Abstract
Maneb and paraquat are known to induce Parkinson’s disease (PD) phenotype, however, caffeine offers neuroprotection. Nitric oxide (NO) acts an important mediator in PD phenotype and tyrosine kinase (TK), nuclear factor kappa B (NF-kB), p38 mitogen activated protein kinase (p38 MAPK) are known to regulate its production. The present study aimed to elucidate the role of caffeine in the regulation of NO production and microglial activation and their subsequent contribution in dopaminergic neuroprotection. The animals were treated with caffeine and/or maneb and paraquat along with controls. In a few sets of experiments, the animals were also treated with aminoguanidine, an inhibitor of inducible NO synthase, pyrrolidine dithiocarbamate (PDTC), an inhibitor of NF-kB, genistein, an inhibitor of TK or SB202190, an inhibitor of p38 MAPK. Tyrosine hydroxylase (TH)-immunoreactivity and anti-integrin αM (OX-42) staining were performed to assess the number of dopaminergic neurons and activation of microglia, respectively. NO was measured in terms of nitrite, however, the expressions of p38 MAPK, interleukin (IL)-1β, NF-kB and TK were checked by western blot analyses. Maneb and paraquat induced the number of degenerating dopaminergic neurons, microglial cells, nitrite content, expressions of IL-1β, p38 MAPK, NF-kB and TK and caffeine co-treatment reduced the level of such alterations. Reductions were more pronounced in the animals co-treated with aminoguanidine, PDTC, genistein or SB202190. The results obtained thus demonstrate that caffeine down-regulates NO production, neuroinflammation and microglial activation, which possibly contribute to neuroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common progressive neurodegenerative disorder after Alzheimer disease and is clinically characterized by muscular rigidity, resting tremor, bradykinesia and postural instability [1, 2]. Clinical symptoms appear only after the degeneration of more than 50% of dopaminergic neurons in the substantia nigra pars compacta, which extends to the striatum through axons [2, 3]. Despite first scientific description of the disease in 1817 by James Parkinson, complete molecular pathogenesis, exact contributory factors and permanent cure are yet elusive [1–3]. However, ageing, genetic factors and environmental exposure to pesticides and heavy metals have emerged as the putative risk factors.
Various chemicals, such as 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine (MPTP), N, N′-dimethyl-4, 4′-bipyridinium dichloride (paraquat), manganese ethylene-bisdithiocarbamate (maneb), rotenone and cypermethrin have been used to develop animal models to understand PD pathogenesis and treatment outcomes [4–8]. Paraquat produces neurodegeneration due to its structural similarity with 1-methyl-4-phenylpyridinium (MPP+), a toxic metabolite of MPTP. Paraquat inhibits electron transport chain complex-1 while maneb inhibits complex III, leading to free radical generation, which ultimately leads to neurodegeneration [4–6, 9, 10]. Maneb and paraquat, either alone or in combination are found to induce PD phenotype in the mouse [6, 11]. Combined exposure exhibits more pronounced changes in the indices of the nigrostriatal dopaminergic neurodegeneration and overall oxidative stress than alone [5, 12]. Maneb- and paraquat-induced animal model is of environmental relevance, as these pesticides are commonly and concurrently used by the farmers worldwide [5, 11–13].
Oxidative stress, caused by enhanced reactive oxygen and nitrogen species and reduced antioxidants machinery, have been found to be one of the main culprits of PD [2, 10]. Nitric oxide (NO), is known to regulate the lipid peroxidation in maneb- and paraquat-induced PD phenotype [14]. Maneb- and paraquat-induced PD phenotype is contributed by NO, which in turn is produced by inducible NO synthase (iNOS) involving nuclear factor kappa B (NF-kB), p38 mitogen activated protein kinase (p38 MAPK) and tyrosine kinase (TK) pathways [14]. An interaction among secondary signalling molecules, which are released after microglial activation and/or during lipid peroxidation, contributes to the nigrostriatal dopaminergic neurodegeneration [14, 15]. The role of NO in the nigrostriatal dopaminergic neurodegeneration is further supported by the fact that NOS inhibitors encounter the progression of PD [14]. Similarly, microglial activation is reported as one of the critical events in the pathogenesis of maneb and paraquat- induced PD phenotype in rodents [15].
Caffeine is one of the most widely consumed psycho-stimulants and is consumed by around 70% of the population globally [16]. An inverse relationship between PD and caffeine consumption is reported in epidemiological and animal based studies [8, 17–20]. The neuroprotective effects of caffeine are reported to be mediated by its antagonistic action on adenosine A2A receptor, leading to enhanced dopamine release into the striatum [13]. Additionally, its phosphodiesterase inhibitory activity leads to pronounced availability of cyclic adenosine monophosphate, which augments the protein kinase activity, thereby enhancing the expression of growth factors, modulation of toxicant responsive genes and anti-apoptotic property [8, 19–25].
The present study was undertaken to investigate the effect of caffeine in the regulation of p38 MAPK, TK and NF-kB and inflammatory molecule interleukin-1β (IL-1β) expressions, microglial activation and nitrite content and the possible contribution of these secondary signalling molecules to the nigrostriatal dopaminergic neuroprotection in maneb- and paraquat-induced PD phenotype in the mouse.
Materials and Methods
Chemicals
Polyvinylidine difluoride (PVDF) membrane was purchased from Millipore, Billerica, MA, USA. Streptavidin peroxidase complex and normal goat serum were procured from Bangalore Genei Private Limited, Bangalore, India. Anti-integrin αM (OX-42), anti-IL-1β, anti-tyrosine hydroxylase (anti-TH), anti-TK, anti-NF-kB and anti-p38 MAPK antibodies and compatible secondary antibodies were procured from Santa Cruz Biotechnology, Santa Cruz, CA, USA. Ammonium chloride (NH4Cl), hydrogen peroxide, ethanol, potassium dihydrogen orthophosphate, potassium permanganate and dimethyl sulfoxide (DMSO) were procured locally either from Qualigens or from SISCO Research Laboratory (SRL), Mumbai, India. All other chemicals were purchased from Sigma–Aldrich, St. Louis, MO, USA.
Animal Treatment
Male Swiss albino mice (20–25 g) were obtained from the animal house of the Council of Scientific and Industrial Research-Indian Institute of Toxicology Research (CSIR–IITR), Lucknow. Animals were housed in a room under the standard conditions of temperature, humidity and 12/12 h light/dark cycle. The CSIR-IITR animal ethics committee approved the study. Animals were fed commercially available pellet diet and drinking water ad libitum. Animals were treated intraperitoneally with paraquat (10 mg/kg) and maneb (30 mg/kg) in combination for 9 weeks along with saline treated controls [14]. In a few sets of experiments, the animals were also treated with caffeine, intraperitoneally (20 mg/kg). In another few sets, the animals were treated with aminoguanidine (30 mg/kg), an inhibitor of iNOS, pyrrolidine dithiocarbamate (PDTC; 50 mg/kg), an inhibitor of NF-kB, genistein (10 mg/kg), an inhibitor of TK and SB202190, an inhibitor of p38 MAPK (5 mg/kg) along with respective controls. The animals were sacrificed via cervical dislocation; the brain was dissected out immediately and kept in liquid nitrogen. The striatum was dissected out for the measurement of nitrite content and preparation of protein samples for western blotting as reported previously [14]. TH-immunoreactivity and immunohistochemistry for microglial activation were performed on the coronal brain sections across the substantia nigra as described elsewhere [7, 19].
Tyrosine Hydroxylase (TH)-Immunoreactivity
TH-immunoreactivity was performed followed by the unbiased counting of the TH- positive neurons in the substantia nigra. In brief, the sections were cut from the perfused brain (n = 3 in each group) dissected coronally through median eminence and non-specific labelling in the sections was blocked. The sections were incubated in monoclonal anti-TH antibody, washed and re-incubated with secondary antibody, followed by streptavidin peroxidase complex for 30 min. The colour was developed with 3, 3′diaminobenzidine (DAB) and the sections were dehydrated in graded ethanol, mounted with DPX and visualized under the microscope (Leica Microscope DM6000B, Germany). The images were captured at lower and intermediate magnification ranges and the TH-positive cells were counted as described previously [7, 26].
Microglial Activation
The microglial activation was performed in the substantia nigra region of the brain (n = 3 in each group) as described elsewhere [27]. In brief, the coronal sections were taken and incubated with 3% v/v hydrogen peroxide for 30 min at room temperature. After this, the sections were washed with phosphate buffered saline and incubated with 0.1% triton X-100 and 5% normal goat serum in phosphate buffered saline for 30 min. The sections were again incubated for 48 h at 4°C with anti-OX42 primary antibody (1:500 dilution in 0.1% triton X-100 and 5% normal goat serum) followed by incubation in biotinylated goat anti-rabbit (1:1000) secondary antibody for 2 h at room temperature. The sections were washed with phosphate buffered saline and transferred in a solution containing streptavidin-horseradish peroxidase (HRP) complex for 30 s and the colour was developed with DAB. The sections were dehydrated in graded ethanol, mounted with DPX and visualized under the microscope as above.
Measurement of Nitrite Content
Nitrite content was estimated (n = 3 in each group) as reported previously [14]. Briefly, 10% w/v tissue homogenate was incubated with NH4Cl (0.7 mM) and Griess reagent [0.1% N-(1-naphthyl) ethylene-diamine dihydrochloride and 1% sulfanilamide in 2.5% orthophosphoric acid] at 37°C for 30 min. The reaction mixture was centrifuged at 10,000×g at 4°C for 10 min and the absorbance of the supernatant was measured at 550 nm. The nitrite content was calculated as μmoles/ml using a standard curve for sodium nitrite (10–100 μM).
Western Blotting of NF-kB, TK, IL-1β, and p38 MAPK
Western blot analysis was performed (n = 3 in each group) as described elsewhere previously [14, 19, 28] with some modifications. In brief, 10% tissue homogenate was prepared in lysis buffer containing 20 mM tris–HCl, pH 7.4, 2 mM ethylene-diamine-tetra-acetic acid (EDTA), 2 mM ethylene glycol-bis (β-aminoethylether)-N, N, N′, N′-tetra-acetic acid (EGTA), 1 mM phenyl methyl sulphonyl fluoride (PMSF), 30 mM sodium fluoride, 30 mM sodium pyrophosphate, 0.1% sodium dodecyl sulphate (SDS), 1% glycerol, 1% triton X-100 and protease inhibitor cocktail. The resultant homogenate was centrifuged at 10,000×g for 10 min and the supernatant was taken. The protein content was measured [29] and ~100 μg protein was denatured with SDS buffer. The denatured protein was separated on (8–12%) polyacrylamide gel and transferred onto PVDF membrane. Non-specific labelling was blocked with tris buffered saline containing 5% non-fat dry milk for 2 h. The blots were incubated with any of the primary antibodies (anti-NF-kB, TK, IL-1β or p38 MAPK; dilution: 1:1000) overnight. Anti-mouse or anti-rabbit alkaline phosphatase-conjugated secondary antibody was used to detect the target proteins, visualized using 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitro blue tetrazolium (NBT) chromogen solution as substrate. The relative band density was calculated with respect to the β-actin blot developed under similar conditions using the computerized densitometry system (Alpha Imager System, Alpha Innotech Corporation, San Leandro, CA, USA).
Statistical Analysis
One-way analysis of variance (ANOVA) followed by Newman-Keuls post hoc test was used for comparisons between various groups. The data are expressed as means ± standard error of means (SEM). The differences between groups were considered statistically significant when the P value was less than 0.05.
The various levels of significance in this study are symbolically depicted as *(P < 0.05); #(P < 0.01) and δ (P < 0.001) and numerically designated as 1, 2, 3 and 4 in comparison with control, maneb- and paraquat-treated, maneb- and paraquat- and caffeine-treated and maneb- and paraquat- and PDTC-treated animals, respectively.
Results
TH-Immunoreactivity
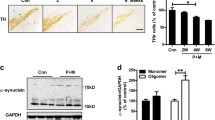
The number of TH-positive cells is increased in the substantia nigra of caffeine and maneb and paraquat co-treated animals as compared with maneb and paraquat alone co-treated animals. However, treatment of caffeine alone did not change the TH-immunoreactivity and the number of TH positive cells in the substantia nigra. Aminoguanidine, PDTC, genistein and SB202190, partially but significantly, restored the level of TH-positive neurons in maneb and paraquat treated mice. The restoration was more pronounced in the animals that were co-treated with caffeine (Fig. 1a, b).
TH immunoreactivity of dopaminergic neurons in maneb- and paraquat-induced PD phenotype in mouse substantia nigra in the presence or absence of caffeine co-treatment along with aminoguanidine, PDTC, genistein and SB202190 (n = 3 in each group) (a). The number of TH-positive cells expressed as percent of control (b). The control values were considered 100% in each replicate and experimental values were calculated accordingly for each experiment, therefore, there is no error bar in the control in b. Significant changes are expressed as 1δ (P < 0.001) in comparison with control; 2δ (P < 0.001) in comparison with maneb and paraquat and 3δ (P < 0.001) in comparison with maneb and paraquat and caffeine
Microglial Activation
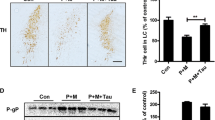
Maneb and paraquat significantly increased the number of activated microglia in the substantia nigra, as evidenced by OX-42 immunostaining; however, caffeine alone did not produce any significant change in the number of activated microglia in the studied tissue. Caffeine reduced the number of activated microglia in maneb and paraquat co-treated animals significantly. Although aminoguanidine, PDTC, genistein and SB202190 produced a significant decrease in the number of activated microglia in maneb and paraquat co-treated animals, the responses were more evident in the animals that were also treated with caffeine (Fig. 2a, b).
OX-42 staining (microglia) in control and maneb- and paraquat-induced PD phenotype in mouse substantia nigra in the presence or absence of caffeine pre-treatment along with aminoguanidine, genistein, PDTC and SB202190 (n = 3 in each group) (a) and the number of microglial cells in various treatment groups expressed as percent of control (b). The control values were considered 100% in each replicate and experimental values were calculated accordingly for each experiment, therefore, there is no error bar in controls in b. Significant changes are expressed as 1δ (P < 0.001) in comparison with control; 2δ (P < 0.001) in comparison with maneb and paraquat; 3* (P < 0.05) and 3# (P < 0.01) in comparison with maneb and paraquat and caffeine
Nitrite Content
Caffeine co-treatment exhibited significant reduction in the nitrite content as compared with maneb and paraquat treated animals. However, caffeine vehicle did not alter the nitrite level significantly. Aminoguanidine, genistein, PDTC and SB202190 significantly attenuated the nitrite level as compared with maneb and paraquat treated animals and the responses were more pronounced in the animals that were also treated with caffeine (Fig. 3).
Nitrite level in control and maneb- and paraquat-induced PD phenotype in mouse in the presence or absence of caffeine pre-treatment along with aminoguanidine, genistein, PDTC and SB202190 (n = 3 in each group). Significant changes are expressed as 1* (P < 0.05), 1# (P < 0.01), 1δ (P < 0.001) in comparison with control; 2δ (P < 0.001) in comparison with maneb and paraquat; 3* (P < 0.05) in comparison with maneb and paraquat + caffeine and 4* (P < 0.05) in comparison with maneb and paraquat and PDTC
IL-1β and p38 MAPK Protein Expressions
Caffeine alone did not produce significant change in the IL-1β expression; however, maneb and paraquat significantly increased its expression level. Caffeine in combination with maneb and paraquat reduced the IL-1β expression as compared with maneb and paraquat treated animals. Aminoguanidine, genistein, PDTC and SB202190 attenuated the IL-1β expression as compared with maneb and paraquat treated animals and the expression level was more pronounced when the animals were also treated with caffeine. Similar trend was seen in the case of p38 MAPK expression but the changes were statistically significant only when the animals were treated with PDTC or SB202190 in combination with caffeine (Fig. 4a, b).
Effect of maneb and paraquat and caffeine on IL-1β and p38 MAPK protein expressions in the presence or absence of aminoguanidine, genistein, PDTC and SB202190. IL-1β and p38 MAPK expressions are shown in a, bar diagram representing the band density ratio with respect to the housekeeping gene β-actin (expressed as percent of control) in b. The data are expressed as means ± SEM (n = 3 in each group). Significant changes are expressed as 1* (P < 0.05), 1# (P < 0.01), 1δ (P < 0.001) in comparison with control; 2* (P < 0.05) and 2# (P < 0.01) in comparison with maneb and paraquat and 3* (P < 0.05) in comparison with maneb and paraquat and caffeine
NF-kB and TK Protein Expressions
The caffeine treatment did not alter NF-kB and TK expressions as compared with the respective controls. Caffeine, even without NF-kB or TK inhibitor, reduced NF-kB and TK protein expressions in maneb and paraquat treated mice. The reductions were more pronounced in the presence of the inhibitors of various pathways used in this study. However, the pronounced change was statistically significant only in the case of NF-kB protein expression (Fig. 5a, b).
Effect of maneb and paraquat and caffeine on NF-kB and tyrosine kinase protein expressions in the presence or absence of aminoguanidine, genistein, PDTC and SB202190. NF-kB and tyrosine kinase expressions with respect to the housekeeping gene β-actin are shown in panel (a) and the corresponding bar diagram representative of the relative band density ratio (expressed as percent of control) in b. The data are expressed as means ± SEM (n = 3 in each group). Significant changes are expressed as 1* (P < 0.05) and 1δ (P < 0.001) in comparison with control; 2* (P < 0.05) and 2# (P < 0.01) in comparison with maneb and paraquat
Discussion
Maneb and paraquat in combination were found to induce degeneration of dopaminergic neurons, which is in accordance with the observations that maneb and paraquat in combination induce PD phenotype in the mouse [5, 6, 11, 12, 14, 26, 30, 31]. Caffeine was found to protect the degeneration of dopaminergic neurons in this study as reported in several chemically induced mice models of PD [8, 13, 19, 20]. In this study, increased nitrite content, an indicator of NO, was seen after maneb and paraquat exposures, as observed previously [14]. The nitrite content and neurodegeneration were significantly reduced in the presence of aminoguanidine, an inhibitor of iNOS, PDTC, an inhibitor of NF-kB, genistein, an inhibitor of TK and SB202190, an inhibitor of p38 MAPK showing the involvement of multiple pathways in the regulation of NO production and neurodegeneration. The study showed that NO is critical in the nigrostriatal dopaminergic neurodegeneration mediated by maneb and paraquat [14]. Caffeine reduced the nitrite content, which is further altered by the inhibitors of various pathways, showing that NO is involved in caffeine-mediated neuroprotection.
Activation of microglial cells, the resident innate immune brain cells, was observed in the substantia nigra region after maneb and paraquat co-exposure. Previous studies have also shown that microglial activation is an important event in chemically-induced [32–36] and sporadic PD [37]. Activation of microglial cells is associated with excessive production of NO as activated microglial cells contain high level of iNOS. NO is known to increase glutamate release, which leads to excitotoxicity via the N-methyl-D-aspartate receptor. Aminoguanidine partially attenuated the effect of maneb and paraquat possibly by reducing the number of activated microglia. Caffeine treatment reduced the number of activated microglial cells as compared with maneb and paraquat treated animals, which could be responsible for the reduction of nitrite content in caffeine treated animals. Increased level of IL-1β in maneb- and paraquat-induced PD and reduction in its extent in caffeine treated animals is in accordance with previous reports as activated microglial cells secrete the pro-inflammatory cytokine IL-1β, which could exacerbate toxicity in dopaminergic neurons [38]. Genistein reduced the nitrite content, thereby reduced the nigrostriatal dopaminergic neurodegeneration as reported in a previous studies [14]. This is further supported by the role of NF-kB in the regulation of IL-1β expression and its subsequent association with neurodegeneration [39–43]. The decreased level of its expression in aminoguanidine treated group as compared with maneb and paraquat treated mice further suggests the involvement of microglial activation in maneb- and paraquat-induced PD phenotype and caffeine-mediated neuroprotection.
Nitrite content, which increased in maneb and paraquat treated animals, was reduced by the caffeine or inhibitors of various pathways, significantly by PDTC, an inhibitor of NF-kB. Reduced nitrite content could be responsible for the reduced loss of TH-positive cells in the nigrostriatal tissues. This observation suggests that NF-kB could be critical in the regulation of NO level in caffeine-mediated effects. Caffeine-mediated neuroprotection in maneb and paraquat treated animals could be contributed by the reduced microglial activation or nitrite content. The results obtained in this study demonstrate that caffeine offers neuroprotection involving NO, which in turn is regulated by multiple pathways, and reduced microglial activation.
Overall, the maneb- and paraquat-induced PD phenotype is contributed by NO (measured in terms of nitrite), which in turn regulates nigrostriatal dopaminergic neurodegeneration. In this study, caffeine was found to reduce the levels of NO, microglial activation and thereby protect dopaminergic neurodegeneration and the changes were more pronounced in aminoguanidine (inhibitor of NO synthase enzyme) and caffeine both treated animals as compared with caffeine alone-treated animals. The NO level is reported to be regulated by NF-kB, p38 MAPK and TK pathways in maneb- and paraquat-induced PD [14], therefore, the roles of these pathways in caffeine-mediated reductions of NO and microglial activation and subsequent nigrostriatal dopaminergic neuroprotection were assessed. The alterations in these parameters were checked in the presence/absence of NF-kB inhibitor-PDTC, TK inhibitor-genistein and p38 MAPK inhibitor-SB202190. Although caffeine-mediated reductions in NO and microglial activation leading to dopaminergic neuroprotection were modulated by all these three inhibitors, more pronounced modulations were seen in PDTC-treated groups. The results obtained thus suggest that caffeine-mediated neuroprotection is regulated by NF-kB, TK and p38 MAPK pathways; however, NF-kB pathway seems to be predominant.
References
Klockgether T (2004) Parkinson’s disease: clinical aspects. Cell Tissue Res 318:115–120
Singh MP, Patel S, Dikshit M, Gupta YK (2006) Contribution of genomics and proteomics in understanding the role of modifying factors in Parkinson’s disease. Indian J Biochem Biophys 43:69–81
Patt S, Gertz HJ, Gerhard L, Cervos-Navarro J (1991) Pathological changes in dendrites of substantia nigra neurons in Parkinson’s disease: a Golgi study. Histol Histopathol 6:373–380
Tanner CM, Langston JW (1990) Do environmental toxins cause Parkinson’s disease? A critical review. Neurology 40:17–30
Thiruchelvam M, Richfield EK, Baggs RB et al (2000) The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci 20:9207–9214
Patel S, Singh V, Kumar A et al (2006) Status of antioxidant defense system and expression of toxicant responsive genes in striatum of maneb and paraquat-induced Parkinson’s disease phenotype in mouse: mechanism of neurodegeneration. Brain Res 1081:9–18
Singh AK, Tiwari MN, Upadhyay G et al (2012) Long-term exposure to cypermethrin induces the nigrostriatal dopaminergic neurodegeneration in adult rats: postnatal exposure enhances the susceptibility during adulthood. Neurobiol Aging 33:404–415
Singh K, Singh S, Singhal NK et al (2010) Nicotine- and caffeine-mediated changes in gene expression patterns of MPTP-lesioned mouse striatum: implications in neuroprotection mechanism. Chem Biol Interact 185:81–93
Thrash B, Uthayathas S, Karuppagounder SS et al (2007) Paraquat and maneb induced neurotoxicity. Proc West Pharmacol Soc 50:31–42
Srivastava G, Singh K, Tiwari MN, Singh MP (2010) Proteomics in Parkinson’s disease: current trends, translational snags and future possibilities. Expert Rev Proteomics 7:127–139
Thiruchelvam M, Richfield EK, Goodman BM et al (2002) Developmental exposure to the pesticides paraquat and maneb and the Parkinson’s disease phenotype. Neurotoxicology 23:621–633
Patel S, Singh K, Singh S, Singh MP (2008) Gene expression profiles of mouse striatum in control and maneb + paraquat-induced Parkinson’s disease phenotype: validation of differentially expressed energy metabolizing transcripts. Mol Biotechnol 40:59–68
Kachroo A, Irizarry MC, Schwarzschild MA (2010) Caffeine protects against combined paraquat and maneb-induced dopaminergic neuron degeneration. Exp Neurol 223:657–661
Gupta SP, Patel S, Yadav S et al (2010) Involvement of nitric oxide in maneb- and paraquat-induced Parkinson’s disease phenotype in mouse: is there any link with lipid peroxidation? Neurochem Res 35:1206–1213
Cicchetti F, Lapointe N, Roberge-Tremblay A et al (2005) Systemic exposure to paraquat and maneb models early Parkinson’s disease in young adult rats. Neurobiol Dis 20:360–371
Fredholm BB, Ijzerman AP, Jacobson KA et al (2001) International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Ross GW, Abbott RD, Petrovitch H et al (2000) Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 283:2674–2679
Ascherio A, Zhang SM, Hernán MA et al (2001) Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol 50:56–63
Singh S, Singh K, Gupta SP et al (2009) Effect of caffeine on the expression of cytochrome P450 1A2, adenosine A2A receptor and dopamine transporter in control and 1-methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine treated mouse striatum. Brain Res 1283:115–126
Singh S, Singh K, Patel S et al (2008) Nicotine and caffeine-mediated modulation in the expression of toxicant responsive genes and vesicular monoamine transporter-2 in 1-methyl 4-phenyl-1, 2, 3, 6-tetrahydropyridine’induced Parkinson’s disease phenotype in mouse. Brain Res 1207:193–206
Richardson PJ, Kase H, Jenner PG (1997) Adenosine A2A receptor antagonists as new agents for the treatment of Parkinson-s disease. Trends Pharmacol Sci 18:338–344
Ochi M, Koga K, Kurokawa M et al (2000) Systemic administration of adenosine A2A receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: a microdialysis study. Neuroscience 100:53–62
Lindskog M, Svenningsson P, Pozzi L (2002) Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature 418:774–778
Chen X, Lan X, Roche I et al (2008) Caffeine protects against MPTP-induced blood–brain barrier dysfunction in mouse striatum. J Neurochem 107:1147–1157
Nakaso K, Ito S, Nakashima K (2008) Caffeine activates the PI3 K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci Lett 432:146–150
Singhal NK, Srivastava G, Patel DK et al (2011) Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson’s disease phenotype in the mouse. J Pineal Res 50:97–109
Singh AK, Tiwari MN, Dixit A et al (2011) Nigrostriatal proteomics of cypermethrin-induced dopaminergic neurodegeneration: microglial activation dependent and independent regulations. Toxicol Sci 122:526–538
Martin PY, Bianchi M, Roger F et al (2002) Arginine vasopressin modulates expression of neuronal NOS in rat renal medulla. Am J Physiol Renal Physiol 283:F559–F568
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
McCormack AL, Thiruchelvam M, Manning-Bog AB et al (2002) Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 10:119–127
Patel S, Sinha A, Singh MP (2007) Identification of differentially expressed proteins in striatum of maneb- and paraquat-induced Parkinson’s disease phenotype in mouse. Neurotoxicol Teratol 29:578–585
Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39:151–170
Kim WG, Mohney RP, Wilson B et al (2000) Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci 20:6309–6316
Wu XF, Block ML, Zhen W et al (2005) The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal 7:654–661
Zhou Y, Wang Y, Kovacs M et al (2005) Microglial activation induced by neurodegeneration: a proteomic analysis. Mol Cell Proteomics 4:1471–1479
Gao HM, Hong JS, Zhang W, Liu B (2002) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291
Pott Godoy MC, Tarelli R, Ferrar CC et al (2008) Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain 131:1880–1894
Qian L, Flood PM (2008) Microglial cells and Parkinson’s disease. Immunol Res 41:155–164
Hunot S, Brugg B, Ricard D et al (1997) Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proc Natl Acad Sci USA 94:7531–7536
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107:7–11
Cogswell JP, Godlevski MM, Wisely GB et al (1994) NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J Immunol 153:712–723
Wilms H, Rosenstiel P, Sievers J et al (2003) Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease. FASEB J 17:500–502
Acknowledgments
We sincerely acknowledge the CSIR, New Delhi, India for providing research fellowships to Sharawan Yadav, Satya Prakash Gupta and Garima Srivastava. This work was supported by the Department of Biotechnology, Government of India. The CSIR–IITR communication number of this article is 2971.
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, S., Gupta, S.P., Srivastava, G. et al. Role of Secondary Mediators in Caffeine-Mediated Neuroprotection in Maneb- and Paraquat-Induced Parkinson’s Disease Phenotype in the Mouse. Neurochem Res 37, 875–884 (2012). https://doi.org/10.1007/s11064-011-0682-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0682-0