Abstract
How the vast majority of nitrous oxide (N2O) in the aerobic zone of nitrogen bio-removal process is produced is still a controversial issue. To solve this issue, this study measured the activities of two key denitrifying enzymes (nitric oxide reductase (Nor) and nitrous oxide reductase (N2OR)) in an A/O SBR with different chemical nitrogen demand (COD)/total nitrogen (TN) ratios. By analyzing the Spearman’s correlations between the N2O production, the enzyme activities, and the factors, the main N2O production process was identified. By comparing the activities of these enzymes, this study analyzed the reasons for the N2O production. Results show that Nor activities had a linear relationship with total N2O concentrations (y = 0.34749 + 31.31365x, R2 = 0.83362) and were not affected by COD (r = 0.299, N = 15, P = 0.279 > 0.05), which showed that most of the N2O released and produced came from the autotrophic denitrification. N2OR activities had a positive correlation with COD (r = 0.692, N = 15, P = 0.004 < 0.01), which showed that heterotrophic denitrification played a role as an N2O consumer. Nor activities were much higher than N2OR activities and the gap between them increased when the total N2O concentration increased, showing that the heterotrophic denitrification was difficult to consume all the N2O produced by the autotrophic denitrification. Reducing autotrophic denitrification is the best way to reduce N2O production in aerobic phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) is a greenhouse gas, and its greenhouse effect is 298 times that of carbon dioxide and 30 times that of methane (Edenhofer et al. 2014). The emissions of N2O from wastewater treatment plants (WWTPs) were estimated of accounting for about 3% of global emissions in 2011 (Chapa 2011). In WWTPs, the aeration tank is considered to be an important unit for the production and emission of N2O (Foley et al. 2010). Reducing the production and emission of N2O at this phase is a key step in reducing the greenhouse gas emissions in WWTPs. Studies suggest that there are three main pathways to produce N2O: incomplete oxidation of hydroxylamine (NH2OH) to nitrite (Cavazos et al. 2018; Zhou et al. 2020), heterotrophic denitrification (Guo et al. 2018; Zhou et al. 2020), and autotrophic denitrification (Wrage-Mönnig et al. 2018; Yan et al. 2021). However, which production pathway is the main production process of N2O under aerobic conditions has been controversial.
Nitric oxide reductase (Nor) and nitrous oxide reductase (N2OR) are two key enzymes that control the production of N2O during denitrification (Guo et al. 2018). Nor is an enzyme that catalyzes the reduction of nitric oxide (NO) to N2O. It is in the inner membrane of gram-negative bacteria and shows catalytic activity under both aerobic and anaerobic conditions (Peder et al. 2013). Meanwhile, N2OR is an enzyme that catalyzes the reduction of N2O to nitrogen gas (N2). It is in the periplasmic of gram-negative bacteria and also has catalytically active under both aerobic and anaerobic conditions (Conthe et al. 2018; Pauleta et al. 2013). By measuring the activities of these two enzymes, the source of N2O and the reason for its production can be effectively determined. Besides, the synthesis and specific activity of Nor and N2OR would be affected by the external environment, especially the dissolved oxygen (DO) (Conthe et al. 2018) and the amount of organic matter (Pan et al. 2013). Therefore, the nitrogen demand (COD)/total nitrogen (TN) ratio and the DO are the two key points for studying how enzymes regulate N2O accumulation.
In this study, the dynamic changes of Nor activities and N2OR activities were measured in an anoxic/aerobic sequential batch reactor (A/O SBR) with different COD/TN ratios. Firstly, from the perspective of key enzymes, the main production process of N2O and the main consumption process of N2O were identified in aerobic phase of A/O SBR. Secondly, from the relationship between enzyme activity and environmental factors (pH conditions, amount of organic matter, and supply rates of DO), this study further analyzed how environmental changes caused by changes in the organic load had an impact on the N2O production. Finally, by comparing the activities of these two enzymes, this study revealed the reasons for the N2O production and gave some suggestions on how to reduce N2O in WWTPs in the future.
Materials and methods
Reactor and operation
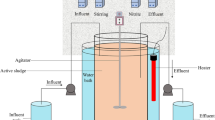
A 6-L lab-scale A/O SBR was used, of which 5 L was used as a reaction space (as shown in Fig. 1). Inoculated sludge was from an aerobic tank of WWTPs in Xi’an, China. The stirring and aeration of the reactor were provided by a magnetic stirrer and bubble air diffuser (1 L·min−1), respectively. And the volumetric exchange ratio, sludge concentration, and operating temperature of the reactor were 50%, 3500 ± 200 mg·L−1, and 27 ± 1 °C, respectively.
To make the relevant bacteria adapt to the environment of different periods and the bacterial population community did not change, there was a 2-week operating cycle between each group of experiments, in which the removal of COD and ammonia nitrogen was stable. Each experiment was carried out three times. An 8-h working period was applied over the entire research, and the cycle time setting of the reactor was shown below: an inflowing phase (10 min), an anoxia phase (120 min), an aerobic phase (240 min), a setting phase (40 min), a decanting phase (10 min), and an idle phase (60 min).
Synthetic wastewater
The reactor had three operating modes in this study, and the difference in COD/TN ratios was the main difference between them. In these three operating modes, the COD/TN ratios of synthetic wastewater were 3.3, 6.5, and 9.3, respectively. The influent components were as follows: CH3COONa 117.50 mg·L−1 (3.3), 235.00 mg·L−1 (6.5), 352.50 mg·L−1 (9.3), C6H12O6 117.50 mg·L−1 (3.3), 235.00 mg·L−1 (6.5), 352.50 mg·L−1 (9.3), NH4Cl 230 mg·L−1, NaHCO3 200 mg·L−1, KH2PO4 22 mg·L−1, MgSO4·7H2O 10 mg·L−1, FeSO4·7H2O 10 mg·L−1, CaCl2·2H2O 10 mg·L−1, CuSO4·5H2O 0.03 mg·L−1, H3BO3 0.15 mg·L−1, MnSO4·H2O 0.12 mg·L−1, KI 0.18 mg·L−1, Na2MoO4·2H2O 0.06 mg·L−1, CoCl2·6H2O 0.15 mg·L−1, ZnSO4·7H2O 0.12 mg·L−1, EDTA·2Na 10 mg·L−1. The pH value of synthetic wastewater was around 7.5 ± 0.5.
Extraction of enzymes
Extraction of N 2 OR
The extraction methods of N2OR were shown below (Ferretti et al. 1999; Hulse and Averill 1990; Yang et al. 2021). The activated sludge sample was harvested by centrifugation at 4000 r·min−1 for 10 min in a high-speed refrigerated centrifuge (Beckman Coulter Co., Ltd) from the liquid sample with a volume of 20 mL. And the activated sludge sample was suspended in 20 mL with buffer solutions (5 mM MgCl2, 20 mM Tris–HCl (pH 8.0) (buffer A)). The resuspended solution was centrifuged again at 4000 r·min−1 for 10 min to separate the activated sludge sample. In this way, the activated sludge sample was washed 3 times with buffer A. After rinsing, the activated sludge sample was suspended in 20 mL with buffer A and was disrupted in a high-pressure homogenizer (Guangdong Juneng Biological Technology Co., Ltd) operating at 4 °C at 160 Mpa. The crushed suspension was collected in 75-mL anaerobic bottles and was centrifuged at 4 °C at 40,000 g for 30 min. The supernatant was used to assay the N2OR activity.
Extraction of Nor
The extraction methods of Nor were shown below (Heiss et al. 1989; Kastrau et al. 2005). The activated sludge sample was harvested by centrifugation at 4000 r·min−1 for 10 min from the liquid sample with a volume of 20 mL. The activated sludge sample was suspended in 20 mL with buffer solutions (200 mM Tris–HCl (pH 8.0) (buffer B)). The resuspended solution was centrifuged again at 4000 r·min−1 for 10 min to separate the activated sludge sample. In this way, the activated sludge sample was washed 3 times with buffer B. After rinsing, the activated sludge sample was suspended in 20 mL with buffer solutions (50 mM Tris–HCl, 150 mM KCl (buffer C)) and was repeatedly crushed 4 times with a high-pressure homogenizer operating at 4 °C at 100 Mpa. The crushed suspension was centrifuged at 4 °C at 5000 g for 10 min. The resulting supernatant with a volume of 10 mL was transferred to a centrifuge tube, and 0.1 mL 0.02% phenylethyl alcohol and 0.1 mL 1% n-Dodecyl-beta-D-maltoside were added while stirring. The sample was reacted in an ice bath for 15 min and was centrifuged at 4 °C at 40,000 g for 90 min. The supernatant was used to assay the Nor activity.
Enzyme assays
N 2 OR assays
The methods of N2OR assays were shown below (Ferretti et al. 1999; Hulse and Averill 1990; Yang et al. 2021). The activity of N2OR was measured under an argon atmosphere in a 13-mL stoppered vial. The mixture reaction within a total volume of 5 mL contained 14 mM Tris–HCl, 1 mM Methyl Viologen, and 20 mM sodium dithionite. And the pH of this mixture reaction was controlled at 8.0. The mixture reaction was shocked vigorously for 20 min after injecting 5 mL N2O (10 mol·m−3) and then stood for 10 min. The appropriate amount of the test enzyme was added to start the reaction. The reaction flask was placed on a shaker and shaken vigorously for 30 min. The gas sample with a volume of 1 mL was extracted from the reaction flask, and it would be diluted with argon to be measured by gas chromatography (GC) (Clarus 600; Singapore (PerkinElmer)). The blank sample was the sample that underwent the above treatment process after replacing the enzyme with an equal volume of buffer solution.
The activity of N2OR was calculated by Eq. (1):
where \({EA}_{{\mathrm{N}}_{2}\mathrm{OR}}\) is the activity of N2OR, U·mL−1, \({n}_{\mathrm{s}}\) is the mole number of N2O in the samples, µmol, \({n}_{\mathrm{b}}\) is the mole number of N2O in the blank samples, µmol, t is the reaction time, min, and \({V}_{\mathrm{E}}\) is the was the volume of enzyme, mL.
Nor assays
The methods of Nor assays were shown below (Heiss et al. 1989; Kastrau et al. 2005). The activity of Nor was measured under an argon atmosphere in 13-mL stoppered vials containing, within a total volume of 3 mL, 300 µmol of sodium acetate, 100 µmol of sodium ascorbate, 0.5 µmol of phenazine methosulfate, and the enzyme sample. An appropriate amount of tested enzyme and NO (25 µmol) was added to the reaction flask. The reaction flask was placed on a shaker and shaken vigorously for 30 min. The gas sample with a volume of 1 mL was extracted from the reaction flask, and it would be diluted with argon to be measured by GC. The blank sample was the sample that underwent the above treatment process after replacing the enzyme with an equal volume of buffer solution.
The activity of Nor was calculated by Eq. (2):
where \({EA}_{Nor}\) is the activity of Nor, U·mL−1, \({n}_{\mathrm{s}}\) is the mole number of N2O in the samples, µmol, \({n}_{b}\) is the mole number of N2O in the blank samples, µmol, t is the reaction time, min, and \({V}_{E}\) is the was the volume of enzyme, mL.
Measurement and calculation of N 2 O and other indicators
The gaseous N2O was measured by GC (Clarus 600; Singapore (PerkinElmer)) and dissolved N2O was measured by the headspace method (He et al. 2017; Yang et al. 2021). A 20 mL liquid sample was transported to a 75-mL headspace bottle. For inhibiting the microbial activity, 2 mL 2 M H2SO4 was added to the bottle. The bottle was shaken for about 1 min and then stand for 1 h. A 1 mL gas sample was extracted from the headspace of this bottle and was used to measure the concentration of N2O by GC (Clarus 600; Singapore (PerkinElmer)). The amount of dissolved N2O could be calculated by Henry’s law (H (25 °C) = 2.47 × 10−7 mol·L−1·Pa−1). And the calculation of dissolved N2O, total N2O emission, and total N2O concentration could refer to Yang et al. (2021). The chemic indicators, ammonia, nitrite, nitrate, total nitrogen (TN), chemical oxygen demand (COD), and mixed liquid suspended solids (MLSS), were determined using Standard Methods (APHA 1998). pH and DO values were determined by pH meters (FE-20, Mettler Toledo Instrument (Shanghai) Co., Ltd.) and DO meters (HQ40d, Hach Company World Headquarters), respectively. The community of activated sludge was determined by Sangon Biotech (Shanghai) Co., Ltd.
The nitrogen reduction was calculated by Eq. (3):
where NR was the nitrogen reduced in the aerobic condition, mg, TN120 and TN360 were the TN in 120 min and 360 min, respectively, mg·L−1, and Vliquid was the volume of the reactor’s reaction space, mL.
The ammonia oxidation rate was calculated by Eq. (4):
where \({v}_{{{\mathrm{NH}}_{4}}^{+}}\) was the ammonia oxidation rate, mg·(L·min) −1, \({S}_{{t}_{1}}\) and \({S}_{{t}_{2}}\) were the concentration of ammonia in \({t}_{1}\) and \({t}_{2}\), respectively, mg·L−1, and \({t}_{1}\) and \({t}_{2}\) were the time point of reactor operation, min.
The proportion of ammonia oxidation was calculated by Eq. (5):
where \({R}_{{{\mathrm{NH}}_{4}}^{+}}\) was the proportion of ammonia oxidation, %, and \({S}_{0}\) and \({S}_{360}\) were the concentration of ammonia in 0 min and 360 min, respectively, mg·L−1.
Data processing
SPSS 13.0 software was used to calculate the standard deviation of several parallel experiment groups and to analyze the correlation between factors. Origin pro 9.0 software was used to perform linear fit on the experimental data. The convex hull points were calculated by the Graham’s scan method (Graham 1972), and the convex hull points were connected to form the shadow part.
Results and discussion
Analysis of the main production pathways of N 2 O
For A/O SBR, the vast majority of N2O was produced in the prophase of the aerobic phase. As shown in Fig. 2D and E, in the prophase of aerobic phase (120 to 240 min), the emissions of N2O accounted for the largest proportion (78.08–88.79%) of the total emissions, and the nitrogen loss accounted for the largest proportion (77.29–85.08%) of the total nitrogen loss. Meanwhile, in the aerobic phase, the aeration intensity was relatively high (1 L·min−1), which caused the N2O produced in the anoxic phase to be quickly blown off after the aeration started. It means that the emitted N2O was mainly produced in the aerobic phase rather than the anoxic phase, especially after 150 min (Foley et al. 2010). Therefore, the vast majority of N2O was produced in the early stages of the aerobic phase.
Conversion rules of ammonia, nitrate, and nitrite under COD/TN ratio of 3.3 (A), 6.5 (B) and 9.3 (C), and the amount of nitrogen loss (D) and the cumulative N2O emissions (E) in the aerobic phase of different periods, and the amount of nitrogen loss in the aerobic phase and its proportion of N2O (F)
For the aerobic phase of A/O SBR, the nitrifier denitrification is the main N2O production process. As shown in Figs. 2A–C and 3, in the prophase of the aerobic phase (120 to 240 min), the ammonia oxidation process, the nitrite accumulation, and the lower DO concentration coexisted, so all three pathways of N2O production might exist in this study. As shown in Fig. 4, when the total N2O concentration was between 0 and 0.6 mg N·L−1, the total N2O concentrations showed positive correlations with the Nor activities. By conducting linear regression based on the research data, most data points stayed within the predicted 95% confidence bounds, which showed that there was a good linear relationship between the Nor activity and the total N2O concentration (y = 0.34749 + 31.31365x, R2 = 0.833). This phenomenon showed that in the aerobic phase, Nor activities determined the amount of N2O produced in the reactor. In other words, most N2O was produced through the denitrification process with Nor as a key enzyme, rather than the incomplete oxidation of NH2OH to nitrite or other ways. Meanwhile, as shown in Fig. 5C, in the aerobic phase, when the concentration of COD was between 0 and 70 mg·L−1, the Nor activities were not affected by the levels of COD (r = 0.299, N = 15, P = 0.279 > 0.05). Therefore, in the aerobic phase of A/O SBR, autotrophic denitrification was the main N2O production process. As shown in Fig. 6, the possible autotrophic denitrifiers included Ferruginibacter and Nitrospira. Ferruginibacter could perform iron-dependent denitrification (Wu et al. 2019), and Nitrospira could perform nitrifier denitrification (Wrage-Mönnig et al. 2018). Since the electron acceptor of iron-dependent denitrification is divalent iron, it is not conducive to its denitrification process under aerobic conditions. The concentration of iron in the reactor was about 18 µmol·L−1, showing that the iron-dependent denitrification process was weak in the aerobic phase. Therefore, in the aerobic phase, nitrifier denitrification might be the main N2O production process. The reasons for “incomplete” nitrifier denitrification might be that N2O has lower toxicity and the related enzyme (N2OR) has weaker electronic competitiveness. In the presence of DO and N2O, nitrifiers were more likely to use oxygen as their electron acceptors (Conthe et al. 2018; Zumft and Kroneck 2006). Besides, under aerobic conditions, NO had an inhibitory effect on the activity of oxidase and N2OR, which would cause that the NO reduction process would take precedence when the electron was limited (Carr and Ferguson 1990). Therefore, N2O was the main final product of nitrifier denitrification.
Spearman correlation between Nor activity and DO (A), Nor activity and pH (B), Nor activity and COD (C), and Spearman’s correlation between N2OR activity and DO (D), N2OR activity and pH (E), N2OR activity, and COD (F) (To show the correlation between the data, convex hull graphs are drawn. The convex hulls are the convex polygon formed by connecting the outermost points, and the convex hull points are calculated by the Graham’s scan method (Graham, 1972))
Analysis of the influence of COD/TN ratio on the N 2 O production
The COD/TN ratio of the rector has a great influence on N2O production. As shown in Fig. 2F, in periods with the COD/TN ratio of 3.3, the amount of nitrogen loss in the aerobic phase was the least, while the proportion of N2O was the highest. In contrast, in periods with the COD/TN ratio of 6.5, the amount of nitrogen loss in the aerobic phase was the highest, while the proportion of N2O was the least. It indicated that the COD/TN ratio of the rector would affect the N2O production and a reasonable COD/TN ratio would reduce its production. Meanwhile, as shown in Fig. 7A–C, in periods with the COD/TN ratios of 3.3 and 9.3, when N2O had higher emissions, the Nor activities showed an upward trend, while the N2OR activities showed a downward trend. In periods with the COD/TN ratio of 6.5, the Nor activities and the N2OR activities both showed an upward trend. This shows that in the periods of COD/TN ratio of 3.3 and 9.3, the reason for the higher N2O production is the decrease of N2OR activities.
The massive production of N2O in periods with lower COD/TN ratios (3.3) was mainly caused by the unsuitable external environment (pH) and the lower supply of organic matter. The lower supply of organic matter affected the supply of electrons in the electron transport chain (ETC) for the heterotrophic denitrification, which was not conducive to this process. And as shown in Fig. 5F, there was a positive correlation between N2OR activities and COD concentrations (r = 0.692, N = 15, P = 0.004 < 0.01), which indicated that the main N2O consumption process was heterotrophic denitrification (aerobic denitrification or micro-zones anoxic denitrification). As shown in Fig. 6, the aerobic denitrifiers included Thauera, Terrimonas, Gemmatimonas, and unclassified Burkholderiales, which accounted for 14.71% of the total sample. And the possible micro-zones anoxic denitrifiers included Defluviicoccus, Meiothermus, unclassified Chitinophagaceae, unclassified Xanthomonadaceae, and unclassified Rhizobiales, which accounted for 27.3% of the total sample. Among these denitrifiers, many bacteria have been proven to consume N2O, especially Gemmatimonas which can consume N2O under aerobic conditions (Park et al. 2017). Compared with other denitrifying enzymes, the N2OR has lower electronic competitiveness (Pan et al. 2013). Therefore, it was detrimental to the N2O consumption process in periods with lower COD/TN ratios, which promoted N2O production. The unsuitable external environment (pH) directly affected the catalytic activities of enzymes and the supply of electrons for the nitrifier denitrification. As shown in Table 1, when the COD/TN of the influent decreased from 9.3 to 3.3, the pH state of the reactor was changed: the pH0 min decreased from 7.93 ± 0.33 to 6.95 ± 0.42, and the pH360 min decreased from 7.4 ± 0.23 to 4.59 ± 0.52. And the main reason for the decrease of pH was the imbalance of nitrification reactions and denitrification reactions because of the reduction of organic matter. The outer membrane of the bacteria could not effectively inhibit the movement of protons from the outside because it has a larger pore size (Lund et al. 2014). The decrease of pH of the external environment would lead the pH of the periplasmic space of bacteria to decrease, which in turn affected the activity of intracellular enzymes. As shown in Fig. 5B and E, the levels of pH had little effect on the Nor activities (r = 0.318, N = 23, P = 0.139 > 0.05), and N2OR had relatively high activity under alkaline pH conditions (r = 0.555, N = 23, P = 0.06 < 0.01). It means that the N2O is more likely to be produced during denitrification under acidic conditions. This result also explains the reason for the higher N2O production in the reactor under low pH conditions in previous studies (Cao et al. 2021). In addition, the lower pH would cause some free nitrous acid (FNA) in the reactor. According to Zhou et al. (2010), the maximum FNA in this study was 1.2 × 10−4 mg N·L−1, which would inhibit the partial N2OR activity and promote the nitrifiers denitrification (Wang et al. 2020). Therefore, the environment with lower pH in periods with a lower COD/TN ratio would promote N2O production. The lower pH environment is also detrimental to the ammonia oxidation process. As shown in Fig. 2A–C, the ammonia oxidation rate of three periods was 0.19 mg·(L·min)−1, 0.28 mg·(L·min)−1, and 0.26 mg·(L·min)−1, respectively, and the proportion of ammonia oxidation (\({R}_{{{\mathrm{NH}}_{4}}^{+}}\)) of three periods was 50.21 ± 1.57%, 95.89 ± 0.36%, and 99.35 ± 0.18%, respectively. Meanwhile, the electric source of nitrifier denitrification was the ammonia oxidation process. Therefore, the environment with lower pH in periods with a lower COD/TN ratio would affect the electronic supply of nitrifier denitrification, which was detrimental to the N2OR activity, and it was more likely to produce more N2O during nitrifier denitrification. In addition, FNA would chemically decompose to produce NO, which also stimulated the denitrification process to produce N2O.
The massive productions of N2O in periods with higher COD/TN ratios (9.3) were mainly caused by the lower DO concentration. The increase of organic load intensified the competition for oxygen by aerobic heterotrophic bacteria, which made the DO concentration in the early aerobic phase relatively low. The lower DO concentration would cause the accumulation of nitrite, thereby promoting the nitrifier denitrification to produce more N2O. As shown in Fig. 3, in the prophase of the aerobic phase (150 to 210 min), the DO concentration was between 1.33 and 5.67 mg L−1. The oxygen half-saturation constant of ammonia-oxidizing bacteria (AOB) is smaller than nitrite-oxidizing bacteria (NOB), which means that the DO concentration has a higher impact on NOB than AOB (Hanaki et al. 1990). As shown in Fig. 2C, the ammonia oxidation rate remained unchanged but the nitrite oxidation rate decreased, which caused that the instantaneous cumulative amount of nitrite increased. As the COD/TN ratio of the reactor increased, the instantaneous cumulative amount of nitrite increased from 0.35 to 3.97 mg·L−1. The accumulation of nitrite would promote nitrifier denitrification, which further caused an increase in N2O production (Harris et al. 2015; Wang et al. 2016).
The reasons for the N 2 O emissions under aerobic conditions
The samples used to extract the two enzyme proteins belonged to the activated sludge of a mixed bacteria system, indicating that the catalytic activities of the two enzymes could reflect the overall metabolic capacity of the reactor on the two substrates. As shown in Fig. 7D, when the total N2O concentration was between 0 and 0.6 mg N·L−1, the Nor activities were higher than the N2OR activities. In other words, the rate of N2O production was higher than the rate of N2O consumption, which indicated that the emissions of N2O were inevitable. In theory, the N2O-reducing capacity of heterotrophic denitrifiers was usually 2–10 times higher than its N2O production capacity (Conthe et al. 2019). However, why can’t the heterotrophic denitrification completely metabolize the N2O produced in the nitrifier denitrification?
The reasons for the unavoidable emissions of N2O were the insufficient supply of electrons, the reduction of the micro-anoxic zone, and the different transcription numbers of enzymes. Firstly, the insufficient electron supply rate of the ETC led to the weakening of the N2O reduction ability of heterotrophic denitrification. As shown in Fig. 5A and D, when the DO concentration was between 0 and 8 mg·L−1, the Nor activities (r = − 0.291, N = 15, P = 0.292 > 0.05) and the N2OR activities (r = − 0.274, N = 15, P = 0.324 > 0.05) were hardly affected by the DO concentrations. This showed that it may not be the DO concentration but the electron supply rate of the ETC that affects the production and consumption of N2O (Conthe et al. 2018; Yang et al. 2021). In this study, heterotrophic denitrification occurred inside two types of bacteria, aerobic denitrifiers and anoxic denitrifiers (the existence of a micro-anoxic zone ensured that the two reactions could coexist because the diameter of the floc of activated sludge ranged from 49 to 306.5 µm (Andreadakis 1993).). For aerobic denitrifiers, the speed of electron supply is an important factor affecting the activity of N2OR (Conthe et al. 2018). Because of the competitive effect of aerobic heterotrophic bacteria on organic matter, the supply number of electrons and the supply rate of electrons for aerobic denitrifiers were limited. Aerobic denitrifiers were more likely to catalyze the reduction of more toxic intermediate products. NO was more toxic to cells than N2O (Abelson 1996) and had inhibitory effects on the oxidase activity and N2OR activity (Carr and Ferguson 1990). Therefore, aerobic denitrifiers would preferentially catalyze the reduction of NO when the electron was limited. For anoxic denitrifiers, it was in the micro-anoxic zone inside the floc. The substrate used by the anoxic denitrifier was the soluble organic transmitted from the outside, which meant that the substrate was limited for the anoxic denitrifier. Similar to aerobic denitrifiers, the activity of N2OR was also inhibited (Perez-Garcia et al. 2017). Secondly, the reduction of the micro-anoxic zone inhibited the anoxic denitrification, inhibiting the N2O reduction ability of the activated sludge. The increase of DO concentration would shrink the micro-anoxic zone inside the floc. The reduction of the micro-anoxic zone would inhibit the metabolism of anoxic denitrifiers. Therefore, the consumption of N2O was naturally suppressed. Finally, the transcription amount of N2OR and that of Nor are imbalanced (Zheng et al. 2019). This also resulted in weaker N2OR activity. Based on the above reasons, the production rate of N2O in the reactor was higher than the consumption rate of N2O.
Countermeasures and suggestions
From the results of this study, in the aerobic phase of the A/O SBR, the production rate of N2O in the reactor was higher than the consumption rate of N2O. In particular, as the total N2O concentration in the reactor increased, the gap between them increased (Fig. 7D), indicating that it is difficult to reach a balance between the two. It is not feasible to increase the N2OR activities by increasing the organic load to reduce the N2O production. The increase in the consumption rate of N2O caused by the increase in organic load is much smaller than the increase in the production rate of N2O caused by it, which means that it cannot reduce the N2O production but rather increase its production (Fig. 2F). In contrast, reducing the N2O production process, autotrophic denitrification, especially the nitrifier denitrification, in the aerobic phase is the key to solving the problem of N2O production in WWTPs. The accumulation of nitrite, low DO and low pH will promote the nitrifier denitrification (Wrage-Mönnig et al. 2018). Therefore, these conditions should be avoided as much as possible in the actual operation of the WWTPs.
Conclusion
In the aerobic phase of A/O SBR, most of the N2O released and produced came from the autotrophic denitrification process, and the reason for the higher N2O production was the weak activity of N2OR. The N2O production rate was higher than the N2O consumption rate, showing that N2O consumers (heterotrophic denitrification) could not completely metabolize the N2O produced by the N2O producers (autotrophic denitrification, especially the nitrifier denitrification). Increasing the N2O consumption capacity of heterotrophic denitrification could reduce the N2O production but could not completely prevent this process. Taking reasonable measures to inhibit autotrophic denitrification, especially the nitrifier denitrification, might completely prevent N2O production.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical approval.
Not applicable.
References
Abelson JN (1996) Nitric Oxide, Part B: Physiological and Pathological Processes. AP, San Diego
Andreadakis AD (1993) Physical and chemical properties of activated sludge floc. Water Res 27:1707–1714
APHA (1998) Standard methods for the examinations of water and wastewater. APHA, Washington
Cao X, Zhou X, Xue M, Chen J, Li S (2021) Evaluation of nitrogen removal and N2O emission in a novel anammox coupled with sulfite-driven autotrophic denitrification system: influence of pH. J Clean Prod 321:128984
Carr GJ, Ferguson SJ (1990) The nitric oxide reductase of Paracoccus denitrificans. Biochem J 269:423–429
Cavazos AR, Taillefert M, Tang Y, Glass JB (2018) Kinetics of nitrous oxide production from hydroxylamine oxidation by birnessite in seawater. Mar Chem 202:49–57
Chapa O (2011) Global anthropogenic non-CO2 greenhouse gas emissions 1990–2020. In. EPA. pp 278
Conthe M, Lycus P, Arntzen MØ, Silva ARd, Frostegård Å, Bakken LR, Kleerebezem R, Loosdrecht MCMv (2019) Denitrification as an N2O sink. Water Res 151:381–387
Conthe M, Parchen C, Stouten G, Kleerebezem R, Loosdrecht MCMv, (2018) O2 versus N2O respiration in a continuous microbial enrichment. Appl Biochem Biotechnol 102:8943–8950
Edenhofer O, Pichs-Madruga R, Sokona Y, Minx JC (2014) Climate change 2014: mitigation of climate change, contribution of working group III to the fifth assessment report of the IPCC. CUP, Cambridge
Ferretti S, Grossmann JG, Hasnain SS, Eady RR, Smith BE (1999) Biochemical characterization and solution structure of nitrous oxide reductase from Alcaligenes xylosoxidans(NCIMB 11015). Eur J Biochem 259:651–659
Foley J, Haas Dd, Yuan Z, Lant P (2010) Nitrous oxide generation in full-scale biological nutrient removal wastewater treatment plants. Water Res 44:831–844
Graham RL (1972) An efficient algorith for determining the convex hull of a finite planar set. Inform Process Lett 1:132–133
Guo G, Wang Y, Hao T, Wu D, Chen G (2018) Enzymatic nitrous oxide emissions from wastewater treatment. Front Env Sci Eng 12:10
Hanaki K, Wantawin C, Ohgaki S (1990) Nitrification at low levels of dissolved oxygen with and without organic loading in a suspended-growth reactor. Water Res 24:297–302
Harris E, Joss A, Emmenegger L, Kipf M, Wolf B, Mohn J, Wunderlin P (2015) Isotopic evidence for nitrous oxide production pathways in a partial nitritation-anammox reactor. Water Res 83:258–270
He Z, Yuan L, Wei Y, Nan Y (2017) N2O emission and hydroxylamine oxidase (HAO) activity in a nitrogen removal process based on activated sludge with three COD/NH4+ ratios. Water Environ Res 89:387
Heiss B, Frunzke K, Zumft WG (1989) Formation of the N-N bond from nitric oxide by a membrane-bound cytochrome bc complex of nitrate-respiring (denitrifying) Pseudomonas stutzeri. J Bacteriol 171:3288–3297
Hulse CL, Averill BA (1990) Isolation of a high specific activity pink, monomeric nitrous oxide reductase from Achromobacter cycloclastes. Biochem Biophys Res Commun 166:729–735
Kastrau DHW, Heiss B, Kroneck PMH, Zumft WG (2005) Nitric oxide reductase from Pseudomonas stutzeri, a novel cytochrome bc complex: phospholipid requirement, electron paramagnetic resonance and redox properties. Eur J Biochem 222:293–303
Lund P, Tramonti A, Biase DD (2014) Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev 38:1091–1125
Pan Y, Ni B-J, Bond PL, Ye L, Yuan Z (2013) Electron competition among nitrogen oxides reduction during methanol-utilizing denitrification in wastewater treatment. Water Res 47:3273–3281
Pauleta SR, Dell’Acqua S, Moura I (2013) Nitrous Oxide Reductase. Coordin Che Rev 257:332–349
Peder C, Hooper AB, Wilmot CM (2013) Structural studies of hydroxylamine oxidoreductase reveal a unique heme cofactor and a previously unidentified interaction partner. Biochemistry-US 52:6211–6218
Perez-Garcia O, Mankelow C, Chandran K, Villas-Boas SG, Singhal N (2017) Modulation of nitrous oxide (N2O) accumulation by primary metabolites in denitrifying cultures adapting to changes in environmental C and N. Environ Sci Technol 51:13678–13688
Wu J, Chen Z, Zhang S, Gao L, Yu R, Zhan M (2019) Mechanistic understanding of predatory bacteria-induced biolysis for waste sludge dewaterability improvement. Water Air Soil Pollut 230:194
Wang S, Zhao J, Ding X, Li X (2020) Nitric oxide and nitrous oxide production in anaerobic/anoxic nitrite-denitrifying phosphorus removal process: effect of phosphorus concentration. Environ Sci Pollut Res 27:45925–45937
Wang Y, Lin X, Zhou D, Ye L, Song C (2016) Nitric oxide and nitrous oxide emissions from a full-scale activated sludge anaerobic/anoxic/oxic process. Chem Eng J 289:330–340
Wrage-Mönnig N, Horn MA, Well R, Müller C, Velthof G, Oenema O (2018) The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol Biochem 123:A3–A16
Yan X, Yang J, Guo D, Ma J, Su X, Sun J (2021) Effect of carbon source on nitrous oxide emission characteristics and sludge properties during anoxic/aerobic wastewater treatment process. Environ Sci Pollut Res 28:57557–57568
Yang R, Yuan L, Wang R, He Z, Chen X (2021) New insight on the regulation of N2O production in aerobic condition: an N2O metabolic perspective based on enzymatic analysis of nitrous oxide reductase. J Water Process Eng 41:102090
Zheng M, Zhou N, Liu S, Dang C, Liu Y, He S, Zhao Y, Liu W, Wang X (2019) N2O and NO emission from a biological aerated filter treating coking wastewater: main source and microbial community. J Clean Prod 213:365–374
Zhou X, Song J, Wang G, Yin Z, Cao X, Gao J (2020) Unravelling nitrogen removal and nitrous oxide emission from mainstream integrated nitrification-partial denitrification-anammox for low carbon/nitrogen domestic wastewater. J Environ Manage 270:110872
Zhou Y, Pijuan M, Yuan Z (2010) Free nitrous acid inhibition on anoxic phosphorus uptake and denitrification by poly-phosphate accumulating organisms. Biotechnol Bioeng 98:903–912
Zumft WG, Kroneck PMH (2006) Respiratory transformation of nitrous oxide (N2O) to dinitrogen by bacteria and archaea. Adv Microb Physiol 52:107–227
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 51878538 and 51808433).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RY, L-jY, RW, Z-xH, LL, and Y-cM. The first draft of the manuscript was written by RY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, R., Yuan, Lj., Wang, R. et al. Analyzing the mechanism of nitrous oxide production in aerobic phase of anoxic/aerobic sequential batch reactor from the perspective of key enzymes. Environ Sci Pollut Res 29, 39877–39887 (2022). https://doi.org/10.1007/s11356-022-18800-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18800-3