and kidney (Fig. 2).
DNA fragmentation
As presented in Fig. 3, a smear ladder formation on agarose gel electrophoresis, revealing random DNA fragmentation, was observed in the kidney tissue of maneb-treated mice. Zn co-treatment decreased the smear formation. DNA damage was not detected in negative and positive control groups.
Kidney histopathological studies
Kidney of control group showed normal features of renal tubules and intact glomeruli (Fig. 4(a)). The histopathological hallmarks of maneb-induced renal injury were evident by glomeruli hypertrophy and fragmentation, Bowman’s space enlargement, hypercellularity and increased nuclear size of the glomeruli, marked leukocytes infiltration between the tubules, and a large area of hemorrhage in the interstitium (Fig. 4(c1, d1, d2)). These alterations were attenuated after Zn co-treatment, indicating the reduction of the pathological changes associated with nephrotoxicity (Fig. 4(e)). Treatment with Zn alone showed a normal appearance of the kidney histoarchitecture (Fig. 4(b)).
Kidney tissue histological aspects of adult mice; control (a), treated with zinc alone (b), maneb (c, c1, d, d1, d2), and zinc along with maneb (e). Optic microscopy; H&E; magnification a, b, c, d, e: (× 200) and c1, d1, d2: (× 400). Arrows indicate:  Glomerular fragmentation;
Glomerular fragmentation;  Bowman’s space enlargement and hypertrophy;
Bowman’s space enlargement and hypertrophy;  Glomerulus exhibiting a hypercellularity and an increased nuclear size;
Glomerulus exhibiting a hypercellularity and an increased nuclear size;  Hemorrhage;
Hemorrhage;  Infiltration of leukocytes
Infiltration of leukocytes
The severity of these histological alterations was evaluated semiquantitatively in the histopathological kidney sections of control and Zn groups, and the damage scores were found to be zero. Kidney damage parameters (glomeruli fragmentation, Bowman’s space enlargement, increased cellularity and nuclear size of glomeruli, leukocytes infiltration, and hemorrhage) for MB and MB + Zn groups were scored and results showed that injury scores for MB group were significantly higher than those for MB + Zn group (Table 4).
Discussion
There is accumulating evidence that Zn has antioxidant properties and can protect kidney against xenobiotics-induced nephrotoxicity. In the present study, we investigated, for the first time, whether Zn administration to maneb-exposed mice would potentially alleviate the adverse effects of this fungicide.
In toxicological studies, body and organ weights are the important endpoints for evaluating xenobiotics-induced organ toxicity (Crissman et al. 2004). The current study revealed that maneb exposure significantly increased the relative kidney weight without affecting the body weight of mice. This rise might be a result of the hypercellularity and the enhanced nuclear size of the glomeruli, as shown in histological kidney sections of MB group (Fig. 4(d2)). Furthermore, El-Damaty et al. (2012) have stated that kidney enlargement and its weight increment in rats treated with pesticides could be due to the accumulation of abnormal cells. In this context, a previous study conducted by De Carvalho et al. (1989) revealed kidney toxicity, illustrated by severe glomerular lesions, in patients after unprotected use of maneb. Recently, Abolaji et al. (2017) have reported an increase in the relative kidney weight of female rats treated with two pesticides, chlorpyrifos and carbendazim.
The kidney morphological changes induced by maneb could be related to the disturbances of the normal redox state in this organ, resulting from oxidative stress installation. The latter has been recognized as a shift in the balance between oxidants and antioxidants in favor of the former, due to increased cellular levels of ROS. In such case, our results revealed that the concentration of malondialdehyde (MDA), the end product of lipid peroxidation, increased in the kidney of MB group. MDA has been accepted as a reliable parameter reflecting an enhanced ROS production, leading to oxidative injury to the body (Kim et al. 2012). Similarly, previous reports of Osman et al. (2011) on rats have shown an increased lipid peroxidation derived from bromuconazole exposure, a triazole fungicide. The enhanced MDA level, recorded in tissues of maneb-exposed animals, could be probably due to the excessive production of ROS caused by this fungicide leading to renal injury, as indicated in our recent study (Sefi et al. 2019) conducted in the liver. Likewise, in the present study, the generation of H2O2 was increased following treatment with maneb, reflecting a possible mitochondrial dysfunction induced by this fungicide. Elevated ROS production and mitochondrial dysfunction seemed to be key factors in maneb-induced toxicity, with potential secondary renal consequences. Previous studies of Todt et al. (2016) have shown that Mn/Zn ethylene-bis-dithiocarbamate fungicide resulted in mitochondrial dysfunction and excessive ROS production. Moreover, Jaballi et al. (2017) have demonstrated that maneb, administered to adult mice at four graded doses (1/8, 1/6, 1/4, and 1/2 of LD50), caused a significant increase in the kidney levels of MDA and H2O2. In the present work, the used dose of maneb (30 mg/kg bw), representing 1/50 of the fungicide LD50, was lower than those tested by Jaballi et al. (2017).
In addition to cell membrane lipids, proteins may also be altered by ROS production, especially at their labile thiol groups. Protein oxidation can be triggered directly by ROS or indirectly through the reactions with oxidative stress secondary-products (Dalle-Donne et al. 2003). In the present work, we have studied two biomarkers of oxidative protein damage, PCO and AOPP. Elevated levels of the above parameters, after maneb exposure, indicated the occurrence of protein oxidative modifications in the kidney of experimental mice. Co-administration of Zn to maneb-treated mice ameliorated MDA, H2O2, PCO, and AOPP levels, reflecting the antiperoxidative and free radical-scavenging properties of Zn, as has been shown previously by Goel et al. (2005). It has also been reported that this element interacts with cell membranes in order to stabilize them against various damaging effects, including those due to oxidative injuries (O’Dell 2000). Another mechanism that may also contribute to the antioxidant role of Zn is its ability to compete with transition metals like Fe and Cu, for the binding sites on the cell membrane. These two metals catalyze the production of lipid peroxides, and thereby replacement of these metals by Zn in the cell membrane could inhibit lipid peroxidation and oxidative stress condition (Barman and Srinivasan 2017). Consistent with these results, Bashandy et al. (2018) have recently reported in rats that zinc oxide nanoparticles exhibit renoprotective effects against thioacetamide, a potent hepatotoxic compound, by reducing lipid peroxidation.
Increased lipid peroxidation and oxidative stress can affect the activities of protective non-enzymatic antioxidants (Yonar and Sakin 2011). In particular, GSH and its metabolizing enzymes provide the major defense against ROS-induced cellular damage (Ozden and Alpertunga 2010). Cellular stress response is often known to involve changes in the content of thiols, which are firstly consumed during their reaction with the deleterious compounds, and then replaced through enzymatic reduction of disulfides, or by de novo biosynthesis. Two enzymes, namely glutamate-cysteine ligase (GCL, rate limiting) and glutathione synthase, are concertedly implicated in GSH synthesis, which is both constitutive and regulated. According to Dickinson and Forman (2002), exposure to ROS-generating compounds can elevate GSH content by increasing its biosynthesis rate, as reported in our experiment after maneb exposure. Concerning ascorbic acid, the main water-soluble antioxidant vitamin able to scavenge free radicals (Molyneux et al. 2002), its increase after maneb exposure resulted probably from the re-activation of enzymatic and non-enzymatic mechanisms of vitamin C synthesis. Our results were in accordance with those of Spodniewska and Zasadowski (2008), who have found an increase of GSH and ascorbic acid contents in the liver of rats treated for 7 days with pyrantel tartrate, an anthelmintic regimen. Moreover, a significant increase of metallothionein (MT) content was found in maneb-treated mice. In fact, the main characteristic of MT is its richness in cysteine residues (Dondero et al. 2005), which are responsible for the metal sequestration capacity of the protein. Thiol groups can also serve as scavengers of highly toxic compounds such as free radicals and reactive metabolites of xenobiotics. In our study, the stressful condition caused by the toxic effect of maneb can be prevented by an elevation of intracellular thiol groups like MT. It is proposed that one of the self-protecting mechanisms of the kidney against toxic compounds is the increase of the MT level and consequently the elevation of naturally occurring ROS scavengers in the cell. The significant improvement of renal non-enzymatic antioxidant (GSH, vitamin C, and MT) contents by Zn treatment supported its potential protective role against maneb-mediated renal injury.
In addition, other antioxidant enzymes, like superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), must be considered in cellular stress condition. Such enzymes act in two steps as follows. First, SOD catalyzes the conversion of superoxide radicals into oxygen and hydrogen peroxide, which is next converted by CAT and GPx to water and oxygen (Sies 1993). In the current study, exposure to maneb decreased the activities of both CAT and GPx, and paradoxally induced a significant increase of SOD activity. Our results were in accordance with the recent study of Jaballi et al. (2017), who have reported that mice exposure to graded maneb doses (1/8, 1/6, 1/4, and 1/2 of LD50) significantly decreases CAT and GPx activities and increases SOD in their renal tissue. In our study, Zn counteracted oxidative damages by modulating these enzymes’ activities, suggesting that this bioelement could act as a potent ROS scavenger, making cells apt to preserve their integrity and function. These effects could be related to the direct and indirect antioxidant properties of Zn (Powell 2000). Indeed, this trace element is a structural component of the SOD enzyme involved in neutralizing free radicals’ attacks through converting the superoxide anion radical (O2−) to hydrogen peroxide (H2O2) (Ling et al. 2016), which is in turn removed by CAT and GPx enzymes (Raat et al. 2009). Also, Zn is able to bind directly to sulfhydryl groups, allowing their protection from oxidation (Powell 2000). Thus, we provide evidence revealing that Zn could mitigate oxidative stress damages owing to its free radical scavenging potential. Our results were in line with those of Swelam et al. (2017) who have reported that Zn supplementation mitigates fipronil-induced oxidative damage by an improvement of GPx, CAT, and SOD activities in the liver and kidney of rats.
Furthermore, kidney impairment was assessed by measuring plasma creatinine, urea, and uric acid concentrations. The current increment in these biochemical parameters proved renal dysfunction in maneb-treated mice. Creatinine and urea are waste products deriving from protein metabolism and they require to be excreted through the kidney. Consequently, an obvious elevation of these compounds, as noticed in our study, was indicative of kidney functional damage, as reported earlier by Gowda et al. (2010). These findings concours with a similar report in a recent study of Jaballi et al. (2017). Renal injury was also confirmed in the present work by LDH activity, which decreased in kidney and increased in plasma, speaking in favor of membrane damage. Co-administration of Zn to maneb-intoxicated mice afforded a significant protection against the fungicide-induced nephrotoxicity, due to its antioxidant properties.
Regarding DNA damage of kidney tissue triggered by maneb exposure, our study demonstrated a higher intensity of DNA laddering on agarose gel electrophoresis as compared to the controls, which might be attributed to oxidative stress induced by this fungicide. Our previous study confirmed the genotoxic effect of maneb in the liver (2019). According to Bertini et al. (2000), ethylene thiourea the main metabolite of maneb, has been proven to have genotoxic effects in Wistar rats. In the present study, Zn co-administration led to a reduced DNA fragmentation induced by maneb. Thereby, Zn could protect DNA and other important molecules from oxidation, improving consequently kidney function. In fact, it exhibited DNA protection, attenuated lipid peroxidation, and decreased free radicals production indicating its strong antioxidant power. Our results were in accordance with other studies (Varghese et al. 2009), showing that Zn reduces the oxidative DNA damage in the kidney of rats exposed to indomethacin, a non-steroidal anti-inflammatory drug.
Overall, the biochemical and molecular changes, observed in the current work following maneb exposure, were confirmed by kidney histopathological findings. The structures of glomeruli as well as renal tubules were affected after maneb treatment. In fact, kidney of mice exposed to maneb showed glomerular hypertrophy and fragmentation (Fig. 4(c1)); and an enlargement of glomeruli Bowman’s space (Fig. 4(c1 and d2)). Such changes in glomerular structure have been observed by Chaâbane et al. (2017) as a result of penconazole fungicide treatment in rats. Moreover, kidney sections of maneb-treated mice showed also some abnormalities in the renal glomerular structure characterized by an hypercellularity and an increased nuclear size (Fig. 4(d2)). These changes might occur as an outcome of direct glomerular cytotoxicity and oxidative stress at the glomerular level caused by maneb. The renal glomeruli are particularly sensitive to toxicity because they have a high oxygen consumption, a vulnerable enzymatic system, and a complicated transport mechanism that may be used for toxins transport and may be damaged by such toxins during their excretion (Chevalier 2016). Similar histopathological observations have been reported in kidney of rats treated with permethrin, a pyrethroid insecticide (Nessiem et al. 2003). Maneb administration also induced a marked leukocyte infiltration between the tubules and a large area of hemorrhage in the interstitium (Fig. 4(d1)). Scoring of kidney histological changes revealed that leukocytes infiltration was the most prominent pathological feature associated with maneb exposure. Interestingly, the reduction of histopathological changes and the low injury scores in the kidney of mice co-treated with both maneb and Zn suggested the ameliorative effect of this essential mineral.
On the basis of this work, maneb administered to adult mice at a dose of 30 mg/kg bw caused a disruption of the redox status, increased MDA and protein oxidation products’ levels, induced DNA fragmentation and histopathological changes in the kidney tissue. Maneb was considered as a pro-oxidant agent. It disturbed the oxidant-antioxidant balance and induced a depletion of renal antioxidant activities. The co-administration of Zn to maneb-treated mice attenuated the toxicity of this fungicide in mice, as objectified by the biochemical and histological improvements. The mechanisms, which contributed to its effectiveness, involved ROS quenching, attenuation of lipid and protein oxidation, prevention of DNA damage, and improvement of the antioxidant status. Thus, Zn appeared to be a promising agent against maneb-induced renal dysfunction.
Conclusion
In summary, we have shown that exposure of mice to maneb resulted in an increased renal cytotoxicity mainly through inducing lipid peroxidation and ROS production. Zn administration alleviated the toxicity rate in the renal tissue of maneb-exposed mice. Understanding the mechanism of the protective role of Zn at the cellular level may provide a rationale for its use in antioxidant therapeutic strategies against nephrotoxicity in human beings exposed to different fungicides.
References
Abolaji AO, Awogbindin IO, Adedara IA, Farombi EO (2017) Insecticide Chlorpyrifos and fungicide carbendazim, common food contaminants mixture, induce hepatic, renal, and splenic oxidative damage in female rats. Hum ExpToxicol 36:483–493. https://doi.org/10.1177/0960327116652459
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Babaknejad N, Moshtaghie AA, Nayeri H, Hani M, Bahrami S (2016) Protective role of zinc and magnesium against cadmium nephrotoxicity in male Wistar rats. Biol Trace Elem Res 174(1):112–120. https://doi.org/10.1007/s12011-016-0671-x
Barman S, Srinivasan K (2017) Attenuation of oxidative stress and cardioprotective effects of zinc supplementation in experimental diabetic rats. Br J Nutr 117:335–350. https://doi.org/10.1017/S0007114517000174
Bashandy SAE, Alaamer A, Moussa SAA, Omara EA (2018) Role of zinc oxide nanoparticle in alleviating hepatic fibrosis and nephrotoxicity induced by thioacetamide in rats. Can J Physio Pharmacol 96:337–344. https://doi.org/10.1139/cjpp-2017-0247
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bertini S, Del Carratore M, Giorgi M, Bronzetti G, Della Croce C (2000) Genotoxic and monooxygenase system effects of the fungicide maneb. Arch Toxicol 74:415–420. https://doi.org/10.1007/s00240000152
Bettger WJ (1993) Zinc and selenium, site-specific versus general antioxidation. Can J Physiol Pharmacol 71:721–724. https://doi.org/10.1139/y93-108
Chaâbane M, Koubaa M, Soudani N, Elwej A, Grati M, Jamoussi K, Boudawara T, EllouzeChaabouni S, Zeghal N (2017) Nitrariaretusa fruit prevents penconazole-induced kidney injury in adult rats through modulation of oxidative stress and histopathological changes. Pharm Biol 55:1061–1073. https://doi.org/10.1080/13880209.2016.1278455
Chevalier RL (2016) The proximal tubule is the primary target of injury and progression of kidney disease: role of glomerulotubular junction. Am J Physiol Renal Physiol 311:F145–F161. https://doi.org/10.1152/ajprenal.00164.2016
Cortese MM, Suschek CV, Wetzel W, Kroncke KD, Kolb-Bachofen V (2008) Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med 44:2002–2012. https://doi.org/10.1016/j.freeradbiomed.2008.02.013
Council of European Communities (1986) Council instructions about the protection of living animals used in scientific investigations. Off J Eur Commun (JO 86/609/CEE) L358:1–18
Crissman JW, Goodman DG, Hildebrandt PK, Maronpot RR, Prater DA, Riley JH (2004) Best practice guideline: Toxicologic histopathology. Toxicol Pathol 32:126–131. https://doi.org/10.1080/01926230490268756
Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A (2003) Protein carbonylation in human diseases. Trends in Mol Med 9:169–176. https://doi.org/10.1016/S1471-4914(03)00031-5
De Carvalho E, Faria V, Loureiro A, Miranda V (1989) Acute renal failure and nephrotic syndrome after maneb exposure. A new case with light and electron microscopic study. Acta Medica Portuguesa 5:215–218
Dickinson DA, Forman HJ (2002) Cellular glutathione and thiols metabolism. BiochemPharmacol 64:1019–1026. https://doi.org/10.1016/S0006-2952(02)01172-3
Ding Q, Li H, Tian X, Shen Z, Wang X, Mo F, Huang J, Shen H (2016) Zinc and imipramine reverse the depression-like behavior in mice induced by chronic restraint stress. J Affect Disord 197:100–106. https://doi.org/10.1016/j.jad.2016.03.017
Domico LM, Zeevalk GD, Bernard L, Cooper K (2006) Acute neurotoxic effect of mancozeb and maneb in mesencephalic neuronal cultures are associated with mitochondrial dysfunction. Neuro Toxicology 27:816–825. https://doi.org/10.1016/j.neuro.2006.07.009
Dondero F, Piacentini L, Banni M, Rebelo M, Burlando B, Viarengo A (2005) Quantitative PCR analysis of two molluscan metallothionein genes unveils differential expression and regulation. Gene 345:259–270. https://doi.org/10.1016/j.gene.2004.11.031
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431. https://doi.org/10.1016/0076-6879(90)86135-I
Edwards IR, Ferry DG, Temple WA (1991) Fungicides & related compounds. Handbook of pesticide toxicology. In: Hayes WJ, Laws ER (eds) Academic press. NY, New York, pp 10–14
El-Damaty EMA, Farrag AH, Rowayshed G, Fahmy HM (2012) Biochemical and Histopathological effects of systemic pesticides on some functional organs of male albino rats. J Appl Sci Res 8:5459–5469
Ellman GL (1959) Tissue sulfhydryl groups. Arch BiochemBiophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121. https://doi.org/10.1016/S0076-6879(84)05015-1
Ghabaee DNZ, Amiri FT, Moghaddam AE, Khalatbary AR, Zargari M (2017) Administration of zinc against arsenic-induced nephrotoxicity during gestation and lactation in rat model. J Nephropathol 6(2):74–80. https://doi.org/10.15171/jnp.2017.13
Goel A, Dani V, Dhawan DK (2005) Protective effects of zinc on lipid peroxidation,antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. ChemBiolInterac 156:131–140. https://doi.org/10.1016/j.cbi.2005.08.004
Gowda S, Desai PB, Kulkarni SS, Hull VV, Math AAK, Vernekar SN (2010) Markers of renal function tests. N Am J Med Sci 2:170–173
Jaballi I, Ben Saad H, Bkhairia I, Kammoun I, Droguet M, Magné C, Boudawara T, Kallel C, Nasri M, Hakim A, Ben Amara I (2017) Increasing maneb doses induced reactive oxygen species overproduction and nephrotoxicity in adult mice. Toxicol Mech Methods 27:382–393. https://doi.org/10.1080/15376516.2017.1300617
Jacques-Silva MC, Nogueira CW, Broch LC, Flores EMM, Rocha JBT (2001) Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. J Pharmacol Toxicol 88:119–125. https://doi.org/10.1034/j.1600-0773.2001.d01-92.x
Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T (2017) Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling Inflammopharmacology 25:11–24. https://doi.org/10.1007/s10787-017-0309-4
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169. https://doi.org/10.1159/000136485
Kayali R, Cakatay U, Akcay T, Altug T (2006) Effect of alpha lipoic acid supplementation on markers of protein oxidation in postmitotic tissues of ageing rat. Cell BiochemFunct 24:79–85. https://doi.org/10.1002/cbf.1190
Kim SH, Lee IC, Lim JH, Moon C, Bae CS, Kim SH, Shin DH, Park SC, Kim HC, Kim JC (2012) Protective effects of pine bark extract on developmental toxicity of cyclophosphamide in rats. Food ChemToxicol 50:109–115. https://doi.org/10.1016/j.fct.2011.10.048
Ling H, Chen H, Wei M, Meng X, Yu Y, Xie K (2016) The effect of autophagy on inflammation cytokines in renal ischemia/reperfusion injury. Inflammation 39:347–356. https://doi.org/10.1007/s10753-015-0255-5
Lowry OH, Rosebrugh NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J BiolChem 193:265–275
Mallem L, Keck G, Franck M, Boulakoud MS (2007) The effect of maneb on thyroid and fertility in the rabbit. Rev Med Vet 158:452–457
Mariani E, Mangialasche F, Feliziani FT, Cecchetti R, Malavolta M, Bastiani P, Baglioni M, Dedoussis G, Fulop T, Herbein G, Jajte J, Monti D, Rink L, Mocchegiani E, Mecocci P (2008) Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Exp Gerontol 43:445–451. https://doi.org/10.1016/j.exger.2007.10.012
Mnif W, Hassine AIH, Bouaziz A, Bartegi A, Thomas O, Roig B (2011) Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 8:2265–2303. https://doi.org/10.3390/ijerph8062265
Molyneux CA, Glyn MC, Ward BJ (2002) Oxidative stress and cardiac microvascular structure in ischemia and reperfusion: the protective effect of antioxidant vitamins. Microvasc Res 64:265–277. https://doi.org/10.1006/mvre.2002.2419
Nessiem AL, Bassily NS, Metwally SA (2003) Comparative histopathological evaluation of permethrin, pirimiphos, methyl and bendiocarb toxicities in testes, liver and kidney of rat. Egypt J Hosp Med 11:58–73. https://doi.org/10.12816/EJHM.2003.18719
O’Dell BL (2000) Role of zinc in plasma membrane function. J Nut 130:1432S–1436S. https://doi.org/10.1093/jn/130.5.1432S
Osman AH, El-Shama SS, Osman AS, Abd El-Hameed AK (2011) Toxicological and pathological evaluation of prolonged bromuconazole fungicide exposure in male rats. Med J Cairo Univ 79:555–564
Ou P, Wolff SP (1996) A discontinuous method for catalase determination at near physiological concentrations of H2O2 and its application to the study of H2O2 fluxes within cells. J BiochemBiophys Methods 31:59–67. https://doi.org/10.1016/0165-022X(95)00039-T
Ozden S, Alpertunga B (2010) Effects of methiocarb on lipid peroxidation and glutathione level in rat tissues. Drug ChemToxicol 33:50–54. https://doi.org/10.3109/01480540903130708
Petrovic S, Ozretic B, Krajnovic-Ozretic M, Bobinac D (2001) Lysosomal membranestability and metallothioneins in digestive gland of mussels (Mytilusgalloprovincialis Lam.) as biomarkers in a field study. Mar Pollut Bull 42:1373–1378. https://doi.org/10.1016/S0025-326X(01)00167-9
Powell SR (2000) The antioxidant properties of zinc. J Nutr 130:1447S–1454S. https://doi.org/10.1093/jn/130.5.1447S
Prashanth L, Kattapagri KK, Chitturi RT, Baddam VRR, Prasad LK (2015) A review on role of essential trace elements in heath and disease. J Dr NTR Univ Health Sci 4:75–85. https://doi.org/10.4103/2277-8632.158577
Raat N, Shiva S, Gladwin M (2009) Effects of nitrite on modulating ROS generation following ischemia and reperfusion. Adv Drug Deliv Rev 61:339–350. https://doi.org/10.1016/j.addr.2009.02.002
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl. Method Enzymol. Academic Press, New York, p 357. https://doi.org/10.1016/S0076-6879(94)33041-7
Salgueiro MJ, Zubillaga M, Lysionek A, Sarabia MI, Caro R, De Paoli T, Hager A, Weill R, Boccio J (2000) Zinc as an essential micronutrient: a review. Nutr Res 20:737–755. https://doi.org/10.1016/S0271-5317(00)00163-9
Sanborn M, Kerr KJ, Sanin LH, Cole DC, Bassil KL, Vakil C (2007) Non-cancer health effects of pesticides. Systematic review and implications for family doctors. Can Fam Physician 53:1712–1720
Sefi M, Elwej A, Chaâbane M, Bejaoui S, Marrekchi R, Jamoussi K, Gouiaa N, Boudawara-Sellami T, El Cafsi M, Zeghal N, Soudani N (2019) Beneficial role of vanillin, a polyphenolic flavoring agent, on maneb-induced oxidative stress, DNA damage, and liver histopathological changes in Swiss albino mice. Hum Exp Toxicol 38(6):619–631. https://doi.org/10.1177/0960327119831067
Sies H (1993) Strategies of antioxidant defense. Eur J Biochem 215:213–219. https://doi.org/10.1111/j.1432-1033.1993.tb18025.x
Smith AF, Loo G (2012) Upregulation of haeme oxygenase-1 by zinc in HCT-116 cells. Free Radic Res 46:1099–1107. https://doi.org/10.3109/10715762.2012.690872
Spodniewska A, Zasadowski A (2008) Content of glutathione and vitamin C in the liver of rats exposed to dimethoate and pyrantel tartrate. Acta Vet Brno 77:355–362. https://doi.org/10.2754/avb200877030355
Suvarna SK, Layton C, Bancroft JD (2013) Bancroft’s theory and practice of histological techniques, 7th edn. Churchill Livingston, New York, p 637
Swelam ES, Abdallah IS, Mossa AH (2017) Ameliorating effect of zinc against oxidative stress and lipid peroxidation induced by fipronil in male rats. J Pharmacol Toxicol 12:24–32. https://doi.org/10.3923/jpt.2017.24.32
Tate DJ Jr, Miceli MV, Newsome DA (1997) Zinc induces catalase expression in cultured fetal human retinal pigment epithelial cells. Curr Eye Res 16:1017–1023. https://doi.org/10.1076/ceyr.16.10.1017.9011
Thakur DS, Khot R, Joshi PP, Pandharipande M, Nagpure K (2014) Glyphosate poisoning with acute pulmonary edema. ToxicolInt 21:328–330. https://doi.org/10.4103/0971-6580.155389
Todt CE, Bailey DC, Pressley AS, Orfield SE, Denney RD, Snapp IB, Negga R, Bailey AC, Montgomery KM, Traynor WL, Fitsanakis VA (2016) Acute exposure to a Mn/Zn ethylene-bis-dithiocarbamate fungicide leads to mitochondrial dysfunction and increased reactive oxygen species production in Caenorhabditis elegans. Neurotoxicology 57:112–120. https://doi.org/10.1016/j.neuro.2016.09.011
Varghese J, Faith M, Jacob M (2009) Zinc prevents indomethacin-induced renal damage in rats by ameliorating oxidative stress and mitochondrial dysfunction. Eur J Pharmacol 614:114–121. https://doi.org/10.1016/j.ejphar.2009.04.053
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic mollusks. Mar Environ Res 44:69–84. https://doi.org/10.1016/S0141-1136(96)00103-1
Yadav S, Gupta SP, Srivastava G, Singh MP (2012) Role of secondary mediators in caffeine-mediated neuroprotection in maneb-and paraquat-induced Parkinson’s disease phenotype in the mouse. Neurochem Res 37:875–884. https://doi.org/10.1007/s11064-011-0682-0
Yonar ME, Sakin F (2011) Ameliorative effect of lycopene on antioxidant status in Cyprinuscarpioduring pyrethroid deltamethrin exposure. Pestic Biochem Physiol 99:226–231. https://doi.org/10.1016/j.pestbp.2010.12.008
Zhou Z, Kang X, Jiang Y, Song Z, Feng W, Mcclain CJ, Kang YJ (2007) Preservation of hepatocyte nuclear factor-4훼 is associated with zinc protection against TNF-훼 hepatotoxicity in mice. ExpBiol Med 232:622–628. https://doi.org/10.3181/00379727-232-2320622
Acknowledgments
This work was supported by the Ministry of Higher Education and Scientific Research [LR/18ES-41] Tunisia. The authors are indebted to Mrs. Raoudha Ben Amar Abdennadher and Mr. Chedli Tmar for their skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Mohamed Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sefi, M., Chaâbane, M., Elwej, A. et al. Zinc alleviates maneb-induced kidney injury in adult mice through modulation of oxidative stress, genotoxicity, and histopathological changes. Environ Sci Pollut Res 27, 8091–8102 (2020). https://doi.org/10.1007/s11356-019-07175-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07175-7