Abstract
Concentration of dissolved nitrate (NO3 −) and stable nitrogen isotopic composition of nitrate in water samples from 19 epikarst springs in the Jinfo Mountain area were analysed in order to understand the temporal–spatial distribution of nitrate in karst spring waters and trace nitrogen sources for the springs that have suffered from nitrate contamination. The results show that hydrochemistry of epikarst spring water is Ca·Mg–HCO3 facies, reflecting the control of water–carbonate rock interaction. NO3 − concentrations in water of springs within the national nature reserve (NNR) in the Jinfo Mountain area range from 0 to 8.16 mg/L, suggesting that these springs within the NNR have good water quality. However, most springs outside the NNR have high NO3 concentrations (c: 10.5–46.18 mg/L) that have exceeded the value of Chinese national drinking water quality standard (NO3–N ≤10 mg/L), revealing that these springs have suffered from nitrate contamination to a significant degree. δ 15N–NO3 values of these contaminated springs outside the NNR range from −1.2 ‰ to 11.27 ‰, higher than those of unpolluted springs within NNR (−5.2 ‰ –8.6 ‰). Two main sources of nitrate in these contaminated springs have been identified, including animal manure/sewage and chemical nitrogen fertilisers that are from anthropogenic activities. Our study also shows that the nitrate fate and origin are associated with the land use in the recharge catchment of individual epikarst springs, and the establishment of national nature reserve can protect the water quality of epikarst spring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities have been increasingly changing the nitrogen cycle in nature, leading to a cascade of environmental problems on soil and water and threatening the human health (Wolfe and Patz 2002; Galloway et al. 2008; Vitousek et al. 2009). Nitrate per se is non-toxic (Mensinga et al. 2003), but approximately 5 % of ingested nitrate in the human body can be converted to nitrite in the saliva and gastrointestinal tract, and the latter is toxic (Spiegelhalder et al. 1976; Pannala et al. 2003). Hence, nitrate are usually related to the pathogenesis of gastric cancers and other malignancies, as it can enhance the generation of carcinogenic N-nitrosamines (Lundberg et al. 2004). In addition, many studies found that the nitrate content in drinking water has a positive relationship with the childhood-onset insulin-dependent diabetes mellitus and methaemoglobinaemia (Parslow et al. 1997; Gupta et al. 2000).
The European Commission’s Scientific Committee on Food (SCF) sets an acceptable daily intake (ADI) for nitrate (NO3 −) of 0–3.7 mg/kg body weight (Speijers and van den Brandt 2003; Mensinga et al. 2003). The reference dose for nitrate from the US Environmental Protection Agency (EPA) is below 7.0 mg NO3 −/kg body weight/day (Mensinga et al. 2003). The Ministry of Health of the People’s Republic of China (MOH) and the Standardization Administration of the People’s Republic of China (SAC) also state that nitrate concentration in drinking water must not exceed 10 mg/L (MOH and SAC 2006).
In the recent years, the stable nitrogen isotope (δ 15N) in nitrate has been widely used to trace the source, migration and transformation of inorganic nitrogen. It has been considered to be an effective way to recognise nitrate sources in waters that have suffered from nitrate contamination to various degrees (Kohl et al. 1971, 1972; Hauck et al. 1972; Kreitler 1975, 1979; Kreitler and Browning 1983; Mariotti and Letolle 1977; Gormly and Spalding 1979; Heaton 1986). Previous studies have identified three main sources of nitrate contaminants based on their distinct δ 15N values: mineralised organic nitrogen from the soil (2–9 ‰), synthetic chemical fertilisers (−4–4 ‰) and animal manure and sewage (4–25 ‰) (Kreitler 1979; Heaton 1986; Aravena et al. 1993). Other studies are focusing on refining these δ 15N values and different nitrogen sources based on conditions of individual study sites.
In many countries and regions, groundwater is the main source of drinking and irrigation water (additional 25 % of the population use the karst water). Generally, natural nitrate concentrations in groundwater are very low (Debernardi et al. 2008) but human activities have input a large amount of excess nitrate to the groundwater via organic or inorganic nitrogen fertilisation in agriculture land, drainage of industrial and domestic sewage and disposal of garbage and other wastes (Chen et al. 2005; Debernardi et al. 2008; Galloway et al. 2008; Vitousek et al. 2009; Zhang et al. 2012). These anthropogenic inputs of nitrogen to the groundwater have resulted in the deterioration of groundwater quality, seriously threatening drinking water security (Guo et al. 2007; Boyer and Pasquarell 1995). Epikarst springs develop, in the epikarst zone, an irregular karst zone occurring in surface carbonate rocks that consists of various individual and micro karst forms resulting from strong karstification processes. Karst aquifer systems, with the thin and discontinuous distribution soil, have developed many sinkholes and fractures. Contamination of agricultural irrigation, industrial waste and domestic waste can be easily brought into karst aquifer by rainfall. Concentrations of nitrate have shown notable increases as a result of the application of large amounts of chemical fertilisers used in agriculture (Compton and Boone 2000; Jiang et al. 2008). Several previous studies have examined the links between human activity and the hydrochemistry of springs in China (Jia and Yuan 2003; Zhang and Yuan 2004). In this paper, concentration of dissolved NO3 − and stable nitrogen isotopic composition of nitrate (δ 15N–NO3 −) in water samples from 19 epikarst springs in the Jinfo Mountain area were tested and analysed, understanding the temporal–spatial distribution of nitrate in karst spring waters and trace nitrogen sources for the springs that have suffered from nitrate contamination.
Site description
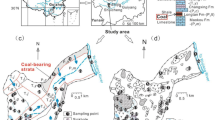
The Jinfo Mountain (Mt. Jinfo) area is located in Nanchuan District, Chongqing, Southwest China (Fig. 1). This area lies in the north-eastern Dalou Mountain ranges and is characterised to be a typical karst area (Wang and Wang 1990). Due to its special landforms and being rich in animal and plant species, the central parts around the main peak of the Mt. Jinfo have been declared a national nature reserve (NNR). The study area covers most of the Mt. Jinfo area, including the NNR (∼441 km2) and outside NNR.
The study area is affected by both south-west and south-east Asian summer monsoon, and thus, the climate is characterised by heavy clouds and little sunshine (Fig. 1(a)). The mean annual temperature in the study area is ∼16.6 °C, and its mean annual precipitation is ∼1287 mm, over 80 % of which occurs during monsoon seasons (April to October).
The structural setting of the study area is an anticline and includes fault structures along the NE–SW-oriented major tectonic line. The exposed strata in the study area are mainly the Cambrian dolostone and the Ordovician and Permian limestone (Fig. 1(c)). In addition, the Silurian fine-grained clastic rocks are sandwiched among the carbonate rocks (Fig. 1(c)). This lithology setting controls the regional shallow groundwater system, with the main groundwater aquifer developed in relation to the occurrence of carbonate rocks. The shallow groundwater aquifer in the study area consists of numerous fractures and caverns well-developed in the carbonate rocks. Clastic rocks (siltstone/mudstone) become the regional aquiclude. Being a national forest garden, the area within the NNR is dominated by forestland (how is the percentage of forest coverage) whereas the area investigated outside the NNR includes cultivated lands and grassland, besides forestland. Small-sized industrial and mining activities are present outside the NNR too. The source recharging the shallow groundwater is mainly atmospheric precipitation which infiltrates through fractures and sinkholes. The discharge of shallow groundwater occurs in forms of karst springs and underground streams, and regional groundwater discharge level lies close to the base of the Jinshan-Toudu valley (Fig. 1(c)).
Methods
Nineteen epikarst springs in the Mt. Jinfo area were investigated on January and July 2009 (Fig. 1(c)). Nine epikarst springs, including springs J04, J05, J08, J09, J10, J12, J13, J14 and J17, are within the NNR, while the other 10 springs, including springs J01, J02, J03, J06, J07, J11, J15, J16, J18 and J19, are outside the NNR. These epikarst springs outside the NNR are important drinking water sources for the local people. Water temperature (T), pH and electric conductivity (EC) were measured using the Hach (USA) Portable Water Quality analysers with any accuracy of the following: 0.1 °C for T, 1 μS/cm for EC and 0.01 unit of pH. HCO3 − was measured in situ using a portable kit produced by Merck (Germany) with an accuracy of 0.1 mmol/L.
Epikarst spring water samples for cation analysis were collected in 50-mL polyethylene bottles with the addition of a small amount of nitric acid (1:1) for preservation. Samples for anion analysis were also collected in 50-mL polyethylene bottles but without acidification. All samples were stored at 4 °C before analysis. Sample cations were determined using an Optima 2100 DV inductively coupled plasma optical emission spectrometry (ICP-OES; Perkin Elmer, USA) and ion chromatography (Shimadzu, Japan).
The nitrogen isotope composition of nitrate (δ 15N–NO3) in water samples taken on January 2009 was analysed at the Isotope Laboratory of the School of Geographical Sciences, Southwest University, China. δ 15N–NO3 values for water samples collected on July 2007 from the same springs were cited in Gao et al. (2008). Three-litre water samples were collected from these 19 springs, and all the water samples were filtered using glass fibre filters with a pore size of 0.45 μm. The samples were then preserved at a temperature of 4 °C until analysis. The pre-treatment for samples was conducted using the method described in Silva et al. (2000). The EA-Conflo-IRMS system was applied to analyse δ 15N–NO3. The international standards IAEA-N1 and IAEA-N2 were used for the calibration, and the analytical precision is ≤±0.2 ‰.
Results
Hydrochemistry of epikarst springs
Table 1 shows that most of the springs in the study area are alkaline with pH values of 7.14–8.35 in winter and 7.0–9.5 in summer. The water temperature ranges from 8.6 to 25.6 °C in winter and from 9.5 to 18.6 °C in summer. The water EC ranges from 123.5 to 688 μS/cm in winter and from 96.1 to 1068 μS/cm in summer. Ca2+ and HCO3 − are the main ions in all springs investigated (Table 1). The concentrations of Ca2+ are 21–143 mg/L in winter and 22–166 mg/L in summer. The concentrations of HCO3 − are 42.50–222.99 mg/L in winter and 18.3–231.8 mg/L in summer. The other ions present in these water samples, including Na+, K+, Cl−, SO4 2− and NO3 −, are commonly of low concentrations, except for SO4 2− and NO3 − in some water samples (Table 1). The piper plot (Piper 1944) shows that all epikarst spring samples are Ca·Mg–HCO3 type (Fig. 2).
Nitrate concentration and stable nitrogen isotope
At present, the NO3 − concentrations in the epikarst spring water are 0–46.18 mg/L (Table 1). The springs within the NNR have NO3 − concentrations of 0–7.77 mg/L in winter and 0.22–9.20 mg/L in summer, while the springs outside the NNR have NO3 − concentrations of 2.21–28.24 mg/L in winter and 3.56–46.18 mg/L in summer. All springs outside the NNR have nitrate concentrations that exceed the national drinking water standard (Table 1, Fig. 3).
δ 15N–NO3 values in all water samples range from −5.1 ‰ to 11.2 ‰ in winter and from −8.6 ‰ to 11.27 ‰ in summer (Table 1; Gao et al. 2008), and the two main distribution intervals ranging from 0 to 5 ‰ and from 10 ‰ to 12.5 ‰ are shown in Fig. 4. δ 15N–NO3 values of the water samples collected within and outside the NNR are different. In winter, the δ 15N–NO3 values of the samples from the NNR range from −5.1 ‰ to 5.2 ‰ and those of the samples from the outside area of the NNR range from 1.2 ‰ to 11.2 ‰. In summer, however, the δ 15N–NO3 values of the samples from the NNR range from −8.6 ‰ to 5.23 ‰ and those of the samples from the outside area of the NNR range from 1.34 ‰ to 11.27 ‰.
Distribution of δ 15N–NO3 − values of water samples from epikarst springs in the Jinfo Mountain area. The shadowed and open histograms show the frequency of δ 15N–NO3 − values for springs within the NNR and outside the NNR. Solid rectangles show the δ 15N–NO3 − values of possible nitrate sources obtained from Freyer (1978), Kreitler (1979), Mariotti (1983), Heaton (1986), Aravena et al. (1993) and BiShlke et al. (1997)
Discussion
Hydrochemical characteristics and controlling factors of epikarst springs
The hydrochemistry of groundwater significantly influences the nitrogen cycle in groundwater systems; hence, understanding the chemical composition of waters is helpful in studies of the fate of nitrate contaminant within shallow karst aquifers. The hydrochemical characteristics of epikarst springs are mainly controlled by the interaction of water with soil minerals and host rock, but human activities can also affect the hydrochemistry of epikarst water (Zhu and Qian 2005). The hydrochemistry of these epikarst spring waters was controlled by the interaction of water–carbonate rock that results in high Ca2+ and HCO3 − concentrations in waters, taking more than 50 % of the total dissolved ions (Yuan et al. 2002; Ford and Williams 2007). Understanding the control mechanism of natural chemical composition in groundwater is very important for distinction of anthropogenic contaminant. In general, SO4 2− and HCO3 − together accounted for 80 % to 95 % of the total anions while Ca2+ and Mg2+ dominated the cation concentrations of the spring waters, accounting for more than 80 % of the total cation concentrations (Xiao et al. 2015). Therefore, the hydrochemical water type for these epikarst springs was Ca–HCO3, suggesting that the weathering of carbonate host rocks primarily controls the natural chemistry of waters. The concentration of Cl− is 2.75–12.39 mg/L in winter and 0.1–14.39 mg/L in summer, which exceeds the national drinking water threshold (2.5 mg/L). Otherwise, the concentration of SO4 2− is 9.2–62.2 mg/L in winter and 12.52–52.12 mg/L in summer, which is lower than the national drinking water threshold.
Nitrate contamination of epikarst springs
Although nitrate concentrations higher than 3 mg/L can be assumed to be a result of anthropogenic sources (Babiker et al. 2004), the baseline of nitrate concentration in groundwater will be different depending on geological setting and environmental conditions. Normally, groundwater away from human influence can be used to represent the regional nitrate baseline. In the 1970s, the nitrate concentration in most shallow groundwater in the study area was lower than 3 mg/L (Xiao et al. 2015).
At present, only seven springs in winter (Nos. J04, J08, J09, J10, J12, J13 and J15) and three springs in summer (Nos. J4, J10 and J12) have nitrate concentrations (ca 0–2.95 mg/L) lower than the regional baseline. This indicates that the rapid development of local communities has significantly increased the regional nitrate background values in the shallow groundwater system (Fig. 3). This is supported by the fact that the water samples collected inside the NNR in the Mt. Jinfo have much lower nitrate concentrations than those collected outside the NNR.
Table 2 shows the nitrate contaminant distribution status, showing that only three water samples (J01, J16 and J19) in winter and five water samples (J01, J06, J16, J18 and J19) in summer have high nitrate concentrations exceeding the national drinking water threshold in winter, accounting for nearly half of all water samples from the outside area of the NNR. The nitrate concentrations of springs inside the NNR are all below the national drinking water standard, much lower than that of springs outside the NNR. Such difference may be attributed to the absence of anthropogenic activities inside the NNR. In addition, large areas of vegetation coverage inside the NNR may contribute to phytoremediation of nitrate contamination (Cunningham and Ow 1996), uptaking the excess nitrate and, in turn, reducing the nitrate concentration in groundwater and soil systems. The absence of nitrate contamination in springs inside the NNR also suggests that the establishment of the NNR is a useful means for protecting the regional groundwater environment.
The origin of nitrate source
Denitrification is a common process within the soil and shallow aquifers. The process involves bacteria that can use the nitrate as a terminal electron acceptor and convert it to gaseous nitrogen (see Eq. 1).
Denitrification may proceed through the action of both heterotrophic and autotrophic bacteria. If pyrite is present in the aquifer rock, the reaction is mediated by the autotrophic bacteria Thiobacillus denitrificans (see Eq. 2; Kölle et al. 1985).
During the processes of autotrophic denitrification (Eq. 2), the nitrate acts as the electron acceptor while the pyrite acts as the electron donor. Thus, the presence of Fe2+ and SO4 2− is commonly linked to a decrease in NO3 −, suggesting that the NO3 − reduction is related to pyrite oxidation in groundwater (Pauwels et al. 2000, 2001). Concentrations of NO3 −, SO4 2− and ∑Fe2+ on January 2009 are shown in Fig. 5. According to Eq. 2, NO3 − concentration should be inversely proportional to SO4 2− and ∑Fe2+, but there is no clear increase of SO4 2− and ∑Fe2+ concentrations with the decrease in NO3 − concentration (Fig. 5a, b). Conversely, a positive correlation between NO3 − and SO4 2− concentrations is revealed (Fig. 5b). The reason could be ascribed to the dilution of nitrate-contaminated water with an unpolluted end member that has low nitrate concentration. Despite these, denitrification can lead to a 15N enrichment of the residual NO3 −, resulting in the increase of δ 15N but decrease of NO3 - concentration (inverse correlation between δ 15N and NO3 − concentration). For the epikarst spring water in the Mt. Jinfo area, the δ 15N–NO3 − value is positively correlated with the NO3 − concentration (Fig. 6). This indicates that denitrification has no or less effects on the δ 15N–NO3 − variation in the epikarst spring water in the Mt. Jinfo area.
The previous literature has published a number of δ 15N values or ranges of major nitrogen sources, including atmospheric N2, rainwater, soil organic nitrogen, natural nitrate minerals, synthetic chemical fertilisers and animal manure. As the standard, the δ 15N values of N2 are considered constantly equal to 0 ‰ (Mariotti 1983) and nitrate and ammonium in fertilisers are derived by industrial fixation of atmospheric nitrogen and have δ 15N values closed to 0 ‰ (Heaton 1986). Normally, the δ 15N values of chemical fertilisers range from −4 ‰ to ±4 ‰, which are very similar to those of natural nitrate minerals (−4.9 ‰ ± 4.1 ‰; Heaton 1986; BiShlke et al. 1997). The typical δ 15N–NO3 values of rainwater range from −12 ‰ to ±2 ‰ with a weighted mean value of −3 ‰ (Freyer 1978; Heaton 1986), while the δ 15N–NO3 − values of mineralised soil organic nitrogen and animal manure/sewage range from 2 ‰ to 9 ‰ and from 4 ‰ to 25 ‰, respectively (Kreitler 1979; Heaton 1986; Aravena et al. 1993). The δ 15N–NO3 values of water from the Mt. Jinfo area have a wide distribution interval, indicating that the nitrate in epikarst springs may have various sources. In addition, dilution of the polluted groundwater and natural denitrification that happened during the infiltrating processes can lead to an isotopic shift, which will make the tracing of nitrate sources more difficult.
Figure 6 shows the correlation between δ 15N–NO3 − value and nitrate concentration. This correlation between δ 15N–NO3 − value and nitrate concentration demonstrates that the regional nitrate is from three main sources: nitrogen fertiliser which has δ 15N–NO3 − values ranging from −4 ‰ to 4 ‰, mineralised soil organic nitrogen which has δ 15N–NO3 − values ranging from 2 ‰ to 9 ‰ and animal wastes/sewage which has δ 15N–NO3 − values ranging from 4 ‰ to 25 ‰. However, the springs with high nitrate concentrations always have the δ 15N–NO3 − values distributed in the range of −4 ‰ to 4 ‰ and 4 ‰ to 25 ‰, indicating that fertiliser and animal wastes/sewage are the main nitrate contamination. This is consistent with the land use and human activities in the catchments of individual springs.
Water sample J01 has high nitrate concentrations of 21.16 mg/L in winter and 17.16 mg/L in summer, but low δ 15N–NO3 − values of −1.8 ‰ in winter and 2.3 ‰ in summer, falling in the δ 15N–NO3 − range of synthetic chemical fertilisers. This spring J01 is located near the Nanchuan City, far from the NNR, and land use is dominated by agricultural land (mainly planting corn) in which chemical nitrogen fertilisers (e.g. urea) are widely used (annually ∼48 g/m2). Therefore, the high nitrate concentration is probably related to the fertilising activities in the agricultural land and the nitrate in spring J01 is probably derived from the chemical nitrogen fertilisers (Fig. 6).
Nitrate concentrations of sample J19 are also high (28.24 mg/L in winter and 32.78 mg/L in summer), and the value of δ 15N–NO3 − is 11.2 ‰; sample J16 had the highest nitrate concentration among all water samples (35.49 mg/L in winter and 46.1 8 mg/L in summer, Table 1). And the value of δ 15N–NO3 − of J16 is 10.8 ‰ in winter and 11.27 ‰ in summer. The main land use in the catchments of springs J16 and J19 is paddy rice farming land. Also, these catchments have some restaurants and small-scale animal husbandry and poultry farming. It is assumed that chemical nitrogen fertiliser and animal wastes/sewage are the main sources of nitrate contaminants in these springs. And the concentration of Cl− and SO4 2− of springs J16 and J19 proved this consistent. The concentration of Cl− of J16 is 12.04 mg/L in winter and 11.31 mg/L in summer, while the concentration of SO4 2− is 16.95 mg/L in winter and 18.14 mg/L in summer. For J19, the concentration of Cl− is 2.75 mg/L in winter and 14.39 mg/L in summer and that of SO4 2− is 37.61 mg/L in winter and 48.61 mg/L in summer. Springs J16 and J19 are located outside the NNR, and the dominant land use in their catchments is significantly influenced by urban industrial activities, including the restaurant industry and small-scale animal husbandry and poultry farming. These untreated animal manure and sewage were unreasonably disposed directly into the catchments of springs J16 and J19, resulting in the nitrate contamination of spring water.
Nitrate concentrations of samples J06 (c:6.02 mg/L in winter and 10.50 mg/L in summer) and J18 (c: 5.63 mg/L in winter and 12.91 mg/L in summer) also exceed the national drinking water standards in summer. And the δ 15N–NO3 − value of J06 is 4.5 ‰ in winter and 3.44 ‰ in summer, and that of J18 is 4.1 ‰ in winter and 2.1 ‰ in summer. Both of the two springs had the higher nitrate concentration and lower δ 15N–NO3 − value in summer, combined with sample J06 and J18 plot in the mixing of fertiliser contamination part and mineralised soil organic nitrogen part. So, the nitrate of these springs may have a contribution of chemical nitrogen fertilisers in summer.
Epikarst spring is the main source of drinking water for local communities in the Mt. Jinfo area. Due to the intrinsic vulnerability of karst groundwater systems, there are greater risks to environmental and human health (Yuan et al. 1993). The soil in the karst area is thin and has discontinuous contribution and with many fractures and sinkholes. The contamination produced by human activities may easily flow into the karst aquifer through the sinkholes by rainfall or surface flow. Nitrate concentration of epikarst springs is influenced by many factors, such as acid rain and fertiliser. With rapid economic growth and population increase, land use has become one of the most important influences on nitrate concentration of epikarst springs. Agricultural irrigation, industrial waste and domestic waste have largely contributed to the nitrate contamination of springs. Concentrations of nitrate have shown notable increases as a result of the application of large amounts of chemical fertilisers used in agriculture (Compton and Boone 2000; Jiang et al. 2008). Waste water and waste residues produced by the construction of factories and homes cause an increase in nitrogen (Wakida and Lerner 2006). In the Mt. Jinfo area, nitrate concentrations of all springs inside the NNR and several springs (J15 and J11) outside the NNR do not exceed the regional baseline and can be considered the unpolluted end member. And the δ 15N–NO3 − values of these samples indicate that their nitrite source is atmospheric precipitation. Springs near the NNR have nitrate concentrations higher compared to springs in the NNR but still below the national limits for drinking water. The greater the distance from the NNR, the greater the contamination by nitrates, and three epikarst springs in winter and five epikarst springs in summer exceeded the national limits: an over-limit ratio of 245.9 % in winter and 361.8 % in summer, which may be attributed to changes in land use changed from forest to farmland or urbanisation and the increase in associated human activity. In particular, the land use is almost forest inside the NNR while the main land use is farmland outside the NNR, and the ecotourism and forestry were developed inside the NNR while the traditional agriculture and industry outside the NNR, which are the main influences of the nitrate concentration of epikarst springs of the Mt. Jinfo area.
Conclusion
The analyses of water hydrochemistry combined with the land use type in the catchments of epikarst springs give an insight into sources and distributions of nitrate contaminants in this study area. According to data analyses and discussion, we draw the following conclusions:
-
1.
The hydrochemistry of epikarst spring waters in the Mt. Jinfo area is controlled by the dissolution of host carbonate rocks and shows a Ca·Mg–HCO3 type. Springs within the NNR have low nitrate contamination, whereas most springs outside the NNR have suffered from nitrate contamination, suggesting that the establishment of the NNR has prevented the epikarst groundwater from anthropogenic nitrate contamination.
-
2.
Nitrogen isotope compositions of nitrate suggest that the main sources of nitrate contaminants in epikarst springs in areas outside the NNR are chemical nitrogen fertilisers and animal manure/sewage. The origin of nitrate contaminates is associated with the land use in the recharge catchment of individual epikarst springs. The influencing factors of the nitrate concentration in springs from the Mt. Jinfo area is mainly land use and human activity. Epikarst springs inside the NNR covered by forest have lower nitrate concentration, while springs outside the NNR with the cover of farmland and traditional agriculture and industry have higher nitrate concentration.
References
Aravena R, Evans ML, Cherry JA (1993) Stable isotopes of oxygen and nitrogen in source identification of nitrate from septic system. Ground Water 31(2):180–186
Babiker IS, Mohamed MAA, Terao H, Kato K, Ohta K (2004) Assessment of groundwater contamination by nitrate leaching from intensive vegetable cultivation using geographical information system. Environ Int 29(8):1009–1017
BiShlke JK, Ericksen GE, Revesz K (1997) Stable isotope evidence for an atmospheric origin of desert nitrate deposits in northern Chile and southern California, U.S.A. Chem Geol 136:135–152
Boyer DG, Pasquarell GC (1995) Nitrate concentrations in karst springs in an extensively grazed area. Water Resour Bull 1(4):29–36
Chen J, Tang C, Sakura Y, Yu J, Fukushima Y (2005) Nitrate pollution from agriculture in different hydrogeological zones of the regional groundwater flow system in the North China Plain. Hydrogeol J 13:481–492
Compton JE, Boone RD (2000) Long-term impacts of agriculture on soil carbon and nitrogen in New England forests. Ecology 81(8):2314–2330
Cunningham SD, Ow DW (1996) Promises and prospects of phytoremediation. Plant Physiol 110:715–719
Debernardi L, Luca DAD, Lasagna M (2008) Correlation between nitrate concentration in groundwater and parameters affecting aquifer intrinsic vulnerability. Environ Geol 55:539–558
Ford DC, Williams PW (2007) Karst hydrogeology and geomorphology. John Wiley and Sons, Chichester, 562 p
Freyer HD (1978) Seasonal trends of NH4 + and NO3 − nitrogen isotope composition in rain collected at Jülich, Germany. Tellus 30:83–92
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle, recent trends, questions, and potential solutions. Science 320:889–892
Gao Y, Li H, Shen L, Yang P (2008) Geochemical characteristics and spatial distribution of spring water at Mt. Jinfo, Chongqing. Geol China 35(2):322–331
Gormly JR, Spalding RJ (1979) Sources and concentrations of nitrate nitrogen in groundwater of the Central Platte Region, Nebraska. Ground Water 17:291–301
Guo F, Jiang GH, Yuan DX (2007) Major ions in typical subterranean rivers and their anthropogenic impacts in southwest karst areas, China. J Environ Geol 53(3):533–541
Gupta SK, Gupta RC, Seth AK, Gupta AB, Bassin JK, Gupta A (2000) Methaemoglobinaemia in areas with high nitrate concentration in drinking water. Natl Med J India 13(2):58–61
Hauck R, Bartholomew W, Bremner J et al (1972) Use of variations in natural nitrogen isotope abundance for environmental studies: a questionable approach. Science 177:453–454
Heaton THE (1986) Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: a review. Chem Geol 59(1):87–102
Jia YL, Yuan DX (2003) The impact of land use change on karst water in Shuicheng basin of Guizhou province. Acta Geograph Sin 58(6):831–838
Jiang YJ, Yuan DX, Zhang C, Zhang G, He R (2008) Impact of land use change on groundwater quality in a typical karst watershed of Southwest China. Hydrogeol J 16(4):727–735
Kohl DH, Shearer G, Commoner B (1971) Fertilizer nitrogen: contribution to nitrate in surface in a corn belt watershed. Science 174:1331–1334
Kohl DH, Commoner B, Shearer GB (1972) Use of variations in natural nitrogen isotope abundance for environmental studies—a questionable approach. Science 177:453–456
Kölle W, Strebel O, Böttcher J (1985) Formation of sulfate by microbial denitrification in a reducing aquifer. Water Supply 3:35–40
Kreitler C W (1975) Determining the source of nitrate in groundwater by nitrogen isotope studies. In: Report of investigation 83. Austin, TX: Bureau of Economic Geology, University of Texas at Austin pp. 57
Kreitler CW (1979) Nitrogen-isotope ratio studies of soils and groundwater nitrate from alluvial fan aquifers in Texas. J Hydrol 42(1–2):147–170
Kreitler CW, Browning LA (1983) Nitrogen-isotope analysis of groundwater nitrate in carbonate aquifers: natural sources versus human pollution. J Hydrol 61:285–301
Lundberg J O, Weitzberg E, Cole JA, Benjamin N (2004) Nitrate, bacteria and human health. Nat Rev Microbiol 2:593–602
Mariotti A (1983) Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature 303:685–687
Mariotti A, Letolle R (1977) Application de l’etude isotopique de l’azote en hydrologieet en hydrogeologie-analyse des resultats obtenus sur un exemple precis: Le bassin de melarchez (seine-et-marne, France). J Hydrol 33:157–172
Mensinga TT, Speijers GJA, Meulenbelt J (2003) Health implications of exposure to environmental nitrogenous compounds. Toxicol Rev 22(1):41–51
Ministry of Health of the People’s Republic of China (MOH), Standardization Administration of the People’s Republic of China (SAC) (2006) National drinking water standard of People’s Republic of China. GB_5749-2006. (in Chinese)
Pannala AS, Mani AR, Spencer JPE, Skinner V, Bruckdorfer KR, Moore KP, Rice-Evans CA (2003) The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med 34:576–584
Parslow RC, McKinney PA, Law GR, Staines A, Williams R, Bodansky HJ (1997) Incidence of childhood diabetes mellitus in Yorkshire, Northern England, is associated with nitrate in drinking water, an ecological analysis. Diabetologia 40(5):550–556
Pauwels H, Foucher J, Kloppmann W (2000) Denitrification and mixing in a schist aquifer, influence on water chemistry and isotopes. Chem Geol 168:307–324
Pauwels H, Lachassagne P, Bordenave P, Foucher J, Martelat A (2001) Temporal variability of nitrate concentration in a schist aquifer and transfer to surface waters. Appl Geochem 16:583–596
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Am Geophys Union 25(6):914–928
Silva SR, Kendall C, Wilkison DH, Ziegler AC, Chang CCY, Avanzino RJ (2000) A new method for collection of nitrate from fresh water and the analysis of nitrogen and oxygen isotope ratios. J Hydrol 228:22–36
Speijers G J A, van den Brandt P A (2003) Nitrate (and potential endogenous formation of N-nitroso compounds). WHO Food Additives Series 50. Available http://www.inchem.org/documents/jecfa/jecmono/v50je06.htm
Spiegelhalder B, Eisenbrand G, Preussmann R (1976) Influence of dietary nitrate on nitrite content of human saliva, possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol 14:545–548
Vitousek PM, Naylor R, Crews T, David MB, Drinkwater LE, Holland E, Johnes PJ, Katzenberger J, Martinelli LA, Matson PA, Nziguheba G, Ojima D, Palm CA, Robertson GP, Sanchez PA, Townsend AR, Zhang F (2009) Nutrient imbalances in agricultural development. Science 324:1519–1520
Wakida FT, Lerner DN (2006) Potential nitrate leaching to groundwater from house building. Hydrol Process 20:2077–2081
Wang B, Wang Y (1990) National key scenic spots—Jingfo Mountain. Sichuan Scientific and Technology, Chengdu, 129p. (in Chinese)
Wolfe AH, Patz JA (2002) Reactive nitrogen and human health, acute and long-term implications. AMBIO 31(2):120–125
Xiao Q, Shen LC, Wu KY (2015) Hydrochemical variations of the springs of Jinfo Mountain, Chongqing, China. Acta Carsologica 44(1):59–69
Yuan D, Zhu D, Weng J, Zhu X, Han X, Wang X, Cai G, Zhu Y, Cui G, Deng Z (1993) Karstology in China. Geological, Beijing (in Chinese)
Yuan D, Liu Z, Lin Y, Shen J, He S, Xu S, Yang L, Li B, Qing J, Cai W, Cao J, Zhang M, Jiang Z, Zhao J (2002) Karst dynamic system of China. Geological Publish House, Beijing, 275p. (in Chinese)
Zhang C, Yuan DX (2004) Hydrochemical variation of typical karst subterranean basin and its relationship with landuse change. J Soil Water Conserv 18(5):134–137
Zhang X, Xu Z, Sun X, Dong W, Ballantine D (2012) Nitrate in shallow groundwater in typical agricultural and forest ecosystems in China, 2004-2010. J Environ Sci 25(5):1007–1014
Zhu XY, Qian XM (2005) Groundwater hydrology. China Environmental Science, Beijing, pp 68–110
Acknowledgments
The authors are grateful to the team of Institute of Karst Environment and Rock Desertification Rehabilitation, Southwest University, for their assistance in the water sampling and analysis as well as their useful suggestions. This study was supported by the National Natural Science Foundation of China (Grant No. 41302213) and the Natural Science Foundation of Guangxi Zhuang Autonomous Region (Grant No. 2013GXNSFBA019221).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, Q., Wu, K. & Shen, L. Nitrate fate and origin in epikarst springs in Jinfo Mountain area, Southwest China. Arab J Geosci 9, 483 (2016). https://doi.org/10.1007/s12517-016-2510-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-016-2510-y