Abstract
The novel nano-flocculants were synthesized through a conjugation of dodecylamine with partly oxidized sodium alginate. The structures of the flocculants were characterized by FTIR, 1HNMR, TGA, and EA. The flocculants possessed amphiphilic structures and formed nano-micelles through self-assembly in water. The nano-micelles showed rod-like shapes about 100 nm. Removal rates of the flocculants for Pb2+ and bisphenol A were determined under different conditions, showing the removal rates as high as 97.20% and 88.66% for Pb2+ and bisphenol A, respectively. The flocculation mechanisms were revealed by X-ray photoelectron spectroscopy (XPS) and scanning electron microscope (SEM), respectively. Isotherm adsorption studies indicated that the flocculation for Pb2+ accorded with the Langmuir single-layer adsorption model, and for bisphenol A accorded with the Freundlich multi-layer adsorption model. The quasi-second-order kinetic model was suitable for describing the adsorption kinetics. The new nano-flocculant was a promising agent for removing both heavy metal ions and organic pollutants of wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are different pollutants in wastewater, including heavy metal ions and inorganic and organic substances. Due to the toxicity and non-excretory, Pb2+ is easy to accumulate in human bodies and causes serious diseases (Li et al. 2015; Zhang et al. 2012). Bisphenol A (BPA) is an endocrine-disrupting organic compound which can affect many aspects of physiological metabolism in human bodies. The content of BPA in the environment increased dramatically because of its wide application in the production of various plastic products (Bhatnagar and Anastopoulos 2017; Melcer and Klečka 2011).
The common methods for wastewater treatment include chemical precipitation, ion exchange, membrane separation, adsorption, and flocculation (Aljuboori et al. 2015; Fu and Wang 2011; Lee et al. 2014; Singh et al. 2013; Yang et al. 2015). But there were some drawbacks in these methods. For example, ion exchange resin was not suitable for wastewater with highly concentrated heavy metal ions; chemical precipitation needed a lot of chemical regents and produced undegradable sludge; membrane filtration were highly costly and complex in water treatment process; post-processing in adsorbents might be highly expensive and often produced secondary pollutions.
Small molecular organic pollutants are difficult to remove in water treatment. Xie et al. (2018) prepared a new supramolecular adsorbent with hydrophobic pores through self-assembly of helical aromatic amphiphilic molecules. It had a good removal effect on ethinyl estradiol and bisphenol A. However, the preparation was complex, and toxic copper catalyst was needed in the preparation. Aziz et al. (2018) reported that they treated the pollutants bisphenol A and 4-di-tert-butylphenol in landfill leach using locust bean gum as a flocculant. The flocculation effect was better than alum, mainly because the flocs produced by locust bean gum had rough cloudy surface and numerous pores. But this flocculant was only be used to pretreat wastewater before deeper treatment. Zhang et al. (2019) reported a processable poly-lipoic ester-based material which had amphiphilic functional groups and had excellent removal efficiency for organic pollutants such as bisphenol A, but this material was only suitable for treating BPA wastewater with high chemical oxygen demand, and the application was limited.
Zeng et al. proposed a micelle-enhanced ultrafiltration (MEUF) method to separate pollutants from water (Zeng et al. 2008). Although MEUF could remove metal ions and organic compounds from water, there were disadvantages such as membrane blockage, complex operation, and high energy consumption. Talens et al. used adsorptive micellar flocculation (AMF) to remove complex pollutants in wastewater (Porras-Rodriguez and Talens-Alesson 1999; Talens et al. 1998). They used micelles based on sodium dodecyl sulfate to solubilize organic pollutants in water, and then applied aluminum and iron ions to combine with anions to form flocculating precipitation for removing pollutants. Talens-Alesson et al. (2010) employed the AMF method to treat phenol containing wastewater, but this method was easy to form residues which caused secondary pollution.
To solve the problems of current methods in water treatment, in this work, we have developed a new kind of nano-flocculants through a conjugation of dodecylamine (DC) with modified sodium alginate. Sodium alginate is a kind of natural macromolecule with abundant sources. It is a degradable, non-toxic, and harmless environment-friendly material (Augst et al. 2006; Lee and Mooney 2012; Pawar and Edgar 2012). Dodecylamine contains hydrophobic carbon chains, and alginate contains hydrophilic sugar rings carrying carboxyl and hydroxyl groups. The conjugation of DC with SA forms amphiphilic polymers and produces nano-micelles through self-assembly in water. It was expected that this novel flocculants can remove heavy metal ions and organic substances simultaneously. The synthetic conditions, characterizations, flocculation behavior for Pb2+ and bisphenol A, and flocculation mechanisms have been investigated. The nano-flocculants demonstrate the advantages such as mild conditions of preparation, low cost, high removal rates for multiple pollutants, and easy post-processing. To our knowledge, this research has not been reported in literatures.
Experimental and methods
Materials
Sodium alginate (SA, viscosity ≥ 0.02 Pa s in an aqueous solution of 1.0 wt%, 25 °C), dodecylamine (DC), Pb(NO3)2, NaIO4, HCl, NaOH, and anhydrous ethanol were purchased from Sinopharm Chemical Reagent Co., Ltd.; bisphenol A (BPA, 98%) was purchased from Damas-beta Company. All the materials were of analytical grade and were used as received without further purification.
Preparation of nano-flocculants (SADC)
Based on a reported method (Balakrishnan et al. 2005), in a glass flask, 4.95 g of SA (0.025 mol) powder was added into 50 mL anhydrous ethanol under magnetic stirring. Then, 50 mL of aqueous solution containing 0.015 mol NaIO4 was added to the solution. The reaction was carried out at room temperature for 6 h, and 5 mL ethylene glycol was added to terminate the reaction; the partly oxidized sodium alginate was obtained after filtration, washing with 70% ethanol and drying.
The oxidized sodium alginate was dissolved in 120 mL deionized water, 4.68 g DC was slowly added to the solution, and the reaction was carried out under stirring at 50 °C for 12 h. After stopping the reaction and cooling to room temperature, 0.82 g NaBH4 was added in three batches, and the reaction was continued for additional 12 h. Anhydrous ethanol was added in a ratio of 1:4 (v:v) for precipitating the product at 4 °C for 8 h. The final flocculants were attained after freeze-drying.
Characterizations of the flocculants SADC
The Fourier transform infrared (FTIR) spectra were carried out with attenuated total reflectance method scanning in a range of 400–4000 cm−1(ATR-FTIR, Nicolet 6700, USA). The 1HNMR was recorded on a nuclear magnetic resonance instrument (Bruker AVANCE III HD 400 MHz). Thermal analysis was performed on a thermogravimetric analysis instrument (TGA/SDTA 851e Mettler Toledo, 1100SF, Switzerland; nitrogen atmosphere flow rate 50 mL/min, heating rate 10 °C/min; temperature 25~600 °C). Elemental analyzer (Vario III Elementar, Germany) was used to analyze contents of the elements. The electron binding energy changes of the flocculants and Pb2+ before and after flocculation were determined by X-ray photoelectron spectroscopy (XPS; Kratos Analytical, AXIS SUPRA). Scanning electron microscope (SEM; Hitachi, S-4800) was used to observe the morphology of flocculant before and after flocculation.
Flocculation property of SADC
The flocculant SADC aqueous solution (wt, 1%) was prepared by dissolving it in deionized water. Lead ion (Pb2+) solution with a concentration of 1000 mg/L was prepared by dissolving Pb(NO3)2 in deionized water. Bisphenol A (BPA) solutions with various concentrations were prepared. The pH could be adjusted using 1 mol/L NaOH and 1 mol/L HCl solutions. All flocculation experiments were carried out on a temperature-controlled rotary shaker (Model DKY-I, Shanghai Duke Automation Equipment Co. Ltd., China). Briefly, a certain amount of flocculant solution was added into a wastewater solution; the mixture was rocked for 30 min at a speed of 110 r/min. In the flocculation for BPA, CaCl2 was added as a coagulant using the same amount of mass as the flocculant, with continuous stirring for 60 min. The products were kept motionless for 30 min, yielding to flocculate precipitate; then, the precipitate was filtered. The concentration of the Pb2+ in the solution was measured by atomic absorption spectrophotometry (Brookhaven Company, SpectrAA-220/220Z, USA), and the concentration of BPA was measured by spectrophotometry (Shimadzu UV-2300) (Cao et al. 2014). The removal rate (Rr) of Pb2+ or BPA was calculated by the following formula (Mallampati and Valiyaveettil 2013):
where C0 and C were concentrations (mg/L) of Pb2+ (or BPA) before and after flocculation, respectively.
Results and discussion
Preparation of SADC nano-flocculants
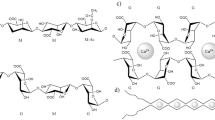
The preparation was carried out in two steps: firstly, the sugar rings of alginate were partly oxidized to produce aldehyde groups at the presence of NaIO4. The oxidation degree was 20%. Then, dodecylamine (DC) was conjugated with SA through a nucleophilic addition reaction between aldehyde groups and amino groups; next, the newly formed C=N bond was converted to CH-NH at the presence of NaBH4. The alginate modified by DC possessed amphiphilic structures with hydrophobic DC chains and hydrophilic sugar rings. The amphiphilic polymers would form nano-micelles through self-assembly in aqueous solutions. The scheme is shown in Fig. 1.
Elemental analysis
Three samples of nano-alginate flocculants (SADC-1, SADC-2, SADC-3) with different components were obtained by changing the dosage of DC. Elemental analysis results are shown in Table 1. It was observed that the modified alginate flocculants contained nitrogen elements, indicating that DC was successfully conjugated with SA. The conjugated rate (CR) was defined as follows:
where m0 and m1 were the weights of alginate before and after conjugation with DC. Table 1 shows that the N percentage and conjugation rate (CR) increased with the increase of DC dosage.
FTIR of the flocculants
Total reflection Fourier transform infrared spectra of the flocculants are presented in Fig. 2. The peak at 3265 cm−1 was the superposition peak of –OH and –NH; the peak at 1602 cm−1 was the stretch of –C=O. Compared with the infrared spectrum of SA, the new absorption peaks at 2922 cm−1 and 2850 cm−1 appeared in SADC, which belonged to the characteristic absorption peaks of –CH3 group; the new peak at 1463 cm−1 belonged to the bending vibration of –CH2, while the new peak at 720 cm−1 was ascribed to the bending of –NH plane. The FTIR spectra proved that DC was successfully introduced to the SA chain. The coexistence of carboxylic acid groups and alkyl long chain indicated that SADC was a new flocculant (Hebeish et al. 2010). Unlike to literatures, we use dodecylamine with long-chain hydrophobic group to modify sodium alginate. The modified flocculant not only has hydrophilic carboxylic acid group, but also has hydrophobic long-chain alkyl group, forming an amphiphilic structure, so it is a new type of flocculant.
1HNMR of the flocculants
The 1HNMR spectra of the flocculants are shown in Fig. 3. Compared with the 1HNMR spectra of SA and DC, all the chemical shift peaks in SA and DC appeared in the SADC spectra, which evidenced that DC was introduced into SA, leading to the formation of conjugated polymers SADC. The detailed chemical shift ascription for different protons is indicated in Fig. 3.
Thermogravimetric analysis
The thermogravimetric analysis (Fig. 4) showed the structure change from SA to SADC. The SA lost weight 13% in the temperature range of 25~109 °C, and the weight loss corresponded to the loss of bound water. After a period of stable temperature range, the SA lost weight rapidly in the temperature range of 230~280 °C, which was mainly ascribed to the thermal decomposition of SA. However, the weight of SADC remained stable in the temperature range of 25~109 °C, indicating that SADC samples had a very little water content and good thermal stability in this temperature range. In the temperature range of 109~210 °C, the SADC slowly lost weight 10%; then, the rapid weight loss began and stopped at 504 °C. The thermogravimetric curves revealed that SADC had a better thermal stability than SA due to the modification (Kumar et al. 2015).
Flocculation properties
The removal rates of the flocculants for heavy metal ions and organic substances were measured using Pb2+ and BPA as model pollutants, respectively. The results are shown in Table 2. It was observed that as CR increased from SADC-1 to SADC-3, the removal rate for Pb2+ decreased gradually, but the removal rate for BPA increased gradually. We explained that two different mechanisms were involved in the flocculation with the two pollutants. The flocculants adsorbed Pb2+ mainly through a chelation process in which COO− and OH groups donated electrons and Pb2+ accepted electrons. The increase of conjugation rate implied that the number of OH groups in the SADC declined, and the chelation ability was reduced, resulting in the decrease of removal rate for Pb2+. However, the flocculants adsorbed organic substance BPA mainly by hydrophobic interaction between DC chains and BPA. Therefore, the higher DC content in the flocculant, the higher removal rate for BPA.
Effect of pH on removal rates
The results of pH effect on the removal rates could be seen in Fig. 5. The removal rates for both Pb2+ and BPA increase gradually. When the pH value of the solution was greater than 6, the heavy metal ion Pb2 + would be hydrolyzed to produce hydroxide precipitation; and when the pH value of the solution was greater than 9, BPA would ionize, affecting the separation. Therefore, no experiments were carried out at higher pH values.
The flocculation for Pb2+ was mainly by the chelation of COOH and OH with heavy metal ions. With the increase of pH value, the number of COO− groups increased due to deprotonation, and the electron-donating ability increased, which resulted in the increase of removal rates. On the other hand, the flocculation for BPA was through hydrophobic interaction of DC in the nano-micelles. With the increase of pH, the amino groups in the molecule were converted from NH3+ to NH2 because of the deprotonation, and the hydrophobicity of the core was enhanced, leading to the increase of the interaction force with the organic molecules, so the removal rates increased.
Effect of initial concentration on the removal rates
The flocculation was carried out with different initial concentrations of Pb2+ and BPA for investigating the effect of initial concentration on the removal rates. After many attempts, we determined that the mass ratio of SADC/Pb2+ was 2:1, and that of SADC/BPA was 50:1, which were the optimal proportions. We fixed the flocculation time 30 min for Pb2+ and 90 min for BPA, because the flocculation basically reached equilibrium within these time according to the flocculation kinetics (Fig. 9). Figure 6 discovers that removal rates for Pb2+ and BPA increased with the increase of initial concentrations of Pb2+ and BPA. Obviously, in concentrated solutions, there were more opportunities for the flocculants to contact with the pollutants, and the flocculants were easier to reach saturated adsorption; consequently, higher removal rates would be attained in these cases.
XPS analysis
To study the mechanism of the adsorption process, we measured the binding energy changes for some atomic orbits of SADC-3 and Pb2+ before and after flocculation using X-ray photoelectron spectroscopy. It is known that the binding energy increases with the loss of electrons and decreases with the gain of electrons (Trochimczuk and Kolarz 2000; Zhang et al. 2009).
Table 3 shows that the binding energy decreased from 138.97 eV to 137.96 eV in Pb2+-4f7/2 orbit and from 143.89 eV to 142.72 eV in Pb2+-4f5/2 orbit, respectively. These binding energy decreases indicated that Pb2+ accepted electrons in the flocculation process. The binding energy changes in some orbits of Pb2+ before and after flocculation with SADC-3 are displayed in Fig. 7.
On the other hand, the binding energy of O1s was 530.89 eV in SADC-3, but it increased to 531.37 eV after flocculation with Pb2+. This increase implied that oxygen provided electrons in the process. The binding energies of C1s and N1s changed very little (Table 3), and this meant that the carbon and nitrogen atoms were not involved in the flocculation process, probably because the carbon and nitrogen atoms were located in the core of the nano-particle and were inaccessible to Pb2+. We could conclude that a stable flocculation product was formed through the electronic interaction of carboxyl and hydroxyl groups with lead ions. The wide spectrum of binding energies of Pb(NO3)2, SADC-3, and flocculants with Pb(II) is shown in Supplementary materials Fig. S1.
SEM observation
The modified alginate was amphiphilic and it formed a nano-particle in aqueous solutions with aggregated DC as the core and hydrophilic sugar rings as the shell. Figure 8 shows the scanning electron microscope (SEM) images of the nano-micelles before and after flocculation with BPA. It was observed that the flocculant formed rod-like micelles with smaller sizes (100 nm) before flocculation; however, the sizes of micelles became larger after the flocculation with BPA, and the shape of nano-micelles also had some changes. This result proved that BPA molecules entered into hydrophobic region of the nano-micelles, leading to size increase of the micelles.
Flocculation mechanism
In order to study the flocculation mechanism, two isotherm adsorption models were used and the results were showed respectively.
Langmuir adsorption isotherm (Dahiya et al. 2008; Dong et al. 2011; Feng et al. 2013) is expressed as follows:
The basic attribute of Langmuir isotherm could be described by a dimensionless parameter RL, which was defined as:
Freundlich adsorption isotherm is expressed as follows:
In the Formulas (3)~(5), C0 and Ce were the initial and equilibrium concentrations of the pollutant, respectively; qe and qm were the equilibrium and maximum adsorption capacities, respectively; KL and KF were the Langmuir and Freundlich constants, respectively.
The content of dodecylamine group in SADC-3 was the highest among the three samples, and it had a typical amphiphilic structure, so it was chosen as a representative to carry out the mechanism and kinetics studies.
The flocculation experiments were carried out at the room temperature. The initial concentration of Pb2+ was fixed at 500 mg/L, the mass ratio of SADC-3 to Pb2+ was varied in a range of m(SADC-3):m(Pb2+) = (0.5~2.5):1, and a group of experimental data of qe related to Ce were obtained. In the flocculation experiments for BPA, the initial concentration of BPA was fixed at 10 mg/L, the mass ratio of SADC-3 to BPA was varied in a range of m(SADC-3):m(BPA) = (10~70):1, and the other procedure was the same as in flocculation experiments for Pb2+.
The linear regressions were performed based on the experimental data of qe at different Ce; the results are displayed in Table 4.
According to a theory (Dahiya et al. 2008), when RL = 0, 0 < RL < 1, RL = 1, and RL > 1 in Formula (4), the adsorption is irreversibly adsorbed, easily adsorbed, linearly adsorbed, and non-adsorbed, respectively. In this study, we calculated RL (Pb2+) = 0.0056 and RL (BPA) = 0.0440; the two data were very close to 0, which meant that Pb2+ or BPA was adsorbed by the flocculants nearly irreversible.
In the Freundlich adsorption isotherm, the constant (n) reflects adsorption strength. The larger the value (n), the easier the adsorption. It was inferred that the flocculation for Pb2+ (n = 49.16) was easier than for BPA (n = 0.460).
According to the correlation coefficients (R2) in Table 4, the Langmuir adsorption model (R2 = 0.9990 for Pb2+) was more suitable to describe the mechanism for Pb2+, indicating that the flocculation process was a single-layer adsorption in which COO– and OH groups chelated Pb2+, while the Freundlich adsorption model (R2 = 0.9700 for BPA) was more suitable to describe the mechanism for BPA, indicating the flocculation process was a multi-layer adsorption in which BPA was solubilized to the nano-micelles.
Sehaqui et al. modified nano-cellulose and prepared nano-cellulose flocculant TOCNF containing carboxylic acid groups (Sehaqui et al. 2014). However, its adsorption capacity for heavy metals was weaker than that of SADC because the content of carboxylic acid groups of modified TOCNF was not high; for example, the adsorption capacity of TOCNF for Cu2+ was 2.1 mmol g/L, while SADC-3 had an adsorption capacity of 2.55 mmol/g for Cu2+, because SADC was a polyanionic flocculant with high content of carboxylic acid groups. Maatar et al. prepared nano-cellulose flocculant NFCo containing hydrophobic alkane chains through a modification (Maatar et al. 2013), which improved the adsorption capacity to aromatic compound pollutants in water. However, the preparation process of NFCo was much more complex than that of SADC, and they are not suitable to deal with complex wastewater systems.
Cellulose is insoluble in water and organic solvents, and the functional groups on the molecular chains are not abundant enough, so it is difficult to prepare amphiphilic flocculants from cellulose (Khalil and Aly 2002; Mahfoudhi and Boufi 2017; Raj et al. 2016; Shak et al. 2018). But SADC has many advantages, such as simple preparation, low cost, biodegradability, environment friendly, and wide applications for treating many pollutants.
Flocculation kinetics
The flocculation speed was studied by measuring flocculation capacity at different time (Fig. 9). It was clear that the initial adsorption for Pb2+ was fast; then, the adsorption gradually slowed down. The equilibrium adsorption was arrived after 20 min. Because the flocculants were uniformly distributed in aqueous solution and nano-sized micelles possessed huge surface areas, the Pb2+ ions were caught by COO– and OH groups on the surfaces of the nano-micelles. However, the adsorption for BPA was slow and it obtained main adsorption at 90 min and reached equilibrium adsorption at 4 h. Because BPA was only adsorbed by hydrophobic DC chains in the core of the nano-micelles, the BPA molecules needed to penetrate the shells of the micelles, resulting in the slow adsorption speed.
Flocculation kinetics were studied using the quasi-first-order and quasi-second-order kinetics models as follows (Chiou and Li 2003; Ho and McKay 1999):
where qe and qt were adsorption capacities at the equilibrium and time t, respectively; k1 is a quasi-first-order kinetic constants, and k2 is a quasi-second-order kinetic constants. The parameters were obtained through regression analysis (Table 5).
According to the correlation coefficients, the quasi-second-order kinetic model (R2 = 0.9997 for Pb2+ and R2 = 0.9960 for BPA) was more suitable for describing the adsorption kinetics than the quasi-first-order model (R2 = 0.8932 for Pb2+ and R2 = 0.9767 for BPA).
It was proposed that three steps were involved in the flocculation process: Firstly, the nano-micelles adsorbed pollutants through electrostatic attraction or chelating action, forming mesh structure polymer; secondly, the mesh structure polymer caught more pollutants by netting and sweeping actions, yielding larger particles; finally, the large particles were converted into solid precipitates after further cohesion and settlement.
Conclusions
A new kind of nano-flocculants was synthesized through a conjugation of dodecylamine with partly oxidized sodium alginate. The flocculants had high removal rates for Pb2+ and bisphenol A. The flocculation mechanisms for Pb2+ were the chelation of COO− and OH with the metal ions, which was proved by XPS, and for BPA was the compatibilization of the micelles, which was proved by SEM. The flocculation process accorded with the Langmuir and Freundlich models for Pb2+ and bisphenol A, respectively. The new kind of nano-flocculants was a promising agent for wastewater purification.
References
Aljuboori AHR, Idris A, Al-Joubory HHR, Uemura Y, Ibn Abubakar BSU (2015) Flocculation behavior and mechanism of bioflocculant produced by Aspergillus flavus. J Environ Manag 150:466–471
Augst AD, Kong HJ, Mooney DJ (2006) Alginate hydrogels as biomaterials. Macromol Biosci 6:623–633
Aziz A, Agamuthu P, Fauziah SH (2018) Removal of bisphenol A and 2,4-di-tert-butylphenol from landfill leachate using plant- based coagulant. Waste Manag Res 36:975–984
Balakrishnan B, Lesieur S, Labarre D, Jayakrishnan A (2005) Periodate oxidation of sodium alginate in water and in ethanol-water mixture: a comparative study. Carbohydr Res 340:1425–1429
Bhatnagar A, Anastopoulos I (2017) Adsorptive removal of bisphenol A (BPA) from aqueous solution: a review. Chemosphere 168:885–902
Cao GP, Zhuang YF, Liu BL (2014) Simultaneous determination of bisphenol A and bisphenol S in environmental water using ratio derivative ultraviolet spectrometry. South Afr J Chem Suid Afr Tydskr Chem 67:99–103
Chiou MS, Li HY (2003) Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 50:1095–1105
Dahiya S, Tripathi RM, Hegde AG (2008) Biosorption of heavy metals and radionuclide from aqueous solutions by pre-treated arca shell biomass. J Hazard Mater 150:376–386
Dong ZB, Liang YR, Fan FY, Ye JH, Zheng XQ, Lu JL (2011) Adsorption behavior of the catechins and caffeine onto polyvinylpolypyrrolidone. J Agric Food Chem 59:4238–4247
Feng J, Yang Z, Zeng G, Huang J, Xu H, Zhang Y, Wei S, Wang L (2013) The adsorption behavior and mechanism investigation of Pb(II) removal by flocculation using microbial flocculant GA1. Bioresour Technol 148:414–421
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418
Hebeish A, Higazy A, El-Shafei A, Sharaf S (2010) Synthesis of carboxymethyl cellulose (CMC) and starch-based hybrids and their applications in flocculation and sizing. Carbohydr Polym 79:60–69
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Khalil MI, Aly AA (2002) Preparation and evaluation of some anionic starch derivatives as flocculants. Starch-Starke 54:132–139
Kumar K, Adhikary P, Karmakar NC, Gupta S, Singh RP, Krishnamoorthi S (2015) Synthesis, characterization and application of novel cationic and amphoteric flocculants based on amylopectin. Carbohydr Polym 127:275–281
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37:106–126
Lee CS, Robinson J, Chong MF (2014) A review on application of flocculants in wastewater treatment. Process Saf Environ Prot 92:489–508
Li P, Lin C, Cheng H, Duan X, Lei K (2015) Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol Environ Saf 113:391–399
Maatar W, Alila S, Boufi S (2013) Cellulose based organogel as an adsorbent for dissolved organic compounds. Ind Crop Prod 49:33–42
Mahfoudhi N, Boufi S (2017) Nanocellulose as a novel nanostructured adsorbent for environmental remediation: a review. Cellulose 24:1171–1197
Mallampati R, Valiyaveettil S (2013) Apple peels--a versatile biomass for water purification? ACS Appl Mater Interfaces 5:4443–4449
Melcer H, Klečka G (2011) Treatment of wastewaters containing bisphenol a: state of the science review. Water Environ Res 83:650–666
Pawar SN, Edgar KJ (2012) Alginate derivatization: a review of chemistry, properties and applications. Biomaterials 33:3279–3305
Porras-Rodriguez M, Talens-Alesson FI (1999) Removal of 2,4-dichlorophenoxyacetic acid from water by adsorptive micellar flocculation. Environ Sci Technol 33:3206–3209
Raj P, Batchelor W, Blanco A, de la Fuente E, Negro C, Garnier G (2016) Effect of polyelectrolyte morphology and adsorption on the mechanism of nanocellulose flocculation. J Colloid Interface Sci 481:158–167
Sehaqui H, de Larraya UP, Liu P, Pfenninger N, Mathew AP, Zimmermann T, Tingaut P (2014) Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment. Cellulose 21:2831–2844
Shak KPY, Pang YL, Mah SK (2018) Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J Nanotechnol 9:2479–2498
Singh RP, Pal S, Rana VK, Ghorai S (2013) Amphoteric amylopectin: a novel polymeric flocculant. Carbohydr Polym 91:294–299
Talens FI, Patón P, Gaya S (1998) Micelar flocculation of anionic surfactants. Langmuir 14:5046–5050
Talens-Alesson F, Svabova M, Svab M (2010) The role of mixing in high performance adsorptive micellar flocculation. Colloids Surf A Physicochem Eng Asp 355:16–22
Trochimczuk AW, Kolarz BN (2000) Synthesis and chelating properties of resins with methylthiourea, guanylthiourea and dithiocarbamate groups. Eur Polym J 36:2359–2363
Xie SY, Wu SS, Bao SH, Wang YQ, Zheng YT, Deng DF, Huang LP, Zhang LL, Lee M, Huang ZG (2018) Intelligent mesoporous materials for selective adsorption and mechanical release of organic pollutants from water. Adv Mater 30
Yang Z, Jia S, Zhang T, Zhuo N, Dong Y, Yang W, Wang Y (2015) How heavy metals impact on flocculation of combined pollution of heavy metals–antibiotics: a comparative study. Sep Purif Technol 149:398–406
Zeng G-M, Xu K, Huang J-H, Li X, Fang Y-Y, Qu Y-H (2008) Micellar enhanced ultrafiltration of phenol in synthetic wastewater using polysulfone spiral membrane. J Membr Sci 310:149–160
Zhang L, Ni C, Zhu C, Jiang X, Liu Y, Huang B (2009) Preparation and adsorption properties of chelating resins from thiosemicarbazide and formaldehyde. J Appl Polym Sci 112:2455–2461
Zhang X, Yang L, Li Y, Li H, Wang W, Ye B (2012) Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ Monit Assess 184:2261–2273
Zhang Z, Liu Q, Sun ZM, Phillips BK, Wang ZZ, Al-Hashimi M, Fang L, Olson MA (2019) Poly-lipoic ester-based coacervates for the efficient removal of organic pollutants from water and increased point-of-use versatility. Chem Mater 31:4405–4417
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20161128), MOE & SAFEA for the 111 Project (B13025), and Research Fund of Central University (JUSRP51626B).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 182 kb)
Rights and permissions
About this article
Cite this article
Tian, Z., Zhang, L. & Ni, C. Preparation and flocculation properties of modified alginate amphiphilic polymeric nano-flocculants. Environ Sci Pollut Res 26, 32397–32406 (2019). https://doi.org/10.1007/s11356-019-06308-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06308-2