Abstract
Livestock wastewater disposal onto rice paddy fields is a cost- and labor-effective way to treat wastewater and cultivate rice crops. We evaluated the influence of nitrogen loading rates on nitrogen assimilation by rice plants and on nitrogen losses (leaching and N2O emission) in forage rice fields receiving liquid cattle waste (LCW). Four forage rice fields were subjected to nitrogen loads of 107, 258, 522, and 786 kg N ha−1 (N100, N250, N500, and N750, respectively) using basal fertilizer (chemical fertilizer) (50 kg N ha−1) and three LCW topdressings (each 57–284 kg N ha−1). Nitrogen assimilated by rice plants increased over time. However, after the third topdressing, the nitrogen content of the biomass did not increase in any treatment. Harvested aboveground biomass contained 93, 60, 33, and 31 % of applied nitrogen in N100, N250, N500, and N750, respectively. The NH4 + concentration in the pore water at a depth of 20 cm was less than 1 mg N L−1 in N100, N250, and N500 throughout the cultivation period, while the NH4 + concentration in N750 increased to 3 mg N L−1 after the third topdressing. Cumulative N2O emissions ranged from −0.042 to 2.39 kg N ha−1; the highest value was observed in N750, followed by N500. In N750, N2O emitted during the final drainage accounted for 80 % of cumulative N2O emissions. This study suggested that 100–258 kg N ha−1 is a recommended nitrogen loading rate for nitrogen recovery by rice plants without negative environmental impacts such as groundwater pollution and N2O emission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The input of reactive nitrogen from human activities is increasingly dominating the nitrogen budget at the global scale (Galloway et al. 2004). Use of nitrogen fertilizer for food production, including livestock farming, has been the biggest input. These activities cause eutrophication, greenhouse gas emissions, and NO x and NH3 emissions (Galloway et al. 2004). In livestock farming areas, the loss of nutrients and farm effluents are of concern with regard to the water quality of rivers and groundwater (Hooda et al. 2000; Mishima et al. 2009).
In recent years, the application of wastewater to rice fields has gained attention as a means of land-based disposal of polluted river water (Zhou and Hosomi 2008), liquid cattle waste (LCW) (Zhou et al. 2009), anaerobic digested cattle slurry (ADCS) (Lu et al. 2012), and rural wastewater (Li et al. 2009). The use of wastewater on rice fields can be a cost-effective way of supplying nutrients for rice growth (Shen and Wu 1998; Zhou and Hosomi 2008). However, the amount of wastewater that can be applied to rice fields may be limited because high nitrogen loading could result in rice plant lodging (Shimono et al. 2007), a phenomenon where the lower part of the stem breaks due to the increasing internode length of rice (Matsushima and Manaka 1961).
In Japan, a new variety of rice for whole-crop silage (forage rice) has been developed (Kato 2008). It is a feed crop featuring high biomass production and a high lodging resistance, and forage rice could be cultivated at a higher nitrogen loading than rice that is cultivated for food production (Zhou et al. 2009). A previous study confirmed the healthy growth of forage rice at 600 kg N ha−1 (Zhou et al. 2009), which is higher than the recommended rate of nitrogen fertilizer (50–100 kg N ha−1) in Ibaraki prefecture, Japan (Ibaraki Prefectural Agricultural Center 2010). Therefore, high amounts of wastewater can be applied to forage rice fields. This is advantageous because this method of fertilization allows for the disposal of high amounts of wastewater onto paddy fields over small areas, which will reduce the land use requirements, costs, and labor for crop management. However, water and air pollution by nitrogen leaching and N2O emission from the excessive application of the wastewater should be taken into account to know application rate with low environmental impact. Nitrogen assimilation by the rice plant plays a central role as a nitrogen sink in the rice crop ecosystem. Therefore, it is important to understand these nitrogen dynamics as a result of plant nitrogen assimilation at different nitrogen loading rate.
Although much research has been conducted to evaluate nitrogen leaching in paddy fields treated with chemical fertilizer (Takamura et al. 1976; Tian et al. 2007; Wang et al. 2004), nitrogen leaching in paddy fields treated with wastewater has rarely been examined. Because livestock wastewater contains high concentrations of NH4 + and is irrigated onto the surface water (Zhou et al. 2009), the heavy application of wastewater causes nitrogen loss through nitrogen leaching and would influence groundwater quality. Zhou et al. (2009) demonstrated that, following the application of LCW at a rate of 83–135 kg N ha−1 onto a forage rice field, the highest NH4 + concentration in pore water at 20 cm depth was 11 mg N L−1. However, contrasting results have been reported. Lu et al. (2012) reported that ADCS application at a rate of 135–540 kg N ha−1 onto rice paddy fields resulted in less than 2 mg N L−1 of NH4 + in pore water. Sasada et al. (2011) reported that NO3 − concentrations in drainage water were consistently low (0.5 mg N L−1) in a paddy field treated with anaerobically digested cattle or pig slurries at a rate of 300 kg N ha−1. Thus, the relationship between the nitrogen loading rate and nitrogen concentration in leached water from rice fields fertilized with livestock wastewater is not straightforward.
Nitrous oxide (N2O) is a strong greenhouse gas and an ozone-depleting substance (Ravishankara et al. 2009). According to the IPCC (2007a), agricultural activity is the primary cause of the increase in N2O in the atmosphere. It has been estimated that anthropogenic N2O emission is 6.7 Tg N year−1, and 42 % of anthropogenic N2O emissions is attributed to agricultural activity (IPCC 2007b). It has been widely known that the application of nitrogen fertilizer increases N2O emissions from agricultural soil (Bouwman 1996; Kim et al. 2013). It has been reported that excessive nitrogen fertilization suppresses the crop yield increment and leads to an exponential increase in N2O emissions once the nitrogen requirement of the crop has been satisfied (McSwiney and Robertson 2005; Zebarth et al. 2008). McSwiney and Robertson (2005) reported that N2O emissions from a maize field increased exponentially with increasing maize yield. Liu et al. (2012) also reported higher rates of increase in N2O emissions compared with crop yields in response to nitrogen fertilization in a wheat and maize cropping system at rates between 270 and 850 kg N ha−1 year−1. Similar results have been reported for rice ecosystems. Ma et al. (2007) reported that an increase in nitrogen fertilization from 0 to 270 kg N ha−1 resulted in ten times higher N2O emissions, while grain yields increased only slightly. Zhou et al. (2011) calculated the nitrogen balance in a forage rice field treated with LCW and showed that the percentage of nitrogen uptake by rice plants decreased, while N2O emission increased in response to an increase in nitrogen loading. Therefore, the optimal nitrogen loading rate for low N2O emissions should be determined. However, the role of the nitrogen loading rate in controlling the relationship between N2O emission and nitrogen uptake by rice plants is not understood.
The aim of this study was to evaluate the influence of the nitrogen loading rate on nitrogen losses to the environment through nitrogen leaching and N2O emissions. In this study, different nitrogen loads of LCW were applied to forage rice fields. We measured temporal changes in nitrogen concentrations in the soil and pore water and the N2O flux from the fields to determine the nitrogen loading threshold that results in minimal nitrogen loss to the environment through leaching and N2O emissions. Additionally, we examined the relationship between the temporal dynamics of nitrogen uptake by rice plants and nitrogen losses.
Materials and methods
Field plot and management
In 2009, a field experiment was conducted in four experimental rice fields at the National Institute for Agro-Environmental Sciences, Tsukuba, Ibaraki, Japan (36°01′ N, 140°07′ E). Two of the four experimental fields had an area of 40 m2 (10 × 4 m for treatments N100 and N250), and the other two had an area of 20 m2 (10 × 2 m for treatments N500 and N750). The experimental fields had an inlet for the irrigation water and an outlet for the overflow of the surface water at the opposite side of the inlet. The soil at the site was a fine-textured lowland soil (IUSS Working Group WRB 2006) and particle size distribution was as follows: 9.5 % coarse sand, 27.8 % fine sand, 23.2 % silt, and 39.5 % clay. Total carbon (TC) and TN contents were 1.96 ± 0.12 and 0.16 ± 0.01 %, respectively, at 0–20 cm depth.
Forage rice cultivation was conducted from May to October (Table 1). On 12 May, chemical fertilizer was applied at a rate of 50 kg N ha−1 as a basal dressing after plowing. Rice seedlings (Oryza sativa L. cv. Takanari; a whole crop silage rice variety) were transplanted at a density of 20 hills m−2 (three rice seedlings per hill) (0 days after transplanting). After 35, 63, and 91 days, different N loads of LCW obtained from the Menuma-machi Organic Center (Saitama, Japan) were applied as supplemental fertilizer to each field (Table 2). The predominant form of nitrogen in the LCW was NH4 +, with a concentration ranging from 1,486 to 2,275 mg N L−1 (Table 2), while the maximum NO3 − concentration was only 2.4 mg N L−1 (data not shown). The dissolved organic carbon (DOC) concentration of the LCW ranged from 1,890 to 2,082 mg C L−1. The total NH4 + loadings from the LCW topdressing in the four forage rice fields were 57, 207, 473, and 736 kg N ha−1 (treatments N100, N250, N500, and N750, respectively) over the experimental period. Following each LCW application, each field was flooded for a week. Midsummer drainage was conducted during the period from days 48 to 63, and intermittent irrigation was conducted during the period from days 70 to 117. After that, final drainage was performed in preparation for the harvest. These water management practices were based on conventional Japanese practices (Suzuki 1997).
Sampling and analysis of the water, soil, and rice plants
Soil pore water at 20 cm depth was sampled periodically from the two locations from each plot. The pore water sampling was conducted using soil water samplers with porous cups permanently inserted into the soil. The concentrations of NH4 + and NO3 − in the water were analyzed using ion chromatography (ICS-90 (for NH4 +) and ICS-1000 (for NO3 −), Dionex, Sunnyvale, CA, USA) after filtration.
Triplicate soil core samples were collected at 0–20 cm depth in each rice field periodically. Soil samples were air-dried and sieved through a 2-mm mesh sieve. Total carbon and nitrogen contents of the soil samples were analyzed with a CHN elemental analyzer (Micro Corder JM10, J-Science, Kyoto, Japan). Adsorbed nitrogen (NH4 + and NO3 −) in the soil was analyzed as described by Zhou et al. (2009).
Rice plants were sampled in triplicate periodically during cultivation. At harvest, rice plants were randomly harvested from three zones of 1 m2 (1 × 1 m) in each plot. The aboveground biomass yield was determined after the harvested rice plants were air-dried. The rice plants were divided into three parts (leaves, stems, and grains) and the nitrogen concentration of each component was determined with a CHN elemental analyzer.
Determination of N2O flux
The closed chamber technique (Hutchinson and Mosier 1981) was used to collect gas samples for measurements of N2O flux. Plexiglas chambers (30 cm diameter, 100 cm height) equipped with a pressure-adjusting bag were employed to collect gas samples at 0-, 10-, and 20-min intervals. Nitrous oxide concentrations in the gas samples were analyzed with a gas chromatograph equipped with an electron capture detector (GC-14A, Shimadzu, Kyoto, Japan). The N2O flux was calculated based on the increase in the gas concentration in the chamber as shown in the following equation:
where F N2O is the N2O flux (g m−2 h−1), ΔC/Δt is the change in N2O concentration in the chamber per unit time (μm3 m−3 h−1), V is the volume of the headspace of the chamber (m3), V 0 is the molar volume of an ideal gas (0.0224 m3 mol−1), T 0 is 273 K, T is the air temperature in the chamber (K), M is the molar weight of N2O (g mol−1), and A is the sectional area of the base (m2). Cumulative N2O emissions during the experimental period were estimated by trapezoidal integration of the mean flux over time.
Statistical analyses
The effect of the LCW application rate on the aboveground biomass and nitrogen content of different parts of the harvested rice plants was analyzed using a one-way analysis of variance (ANOVA). Statistical analyses of the amount of soil adsorbed nitrogen on the different sampling days were performed using a two-tailed t test. All statistical analyses were completed with SPSS 16.0 Family (SPSS Inc., Chicago, IL, USA).
Results and discussion
Plant nitrogen uptake and soil nitrogen dynamics
The nitrogen content in the different parts of the forage rice plants and the weight and the amount of nitrogen in the aboveground biomass are shown in Table 3. No significant differences were found in the weight of aboveground biomass between treatments; values ranged from 14.6 ± 3.0 to 18.5 ± 1.1 t ha−1. We observed lodging of a part of the area of N500 and N750 after a typhoon occurred on 8 October, significantly affecting forage productivity.
The amount of nitrogen in aboveground biomass in the N750 treatment was 2.3 times higher than that in the N100 treatment. One-way ANOVA showed a significant effect of the LCW application rate on the nitrogen content of the aboveground biomass. The percentage of nitrogen present in the different parts of the forage rice was also affected by the LCW application rate (P < 0.05), indicating that higher nitrogen loading enhances nitrogen uptake (Zhou et al. 2009).
In this study, aboveground biomass (Table 3) was similar to that (13–18 t ha−1) of forage rice treated with aerated cattle slurry or slurry anaerobically digested at a rate of 100–170 kg N ha−1 (Doi and Kawamoto 2007; Ishii et al. 2005; Kinoshita et al. 2013). The comparable aboveground biomass despite the higher nitrogen loading rate in this study may be due to the lodging that occurred in N500 and N750 or the growth suppression of the excessive nitrogen application in N500 and N750. It has been known that excessive nitrogen application enhances the growth of leaves and stems, where nitrogen is accumulated (Ikeda 1995). In this study, the nitrogen contents of the grain were the highest in N100, N250, and N750, while the nitrogen content of the leaf was the highest in N750 (Table 3), suggesting the accumulation of nitrogen in the leaves in N750. Furthermore, the excessive growth of leaves intercepts sunlight penetration into the canopy through mutual shading (San-Oh et al. 2008). As a result, photosynthesis is reduced and the growth of aboveground biomass is suppressed.
Nitrogen immobilization by rice plants plays a central role as a nitrogen sink in the rice crop ecosystem. In another study, it was found that harvested aboveground biomass including grain and straw contains 100–200 kg N ha−1, which accounted for more than 30–90 % of the fertilizer nitrogen applied to rice fields (Takamura et al. 1976; Zhao et al. 2009; Zhou et al. 2009; Qiao et al. 2012). Our results are consistent with these ranges. Meanwhile, since there was lower nitrogen recovery in N500 and N750 (33 and 31 % of applied nitrogen, respectively) compared with the other treatments (Table 3), we speculate that the relationship between nitrogen assimilated by rice plants and the nitrogen loading rate is not linear. This is partly explained by the relationship between the NH4 + assimilation rate and NH4 + concentration around roots, which is governed by the Michaelis–Menten saturation pattern (Kronzucker et al. 2000). Furthermore, the production of dead leaves or stems also regulates the amount of nitrogen in harvested plants. Figure 1 shows temporal changes in the nitrogen content of aboveground biomass including grains, leaves, and stems. Figure 1 clearly shows an increase followed by a decrease in the nitrogen content of aboveground biomass in treatments with higher nitrogen loading rates. Nitrogen contents sharply increased prior to the third topdressing in N500 and N750, then decreased (Fig. 1). Changes in the nitrogen contents of aboveground biomass after the third topdressing would be due to the low nitrogen uptake activity of rice plants (Riya et al. 2012) and the occurrence of dead leaves and stems (data not shown). According to a review by Kimura et al. (2004), the amount of dead leaves starts to increase after 42–49 days and reaches a maximum around 105 days after transplanting. Ishikawa (2012) also reported the occurrence of dead leaves after the heading stage.
Temporal change in nitrogen content of aboveground biomass (g N plant−1) (sum of leaf, stem, and panicles). Vertical dotted lines indicate LCW application. Horizontal dotted, hollow, and solid arrows indicate the periods of midsummer drainage, intermittent irrigation, and final drainage, respectively
Before transplantation, the mean soil adsorbed nitrogen content was 7.8 ± 1.8 mg N kg−1 soil in all treatments (Fig. 2). After the application of LCW, increases in adsorbed nitrogen contents were observed, and ranged from 5.3 ± 0.4 to 36.2 ± 13.7 mg N kg−1 soil (50, 65, and 93 days after transplanting). Interestingly, increases in the soil adsorbed nitrogen contents induced by topdressing were reduced to less than 5 mg N kg−1 soil until the next topdressing in all treatments (Fig. 2). Adsorbed nitrogen contents dropped to 3.6 ± 1.0 and 4.2 ± 0.3 mg N kg−1 soil before the second and third LCW applications (63 and 90 DAT). These values were significantly lower than those before transplanting (0 DAT) (P = 0.008), suggesting that most nitrogen applied by topdressing was removed from the soil. The only treatment to exhibit a higher adsorbed soil nitrogen content following a nitrogen application was N750 after the third application. The daily mean decrease in adsorbed nitrogen between 93 and 111 DAT (1.5 kg N ha−1 day−1) was also lower than those between the first and second and second and third applications (2.4 and 2.6 kg N ha−1 d−1) in N750. Given the restricted plant nitrogen uptake in N750 in the late growing period (Fig. 1) and the soil nitrogen dynamics (Fig. 2), 786 kg N ha−1 (N750) may be an excessive amount of fertilizer for forage rice growth.
NH4 + and NO3 − concentrations in the pore water
Concentrations of NH4 + and NO3 − in pore water at 20 cm depth are shown in Fig. 3. Before the first topdressing (until 35 days), NH4 + concentrations were less than 1 mg N L−1 in all treatments (Fig. 3a). After the LCW topdressing, trends in NH4 + concentrations varied in response to the nitrogen loading rate and the topdressing period. At the first topdressing, NH4 + concentrations were not influenced by LCW application in any treatments. On the day after second topdressing, increases in NH4 + concentration were observed and reached around 0.5 mg N L−1 in N250, N500, and N750 within 5 days after topdressing. In N250 and N500, the increased NH4 + concentration decreased to an undetectable level at 80 days. In contrast to N250 and N500, the NH4 + concentration in N750 reached 0.60 mg N L−1 at 70 days and had slightly decreased at 80 days. The NH4 + concentration reached its highest value (2.97 mg N L−1) 3 days after the third topdressing (94 days) in N750, and decreased to 0.41 mg N L−1 at 122 days.
Nitrate concentrations tended to decrease over time, particularly between 7 days and 42 days (a week after the first topdressing) (Fig. 3b). At 7 days after transplanting, 0.08–0.42 mg N L−1 of NO3 − was detected. Nitrate concentrations had decreased to less than 0.1 mg N L−1 except for N250, in which the NO3 − concentration increased to 0.14 mg N L−1 at 35 days. After that, NO3 − concentrations were consistently low (less than 0.1 mg N L−1) with a small increase after topdressing or during drainage regardless of the nitrogen loading rate (Fig. 3b).
In previous studies, 0–20 mg N L−1 of NH4 + and 0–3 mg N L−1 of NO3 −1 in pore water were observed in a paddy field treated with 30–540 kg N ha−1 as basal fertilization or topdressing (Takamura et al. 1976; Wang et al. 2004, 2011; Kyaw et al. 2005; Tian et al. 2007; Lu et al. 2012). In this study, the NH4 + and NO3 − concentrations in the pore water were within the ranges of those studies. Furthermore, compared with unsaturated soil, the effect of the nitrogen loading rate seemed small. For example, Tian et al. (2007) observed less than 1 mg N L−1 of total inorganic nitrogen in the pore water at a depth of 90 cm in a rice field treated with 0–330 kg N ha−1, while the concentration at the same depth in wheat fields treated with 0–248 kg N ha−1 was 8 mg N L−1 of total inorganic nitrogen. The range of the observed soil water NO3 − concentration in rice fields receiving nitrogen inputs of 0–360 kg N ha−1 was 1–2 mg N L−1, whereas the soil water NO3 − concentration in wheat fields receiving nitrogen inputs of 0–315 kg N ha−1 was 0.4–11 mg N L−1 (Wang et al. 2004). These values for rice fields were consistent with the NH4 + and NO3 − concentrations observed in our study (Fig. 3). Furthermore, the concentrations observed in this study were much lower than the environmental standard for groundwater in Japan (10 mg N L−1 of NO3 − and NO2 −) (Ministry of the Environment 1997). However, Zhou et al. (2009) reported 11 mg N L−1 of NH4 + at a depth of 20 cm after LCW topdressing at a rate of ca. 130 kg N ha−1 in other forage rice fields with higher percolation rates than those of this study. Ishikawa et al. (2003) found that a soil column with a higher percolation rate resulted in higher NH4 + concentration in the percolated water. According to Razavipour and Farrokh (2014), the percolation rate is negatively and positively correlated with clay and sand content, respectively. In this study, clay content was 39.5 %, while it was 27.6 % in Zhou et al. (2009). Therefore, this partially explains the differences in the NH4 + concentration in the pore water in the different sites. This suggests that the impact of an N loading rate up to 786 kg N on nitrogen contamination of groundwater is small, but it depends on the soil type.
The dynamics of the NH4 + and NO3 − concentrations in the pore water had different temporal patterns (Fig. 3). Nitrate leaching after irrigation has been reported previously in rice fields (Wang et al. 2004; Zhou et al. 2009). Because the main form of nitrogen in basal fertilizer was NH4 +, NO3 − during this stage may be produced by nitrification in unsaturated conditions before irrigation. The shift in the dominant form of nitrogen from NO3 − to NH4 + after the first topdressing is likely to have been caused by the denitrification of NO3 − under anaerobic conditions (data not shown) and the addition of LCW rich in NH4 + (Table 2). Zhou et al. (2009) also reported that NH4 + was the main form of nitrogen leached from forage rice fields treated with LCW. Interestingly, the NH4 + concentrations of pore water after topdressing gradually increased between the second and third topdressing in N750 (Fig. 3a). Since the amount of NH4 + applied in each topdressing was the same (Table 2), increases in the NH4 + concentration of pore water in N750 are likely due to temporal changes in the mechanism of nitrogen transformation in the rice field. From the data in Figs. 1 and 3a, we can speculate that a decrease in the nitrogen uptake by forage rice plants would lead to an increase in nitrogen leaching after the third topdressing in N750. These results suggest that the NH4 + concentration of the pore water is affected by the soil properties and temporal dynamics of nitrogen uptake of the rice plant.
Nitrous oxide emission and plant nitrogen uptake
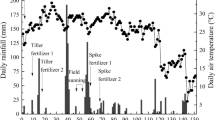
Table 4 shows the cumulative N2O emission during the cultivation period and percentage of applied NH4 +. Nitrous oxide emissions ranged from −0.042 to 2.39 kg N ha−1 and likely increased with increasing nitrogen loading rate (Table 4). It has been well known that N2O emission increases with the nitrogen fertilization rate (Hua et al. 1997; Yao et al. 2012; Zou et al. 2005). In Japanese conventional rice management, 30–90 kg N ha−1 of nitrogen fertilization resulted in N2O emissions ranging from near zero to 0.2 kg N ha−1 in most cases (Itoh et al. 2011; Nishimura et al. 2004). Higher nitrogen fertilization rates (150–450 kg N ha−1) have been evaluated outside of Japan in China and the Philippines, and resulted in 0.64–6.17 kg N ha−1 of N2O emission (Bronson et al. 1997a; Yao et al. 2012; Zou et al. 2005). The trends in N2O emissions and the nitrogen fertilization rate in this study were consistent with those studies, suggesting that 107 kg N ha−1 of N loading rate (N100), which is same to Japanese conventional rice management, has a low impact on N2O emission. We also emphasize that extent of N2O emission in N250 is similar level to N100, suggesting forage rice field can receive more nitrogen than Japanese standard nitrogen loading rate without increasing N2O emission.
In contrast to the percentages of uptake of applied nitrogen by rice plants (Table 3), percentages of N2O emissions to applied nitrogen were proportional to nitrogen loading rates from N250 to N750 (Table 4). The nitrous oxide fluxes from the forage rice fields during cultivation are shown in Fig. 4. In N500 and N750, the amount of N2O emitted during the final drainage (117 days after transplanting to harvest) accounted for 50 and 80 % of cumulative N2O emissions, respectively, indicating that final drainage accelerated N2O emission. As Bronson et al. (1997b) and Zhou et al. (2009) noted, drainage allowed atmospheric O2 to diffuse into the surface soil rich in ammonia-oxidizing bacteria (Nicolaisen et al. 2004) and produced NO2 − or NO3 − that would be denitrified in the anaerobic subsoil. Therefore, the available nitrogen content in the soil would determine the amount of N2O produced during the final drainage. Evidently, the N2O emissions during the final drainage in N750 (Fig. 4) were the result of the high soil nitrogen availability after the third topdressing (Figs. 2 and 3a), exhibiting the reverse trend observed for the temporal change in aboveground nitrogen content (Fig. 1).
Figure 5 shows the relationship between the nitrogen loading rate and the ratio of N2O emission to nitrogen uptake by rice plants. McSwiney and Robertson (2005) reported that maize grain yields reached a plateau, while N2O emissions increased sharply, if nitrogen fertilization exceeded 101 kg N ha−1. Liu et al. (2012) demonstrated that an increase in nitrogen fertilization from 0 to 850 kg N ha−1 caused higher ratios of cumulative N2O emission to wheat and maize yields in a wheat–maize rotation system. In our study, the ratio of N2O emission to nitrogen uptake was higher with higher nitrogen loading rates, consistent with the results of Liu et al. (2012) and McSwiney and Robertson (2005). Nitrogen application over 258 kg N ha−1 is likely in excess of the crop requirement, leading to higher soil available nitrogen content (Fig. 2) and subsequent losses by N2O emission (Fig. 4). Figure 5 also shows that nitrogen fertilization up to 258 kg N ha−1 does not influence N2O emission, implying that this level of nitrogen fertilization meets the nitrogen requirement of forage rice and that the associated N2O emissions are low.
The relationship between the nitrogen loading rate (basal fertilizer plus LCW) and the ratio of N2O emission to nitrogen uptake by rice plants. The ratios were calculated from cumulative N2O emissions during the cultivation period and the nitrogen contents in aboveground biomass (Table 3)
Recommended nitrogen loading rate for forage rice cultivation
In this study, the evaluated nitrogen loading rates (107–786 kg N ha−1) go from matching the Japanese standard level for food rice and forage rice (each around 100 kg N ha−1) to far exceeding them. From the point of the view of sustainable agriculture, productivity as well as environmental impacts should be taken into account in recommendations of the nitrogen loading rate from LCW for forage rice production. From the data presented above, 107–258 kg N ha−1 (between N100 and N250) had the same productivity and environmental impacts as Japanese conventional food and forage rice management. In this study, we cultivated forage rice by Japanese conventional water management. In addition, LCW application for forage rice production has already been conducted by farmers in some regions of Japan at a rate of 100 kg N−1. Therefore, our findings suggest that the presently conducted LCW application (100 kg N ha−1) for forage rice production in Japan had low impacts on nitrogen pollution of groundwater and N2O emissions. In Japan, 258 kg N ha−1 is not recommended; however, this nitrogen loading rate is expected to increase biomass production due to higher nitrogen uptake (Table 3) without an increase in the risk of water pollution and global warming compared with 107 kg N ha−1. In addition, the application of 258 kg N ha−1 allowed us to dispose of livestock wastewater more efficiently than 107 kg N ha−1. In order to apply 258 kg N ha−1 in actual forage rice production, more field trials will be required.
Conclusions
Although nitrogen leaching or N2O emission from rice paddy field fertilized with wastewater has been reported in recent years, relationship between them and nitrogen assimilation by rice plant has not been reported. Nitrogen assimilation by rice plant plays a central role as a nitrogen sink in the rice crop ecosystem. Therefore, it is important to understand nitrogen dynamics as a result of plant nitrogen assimilation. We investigated the nitrogen assimilation by forage rice plants, inorganic nitrogen concentration of the pore water, and N2O emission in the forge rice fields treated with LCW at a different nitrogen loading rate. Our results suggested that higher nitrogen loading rate would exceed nitrogen requirement of the forage rice, leading to increase in the inorganic nitrogen concentration of the pore water and N2O emissions. The highest percentage of N uptake by rice plant to N loading rate was 93 % in N100 and the percentage was decreased with increasing N loading rate. In N750 (786 kg N ha−1), increases in the NH4 + concentration of the pore water and N2O emission after third topdressing would result from the higher amount of soil available nitrogen that was not utilized by the rice plants. Therefore, 786 kg N ha−1 would be an excessive nitrogen loading rate, leading to the loss of applied nitrogen via nitrogen leaching and N2O emissions. We therefore disagree with previous recommendation for a higher N application rate of 600 kg N ha-1 for forage rice (Zhou et al. 2009).
This study also suggested that 100–258 kg N ha−1 is a recommended nitrogen loading rate for nitrogen recovery by rice plants without having a negative environmental impact, such as groundwater pollution and N2O emissions. The range of the recommended nitrogen loading rate extends from the standard Japanese nitrogen fertilization rate (100 kg N ha−1) to 2.5 times that rate. Therefore, our study implies that the presently conducted LCW application (100 kg N ha−1) for forage rice production in Japan has a low impact on groundwater and global warming. Furthermore, a higher rate of LCW loading (258 kg N ha−1) can be used to treat livestock wastewater with a low environmental impact.
References
Bouwman AF (1996) Direct emission of nitrous oxide from agricultural soils. Nutr Cycl Agroecosyst 46:53–70
Bronson KF, Neue HU, Singh U, Abao EB (1997a) Automated chamber measurements of methane and nitrous oxide flux in a flooded rice soil. 1. Residue, nitrogen, and water management. Soil Sci Soc Am J 61:981–987
Bronson KF, Singh U, Neue HU, Abao EB (1997b) Automated chamber measurements of methane and nitrous oxide flux in a flooded rice soil. 2. Fallow period emissions. Soil Sci Soc Am J 61:988–993
Doi S, Kawamoto Y (2007) Utilization of anaerobically digested slurry for forage rice cultivation (in Japanese). Bulletin of Shiga Prefectural Livestock Technology Promotion Center. http://www.pref.shiga.lg.jp/g/nogyo-chikugi/seika/
Galloway JN et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Hooda PS, Edwards AC, Anderson HA, Miller A (2000) A review of water quality concerns in livestock farming areas. Sci Total Environ 250:143–167
Hua X, Guangxi X, Cai ZC, Tsuruta H (1997) Nitrous oxide emissions from three rice paddy fields in China. Nutr Cycl Agroecosyst 49:23–28. doi:10.1023/a:1009779514395
Hutchinson GL, Mosier AR (1981) Improved soil cover method for field measurement of nitrous-oxide fluxes. Soil Sci Soc Am J 45:311–316
Ibaraki Prefectural Agricultural Center (2010) Guideline of food crop cultivation (in Japanese), Ibaraki, Japan
Ikeda T (1995) Crop physiology and ecology (in Japanese). Yokendo, Tokyo
IPCC (2007a) Climate Change 2007: Synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva
IPCC (2007b) Climate Change 2007: The physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Cambridge
Ishii H, Sato K, Hata K, Sakio S, Udagawa K, Iijima Y, Sakai K, Hatahara M (2005) Aerated cattle slurry can be used as topdressing for forage rice (in Japanese). Bulletin of the Saitama Prefectural Agriculture and Forestry Research Center. http://www.pref.saitama.lg.jp/uploaded/attachment/51679.pdf
Ishikawa T (2012) Effect of nitrogen application on dry matter production of high yielding rice (Oryza sativa L.) cultivar takanari. Jpn J Crop Sci 81:89–92
Ishikawa M, Tabuchi T, Yamaji E (2003) Clarification of adsorption and movement by predicting ammonia nitrogen concentrations in paddy percolation water. Paddy Water Environ 1:27–33
Itoh M et al (2011) Mitigation of methane emissions from paddy fields by prolonging midseason drainage. Agric Ecosyst Environ 141:359–372
IUSS Working Group WRB (2006) World reference base for soil resources 2006. 2nd edition. World Soil Resources Reports No. 103. FAO, Rome
Kato H (2008) Development of rice varieties for whole crop silage (WCS) in Japan. JARQ-Jpn Agric Res Q 42:231–236
Kim DG, Hernandez-Ramirez G, Giltrap D (2013) Linear and nonlinear dependency of direct nitrous oxide emissions on fertilizer nitrogen input: a meta-analysis. Agric Ecosyst Environ 168:53–65
Kimura M, Murase J, Lu YH (2004) Carbon cycling in rice field ecosystems in the context of input, decomposition and translocation of organic materials and the fates of their end products (CO2 and CH4). Soil Biol Biochem 36:1399–1416
Kinoshita T, Nagamine T, Akutsu M, Hukushima M, Tazawa N (2013) Establishment of the application method of the methane fermentation digestion liquid in the rice cultivation for forage (in Japanese with English abstract). Bulletin of Tochigi Prefectural Dairy Farming Experiment Station. http://www.pref.tochigi.lg.jp/g70/press_etc/documents/p45-54.pdf
Kronzucker HJ, Glass ADM, Siddiqi MY, Kirk GJD (2000) Comparative kinetic analysis of ammonium and nitrate acquisition by tropical lowland rice: implications for rice cultivation and yield potential. New Phytol 145:471–476
Kyaw KM, Toyota K, Okazaki M, Motobayashi T, Tanaka H (2005) Nitrogen balance in a paddy field planted with whole crop rice (Oryza sativa cv. Kusahonami) during two rice-growing seasons. Biol Fertil Soils 42:72–82
Li S, Li H, Liang XQ, Chen YX, Cao ZH, Xu ZH (2009) Rural wastewater irrigation and nitrogen removal by the paddy wetland system in the Tai Lake region of China. J Soil Sediment 9:433–442
Liu C, Wang K, Zheng X (2012) Responses of N2O and CH4 fluxes to fertilizer nitrogen addition rates in an irrigated wheat-maize cropping system in northern China. Biogeosciences 9:839–850
Lu J, Jiang L, Chen D, Toyota K, Strong PJ, Wang H, Hirasawa T (2012) Decontamination of anaerobically digested slurry in a paddy field ecosystem in Jiaxing region of China. Agric Ecosyst Environ 146:13–22
Ma J, Li XL, Xu H, Han Y, Cai ZC, Yagi K (2007) Effects of nitrogen fertiliser and wheat straw application on CH4 and N2O emissions from a paddy rice field. Aust J Soil Res 45:359–367
Matsushima S, Manaka T (1961) Analysis of developmental factors determining yields and its application to yield prediction and culture improvement of lowland rice. LVIII. Effects of an extraordinary heavy-dressing with ammonium sulphate at various growth stages on the yield, yield-components, growth, morphological characters and the chemical composition of the rice plant. Jpn J Crop Sci 29:202–206 (in Japanese with English abstract)
McSwiney CP, Robertson GP (2005) Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Glob Chang Biol 11:1712–1719
Ministry of the Environment (1997) Environmental quality standards for groundwater pollution (in Japanese). http://www.env.go.jp/kijun/tikat1.html
Mishima S, Endo A, Kohyama K (2009) Recent trend in residual nitrogen on national and regional scales in Japan and its relation with groundwater quality. Nutr Cycl Agroecosyst 83:1–11
Nicolaisen MH, Risgaard-Petersen N, Revsbech NP, Reichardt W, Ramsing NB (2004) Nitrification-denitrification dynamics and community structure of ammonia oxidizing bacteria in a high yield irrigated Philippine rice field. FEMS Microbiol Ecol 49:359–369
Nishimura S, Sawamoto T, Akiyama H, Sudo S, Yagi K (2004) Methane and nitrous oxide emissions from a paddy field with Japanese conventional water management and fertilizer application. Glob Biogeochem Cycle 18, GB2017
Qiao J, Yang L, Yan T, Xue F, Zhao D (2012) Nitrogen fertilizer reduction in rice production for two consecutive years in the Taihu Lake area. Agric Ecosyst Environ 146:103–112
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Razavipour T, Farrokh AR (2014) Measurement of vertical water percolation through different soil textures of paddy field during rice growth season. Int J Adv Biol Biomed Res 2:1379–1388
Riya S, Zhou S, Sagehashi M, Terada A, Hosomi M (2012) Mechanisms of nitrogen removal in forage rice field applied with liquid cattle waste at high nitrogen loading. Kagaku Kogaku Ronbun 38:290–298 (in Japanese)
San-Oh Y, Kond M, Ookawa T, Hirasawa T (2008) Ecophysiological analysis on effect of planting pattern on biomass production and grain yield in rice. Jarq - Jpn Agric Res Q 42:79–89
Sasada Y et al (2011) Methane and N2O emissions, nitrate concentrations of drainage water, and zinc and copper uptake by rice fertilized with anaerobically digested cattle or pig slurry. Biol Fertil Soils 47:949–956
Shen DZ, Wu JZ (1998) Farmland treatment and utilization of straw pulp mill effluents. Ecol Eng 11:121–128
Shimono H, Okada M, Yamakawa Y, Nakamura H, Kobayashi K, Hasegawa T (2007) Lodging in rice can be alleviated by atmospheric CO2 enrichment. Agric Ecosyst Environ 118:223–230
Suzuki A (1997) Fertilization of rice in Japan, edited by A. Suzuki, Jpn. FAO Assoc., Tokyo
Takamura Y, Tabuchi T, Suzuki S, Harigae Y, Ueno T, Kubota H (1976) The fates and balance sheets of fertilizer nitrogen and phosphorus: applied to a rice paddy field in the Kasumigaura Basin. Jpn J Soil Sci Plant Nutr 47:398–405 (in Japanese)
Tian YH, Yin B, Yang LZ, Yin SX, Zhu ZL (2007) Nitrogen runoff and leaching losses during rice-wheat rotations in Taihu Lake Region, China. Pedosphere 17:445–456
Wang DJ, Liu Q, Lin JH, Sun RJ (2004) Optimum nitrogen use and reduced nitrogen loss for production of rice and wheat in the Yangtse Delta region. Environ Geochem Health 26:221–227
Wang X, Suo Y, Feng Y, Shohag MJI, Gao J, Zhang Q, Xie S, Lin X (2011) Recovery of 15 N-labeled urea and soil nitrogen dynamics as affected by irrigation management and nitrogen application rate in a double rice cropping system. Plant Soil 343:195–208
Yao ZS, Zheng XH, Dong HB, Wang R, Mei BL, Zhu JG (2012) A 3-year record of N2O and CH4 emissions from a sandy loam paddy during rice seasons as affected by different nitrogen application rates. Agric Ecosyst Environ 152:1–9
Zebarth BJ, Rochette P, Burton DL (2008) N2O emissions from spring barley production as influenced by fertilizer nitrogen rate. Can J Soil Sci 88:197–205
Zhao X, Xie YX, Xiong ZQ, Yan XY, Xing GX, Zhu ZL (2009) Nitrogen fate and environmental consequence in paddy soil under rice-wheat rotation in the Taihu lake region, China. Plant Soil 319:225–234
Zhou S, Hosomi M (2008) Nitrogen transformations and balance in a constructed wetland for nutrient-polluted river water treatment using forage rice in Japan. Ecol Eng 32:147–155
Zhou S, Nishiyama K, Watanabe Y, Hosomi M (2009) Nitrogen budget and ammonia volatilization in paddy fields fertilized with liquid cattle waste. Water Air Soil Poll 201:135–147
Zhou S, Iino H, Riya S, Nishikawa M, Watanabe Y, Hosomi M (2011) Nitrogen transformations in paddy fields treated with high loads of liquid cattle waste. J Chem Eng Jpn 44:713–719
Zou JW, Huang Y, Jiang JY, Zheng XH, Sass RL (2005) A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: effects of water regime, crop residue, and fertilizer application. Glob Biogeochem Cycle 19, GB2021
Acknowledgments
We thank the National Institute for Agro-Environmental Sciences (NIAES) for permission to use their experimental fields. We also thank Mr. Katsuo Abe and Mr. Takahiro Ara for their help in the field management, and Mr. H. Shitara (Saitama Prefecture Agriculture and Forestry Research Center, Saitama Prefecture, Japan) for providing LCW. This study was supported in part by a Strategic International Cooperative Program from the Japanese Science and Technology Agency (JST).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Riya, S., Zhou, S., Kobara, Y. et al. Influence of nitrogen loading and plant nitrogen assimilation on nitrogen leaching and N2O emission in forage rice paddy fields fertilized with liquid cattle waste. Environ Sci Pollut Res 22, 5762–5771 (2015). https://doi.org/10.1007/s11356-014-3811-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3811-x