Abstract
Ammonia is one of the most common aquatic pollutants. To analyze the effect of ammonia exposure on the glutathione redox system, we investigated the levels of hydrogen peroxide (H2O2) and glutathione, and transcription and activities of glutathione-related enzymes in liver and gills of FFRC strain common carp (Cyprinus carpio L.) exposed to 0, 10, 20, and 30 mg/L of ammonia. The results showed that H2O2 content reached a maximum level at 48 h of exposure in the liver of fish. In gills, H2O2 increased rapidly at 6 h and reached to maximum levels at 24 h of exposure, indicating that gills experienced oxidative stress earlier than the liver of fish exposed to ammonia. Reduced glutathione (GSH) content and reduced glutathione/oxidized glutathione (GSH/GSSG) ratio increased significantly within 24 h of exposure. Meanwhile, the transcription and activities of glutathione S-transferase (GST) and glutathione reductase (GR) increased significantly in the liver, and glutathione peroxidase (GSH-Px) and GST increased in the gills of fish exposed to ammonia. Malondialdehyde (MDA) content kept at a low level after exposure to low concentration of ammonia, but increased significantly after exposure to 30 mg/L ammonia for 48 h along with a decrease in GSH content and GSH/GSSG ratio. These data showed that the glutathione redox system played an important role in protection against ammonia-induced oxidative stress in the liver and gills of FFRC strain common carp, though the defense capacity was not able to completely prevent oxidative damage occurring after exposure to higher concentration of ammonia. This research systematically studied the response of the glutathione redox system to ammonia stress and would provide novel information for a better understanding of the adaptive mechanisms of fish to environmental stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In aquaculture systems, ammonia is one of the major environmental pollutants of concern. It has been reported that the concentration of ammonia increased directly with culture period, and might reach as high as 46 mg/L in intensive aquaculture systems (Cheng et al. 2015). High concentration of ammonia can cause growth reduction, histological damage, immune suppression, and high mortality (Qi et al. 2017; Liang et al. 2018). Exogenous ammonia enters into fish mainly through gills, and is then transported through the blood to the liver and other organs. One of the known mechanisms of ammonia toxicity is the induction of oxidative stress, through increasing the concentration of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radical (HO·), and superoxide radical (O2·−) (Murthy et al. 2001; Rama and Manjabhat 2014). Overproduction of ROS in cells can result in the oxidation of proteins, DNA, and lipids, eventually leading to cell death (Martinez-Alvarez et al. 2005; Hegazi et al. 2010). To cope with oxidative stress and keep cellular redox state in balance, aquatic organisms evolved both non-enzymatic and enzymatic antioxidant defense system to convert ROS to harmless metabolites (Sinha et al. 2015; Ramirez-Duarte et al. 2016). It has been reported that ammonia exposure can alter activities of antioxidant enzymes to prevent oxidative damage in the gills, muscle, liver, and brain of fish (Qi et al. 2017; Hegazi et al. 2010; Lorenzo et al. 2017). The induction of antioxidant defense is thought to be a protective reaction against ammonia stress in fish, but the exact defense mechanism is not clear.
The glutathione redox system, including glutathione, glutathione peroxidase (GSH-Px), glutathione S-transferases (GST), and glutathione reductase (GR), plays important roles in maintaining cellular redox homeostasis and protecting cells from oxidative damage during environmental stress (Lesser 2011; Lin et al. 2018). Glutathione, including reduced glutathione (GSH) and oxidized glutathione (GSSG), is an effective non-enzymatic antioxidant that can modulate the redox status of protein thiols, and directly scavenge singlet oxygen and hydroxyl radicals (Li et al. 2003; Al-Ghais 2013; Xia and Wu 2018). GSH-Px detoxifies the cytosolic H2O2 and hydroperoxides using GSH as a cofactor (Arthur 2001; Srikanth et al. 2013). GST functions to detoxify xenobiotics or lipid peroxidation end products by conjugation GSH (Comakli et al. 2011). GR is a NADPH-dependent oxidoreductase which can catalyze the conversion of GSSG to GSH (Lin et al. 2018). The dynamic balance between GSH and GSSG is important for ROS detoxification and cellular survival, and GSH/GSSG ratio has been widely used as an indicator of cellular redox status (Sies 1999). Previous studies have reported that glutathione and other glutathione-related enzymes were involved in the detoxification of harmful ROS in ammonia-exposed fish (Sinha et al. 2014). However, there are no detailed studies on the molecular patterns of these antioxidative enzymes in fish under ammonia stress.
FFRC strain common carp, a new strain of Cyprinus carpio with the advantages of fast growth, good body shape, strong adaptability, and stable genetic traits, has been cultured widely in many provinces of China (Dong 2011). However, the stress response of FFRC strain common carp to environmental pollutants has not been studied. In this study, a comprehensive analysis of the glutathione redox system, including glutathione, GSH-Px, GST, and GR, was conducted in the gills and liver to understand the antioxidant response of FFRC strain common carp to ammonia pollution. These results will offer a better insight into the mechanism of fish adaption to ammonia.
Materials and methods
Animals
FFRC strain common carps (50 ± 0.16 g body mass, 1 month old) were obtained from a fish farm in Qinxian (Shanxi, China), and acclimated for 2 weeks in cycling-filtered plastic tanks containing continuously circulating dechlorinated tap water at 25 °C (pH 7.5; dissolved oxygen 6.0–7.0 mg/L) with natural light and photoperiod. The fish were fed twice a day with commercial pellets, and were fasted for 24 h prior to experimentation.
Experimental design and sample collection
Ammonium chloride (NH4Cl) was used as a source of the total ammonia-nitrogen (T-AN). Based on the value of 96 h median lethal concentration (96 h LC50, T-AN: 35.6 mg/L) established by our group, test concentrations of ammonia were 0 (control), 10 (low), 20 (middle), and 30 (high) mg/L. The exposure was conducted for 48 h with three replicates of four treatments (total of twelve tanks). Twenty fish were selected randomly and placed in a 150L plastic tank containing 100 L of test solution. Ammonia concentration was measured by nesslerization (Hegazi et al. 2010) every 6 h and adjusted by adding the calculated amount of the NH4Cl solution. Fish were not fed during the whole experimental period. Each tank was cleaned to remove feces and approximately 50% of the water was changed daily with dechlorinated tap water containing the respective amount of ammonia.
After exposure to ammonia for 6, 24, and 48 h, three fish from each tank were randomly sampled and dissected after anesthesia with tricaine methanesulfonate MS-222 (0.2%). The gills and liver were quickly excised within 2 min on ice, and frozen in liquid nitrogen for further analysis.
In this study, all fish were administered in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (China).
Quantification of oxidative stress markers
The frozen samples were homogenized with ice-cold normal saline at a ratio of 10% (w:v). The homogenates were centrifuged at 12,000×g for 15 min at 4 °C, and the supernatants were used for determination of biochemical parameters.
H2O2 content was assessed using a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). H2O2 binds with molybdenic acid to form a complex, which was measured at 405 nm, and the content of H2O2 was then calculated.
MDA content was determined using a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the thiobarbituric acid (TBA) reactivity. Briefly, the supernatants were collected and reacted with an equal volume of 0.67% (w/v) thiobarbituric acid (TBA) in a boiling water bath for 30 min. After cooling, the mixture was centrifuged at 3000×g for 10 min. The absorbance of supernatant was measured at 532 nm and corrected for non-specific turbidity by subtracting the absorbance at 600 nm and 450 nm.
Determination of glutathione
Glutathione content was assessed using a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). For total glutathione (T-GSH), the supernatant was added to a reaction mixture containing 100 mM sodium phosphate buffer (pH 7.5), 0.6 mM 5,5-dithiobis-2-nitrobenzoic acid (DTNB), 0.25 mM NADPH, and 4 mM EDTA .The reaction was initiated by the addition of glutathione reductase and the absorbance was monitored at 412 nm. For oxidized glutathione (GSSG), the supernatant was incubated with 170 mM 2-vinylpyridine for 1 h at 30 °C to derivatize any reduced glutathione (GSH) present in the sample. GSSG in this portion was quantified using the same method. Reduced glutathione (GSH) content was calculated as the difference between the content of T-GSH and GSSH.
Enzyme assays
The activities of glutathione S-transferase (GST), glutathione peroxidase (GPX), and glutathione reductase (GR) were determined with commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions, respectively.
GR activity was measured by the method of Murshed et al. (2008). The reaction was started by adding the supernatant to an assay containing mM sodium phosphate buffer (pH 7.4), 1 mM EDTA, 0.2 mM NADPH, and 5 mM GSSG. GR activity was expressed as units per milligram of protein.
GST activity was measured by measuring the conjugation of GSH with 1-chloro-2,4-dinitrobenzene (CDNB) (Habig et al. 1974). The reaction was started by adding the supernatant to an assay containing 50 mM sodium phosphate buffer (pH 6.5), 1 mM EDTA, 5 mM GSH, and 2 mM CDNB. GST activity was expressed as units per milligram of protein.
GPX activity was measured by the method of Ahmad and Pardini (1988). The reaction was initiated by adding 0.5 mM hydrogen peroxide (H2O2) to the assay mixture of supernatant, 50 mM sodium phosphate buffer (pH 7.0), 1 mM EDTA, 2 mM NaN3, 0.2 mM NADPH, glutathione reductase, and 5 mM GSH. GPX activity was expressed as units per milligram of protein.
Protein amounts were determined by the method of Bradford (1976) with bovine serum albumin (BSA) as the standard.
Total RNA extraction and real-time PCR
Total RNA was isolated from fish tissues using a Trizol kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions and then dissolved in DEPC-treated water. The expression of glutathione S-transferase (GSTP1), glutathione peroxidase (GPX1), and glutathione reductase (GR) was analyzed by quantitative real-time PCR (qPCR). Briefly, 1 μg of total RNA was reverse transcribed into cDNA using the PrimeScriptTM RT Master Mix (TaKaRa, Dalian, China), and qPCR was carried out using the SYBR Premix Ex TaqTM II (TaKaRa, Dalian, China), with β-actin as the internal control. The primers used for qPCR were listed in Table 1. All the qPCR analyses were performed on an Applied Biosystems 7500 Real-Time PCR System. The cycling procedure for qPCR was 94 °C for 1 min, followed by 40 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min.
Three independent biological replicates and three technical replicates of each biological replicate for each sample were analyzed by qPCR. The differences in expression levels were calculated using the 2–ΔΔCT method (Schmittgen and Livak 2008).
Statistical analysis
All data were expressed as mean ± SE (standard error). Statistical analyses were performed using one-way ANOVA followed by LSD test. The significance level was set at P < 0.05.
Results
Ammonia-induced H2O2 generation and lipid peroxidation
Exposure to ammonia induced a production of H2O2 in both the liver and gills of FFRC strain common carp (Fig. 1). In the liver, H2O2 content showed no significant change after exposure to 10 mg/L ammonia, but increased gradually within 48 h of exposure to 20 and 30 mg/L ammonia (Fig. 1A). In the gills, H2O2 content increased rapidly at 6 h of ammonia exposure and reached a maximum at 24 h in all treatment groups of fish (P < 0.05), and then recovered to the control level at 48 h in the 20 and 30 mg/L groups (Fig. 1B).
MDA content significantly increased with increasing ammonia concentration and exposure duration in both the liver and gills of FFRC strain common carp (Fig. 1). In the liver, MDA content showed no significant change within 48 h of exposure to 10 and 20 mg/L ammonia, but increased significantly after exposure to 30 mg/L ammonia as a function of time (P < 0.05) (Fig. 1C). In the gills, MDA content did not have significant change within 48 h of exposure to 10 mg/L ammonia, but increased significantly after exposure to 20 and 30 mg/L ammonia for 48 h (P < 0.05) (Fig. 1D).
Ammonia-induced changes in GSH content and GSH to GSSG ratio
GSH content increased significantly within 24 h of ammonia exposure, following by a reduction at 48 h of exposure in both the liver and gills of FFRC strain common carp (Fig. 2). In the liver, GSH content increased rapidly at 6 h (P < 0.05) and remained at a high level after 24 h of ammonia exposure (P < 0.05). After exposure to ammonia for 48 h, GSH content recovered to the control level in the 10 and 20 mg/L ammonia treatment groups, but decreased significantly in the 30 mg/L treatment group (P < 0.05) (Fig. 2A). In the gills, GSH content did not have significant change during 48 h of exposure to 20 mg/L ammonia. However, a significant increase in GSH content occurred after exposure to 10 and 30 mg/L ammonia for 24 h, respectively (P < 0.05). After exposure to ammonia for 48 h, GSH content recovered to the control level in the 30 mg/L treatment group, but decreased significantly in the 10 mg/L group (P < 0.05) (Fig. 2B).
GSH/GSSG ratio displayed a comparable pattern as seen for GSH content in both the liver and gills of FFRC strain common carps (Table 2). In the liver, GSH/GSSG ratios increased significantly at 6 and 24 h of ammonia exposure (P < 0.05). However, a significant increase occurred after 24 h of ammonia exposure in the gills. After exposure to ammonia for 48 h, GSH/GSSG ratio recovered to the control level in both the liver and gills.
Ammonia-induced responses of glutathione-related enzymes
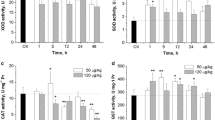
The glutathione-related enzymes in the glutathione redox system responded differently to ammonia exposure in the liver and gills of FFRC strain common carp (Fig. 3). In the liver, the activities of GST and GR showed a gradual increasing trend after exposure to 30 mg/L ammonia, but no significant change was found during exposure to 10 or 20 mg/L ammonia (Fig. 3A and E). The GSH-Px activity had no significant change in any of the treatment groups (Fig. 3C).
In the gills, GST activity increased significantly within 24 h of ammonia exposure, and then recovered to the control level at 48 h (Fig. 3B). The GSH-Px activity increased gradually and reached maximum values at 48 h of ammonia exposure (Fig. 3D). However, there were no significant changes in GR activity during the 48 h of ammonia exposure in any of the treatment groups (Fig. 3F).
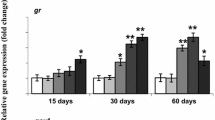
Ammonia-induced changes in the transcription levels of glutathione-related enzymes
The transcriptional levels of genes encoding for the GSH-related enzymes (GSTP1, GPX1, and GR) were analyzed at 24 h of ammonia exposure, which seemed to be the most responsive time point for most of the doses of ammonia (Fig. 4). In the liver, the expression of GSTP1 was significantly upregulated in the 30 mg/L treatment group, and the expression of GR was upregulated in a dose-dependent manner, but the expression of GPX1 was not changed during ammonia exposure. In the gills, the expression of GSTP1 was significantly higher as compared with the control in all treatment groups, and the expression of GPX1 was increased in a dose-dependent manner, while the expression of GR did not show a significant difference between the control and all the treatment groups.
Discussion
Oxidative stress is one of the toxicity mechanisms of environmental pollutants on aquatic organisms (Benli et al. 2008; Liew et al. 2013). In this study, ammonia induced significant increases in H2O2 content in the liver and gills of FFRC strain common carp and caused oxidative stress, which was manifested by an increase in MDA content. However, there were differences in cellular response to ammonia stress between the liver and gills of fish. In the gills, H2O2 content increased rapidly at 6 h, and the content of GSH, the levels of GST, and GSH-Px increased significantly during ammonia exposure. In the liver, H2O2 content enhanced after exposure to ammonia for 24 h, and GSH content increased significantly at 6 h and maintained at a high level at 24 h. The rapid increase in GSH level might be due to the fact that this peptide was synthesized in the liver and then transported to other organs of fish (Kaplowitz et al. 1985). Meanwhile, the levels of GST and GR increased gradually after exposure to high concentration of ammonia (30 mg/L). Based on the accumulation of H2O2 content and change in the glutathione redox system, it can be deduced that the gills experienced oxidative stress earlier than the liver of fish during ammonia exposure. This result was consistent with the previous report that exposure to high concentration ammonia induced oxidative stress in the gills earlier than that in the brain of the mudskipper (Ching et al. 2009). This might be due to that the gills, but not other organs, were exposed to environmental ammonia instantly under ammonia stress. Moreover, fish gills serve as the dynamic respiratory and osmoregulatory organ, and are likely to possess a high capacity to produce ROS (Sinha et al. 2015). Therefore, it is necessary to investigate the injury of gills in fish under various environmental stresses.
Ammonia exposure induces oxidative stress, and further leads to alterations in antioxidant defense system in fish (Sinha et al. 2014; Cong et al. 2018). The glutathione redox system, including GSH and glutathione-related enzymes, has been reported to provide protection against ROS in fish exposed to varied environmental pollutants (Anjum et al. 2014; Radovanovic et al. 2015). Hegazi et al. (2010) have found that glutathione-related enzymes played important roles in preventing ammonia-induced oxidative stress in the liver and white muscle of Nile tilapia juveniles. In the present study, the glutathione redox system was found to be involved in response to ammonia stress in both the liver and gills of FFRC strain common carp. The GSH level and GSH/GSSG ratio increased rapidly after exposure to ammonia. Meanwhile, the expression levels and activities of GSH-Px, GR, and GST enhanced significantly, suggesting a transcriptional activation of genes for antioxidant enzymes in response to ammonia stress. This result was different from a previous study in which the glutathione redox system was nearly unaffected in carp exposed to environmental ammonia (Sinha et al. 2014). A possible explanation for this discrepancy is that fish used in the present study is a new strain of common carp with a well-developed defense system. Present finding suggested that FFRC strain common carp might have an effective antioxidant system to deal with ammonia exposure.
GSH-Px catalyzes the reduction of H2O2 and organic hydroperoxides, and GST detoxifies metabolites from oxidative reactions (Elia et al. 2006). The strong increase in the activities of GSH-Px and GST is expected to play important roles in protection of fish against ammonia-induced oxidative stress, which might lead to a decrease in cellular GSH content. However, both the liver and gills maintained a high level of GSH during ammonia exposure (24 h). In the liver, parallel increment in GR activity provided an efficient replenishment of GSH. However, it could maintain a relatively high GSH content in spite of a lack of increment in GR activity in gills. Likewise, an increase in GSH content and GSH/GSSH ratio with a decline in GR activity was reported in the brain of mudskippers exposed to ammonia (Ching et al. 2009). It has been found that the activity of cysteine synthetase, an enzyme producing GSH in cells, was increased under acute hyperammonemic conditions (Murthy et al. 2000). Presumably, these additional routes could have contributed to the increase in GSH levels in FFRC strain common carp exposed to ammonia.
MDA, an end product of lipid peroxidation, is a marker of radical-induced tissue damage (Papadimitriou and Loumbourdis 2002; Del Rio et al. 2005; Lushchak 2011). The content of MDA showed no significant change in both the liver and gills during exposure to 10 mg/L ammonia, which may be due to the antioxidant defense of the glutathione redox system to ammonia-induced oxidative stress. However, after exposure to 30 mg/L ammonia for 48 h, MDA content increased significantly along with the decrease in GSH content and GSH/GSSG ratio. It is possible that an increase in both GSH-Px and GST activities continuously utilized the GSH to scavenge ROS, leading to a depleted GSH pool, resulting in oxidative damage in the liver and gills of FFRC strain common carp. These data showed that the glutathione redox system could be induced under ammonia stress, but the antioxidant response was not sufficient to prevent oxidative damage from the increasing ammonia concentrations.
In conclusion, this is a systematical study which provided a particular insight into the change in H2O2 production and the response of the glutathione redox system in the liver and gills of FFRC strain common carp under ammonia stress. Ammonia induced fine-tuning in the levels of GSH, and the transcription and activities of glutathione-related enzymes, which played important roles in scavenging H2O2 and preventing lipid peroxidation during exposure to low concentration of ammonia. However, high concentration of ammonia disrupted the glutathione redox system, and the GSH pool and the glutathione-related enzymes could not fully counteract ammonia-induced oxidative damage in both the liver and gills of fish. The glutathione redox system may play an important role in the defense against ammonia stress in the new strain of common carp.
References
Ahmad S, Pardini RS (1988) Evidence for the presence of glutathione peroxidase activity towards an organic hydroperoxide in larvae of the cabbahe looper moth, Trichoplusia ni. Insect Biochem 18:861–866
Al-Ghais SM (2013) Acetylcholinesterase, glutathione and hepatosomatic index aspotential biomarkers of sewage pollution and depuration in fish. Mar Pollut Bull 74:183–186
Anjum NA, Srikanth K, Mohmood I, Sayeed I, Trindade T, Duarte AC, Pereira E, Ahmad I (2014) Brain glutathione redox system significance for the control of silica-coated magnetite nanoparticles with or without mercury co-exposures mediated oxidative stress in European eel (Anguilla anguilla L.). Environ Sci Pollut Res 21:7746–7756
Arthur JR (2001) The glutathione peroxidases. CMLS Cell. Mol Life Sci 57:1825–1835
Benli ACK, Koksal G, Ozkul A (2008) Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): effects on gill, liver and kidney histology. Chemosphere 72:1355–1358
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantity of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cheng CH, Yang FF, Ling RZ, Liao SA, Miao YT, Ye CX, Wang AL (2015) Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat Toxicol 164:61e71
Ching B, Chew SF, Wong WP, Yuen K, Ip YK (2009) Environmental ammonia exposure induces oxidative stress in gills and brain of Boleophthalmus boddarti (mudskipper). Aquat Toxicol 95:203–212
Comakli V, Ciftci M, Kufrevioglu O (2011) Purification of glutathione S-transferase enzyme from rainbow trout erythrocytes and examination of the effects of certain antibiotics on enzyme activity. Hacettepe J Biol Chem 39:413–419
Cong M, Wu H, Cao T, Lv J, Wang Q, Ji C, Zhao J (2018) Digital gene expression analysis in the gills of Ruditapes philippinarum exposed to short- and long-term exposures of ammonia nitrogen. Aquat Toxicol 194:121–131
Del Rio D, Stewart AJ, Pellegrini N (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 15:316–328
Dong ZJ (2011) FFRC strain common carp breeding and cultivation technology comparison test. Sci Fish 6:41–42 (in Chinese)
Elia AC, Anastasi V, Dorr AJM (2006) Hepatic antioxidant enzymes and total glutathione of Cyprinus carpio exposed to three disinfectants, chlorine dioxide, sodium hypochlorite and peracetic acid for superficial water potabilization. Chemosphere 64:1633–1641
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hegazi MM, Attia MM, Ashour MM (2010) Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat Toxicol 99:118–125
Kaplowitz N, Aw TY, Ookhtens M (1985) The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol 25:715–744
Lesser MP (2011) Oxidative stress in tropical marine ecosystems. Oxidative Stress in Aquatic Ecosystems. John Willey & Sons, Itd, pp 7–19
Li Y, Hugenholtz J, Abee J, Molenaar D (2003) Glutathione protects Lactococcus lactis against oxidative stress. Appl Environ Microbiol 69:5739–5745
Liang Y, Lu X, Min Y, Liu L, Yang J (2018) Interactive effects of microcystin and ammonia on the reproductive performance and phenotypic traits of the rotifer Brachionus calyciflorus. Ecotoxicol Environ Saf 147:413–422
Liew HJ, Sinha AK, Nawata CM, Blust R, Wood CM, De Boeck G (2013) Differential responses in ammonia excretion, sodium fluxes and gill permeability explain different sensitivities to acute high environmental ammonia in three freshwater teleosts. Aquat Toxicol 126:63–76
Lin Y, Miao LH, Pan WJ, Huang X, Dengua JM, Zhang WX, Ge XP, Liu B, Ren MC, Zhou QL, Xie J, Pan LK (2018) Effect of nitrite exposure on the antioxidant enzymes and glutathione system in the liver of bighead carp, Aristichthys nobilis. Fish Shellfish Immunol 76:126–132
Lorenzo TD, Melita M, Cifoni M, Galassi DMP, Iannucci DMP, Biricolti S, Gori M, Baratti M (2017) Effect of ammonia on the gene expression levels of the freshwater cyclopoid Eucyclops serrulatus. Environ Toxicol Pharmacol 51:138–141
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Martinez-Alvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15:75–88
Murshed R, Lopez-Lauri F, Sallanon H (2008) Microplate quantification of enzymes of the plant ascorbate-glutathione cycle. Anal Biochem 383:320–322
Murthy CR, Bender AS, Dombro R, Bai G, Norenberg MD (2000) Elevation of glutathione levels by ammonium ions in primary cultures of rat astrocytes. Neurochem Int 37:255–268
Murthy CRK, Rama Rao KV, Bai G, Norenberg MD (2001) Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res 66:282–288
Papadimitriou E, Loumbourdis NS (2002) Exposure of the frog Rana ridibunda to copper: impact on two biomarkers, lipid peroxidation, and glutathione. Bull Environ Contam Toxicol 69:885–891
Qi XZ, Xue MY, Yang SB, Zha SB, Wang GX, Ling F (2017) Ammonia exposure alters the expression of immune-related and antioxidant enzymes-related genes and the gut microbial community of crucian carp (Carassius auratus). Fish Shellfish Immunol 70:485–492
Radovanovic TB, Prokic MD, Gavric JP, Despotovic SG, Gavrilovic BR, Borkovic-Mitic SS, Pavlovic SZ, Saicic ZS (2015) Glutathione-dependent enzyme activities and concentrations of glutathione, vitamin E and sulfhydryl groups in barbel (Barbus barbus) and its intestinal parasite Pomphorhynchus laevis (Acanthocephala). Ecol Indic 54:31–38
Rama S, Manjabhat SN (2014) Protective effect of shrimp carotenoids against ammonia stress in common carp, Cyprinus carpio. Ecotoxicol Environ Saf 107:207–213
Ramirez-Duarte WF, Jin J, Kurobe T, Teh SJ (2016) Effects of prolonged exposure to low pH on enzymatic and non-enzymatic antioxidants in Japanese Medaka (Oryzias latipes). Sci Total Environ 568:26–32
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921
Sinha AK, AbdElgawad H, Giblen T, Zinta G, De Rop M, Asard H, Blust R, De Boeck G (2014) Anti-oxidative defences are modulated differentially in three freshwater teleosts in response to ammonia-induced oxidative stress. PLoS ONE 9:e95319
Sinha AK, Zinta G, AbdElgawad H, Asard H, Blust R, De Boeck G (2015) High environmental ammonia elicits differential oxidative stress and antioxidant responses in five different organs of a model estuarine teleost (Dicentrarchus labrax). Comp Biochem Physiol C 174-175:21–31
Srikanth K, Pereira E, Duarte AC, Ahmad I (2013) Glutathione and its dependent enzymes’ modulatory responses to toxic metals and metalloids in fish—a review. Environ Sci Pollut Res 20:2133–2149
Xia Z, Wu S (2018) Effects of glutathione on the survival, growth performance and non-specific immunity of white shrimps (Litopenaeus vannamei). Fish Shellfish Immunol 73:141–144
Funding
This work was financially supported by the Doctoral Startup and Research Fund of Jinzhong University (grant no. 1000107), the China Postdoctoral Science Founded Project (grant no. 2014M551056), and the Natural Science Foundation for Young Scientists of Shanxi Province (grant no. 2013021022-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Responsible editor: Markus Hecker
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, LH., Qi, HX. Effect of acute ammonia exposure on the glutathione redox system in FFRC strain common carp (Cyprinus carpio L.). Environ Sci Pollut Res 26, 27023–27031 (2019). https://doi.org/10.1007/s11356-019-05895-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05895-4